Abstract
Background
Gender can influence post-infarction cardiac remodeling.
Objective
To evaluate whether gender influences left ventricular (LV) remodeling and integrin-linked kinase (ILK) after myocardial infarction (MI).
Methods
Female and male Wistar rats were assigned to one of three groups: sham, moderate MI (size: 20-39% of LV area), and large MI (size: ≥40% of LV area). MI was induced by coronary occlusion, and echocardiographic analysis was performed after six weeks to evaluate MI size as well as LV morphology and function. Real-time RT-PCR and Western blot were used to quantify ILK in the myocardium.
Results
MI size was similar between genders. MI resulted in systolic dysfunction and enlargement of end-diastolic as well as end-systolic dimension of LV as a function of necrotic area size in both genders. Female rats with large MI showed a lower diastolic and systolic dilatation than the respective male rats; however, LV dysfunction was similar between genders. Gene and protein levels of ILK were increased in female rats with moderate and large infarctions, but only male rats with large infarctions showed an altered ILK mRNA level. A negative linear correlation was evident between LV dimensions and ILK expression in female rats with large MI.
Conclusions
Post-MI ILK expression is altered in a gender-specific manner, and higher ILK levels found in females may be sufficient to improve LV geometry but not LV function.
Keywords: Atrial Remodeling; Myocardial Infarction, Gender Identity; Protein-Serine Threonine Kinases/metabolism; Rats
Introduction
It is well established that premenopausal women have a lower risk of cardiovascular disease than men of the same age1. However, there are conflicting data about gender differences with regard to the adaptive response of the heart during the development of heart failure after myocardial infarction (MI). Thus, although some studies have shown worse cardiac remodeling in male than female rodents post-MI, other researchers have found no gender differences or worse cardiac remodeling in females2,3.
The female sex hormone estrogen has been the most frequently studied mechanism for gender-based left ventricular (LV) remodeling post-MI4-6. The cardiovascular system is influenced not only by estrogen but also by multiple molecular factors that may determine significant gender differences that already exist in the normal heart7. In fact, the different characteristics of the normal myocardium suggest that different molecular responses occur between males and females in response to MI. In this regard, a wealth of data supports a key role of integrin-linked kinase (ILK) in the ischemic myocardium. ILK is a critical effector in the Akt signaling pathway8,9 and has been shown to be up-regulated10 as well as to promote cardiac repair after MI11. Moreover, ILK gene therapy has been shown to improve cardiac remodeling in rats after MI and was associated with increased angiogenesis, reduced apoptosis, and increased cardiomyocyte proliferation12.
Since gender differences in post-MI LV remodeling are unclear, we conducted this study using a rat MI model with a reliable and comprehensive method to examine gender-based differences in myocardial remodeling13-15. Given that previous studies have established that ILK influences LV remodeling, we also examined whether gender affects the myocardial expression of ILK after MI.
Methods
Animals and MI surgery
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1985) as well as to the policy and UK regulations described in the Journal of Physiology16 and Ethical Principles in Animal Experimentation of the Brazilian College of Animal Experimentation (COBEA). The protocol was approved by the Institutional Research Ethics Committee of the Federal University of São Paulo, Brazil. Ten-week-old female and male Wistar rats were subjected to surgical induction of MI or sham operation. Under ketamine (50 mg/kg) plus Xylazin (10 mg/kg) anesthesia the left anterior descending coronary artery was occluded near its origin following a previously described method13-15. Sham-operated rats were treated similarly except that the ligature was not tied. Female and male rats were assigned to either a sham group, a moderate MI group (infarct size between 20-39% of LV area), or a large MI group (infarct size ≥ 40% of LV area).
Assessment of MI size, LV geometry and function
Six weeks after the surgical ligation of the coronary artery or sham operations, the animals were anesthetized as described above and measurements were performed using a 12-MHz transducer connected to a HP Sonos-5500 echocardiograph (Hewlett-Packard, California, USA) as previously described14,15. The MI size was measured by transversal 2-dimensional view of the left ventricle on the basal, midtransversal, and apical planes. Measurements of the endocardial perimeter (EP) and length of the infarcted segment (ISe) for each transverse view were obtained in diastole. The infarct size for each segment (ISi), expressed as the proportion of the left ventricle perimeter of each transverse view, was calculated by the equation ISi (%) = ISe/EP x 100. The MI was defined by echocardiography as any segment with increased echogenicity and/or change in myocardial thickening or systolic movement (hypokinesia, akinesia, or dyskinesia). The diastolic (DA) and systolic (SA) transverse areas of the left ventricle were measured by 2-dimensional images at the basal, midview, and apical view. The final value was the arithmetic mean of the measures of the three views. Systolic function was analyzed by the fractional area change (FAC) as a function for the following equation: FAC = DA-SA/DA x 100.
After echocardiography study, the animals received a urethane overdose (i.p. 4.8 g/kg) and the hearts were immediately excised from the thorax and dissected. Samples from remote MI area and the corresponding part in the sham-operated rats were collected, frozen in dry ice, and stored at −80°C until processing.
Quantitative real-time RT-PCR analysis
Total RNA was isolated from myocardial tissue using TRIzol as previously described17. Reverse transcription was performed in a 200 µl reaction in the presence of 50 mM Tris-HCl pH 8.3, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM dNTPs, and 50 ng of random primers with 200 units of Moloney murine leukemia virus-reverse transcriptase (Invitrogen). The reaction product was amplified by real-time PCR on the 7000 Sequence Detection System (ABI Prism, Applied Biosystems, Foster City, CA) using the SYBRGreen core reaction kit (Applied Biosystems, Foster City, CA, USA). The primers used were the followings: rat ILK (GenBankTM accession number NM 133409), forward primer 5'-ACCCAACCCTCATCACACACT-3' and reverse primer 5'-GCCTCTTGCCATGTCCAAA-3'; rat GAPDH (GenBankTM accession number NM 017008) forward primer 5'-TGCACCACCAACTGCTTAGC-3' and reverse primer 5'-GCCCCACGGCCATCA-3'.
Western blot analysis
Samples were homogenized in lysis buffer containing 0.1M NaCl, 0.01 M Tris-HCl pH 7.6, 0.001M EDTA pH 8.0, 1% NP-40, 10% Glycerol, 10 µM PMSF, 1 mM sodium metavanadate, 0.05 M NAF; 22 nM okadaic acid with protease inhibitor cocktails. The Lowry method was used to determine protein content. Tissue homogenate was diluted in Laemmli buffer and boiled for 5 min; 60µg of protein were loaded on 10% polyacrylamide mini-gels, separated by electrophoresis and transferred to nitrocellulose membranes by electroblotting. Blocking was performed in 5% nonfat milk for 2 h at room temperature. Blots were then probed with an affinity-purified rabbit polyclonal antibody against ILK (Santa Cruz Biotechnology, CA, USA) in TBS-T (50 mM Tris-HCl, 154 mM NaCl, pH 7.5 + 0.1% Tween 20) 2% nonfat milk. After 12 h incubation at room temperature, blots were washed in TBS-T (3 times for 10 min) and incubated for 2 h in biotinylated anti-rabbit immunoglobulins (Vector Laboratories, CA, USA) diluted 1:200 in TBS-T 2% nonfat milk. Blots were washed in TBS-T (3 times for 10 min) and incubated for 1 h in streptavidin-horseradish peroxidase (Vector Laboratories, CA, USA). After washing, the blots were incubated with enhanced chemiluminescence reagents (ECL, Amersham Pharmacia Biotech), exposed to X-ray film (Amersham), and quantified by densitometric analysis. Equal protein loading was shown by stripping and incubation with an anti-GAPDH antibody (1:2000, Proteimax Biotecnologia, Brazil).
Statistical analysis
Data were analyzed by the SPSS software 13.0 for PC (Analytical Software, Chicago, IL, USA), and values are expressed as mean ± S.D. Shapiro-Wilk and Levene tests were used to verify approximately normal statistic distributions and equality of variances, respectively. Two-way ANOVA followed by Bonferroni post-hoc test was used to detect significant differences in sample normality. Kruskal-Wallis followed by Dunn's multiple comparison tests was applied for no-normality samples. Relationships between ILK levels and LV areas post-MI were determined by Pearson's correlation coefficient. In addition, linear multivariable regression models were performed (1) to evaluate the association between gender and LV structural and functional parameters after IM and (2) to examine the association between ILK protein level and LV areas after IM. Significance for statistical analyses was determined using an alpha level of ≤ 0.05.
Results
Survival after coronary occlusion
A total of 41 male and 28 female rats underwent coronary occlusion. After 24 h, 18 (44%) males and two females (7%) rats died. Six weeks after coronary occlusion, two males and two females died. Moreover, one male and three female rats were excluded for not showing signs of MI.
MI size, LV geometry and function
Echocardiographic data are shown in Table 1. The size of MI was similar in female and male rats of the moderate and large infarct size groups. The female sham rats showed significant differences in LV chamber dimensions compared with male sham rats. However, when the size of the cavity was indexed by body weight, it did not differ between genders. Six weeks of MI resulted in end-diastolic as well as end-systolic dimensions, which were significantly enlarged. Moreover, LV areas were significantly increased in both female and male rats as a function of the size of MI. In the following analysis, we observed that female gender favorably influences LV chamber remodeling in response to MI. Thus, when considering animals with a large MI, female rats showed a lower diastolic and systolic LV dilation. Indeed, when related to body weight, male rats also exhibited a LV cavity larger than female rats. Data on LV function identified a depression in both female and male rats with moderate and large infarctions. These changes were similar in both genders.
Table 1.
Echocardiographic characteristics of the animals
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Sham | Moderate MI | Large MI | Sham | Moderate MI | Large MI | |
| N | 7 | 9 | 12 | 8 | 8 | 12 |
| BW (g) | 232 ± 23* | 227 ± 8# | 262 ± 22$† | 343 ± 56 | 322 ± 12 | 300 ± 24‡ |
| IM size (%) | --- | 32 ± 7 | 47 ± 6 | --- | 33 ± 9 | 46 ± 6 |
| LVDA (mm2) | 2.5 ± 0.4 | 4.6 ± 0.8‡ | 5.4 ± 1‡$† | 3.2 ± 0.3 | 5.3 ± 0.6‡ | 8.2 ± 0.7$‡ |
| LVSA (mm2) | 0.8 ± 0.1 | 3 ± 0.8‡ | 3.9 ± 0.9‡$† | 1.2 ± 0.1 | 3.4 ± 0.8‡ | 5.7 ± 1.1$‡ |
| LVDA/BW (mm2/g) | 0.011 ± 0.002 | 0.020 ± 0.003‡ | 0.020 ± 0.004‡† | 0.010 ± 0.001 | 0.016 ± 0.002‡ | 0.027 ± 0.002$‡ |
| LVSA/BW (mm2/g) | 0.003 ± 0.0006 | 0.013 ± 0.003‡ | 0.015 ± 0.004‡† | 0.003 ± 0.0006 | 0.010 ± 0.002‡ | 0.019 ± 0.004$‡ |
| FAC (%) | 67 ± 2 | 34 ± 10‡ | 26 ± 16‡ | 63 ± 4 | 36 ± 11‡ | 29 ± 14‡ |
BW: body weight; LVDA: left ventricular diastolic area; LVSA: left ventricular systolic area; FAC: fractional area change; MI: myocardial infarction
p < 0.05 compared with sham males;
p < 0.05 compared with moderate MI males
p < 0.05 compared with large MI males;
p < 0.05 compared with the respective sham;
p < 0.05 compared with respective moderate MI size.
Linear multivariable regression analyses were performed to assess the association between gender and structural and functional parameters of LV after MI (Table 2).
Table 2.
Multivariable analysis between gender and structural and functional parameters of the left ventricle after myocardial infarction
| Standardized coefficient β | t-value | p value | |
|---|---|---|---|
| Gender – MI sizea | - 0.13 | - 0.93 | 0,9 |
| Gender – LVDAb | 0.530 | 5.533 | < 0.001 |
| Gender – LVSAc | 0.483 | 4.931 | < 0.001 |
| Gender – FACd | - 0.14 | - 0.174 | 0.8 |
Model 1: multivariate linear regression for association of gender and myocardial infarction (MI) size;
Model 2: multivariate linear regression for association of gender and left ventricular diastolic area (LVDA) after adjusting for MI size and body weight;
Model 3: multivariate linear regression for association of gender and left ventricular systolic area (LVSA) after adjusting for MI size and body weight;
Model 4: multivariate linear regression for association of gender and fractional area change (FAC) after adjusting for MI size, body weight, LVDA, and LVSA.
The MI size was not significantly associated with gender. However, male gender was significantly associated with larger LV diastolic area (LVDA) and systolic area (LVSA) after adjustment for MI size and body weight. There was no significant association between gender and FAC after adjustment for MI size, LV diastolic and systolic area, and body weight.
Gender determines differential expression of ILK after MI
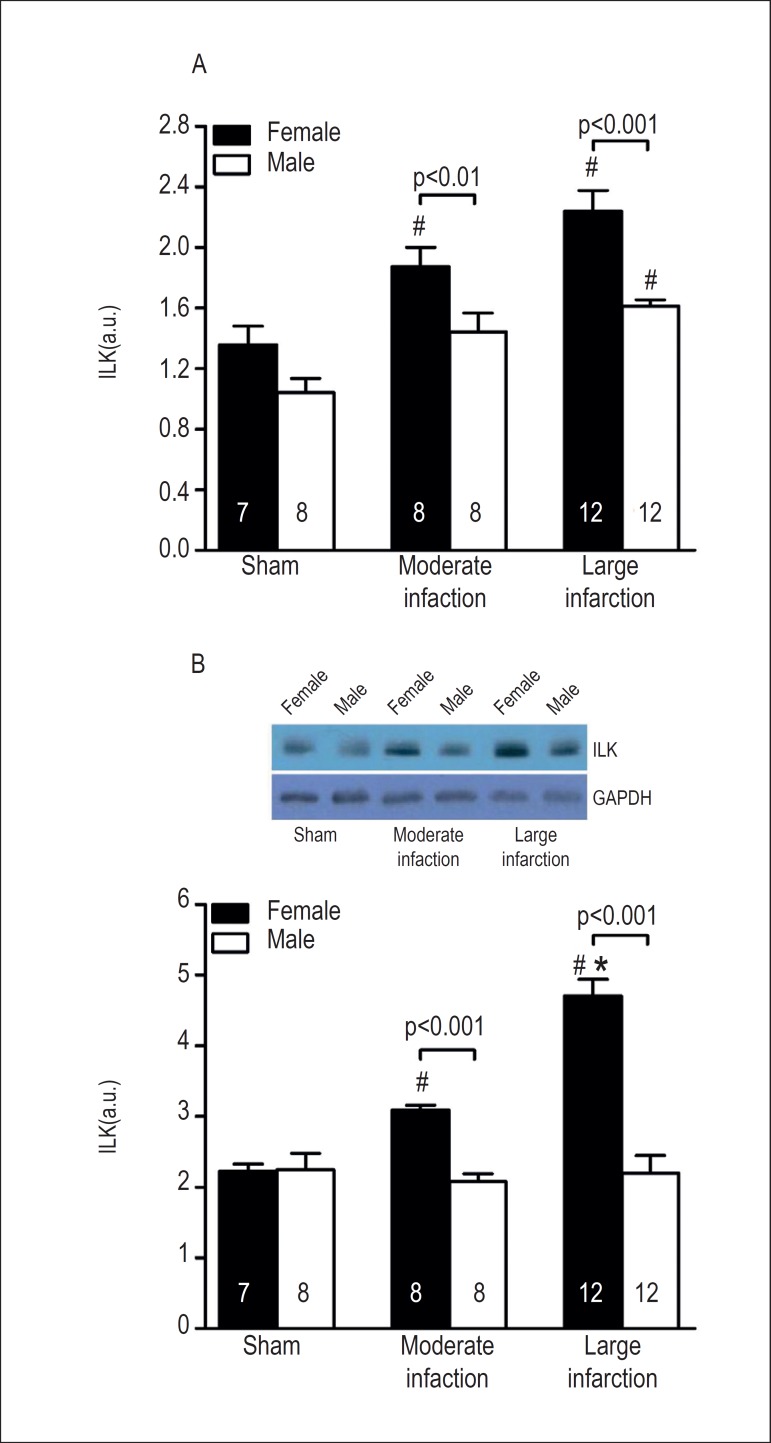
There was no significant difference in ILK expression between female and male sham-operated rats (Figure 1A). MI significantly increased ILK mRNA in female rats compared with the corresponding sham-operated female rats. ILK mRNA expression was higher in females with large infarcts than with moderate infarcts; however, this difference was not statistically significant. In male rats, ILK levels were significantly increased in animals with large MI area. More importantly, gender significantly affected ILK mRNA expression after MI. Female rats with moderate and large MI size showed higher ILK levels compared with male rats with moderate and large MI size. In Figure 1B, ILK protein levels examined by Western blot are shown. As observed for the gene expression, gender did not affect ILK content in the myocardial tissue of sham-operated groups. However, ILK protein levels were upregulated as a function of MI size in females. On the other hand, ILK content was not altered in the myocardial tissue of infarcted male rats. Remarkably, given that a significantly higher amount of ILK protein was found in female compared with male rats, gender determined differential expression of ILK after MI.
Figure 1.
Quantification of (A) ILK mRNA and (B) ILK protein expression in the myocardium of sham-operated and infarct-operated female and male rats, respectively. ILK levels are plotted as a function of MI size. The sample size is shown into the bars. #p < 0.001 vs. respective sham; *p < 0.001 vs. respective moderate infarction.
Correlation between ILK expression and LV geometry
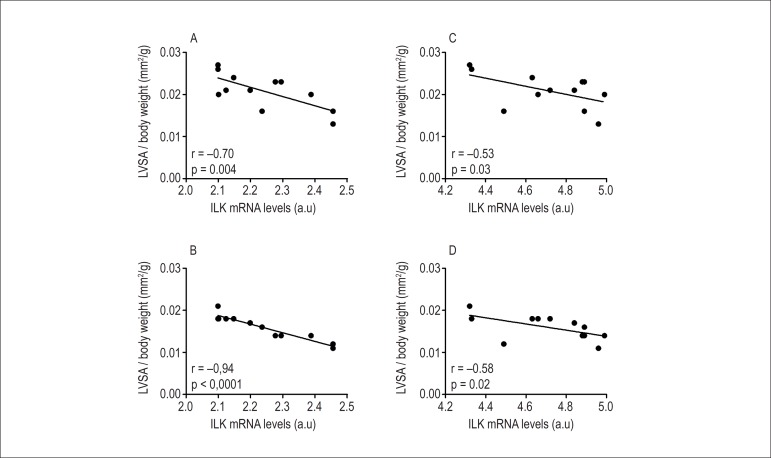
As previously described, female rats with large infarcts showed a lower LV dilatation. In addition, following MI there was increased ILK expression in female rats compared with male rats. Since previous studies with gene therapy approaches have shown that increased myocardial expression of ILK may reduce LV dilation following MI12, correlation analysis between LV areas and ILK levels were performed in female rats with large MI size (Figure 2). We determined that a negative linear correlation existed between LVDA, LVSA and ILK expression. The association between LV areas and ILK remained statistically significant even after the application of a linear multivariable regression model (Table 3). Thus, an increased ILK expression was associated with smaller LVDA as well as LVSA in female rats. However, a similar result was not observed in male rats.
Figure 2.
Correlation analysis between ILK gene (panels: A-B) and protein (panels: C-D) expression and LV diastolic (LVDA) and systolic (LVSA) areas indexed to body weight in female rats with large MI size.
Table 3.
Multivariable analysis between ILK protein expression and left ventricular areas after myocardial infarction in both genders
| Standardized coefficient β | t-value | p value | |
|---|---|---|---|
| LVDA (female) | 0.716 | 5.228 | < 0.001 |
| LVDA (male) | - 0.088 | - 0.459 | 0.6 |
| LVSA (female) | 0.749 | 5.769 | < 0.001 |
| LVSA (male) | - 0.204 | - 1.064 | 0.2 |
LVDA: left ventricle diastolic; LVSA: left ventricle systolic area.
Discussion
It is well known that premenopausal women are less likely to develop coronary heart disease than men of the same age and that heart failure appears to be less common and less severe in premenopausal women than in men1. Although previous studies have shown post-MI gender differences in LV remodeling, the data are inconclusive. Thus, Chen et al18 have reported that female rats had more prominent LV dilatation and systolic dysfunction on echocardiographic analysis four weeks post-MI compared with male rats. In contrast, Litwin et al3 found comparable LV dilatation and systolic dysfunction between female and male rats six weeks post-MI, and similarly Bridgman et al19 found comparable LV dilatation and systolic dysfunction between female and male mice six weeks post-MI. Conversely, we reported that female rats with large MI size resulted in minor LV dilatation six weeks post-MI compared with male rats of similar MI size. These results are in agreement with studies in mice. Cavasin et al2 found that male mice had more pronounced LV dilatation as well as systolic dysfunction 12 weeks post-MI. Similarly, Shioura et al20 found that male mice had more pronounced LV dilatation as well as systolic dysfunction 10 weeks post-MI. The diverse results in the literature comparing post-MI LV remodeling between females and males are unclear. It is possible that the use of different species (e.g., mice or rats), ovariectomized or ovary-intact animals, different observation time points, separation of groups according to MI size, and different ages of the animals could account for the disparate results in the literature.
As determined in our study, although female rats with large MI showed a minor LV cavity compared to male rats with similar MI size, this was not associated with an improved LV systolic function. Thus, these data suggest that despite infarcts of similar size and severe maladaptive remodeling of the LV cavity in male rats, systolic function was comparable between genders.
The underlying mechanisms for a lower post-MI LV dilation in females than in males are not completely understood. It has been reported that female sex hormones (e.g., estrogen) may have protective effects in rats and mice4-6. In this respect, we found that myocardial expression of ILK was significantly increased six weeks after MI. To our knowledge, only limited numbers of studies have investigated the effects of MI on ILK expression10, and the molecular mechanism responsible for the differential expression of ILK between genders has not been clarified. Thus, it should be pointed out that female rats with moderate and large MI showed an increase in ILK gene and protein expression compared with male rats with the same MI size. Given that ILK gene and protein levels may have favorable effect on cardiac remodeling, one might expect that higher ILK content could result in a lower LV dilatation response to MI in female rats. The fact that the concentration of ILK is related to a lower LV cavity is further supported by correlation analysis as documented in this study. The assumption that ILK has a key role in preserving LV remodeling is supported by several evidences in the literature. Ding et al12 injected an adenoviral vector expressing ILK into the myocardial tissue of rats following MI and the gene therapy was associated with preserved LV diameter, enhanced angiogenesis and cardiomyocyte proliferation, reduced infarct scar size, reduced fibrosis, and reduced apoptosis. Moreover, studies of MI in mice have shown that thymosin β4, a monomeric G-actin protein, targets the ILK-PINCH-1 pathway leading to the activation of Akt for cardiac protection after ischemic insult21,22. These studies have shown several beneficial anti-remodeling effects, including smaller infarct sizes, reduced LV dilatation, and improved cardiac function in thymosin β4-treated MI animals compared with MI only animals.
Unfortunately, we did not investigate the mechanism leading to a differential expression of ILK in post-MI female rats versus male rats; to our knowledge, there is no evidence in the literature on this issue. The mechanisms leading to lower ILK transcription in post-MI males than females as well as translation inhibition in males need to be determined. ILK has been shown to activate multiple cardioprotective signaling pathways that can result in an improved myocardial remodeling post-ischemic injury. In this regard, the myocardial ILK expression was shown to be associated with vascular endothelial growth factor expression and phosphorylation of endothelial nitric oxide synthase after MI23. Moreover, it has been taken into account that cardioprotection in female may occur through the PI3-K/AKT signaling pathway24,25. Unfortunately, we were unable to demonstrate that the relationship among vascular endothelial growth factor, endothelial nitric oxide synthase, PI3-K/AKT, and ILK after MI could favorably influence LV remodeling in female rats. This issue is very intriguing and needs to be investigated in future.
Conclusion
Our study is the first to demonstrate a gender-specific differential expression pattern of ILK following MI. Although increased ILK protein levels were shown to correlate with a minor LV chamber area, we did not find significant differences in LV performance between genders. Further studies are necessary to explore the association among ILK level, LV geometry, and cardiac function. This issue should be addressed as a relationship of cause and effect dependent on gender.
Footnotes
Author contributions
Conception and design of the research: Sofia RR, Antonio EL, Manchini MT, Tucci PJF; Acquisition of data: Silva Jr JA, Antonio EL, Manchini MT, Oliveira FAA, Teixeira VPC; Analysis and interpretation of the data: Sofia RR, Silva Jr JA, Oliveira FAA, Teixeira VPC; Statistical analysis: Serra AJ; Obtaining financing: Tucci PJF; Writing of the manuscript: Sofia RR, Serra AJ; Critical revision of the manuscript for intellectual content: Serra AJ, Tucci PJF.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by FAPESP.
Study Association
This article is part of the thesis of Doctoral submitted by Renato Rodrigues Sofia, from Universidade Federal de São Paulo.
References
- 1.Mercuro G, Deidda M, Bina A, Manconi E, Rosano GM. Gender-specific aspects in primary and secondary prevention of cardiovascular disease. Curr Pharm Des. 2011;17(11):1082–1089. doi: 10.2174/138161211795656954. [DOI] [PubMed] [Google Scholar]
- 2.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004;75(18):2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. Gender differences in post-infarction left ventricular remodeling. Cardiology. 1999;91(3):173–183. doi: 10.1159/000006906. [DOI] [PubMed] [Google Scholar]
- 4.Smith PJ, Ornatsky O, Stewart DJ, Picard P, Dawood F, Wen WH, et al. Effects of estrogen replacement on infarct size, cardiac remodeling, and the endothelin system after myocardial infarction in ovariectomized rats. Circulation. 2000;102(24):2983–2989. doi: 10.1161/01.cir.102.24.2983. [DOI] [PubMed] [Google Scholar]
- 5.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284(5):H1560–H15H9. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 6.Beer S, Reincke M, Kral M, Callies F, Strömer H, Dienesch C, et al. High-dose 17beta-estradiol treatment prevents development of heart failure post-myocardial infarction in the rat. Basic Res Cardiol. 2007;102(1):9–18. doi: 10.1007/s00395-006-0608-1. [DOI] [PubMed] [Google Scholar]
- 7.Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I. Gender differences in cardiac ischemic injury and protection - experimental aspects. Exp Biol Med. 2009;234(9):1011–1019. doi: 10.3181/0812-MR-362. [DOI] [PubMed] [Google Scholar]
- 8.Ishii T, Satoh E, Nishimura M. Integrin-linked kinase controls neurite outgrowth in N1E-115 neuroblastoma cells. J Biol Chem. 2001;276(46):42994–43003. doi: 10.1074/jbc.M105198200. [DOI] [PubMed] [Google Scholar]
- 9.Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155(4):505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sopko N, Qin Y, Finan A, Dadabayev A, Chigurupati S, Qin JPenn MS, et al. Significance of thymosin ß4 and implication of PINCH-1-ILK-a-parvin (PIP) complex in human dilated cardiomyopathy. PLoS One. 2011;6(5): doi: 10.1371/journal.pone.0020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ Res. 2007;100(10):1408–1414. doi: 10.1161/01.RES.0000265233.40455.62. [DOI] [PubMed] [Google Scholar]
- 12.Ding L, Dong L, Chen Z, Zhang L, Xu X, Ferro A, et al. Increased expression of integrin-linked kinase attenuates left ventricular remodeling and improves cardiac function after myocardial infarction. Circulation. 2009;120(9):764–773. doi: 10.1161/CIRCULATIONAHA.109.870725. [DOI] [PubMed] [Google Scholar]
- 13.Veiga EC, Antonio EL, Bocalini DS, Murad N, Abreu LC, Tucci PJ, et al. Prior exercise training does not prevent acute cardiac alterations after myocardial infarction in female rats. Clinics. 2011;66(5):889–893. doi: 10.1590/S1807-59322011000500028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.dos Santos L, Serra AJ, Antonio EL, Hull HF, Tucci PJF. Hyperbaric oxygenation applied immediately after coronary occlusion reduces myocardial necrosis and acute mortality in rats. Clin Exp Pharmacol Physiol. 2009;36(5-6):594–598. doi: 10.1111/j.1440-1681.2008.05118.x. [DOI] [PubMed] [Google Scholar]
- 15.Santos AA, Helber I, Flumignan RL, Antonio EL, Carvalho AC, Paola AA, et al. Doppler echocardiographic predictors of mortality in female rats after myocardial infarction. J Card Fail. 2008;15(2):163–168. doi: 10.1016/j.cardfail.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. Pt 4J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra AJ, Santos MH, Bocalini DS, Antônio EL, Levy RF, Santos AA, et al. Exercise training inhibits inflammatory cytokines and more than prevents myocardial dysfunction in rats with sustained beta-adrenergic hyperactivity. Pt 13J Physiol. 2010;588:2431–2442. doi: 10.1113/jphysiol.2010.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YF, Redetzke RA, Sivertson RM, Coburn TS, Cypher LR, Gerdes AM. Post-myocardial infarction left ventricular myocyte remodeling: are there gender differences in rats? Cardiovasc Pathol. 2011;20(5):e189–ee95. doi: 10.1016/j.carpath.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridgman P, Aronovitz MA, Kakkar R, Oliverio MI, Coffman TM, Rand WM, et al. Gender specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotensin II type 1a receptor. Am J Physiol Heart Circ Physiol. 2005;289(2):H586–HH92. doi: 10.1152/ajpheart.00474.2004. [DOI] [PubMed] [Google Scholar]
- 20.Shioura KM, Geenen DL, Goldspink PH. Sex-related changes in cardiac function following myocardial infarction in mice. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R528–RR34. doi: 10.1152/ajpregu.90342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bock-Marquette I, Saxena A, White MD, DiMaio M, Srivastava D. Thymosin ß4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava D, Saxena A, Dimaio M J, Bock-Marquette I. Thymosin ß4 is cardioprotective after myocardial infarction. Ann N Y Acad Sci. 2007;1112:161–170. doi: 10.1196/annals.1415.048. [DOI] [PubMed] [Google Scholar]
- 23.Xie J, Lu W, Gu R, Dai Q, Zong B, Ling L, et al. The impairment of ILK related angiogenesis involved in cardiac maladaptation after infarction. PLoS One. 2011;6(9): doi: 10.1371/journal.pone.0024115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KDR, Schaefer E, et al. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88(10):1020–1027. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 25.Sovershaev MA, Egorina EM, Andreasen TV, Jonassen AK, Ytrehus K. Preconditioning by 17ß-estradiol in isolated rat depends on PI3-K/PKB pathway, PKC, and ROS. Am J Physiol Heart Circ Physiol. 2006;291(4):H1554–H1H62. doi: 10.1152/ajpheart.01171.2005. [DOI] [PubMed] [Google Scholar]