Abstract
Vestibular schwannomas (VSs) arise from Schwann cells (SCs) and result from the loss of function of merlin, the protein product of the NF2 tumor suppressor gene. In contrast to non-neoplastic SCs, VS cells survive long-term in the absence of axons. We find that p75NTR is over-expressed in VSs compared with normal nerves, both at the transcript and protein level, similar to the response of non-neoplastic SCs following axotomy. Despite elevated p75NTR expression, VS cells are resistant to apoptosis due to treatment with pro-NGF, a high affinity ligand for p75NTR. Furthermore, treatment with proNGF protects VS cells from apoptosis due to c-Jun N-terminal kinase (JNK) inhibition indicating that p75NTR promotes VS cell survival. Treatment of VS cells with proNGF activated NF-κB while inhibition of JNK with SP600125 or siRNA-mediated knockdown reduced NF-κB activity. Significantly, proNGF also activated NF-κB in cultures treated with JNK inhibitors. Thus, JNK activity appears to be required for basal levels of NF-κB activity, but not for proNGF-induced NF-κB activity. To confirm that the increase in NF-κB activity contributes to the prosurvival effect of proNGF, we infected VS cultures with Ad.IκB.SerS32/36A virus, which inhibits NF-κB activation. Compared to control virus, Ad.IκB.SerS32/36A significantly increased apoptosis including in VS cells treated with proNGF. Thus, in contrast to non-neoplastic SCs, p75NTR signaling provides a prosurvival response in VS cells by activating NF-κB independent of JNK. Such differences may contribute to the ability of VS cells to survive long-term in the absence of axons.
Keywords: acoustic neuroma, apoptosis, merlin, Schwann cell, neurotrophin
Introduction
Vestibular schwannomas (VS) originate from Schwann cells (SCs) of the vestibular nerve and typically occur as either unilateral, sporadic tumors or as bilateral tumors in patients with neurofibromatosis type 2 (NF2) (Evans 2009; Roosli et al. 2012). Both sporadic and NF2-associated VSs result from loss of function of the NF2 tumor suppressor gene (Rouleau et al. 1993; Stemmer-Rachamimov et al. 1997; Trofatter et al. 1993). Merlin, the protein product of the NF2 gene, regulates several signaling events that control tumor growth (Xiao et al. 2003; Zhou and Hanemann 2012). Merlin appears to associate transmembrane and signaling molecules with cytoskeletal actin thereby affecting cell-cell attachments, cell motility, and the subcellular localization and activity of transmembrane receptors and signaling molecules in response to cell contact inhibition (McClatchey and Giovannini 2005; Scoles 2008; Welling et al. 2007; Xiao et al. 2003).
Recent evidence suggests that merlin suppresses mitogenic signaling at the cell membrane and in the nucleus (Li et al. 2012; Zhou and Hanemann 2012). At the membrane, merlin inhibits signaling by integrins and tyrosine receptor kinases (RTKs) and the activation of downstream pathways, including the Ras/Raf/MEK/ERK, FAK/Src, PI3K/AKT, Rac/PAK/JNK, mTORC1, and Wnt/β-catenin pathways (Bosco et al. 2010; Chadee and Kyriakis 2004; Chadee et al. 2006; Flaiz et al. 2009; Fraenzer et al. 2003; Houshmandi et al. 2009; James et al. 2009; James et al. 2012; Kaempchen et al. 2003; Kissil et al. 2003; Lim et al. 2003; Lopez-Lago et al. 2009; Rong et al. 2004; Yi et al. 2008; Zhou et al. 2011). Merlin also acts upstream of the Hippo pathway to suppress the function of Yes-associated protein 1 (YAP1), an oncogene implicated in meningioma tumor growth (Baia et al. 2012; Hamaratoglu et al. 2006; Striedinger et al. 2008; Zhang et al. 2010). In the nucleus, merlin suppresses the E3 ubiquitin ligase CRL4 (DCAF1) to inhibit proliferation (Li et al. 2010).
p75NTR
p75NTR is the founding member of the TNF receptor superfamily and was the first identified nerve growth factor receptor (Bothwell 1995). p75NTR binds mature neurotrophins with low affinity, while proneurotrophins bind avidly to p75NTR (Chao 2003; Lee et al. 2001). Neurotrophins also signal through Trk receptors to promote cell survival, which are capable of forming high affinity binding sites with p75NTR (Hempstead et al. 1991).
Activation of p75NTR elicits a variety of responses, including apoptosis or cell survival, depending on the cellular context. In the absence of Trk receptors p75NTR activates NF-κB, the sphingomyelin cycle, and c-Jun N-terminal kinase (JNK) (Dobrowski et al. 1994; Gentry et al. 2000; Harrington et al. 2002; Roux and Barker 2002). Consistent with the notion that p75NTR signaling initiates cell death, pro-nerve growth factor (NGF) and pro-brain derived neurotrophic factor (BDNF) induce apoptosis in cells expressing p75NTR (Clewes et al. 2008; Koshimizu et al. 2010; Masoudi et al. 2009; Provenzano et al. 2011). This pro-apoptotic function of p75NTR requires binding of the co-receptor sortilin as well as γ-secretase-dependent intramembranous cleavage and release of the intracellular domain (Jansen et al. 2007; Kenchappa et al. 2006; Parkhurst et al. 2010; Skeldal et al. 2012). In other cells, p75NTR signaling promotes cell survival. What determines whether p75NTR activation leads to cell death or survival remains unknown. However, p75NTR activation of the nuclear transcription factor κB (NF-κB) has been implicated in the pro-survival response (Gentry et al. 2000), whereas activation of JNK is required for the pro-death signal (Friedman 2000; Harrington et al. 2002; Koshimizu et al. 2010; Yoon et al. 1998).
p75NTR and JNK signaling in SCs
Following axotomy, SCs upregulate p75NTR expression (Provenzano et al. 2008; Taniuchi et al. 1986). In the absence of reinnervation, denervated SCs ultimately undergo p75NTR-mediated apoptosis (Ferri and Bisby 1999; Petratos S 2003; Syroid et al. 2000). Consistent with a pro-apoptotic function of p75NTR and JNK in SCs (Parkinson et al. 2001), pro and mature isoforms of NGF activate JNK and induce apoptosis in SCs (Hirata et al. 2001; Provenzano et al. 2011; Soilu-Hanninen et al. 1999; Yeiser et al. 2004). As VSs arise from cells of the SC lineage, they express p75NTR similar to denervated SCs (Bonetti et al. 1997; Laskin et al. 2005; Miettinen et al. 2001). In contrast to non-neoplastic SCs, VS cells have the ability to survive long-term in the absence of axonal contact. Recent reports indicate that JNK is persistently active in human VS cells due to the lack of merlin expression (Hilton et al. 2009; Kaempchen et al. 2003; Yue et al. 2011). Significantly, this elevated JNK activity contributes to VS cell proliferation and survival (Yue et al. 2011).
To better understand the factors that contribute to the ability of VS cells to survive in the absence of axonal contact, we investigated the expression level of p75NTR in primary VS specimens and the responses of primary cultures derived from acutely resected human VSs to high affinity p75NTR ligands. We find that VS cells fail to die in the presence of proNGF unlike their non-neoplastic SC counterparts. Further, proNGF rescues VS cells with suppressed JNK signaling suggesting that, in contrast to its role in normal SCs, p75NTR promotes VS cell survival. We also find that proNGF activates NF-κB to promote survival.
Materials and Methods
VS and nerve collection and primary VS cultures
The institutional review board of the University of Iowa approved the study protocol. VS were collected from patients undergoing microsurgery for removal of sporadic VS and immediately placed in ice-cold Hank's balanced salt solution until used for cultures or snap frozen in liquid nitrogen until used for RNA or protein extracts. None of the specimens were derived from NF2-associated tumors. Histological analysis confirmed typical schwannoma in each instance. Greater auricular nerve (GAN) and vestibular nerve (VN) specimens were collected after surgical removal from separate patients undergoing neck dissection or vestibular nerve section, respectively, and immediately snap frozen in liquid nitrogen until used for RNA (VN) or protein (GAN) extraction.
Primary human VS cultures
Primary human VS cultures were prepared from acutely resected tumors as previously described (Hansen et al. 2006; Yue et al. 2011). The cultures were not passaged prior to experimental manipulation. Adenoviral-mediated gene transfer was also performed as previously described with Ad5.empty vector (EV), Ad5.merlin, or Ad5.IκBαS (gift from Dr. Isaac Samuel), each at 2 × 108 pfu/mL (Yue et al. 2011). To knockdown JNK expression cultures were transfected using RNAiMax (Life Technologies, Invitrogen, Carlsbad, CA) with siRNA oligonucleotides targeting JNK1 and JNK2 (Cell Signaling, #6232, Beverly, MA), as previously described (Yue et al. 2011). I-JIP (30-100 μM) and SP600125 (20 μM) (both from EMD Millipore, Billerica, MA) were used as JNK inhibitors, as before (Yue et al. 2011). At the conclusion of the experiments, the cultures were fixed for 10 min with 4% paraformaldehyde and immunolabeled with anti-S100 (Sigma, St. Louis, MO), p75NTR (kindly provided by Dr. Moses Chao) and/or sortilin (Abcam, Cambridge, MA) antibodies followed by secondary detection with Alexa 488, 568, or 647-conjugated secondary antibodies (Life Technologies).
Non-neoplastic Schwann cell cultures
Non-neoplastic Schwann cell cultures were prepared from neonatal rat or mouse sciatic nerve as previously described (Provenzano et al. 2011; Provenzano et al. 2008). Briefly, sciatic nerves were dissected from P5 pups, washed in ice-cold PBS, and enzymatically digested in 0.125% typsin with EDTA (Life Technology) and 0.2% collagenase (Sigma) in Hanks balanced salt solution without calcium and magnesium (Life Technology), for 20 minutes in 37°C. Fetal bovine serum at 10% final concentration (Life Technology) was used to quench the trypsin, the tissue was washed twice in DMEM (Life Technology) and then suspended in DMEM with N2 supplements (Life Technology) and 10 μg/ml insulin (Sigma). The cells were gently dissociated by titration through fire-polished narrow bored glass pipettes and cultured on laminin-coated 8 well plastic culture slides (Labtek, Campbell, California, USA) or 35 mm tissue culture dishes. Cultures were maintained in serum-free N2 medium until the cells were 70-90% confluent containing over 98% SCs as determined by S100 immunolabeling.
Schwann cell and schwannoma cell apoptosis
Primary human VS cultures and mouse sciatic nerve Schwann cells maintained in the presence or absence I-JIP or SP600125 (JNK inhibitors) were treated with cleavage resistant proNGF (Alomone Labs, Jerusalem, Israel). After 24 h the cultures were fixed and immunolabeled for S100. Apoptotic nuclei were detected using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method as previously described (Hansen et al. 2008; Yue et al. 2011). Nuclei were labeled with DAPI. Criteria for scoring an apoptotic cell included: S100-positive, TUNEL-positive nucleus, and condensed or fragmented nucleus. The percent of TUNEL–positive VS cells was scored from10 randomly selected 20X fields for each well, as described elsewhere (Hansen et al. 2008; Yue et al. 2011). Only S100-positive cells were scored. Given the variability in basal apoptotic rates for each primary tumor, the percent of TUNEL-positive VS cells was expressed as a percentage of the control condition, defined as 100%. Apoptosis was confirmed in a subset of cultures by immunoblotting for cleaved caspase 3. Each condition was performed in duplicate and was repeated on ≥3 VS cultures derived from separate tumors. Statistical significance of differences in the average percent of apoptotic cells among the various treatment conditions was determined by one way ANOVA followed by Dunn's method for normally distributed data or by Kruskal-Wallis one way ANOVA on ranks for nonparametric data using SigmaStat software (Systat Software Inc, Richmond, CA).
Real-time reverse transcriptase polymerase chain reaction
Total RNA was extracted from 5 VSs and 4 VNs, each treated as a separate specimen. Real-time RT-PCR was performed with the TaqMan® and 7500 Real-Time PCR systems (Life Technologies, Applied Biosystems, Carlsbad, CA) according to manufacturer instructions using FAM-labeled probes. We used primer pairs #4331182 for human p75NTR and #4326317E for human GAPDH (Life Technologies, Applied Biosystems). Quantification of gene expression was performed using the ddCt method according to the manufacturer's instructions and expressed as the ratio p75NTR to GAPDH transcript levels. A student's non-paired, two-tailed t-test was used to determine statistical differences of transcript levels.
Western blots
Western blots of protein extracts prepared from VS or GAN tissue or culture lysates were performed as described previously (Brown and Hansen 2008; Hansen et al. 2006; Yue et al. 2011). The primary antibodies used were anti-p75NTR (kindly provided by Dr. Moses Chao), phosphorylated JNK (pJNK, Cell Signaling), JNK (Cell Signaling), phosphorylated JUN (pJUN, Cell Signaling), merlin (Santa Cruz), cleaved caspase-3 (Cell Signaling), RIP2 (Enzo Lifesciences, Farmingdale, NY), sortilin (Abcam), β-actin (Sigma), and Rho-GDI (Cell Signaling). Secondary antibodies (dilution,1:5000-50,000; Santa Cruz) were conjugated with horseradish peroxidase. Blots were developed using Super Signal West Femto kit (Thermo Fisher Scientific, Rockford, IL) and exposed to film (Amersham Hyperfilm TM ECL; GE Healthcare). As needed, membranes were stripped and re-probed with other antibody combinations. Densitometry to quantify protein levels was performed as previously described and statistical significance was determined with a student's non paired, two-tailed t- test.
NF-κB assay
Protein lysates were prepared 2 h following treatment of primary VS cultures with proNGF or sham. JNK inhibitors were added 30 min prior to treatment with or without proNGF. To determine NF-κB activity, we used a chemiluminescent capture assay (Thermo Fisher Scientific, Pierce, #89858) to capture activated NF-κB bound to the consensus DNA binding site according to the manufacturer's protocol. Activated NF-κB binds to the DNA sequence coating the wells and the bound NF-κB is detected with anti-p50 antibody followed by peroxidase conjugated secondary antibody and chemiluminescent quantification with a luminometer. Results are expressed as a fold change in activity relative to the control condition for each repetition. Each condition was performed in duplicate and repeated on ≥3 cultures derived from separate tumors. Statistical significance of differences among the treatment conditions was determined by one way ANOVA followed by Dunn's method.
Results
Vestibular schwannoma cells express high levels of p75NTR
SCs upregulate p75NTR expression following denervation by axotomy and in the absence of reinnervation, ultimately undergo p75NTR-mediated apoptosis (Ferri and Bisby 1999; Petratos S 2003; Provenzano et al. 2008; Syroid et al. 2000; Taniuchi et al. 1986). Since VS cells lack axonal contact, we compared the expression level of p75NTR in VSs with normal nerve. We used real-time RT-PCR to compare the ratio of p75NTR to GAPDH transcript levels in 5 VS tumors and 4 normal human vestibular nerves. As shown in Fig. 1A, there was an >3 fold increase in the mean ratio of p75NTR to GAPDH transcript levels in VSs compared to normal vestibular nerve. To confirm increased p75NTR protein expression in VSs compared to normal nerves, we quantified p75NTR levels in immunoblots of protein lysates from 5 VS and 4 human great auricular nerve (GAN) specimens by densitometry. The blots were reprobed with anti-b-actin to assess protein loading levels. The mean ratio of p75NTR/β-actin band intensity was significantly increased in lysates from VSs compared with GAN (Fig. 1B&C). We also immunolabeled frozen sections of VSs and normal vestibular nerves with anti-neurofilament 200 (NF200, neuronal/axon marker) and anti-p75NTR antibodies. p75NTR immunolabeling was relatively weak in the SCs and neuronal structures in normal vestibular nerves and was more intense in VS tissue, which lacked NF200 labeling (Fig. 1D&E). These results confirm that VSs express higher levels of p75NTR compared to normal nerve.
Figure 1.
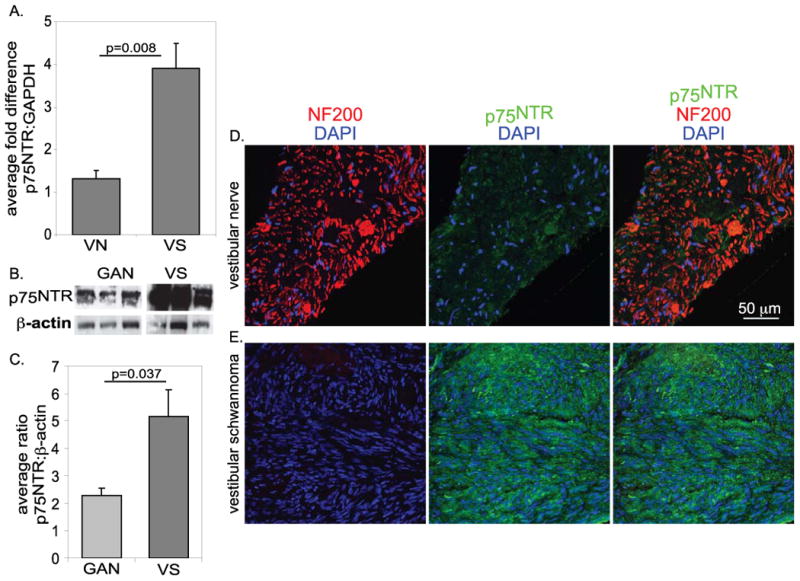
Vestibular schwannomas express high levels of p75NTR. A. Real-time RT-PCR comparing p75NTR expression to GAPDH in normal human vestibular nerve (VN, n=4) and in human vestibular schwannoma (VS, n=5) specimens. B&C. Western blot and comparison of anti-p75NTR band intensity relative to anti-b actin band intensity in protein lysates from normal human great auricular nerve (GAN, n=4) and human VS (n=5). The differences in relative p75NTR expression levels in A and C were statistically significant by Student's unpaired t-test. D&E. Immunostaining of frozen sections of normal human VN (D) and human VS (E) with anti-NF200 (red) and anti-p75NTR (green) antibodies. Nuclei were labeled with DAPI (blue).
ProNGF fails to induce apoptosis in VS cells
Pro isoforms of neurotrophins, such as proNGF and proBDNF, are high affinity ligands for p75NTR and induce apoptosis in SCs in vitro and in vivo (Petratos S 2003; Provenzano et al. 2011; Provenzano et al. 2008). To determine whether VS cells are likewise susceptible to proNGF-mediated apoptosis, we treated primary VS cultures with escalating doses of cleavage-resistant proNGF. After 24 h the cultures were fixed, immunolabeled with anti-S100 antibodies, and apoptotic nuclei were detected with the TUNEL method. The percent of TUNEL-positive VS cell nuclei was determined. Treatment of cultures of primary human VS cells with proNGF (0.1-3 nM) failed to significantly increase the percent of TUNEL-positive VS cell nuclei (Fig. 2). We confirmed that proNGF (0.1 nM) induces apoptosis in non-neoplastic mouse SCs (Fig 2D). These results suggest that VS cells are resistant to proNGF-mediated apoptosis despite high levels of p75NTR expression.
Figure 2.
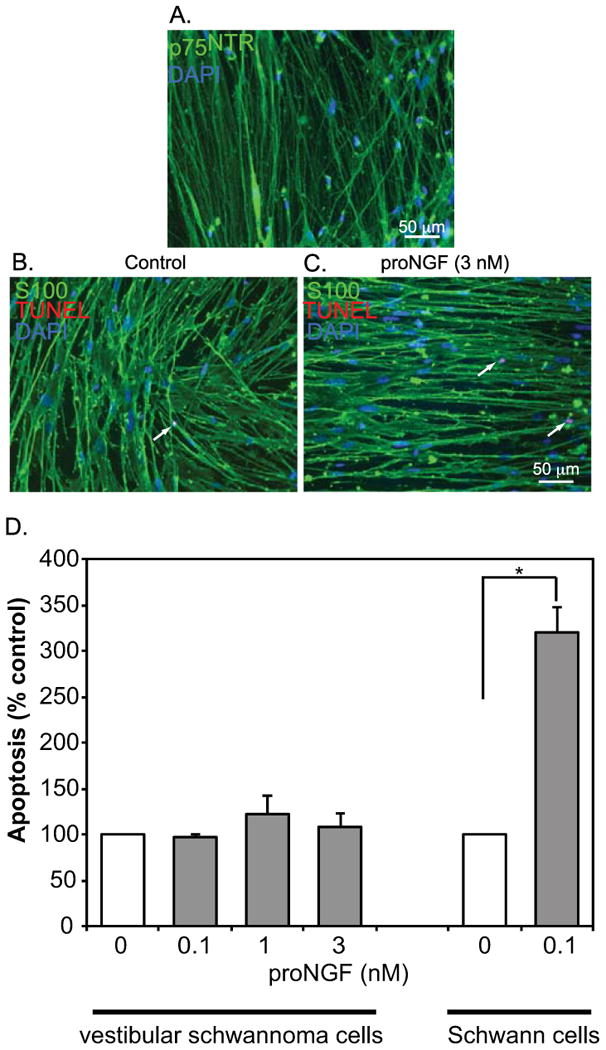
ProNGF fails to induce apoptosis in primary vestibular schwannoma cultures. A. Primary VS cultures immunostained with anti-p75NTR antibody (green). Nuclei are labeled with DAPI (blue). B,C. Primary human VS cultures were treated with escalating doses of proNGF, fixed, immunostained with anti-S100 (green) antibodies and labeled with TUNEL (red) and DAPI (blue). D. The average percent of TUNEL-positive, S100-positive condensed nuclei from primary human vestibular schwannoma cultures or non-neoplastic mouse Schwann cell cultures were scored and plotted relative to cultures not treated with proNGF (control). Error bars present standard error of the mean. Vestibular schwannoma data are from cultures derived from six separate patients while data for non-neoplastic Schwann cells were derived from 3 separate cultures. There was no significant difference between treatment conditions for the vestibular schwannoma cultures by one way ANOVA. *p<0.01 by Student's two tailed t-test.
Sortilin functions a p75NTR co-receptor critical for proNGF-mediated apoptosis (Jansen et al. 2007; Skeldal et al. 2012). Immunoblots and immunostaining confirmed that cultured VS cells express sortilin (Fig. 3A-D) Likewise, we confirmed that cultured VS cells and normal rat sciatic nerve SC cultures express receptor-interacting protein 2 (RIP2) (Fig. 3E), an adaptor protein with a carboxy-terminal caspase activation and recruitment domain (CARD) that is necessary for p75NTR-mediated SC apoptosis (Khursigara et al. 2001). These results indicate that cultured VS cells express the necessary co-receptor and RIP2 for proNGF-mediated apoptosis.
Figure 3.
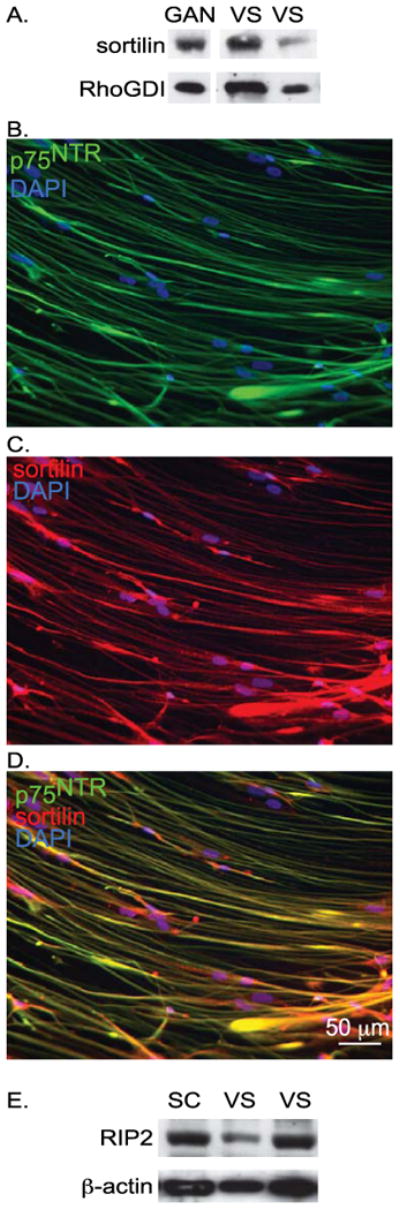
Primary vestibular schwannoma cultures express sortilin and RIP2. A. Western blot of protein lysates from human GAN and human VS specimens probed with anti-sortilin antibodies. The blots were stripped and reprobed with anti-Rho-GDI antibodies to compare protein loading. Blots present the lysates with the highest and lowest sortilin expression compared with Rho-GDI. Bots for lysates from 3 additional tumors demonstrated similar expression levels (not shown). B-D. Immunostaining of primary VS cultures with anti-p75NTR (B, green) and anti-sortilin (C, red) antibodies (D, combined). Nuclei were labeled with DAPI (blue). E. Western blot of protein lysates from rat SC and primary human VS cultures probed with anti-RIP2 antibodies. Blots present the lysates with the highest and lowest sortilin expression compared with b-actin. Bots for lysates from 2 additional cultures from separate tumors demonstrated similar expression levels (not shown).
ProNGF protects VS cells from apoptosis due to inhibition of JNK
Because ProNGF induces apoptosis in sympathetic neurons and SCs by activating JNK whereas JNK activity promotes VS cell survival (Linggi et al. 2005; Yue et al. 2011), we next sought to characterize the interaction of p75NTR and JNK signaling on VS cell survival. Primary human VS cultures were treated with I-JIP, a peptide inhibitor that blocks JNK activation by disrupting binding to the JNK scaffolding protein, JIP, or SP600125, a small molecule JNK inhibitor that competitively blocks kinase activity. We have previously shown that these inhibitors effectively and specifically reduce JNK signaling in VS cultures (Yue et al. 2011). A subset of cultures were simultaneously treated with proNGF. As previously reported, I-JIP (30 or 100 μM) and SP600125 (20 μM), significantly increased VS cell apoptosis (Fig 4A-D). We confirmed apoptosis in cultures by immunoblotting protein lysates from the cultures with anti-active caspase 3 antibody (Fig. 4E). Treatment with proNGF significantly reduced the percent of TUNEL-positive VS cells and the extent of caspase 3 activation in the presence of the JNK inhibitors (Fig. 4A-E). To further verify that p75NTR ligands protect VS cells from apoptosis we treated cultures maintained in the presence of SP600125 with proBNDF. As with proNGF, proBDNF failed to induce VS cell apoptosis and protected the cells from apoptosis due to SP600125 (Fig. 4D). We have previously confirmed the ability of proBDNF to induce apoptosis in non-neoplastic SCs (Provenzano et al. 2011; Provenzano et al. 2008). To determine whether other potential survival factors could likewise prevent VS cell apoptosis due to JNK inhibition we treated cultures with 3 nM neuregulin β-1, a high affinity ErbB2/B3 ligand and potent growth factor for SC and VS cells (Hansen et al., 2006; (Hansen et al. 2008; Yue et al. 2011). In contrast to proNGF, neuregulin β-1 significantly increased the percent of apoptotic VS cells (Fig. 4H).
Figure 4.
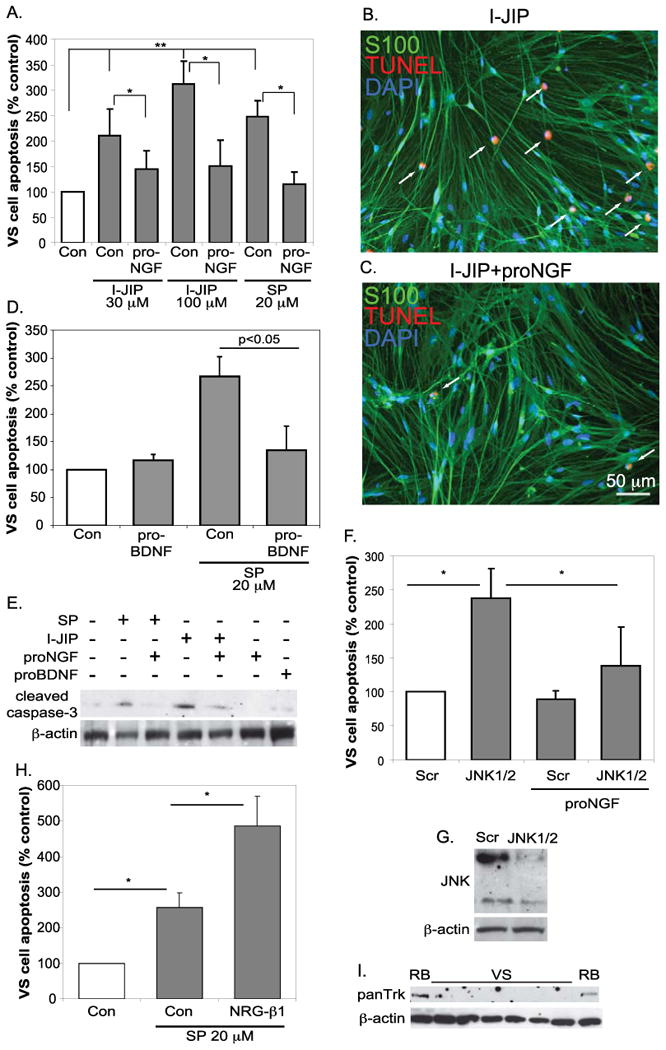
ProNGF protects VS cells from apoptosis due to JNK inhibition. A-D. Primary VS cultures were maintained in the presence of the JNK inhibitors, I-JIP or SP600125, with or without treatment with proNGF (3 nM) (A) of proBDNF (3 nM) (D). Cultures were immunostained with anti-S100 (green) and labeled with TUNEL (red). Nuclei were labeled with DAPI (blue). The average percent of TUNEL-positive, S100-positive condensed nuclei were scored and plotted relative to cultures not treated with JNK inhibitors or proNGF (control). Data are from cultures derived from six separate patients. *p<0.05, **p<0.01 by one way ANOVA followed by Dunn's method. E. Western blot of protein lysates from primary VS cultures treated with the indicated reagents probed with anti-cleaved caspase-3 antibodies. Blots were stripped and reprobed with anti-β-actin antibodies. F. Average percent apoptosis in primary VS cultures transfected with scrambled or anti-JNK1/2 siRNA oligonucleotides and maintained in the presence or absence of proNGF. Comparison for differences among conditions by one way ANOVA followed by Dunn's method. G. Western blot of protein lysates from primary VS cultures transfected with scrambled (Scr) or JNK1/2 targeted siRNA oligonucleotides and probed with anti-JNK antibodies. Blots were stripped and reprobed with anti-b-actin antibodies. H. Average percent apoptosis in primary VS cultures treated with or without neuregulin (NRG) β-1 and maintained in the presence or absence of proNGF. Comparison for differences among conditions by Kruskal-Wallis one way ANOVA on ranks. I. Western blot of protein lysates from primary VS and rat brain (RB) specimens with an anti-panTrk antibody. The blots were stripped and reprobed with anti-β actin antibodies.
To confirm that proNGF rescues VS cells from apoptosis due to loss of JNK activity, we transfected VS cultures with siRNA oligonucleotides targeting JNK1 and JNK2 and maintained the cultures in the presence or absence of proNGF. We verified that these oligonucleotides effectively and specifically reduce JNK1/2 expression in VS cells (Fig. 4G) as previously shown (Yue et al. 2011). Control cultures were transfected with a scrambled oligonucleotide. As before, treatment with proNGF significantly reduced the percent of VS cells undergoing apoptosis due to inhibition of JNK signaling (Fig. 4F). We considered the possibility that proNGF could be signaling through Trk receptors, the high affinity receptor tyrosine kinases for mature neurotrophins such as NGF, to promote VS cell survival. However, Trks were not detected in immunoblots of protein lysates from VS cultures using an anti-panTrk antibody capable of detecting all Trk isoforms including TrkA, TrκB, and TrkC (Fig. 4I). The anti-panTrk did detect Trk expression in protein lysates from the rat brain (cerebral cortex). Taken together, these results suggest that p75NTR, but not Erb2, signaling promotes survival of VS cells with suppressed JNK activity.
ProNGF activates NF-κB independent of JNK
In neuroblastoma cells, p75NTR signaling activates the transcription factor, NF-κB, in a JNK dependent fashion (Costantini et al. 2005). As previously reported (Yue et al. 2011), we confirmed that JNK activity is persistently high in VS cultures due to lack of functional merlin by immunoblotting protein lysates of VS cultures, treated with Ad5.empty vector or Ad5.merlin, with antibodies that detect phosphorylated JNK (Fig. 5). Treatment with proNGF led to an increase in JNK activity in VS cultures that had not been transduced with adenoviral vectors, reflected by a slight increase in JNK phosphorylation and a further increase in c-Jun phosphorylation (Fig. 5B). We next assayed NF-κB activity in VS cultures. Treatment with proNGF increased NF-κB activity by 2-fold in VS cells (Fig. 5C) whereas the JNK inhibitor SP600125 decreased NF-κB activity by 2-fold (Fig. 5C). Significantly, the increase in NF-κB activity induced by proNGF was not attenuated by SP600125. Similarly, transfection of VS cultures with siRNA oligonucleotides targeting JNK1/2 decreased basal levels of NF-κB activity but did not reduce NF-κB activation by proNGF (Fig. 5D). These results suggest that basal levels of NF-κB activity depend on JNK in VS cells whereas proNGF can activate NF-κB independent of JNK signaling.
Figure 5.
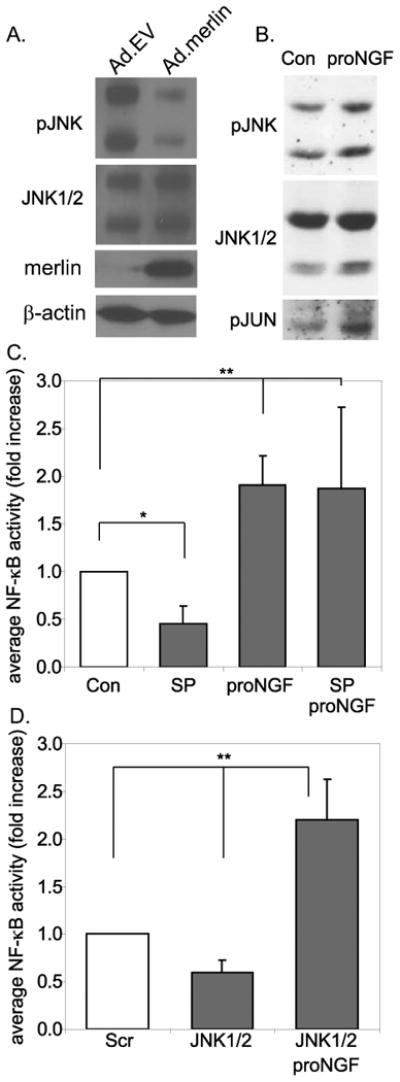
ProNGF activates JNK and NF-κB in primary VS cultures. A. Western blots of protein lysates from primary VS cultures treated Ad5.empty vector (Ad.EV) or Ad5.merlin (Ad.merlin) and probed with anti-phosphorylated JNK antibodies. The blots were stripped and reprobed with non phospho-specific anti-JNK. Parallel blots were probed with anti-merlin and subsequently with anti-b-actin antibodies.B. Western blots of protein lysates from primary VS cultures treated with proNGF and probed with anti-phosphorylated JNK. The blots were stripped and reprobed with non phospho-specific anti-JNK and subsequently with anti-phosphorylated c-JUN antibodies. C&D. Average NF-κB activity, relative to control, in primary VS cultures treated with SP600125 (SP) or proNGF or transfected with scrambled or JNK1/2 specific siRNA oligonucleotides. Data in B are from cultures derived from six separate patients and in C from four separate patients. *p<0.05, **p<0.01 by one way ANOVA followed by Dunn's method.
NF-κB is required for the prosurvival effects of p75NTR signaling
We next sought to determine whether the activation of NF-κB by proNGF contributes to the ability of p75NTR signaling to promote VS cell survival. To inhibit NF-κB function, we transduced VS cultures with an adenoviral vector that expresses a mutant isoform of IκBα (Ad5.IκBαS. This isoform is not able to be phosphorylated on S32 and S36 and thus blocks NF-κB activation by preventing the dissociation IκBα from NF-κB subunits and their subsequent translocation to the nucleus. We have previously shown that Ad5 vectors transduce >85% of VS cells at the titer used here (Yue et al. 2011). Transfection of VS cultures with Ad5.IκBαS effectively inhibited activation of NF-SκB by proNGF and reduced basal levels of NF-κB activity (Fig. 6). The fold increase in NF-κB by proNGF was greater in cultures treated with Ad5.empty vector (Fig. 6) compared with cultures maintained without viral vectors (Fig. 5B) raising the possibility that adenoviral vectors sensitize VS cell cultures to upstream activators of NF-κB. In cultures transduced with an Ad5.empty vector and maintained in SP600125, proNGF significantly reduced the percent of TUNEL-positive VS cells (Fig. 7). The percent of TUNEL-positive VS cells was significantly increased in cultures transduced with Ad5.IκBαS compared with cultures transduced with Ad5.empty vector even in the presence of proNGF (Fig. 7). Thus, inhibition of NF-κB abolishes the ability of proNGF to rescue VS cells indicating that activation of NF-κB is necessary for the prosurvival effect of p75NTR on VS cells.
Figure 6.
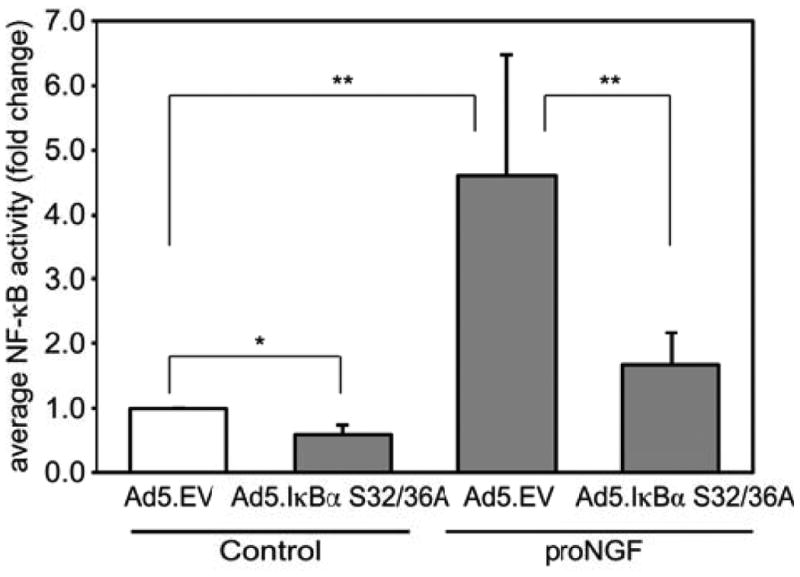
Expression of IκBαS32/36A inhibits NF-κB in primary VS cultures. Average NF-κB activity in primary VS cultures transduced with with Ad5.empty vector (EV) or Ad5.IκBαS32/36A and treated with proNGF or without proNGF. Activity is expressed as fold change relative to control (Ad5.EV without proNGF). Data are from cultures derived from four separate patients. *p<0.05, **p<0.01 by one way ANOVA followed by Holm Sidak post hoc method.
Figure 7.
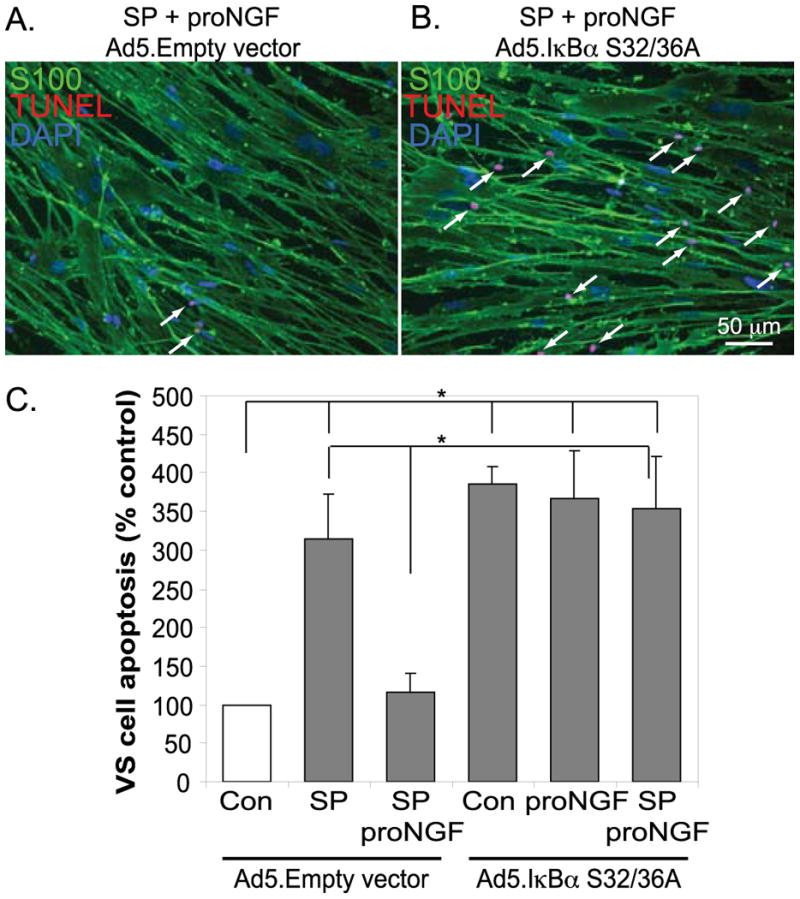
Inhibition of NF-κB limits the ability of proNGF to rescue of VS cell from apoptosis. Primary VS cultures were transduced with Ad5.Empty vector or Ad5.IκBαS32/36A and maintained in the presence or absence of SP600125 (SP, 20 μM) with or without treatment with proNGF (3 nM). A&B. Cultures were immunostained with anti-S100 (green) and labeled with TUNEL (red). Nuclei were labeled with DAPI (blue). C. The average percent of TUNEL-positive, S100-positive condensed nuclei were scored and plotted relative to control (Con) cultures. Data are from cultures derived from four separate patients. *p<0.05 by one way ANOVA followed by Dunn's method.
Discussion
Limited access to primary tissue has hampered the investigation of human schwannoma tumorigenesis. Transgenic mouse models and transformed cell lines provide useful tools to investigate merlin-dependent tumorigenesis however, they fail to fully recapitulate human disease including VS formation (Giovannini et al. 1999; Giovannini et al. 2000; Gutmann and Giovannini 2002; Hung et al. 2002). Here we used primary tissue derived from acutely resected VSs to investigate the contribution of p75NTR to VS cell responses. Although primary cultures limit some of the analyses that can be performed, they provide a more realistic model of human disease compared with transformed cell lines (e.g. HEI193 cells). We find that p75NTR expression is elevated in VS cells. This elevated expression represents an increase both in transcript and protein levels. Non-neoplastic SCs likewise increase p75NTR expression following axotomy and this increase in p75NTR contributes to SC apoptosis following loss of axonal contact (Ferri and Bisby 1999; Petratos S 2003; Provenzano et al. 2008; Syroid et al. 2000; Taniuchi et al. 1986). By contrast, activation of p75NTR fails to induce VS cell apoptosis. Further, p75NTR provides an anti-apoptotic response in VS cells in contrast to its pro-apoptotic function in non-neoplastic SCs. This key difference in the response of VS cells to p75NTR likely contributes to the ability of VS cells to proliferate and survive in the absence of axons, whereas non-neoplastic SCs are eventually lost following nerve injury.
Merlin has previously been shown to decrease the expression and, in some cases, alter the subcellular localization of receptor tyrosine kinases such as ErbB1, ErbB2 and PDGF receptors in schwannoma cells and non-neoplastic SCs (Ammoun et al. 2010; Ammoun et al. 2008; Brown and Hansen 2008; Doherty et al. 2008; Fernandez-Valle et al. 2002; Fraenzer et al. 2003; Hansen and Linthicum 2004; Hansen et al. 2006; Houshmandi et al. 2009; Lallemand et al. 2009; Stonecypher et al. 2006; Torres-Martin et al. 2013; Wickremesekera et al. 2007). Thus, ErbB2 expression remains high in VS cells and contributes to cell proliferation and possibly cell survival and tumor growth (Ahmad et al. 2011; Ammoun et al. 2010; Bush et al. 2012; Clark et al. 2008; Doherty et al. 2008; Hansen et al. 2006; Lallemand et al. 2009; Stonecypher et al. 2006; Torres-Martin et al. 2013). In addition to receptor tyrosine kinases, merlin reduces the expression of other transmembrane receptors such as CD44 (Ahmad et al. 2010). The increased p75NTR expression in VS tissue compared to normal nerve raises the possibility that merlin likewise regulates p75NTR expression levels.
In addition to regulating the expression of transmembrane receptors, merlin also suppresses cell motility and proliferation by inhibiting the activity of several intracellular kinase signaling cascades including Ras-Raf-MEK-ERK, PI3-K-Akt, JNK, and mTORC1 (Bosco et al. 2010; Chadee and Kyriakis 2004; Chadee et al. 2006; Flaiz et al. 2009; Fraenzer et al. 2003; Houshmandi et al. 2009; Jacob et al. 2008; James et al. 2009; James et al. 2012; Kaempchen et al. 2003; Kissil et al. 2003; Lim et al. 2003; Lopez-Lago et al. 2009; Rong et al. 2004; Yi et al. 2008; Zhou et al. 2011). Of these, the Ras-Raf-MEK-ERK and PI3-K-Akt and mTORC1 cascades appear to principally promote proliferation in schwannoma cells such that MEK, Akt, and mTORC1 inhibitors provide a cytostatic response, but do not consistently lead to cell death. However, treatment of VS cells with OSU-03012, a celecoxib-derived small-molecule inhibitor of phosphoinositide-dependent kinase-1 and Akt, increased cell death (James et al. 2009; Lee et al. 2009; Yue et al. 2011). Thus, whether Akt promotes VS cell survival in addition to proliferation requires confirmation with more specific, non-pharmacological methods to suppress Akt activity. Merlin also suppresses proliferation by inhibiting the function of YAP1 and, in the nucleus, CRL4DCAF1 E3 ubiquitin ligase (Baia et al. 2012; Hamaratoglu et al. 2006; Li et al. 2010; Striedinger et al. 2008; Zhang et al. 2010).
Recent reports confirm that JNK is activated in VS cells in a merlin-dependent fashion and contributes to cell proliferation and survival (Hilton et al. 2009; Kaempchen et al. 2003; Yue et al. 2011). Suppression of mitochondrial superoxide accumulation represents one mechanism by which JNK reduces VS cell apoptosis (Yue et al. 2011). Here we demonstrate a requirement for JNK activity to sustain basal levels of NF-κB in VS cells and this likely also contributes to the necessity of JNK activity to support VS cell survival.
VS cells are resistant to the pro-apoptotic effect of p75NTR signaling
The response of cells to p75NTR depends on several factors including expression of co-receptors and the differentiation state of the cell (Casaccia-Bonnefil et al. 1999). In neurons and some carcinoma cells (e.g. breast) that express Trk receptors, activation of p75NTR inhibits apoptosis (Descamps et al. 2001; Hondermarck 2012; Koshimizu et al. 2010; Lu et al. 2005). In these cases, p75NTR appears to function by facilitating neurotrophin binding to the Trk receptors and Trk signaling (Chao et al. 1998). In cells that lack Trk receptors, including non-neoplastic SCs, mature and pro isoforms of NTs induce apoptosis (Kuchler et al. 2011; Provenzano et al. 2011; Truzzi et al. 2011). In these cells p75NTR appears to activate JNK to induce apoptosis and requires the co-receptor, sortilin (Bhakar et al. 2003; Linggi et al. 2005; Skeldal et al. 2012; Truzzi et al. 2011; Yoon et al. 1998). By contrast, proNGF, which increases JNK activity in VS cells, fails to induce apoptosis in VS cells. One key difference between non-neoplastic SCs and VS cells is that JNK activity induces apoptosis in the former but promotes cell proliferation and survival in the later (Yue et al. 2011). This ability of VS cells to survive in the presence of increased JNK activity may explain, at least in part, the failure of proNGF to induce VS cell apoptosis. Importantly, we confirmed that VS cells express the p75NTR co-receptor, sortilin, and adaptor protein, RIP2, suggesting that the failure of proNGF to induce VS cell apoptosis is not due to the lack of expression of these proteins essential for p75NTR-mediated apoptosis (Charalampopoulos et al. 2012; Khursigara et al. 2001).
p75NTR mediates an anti-apoptotic response in VS cells
The data presented here suggest that proNGF/p75NTR signaling elicits an anti-apoptotic response in VS cells and thereby may contribute to tumorigenesis. In addition to their effects on neuronal development, plasticity and injury, there has been a recent recognition that neurotrophins and their receptors contribute to tumorigenesis. For example proNGF stimulates invasion of melanoma cells through a mechanism involving p75NTR and sortilin (Truzzi et al. 2008). In this study, proNGF did not influence melanoma cell survival or proliferation, but did promote melanoma cell migration. On the other hand, inhibition of Trks, all of which are expressed in melanoma cells, led to apoptosis in addition to decreasing migration and proliferation in response to neurotrophins. Similarly, p75NTR has been implicated in glioma cell invasion (Johnston et al. 2007; Wang et al. 2010). In breast carcinoma, p75NTR appears to promote cell migration and invasion and, at least in some cell lines, exerts anti-apoptotic effects via activation of NF-κB and p21(waf1) (Descamps et al. 2001; Hondermarck 2012; Verbeke et al. 2010). By contrast, p75NTR inhibits the invasive and metastatic abilities of gastric cancer cells, at least in part, by NF-κB-dependent up-regulation of tissue inhibitor of matrix metalloproteinase (TIMP)-1 (Jin et al. 2007).
proNGF activates NF-κB independent of JNK to promote VS cell survival
NF-κB plays a critical role in immune responses. It is also widely recognized as a key mediator of tumorigenesis (Baldwin 2012). Generally, NF-κB promotes tumor formation and growth by supporting cell survival although it has been found to have pro-apoptotic functions in some circumstances. Likewise, in the nervous system, NF-κB appears to exert either pro-apoptotic or anti-apoptotic effects, depending on the cell type and context (Maggirwar et al. 1998; Mattson et al. 1997; Schneider et al. 1999).
In addition to its role in immune responses and tumorigenesis, NF-κB appears to contribute to normal SC development and response to injury. For example, activation of NF-κB is essential for driving immature SCs into a promyelinating phenotype in dorsal root ganglion-SC co-cultures (Limpert and Carter 2010; Nickols et al. 2003; Yoon et al. 2008). Peripheral nerve injury significantly increases NF-κB activity and inhibition of NF-κB activation in SCs transiently delays axonal regeneration and compact remyelination (Fernyhough et al. 2005; Fu et al. 2010; Ma and Bisby 1998; Morton et al. 2012; Pollock et al. 2005). Further, activation of p75NTR by NGF treatment increases NF-κB activity in SCs in a TRAF6 and RIP2-dependent fashion (Carter et al. 1996; Khursigara et al. 2001; Khursigara et al. 1999; Yeiser et al. 2004). The extent to which the increase of NF-κB activity in SCs following nerve injury results from the concurrent upregulation of p75NTR and activation of JNK remains unknown.
Here we find that p75NTR activates JNK and NF-κB in primary VS cells. Typically, NF-κB activation by p75NTR is TRAF6 and JNK dependent (Costantini et al. 2005). By contrast, p75NTR activates JNK and promotes apoptosis in keratinocytes, but suppresses NF-κB activity (Truzzi et al. 2011). The effect of NF-κB activation by p75NTR on cell survival appears to depend on the cell type. For example, p75NTR activates JNK and NF-κB to induce apoptosis in neuroblastoma cells (Bai et al. 2008; Costantini et al. 2005; Kuner and Hertel 1998). By contrast, NGF activates JNK and NF-κB in the RN22 schwannoma cell line and induces apoptosis in cells with suppressed NF-κB activity (Gentry et al. 2000). Our results indicate that basal NF-κB activity in primary VS cells depends on JNK activity as both SP600125 and siRNA mediated JNK knock-down reduced NF-κB activity. However, we find that p75NTR can activate NF-κB independent of JNK in VS cells. This activation of NF-κB, even in cells with suppressed JNK activity, appears to contribute to ability of p75NTR to prevent VS cell apoptosis.
NF-κB activation promotes survival of cells treated with many different classes of chemotherapeutics or ionizing radiation and inhibition of NF-κB potently enhances chemo- and radiotoxicity (Wang et al. 1999; Wang et al. 1996). JNK inhibitors represents one class of chemotherapeutics that reduce VS cell proliferation and survival (Yue et al. 2011). The ability of p75NTR to activate NF-κB independent of JNK appears to provide a cytoprotective effect in VS cells treated with JNK inhibitors. Parallel targeting of the p75NTR/NF-κB signaling axis offers the prospect of enhancing the response of VS cells to JNK inhibitors and perhaps to other chemotherapeutics and radiation therapy. One advantage of therapeutically targeting p75NTR function in VS cells is that it would represent a fairly specific target since inhibition of p75NTR signaling supports the survival of non-neoplastic SCs.
Main points.
Vestibular schwannoma (VS) cells express high levels of p75NTR and, in constrast to non-neoplastic Schwann cells, are resistant to p75NTR and JNK-mediated apoptosis.
p75NTR ligands protect VS cells from apoptosis by activating NF-κB independent of JNK.
The paradoxical pro-survival effects of p75NTR and JNK in VS cells compared to their pro-apoptotic function in non-neoplastic Schwann cells raises the possibility of therapeutically targeting p75NTR and/or JNK to specifically limit schwannoma growth.
Acknowledgments
Support: NIDCD R01DC009801, P30DC010362
References
- Ahmad Z, Brown CM, Patel AK, Ryan AF, Ongkeko R, Doherty JK. Merlin knockdown in human Schwann cells: Clues to vestibular schwannoma tumorigenesis. Otol Neurotol. 2010;31:467–472. doi: 10.1097/MAO.0b013e3181d2777f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad ZK, Brown CM, Cueva RA, Ryan AF, Doherty JK. ErbB expression, activation, and inhibition with lapatinib and tyrphostin (AG825) in human vestibular schwannomas. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011;32:841–7. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Cunliffe CH, Allen JC, Chiriboga L, Giancotti FG, Zagzag D, Hanemann CO, Karajannis MA. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro-oncology. 2010;12:834–43. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–45. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- Bai Y, Li Q, Yang J, Zhou X, Yin X, Zhao D. p75(NTR) activation of NF-kappaB is involved in PrP106-126-induced apoptosis in mouse neuroblastoma cells. Neuroscience research. 2008;62:9–14. doi: 10.1016/j.neures.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Baia GS, Caballero OL, Orr BA, Lal A, Ho JS, Cowdrey C, Tihan T, Mawrin C, Riggins GJ. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Molecular cancer research : MCR. 2012;10:904–13. doi: 10.1158/1541-7786.MCR-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunological reviews. 2012;246:327–45. doi: 10.1111/j.1600-065X.2012.01095.x. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA. Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:11373–81. doi: 10.1523/JNEUROSCI.23-36-11373.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti B, Panzeri L, Carner M, Zamboni G, Rizzuto N, Moretto G. Human neoplastic Schwann cells: changes in the expression of neurotrophins and their low-affinity receptor p75. Neuropathol Appl Neurobiol. 1997;23:380–6. [PubMed] [Google Scholar]
- Bosco EE, Nakai Y, Hennigan RF, Ratner N, Zheng Y. NF2-deficient cells depend on the Rac1-canonical Wnt signaling pathway to promote the loss of contact inhibition of proliferation. Oncogene. 2010;29:2540–9. doi: 10.1038/onc.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annual review of neuroscience. 1995;18:223–53. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Brown KD, Hansen MR. Lipid raft localization of erbB2 in vestibular schwannoma and Schwann cells. Otol Neurotol. 2008;29:79–85. doi: 10.1097/mao.0b013e31815dbb11. [DOI] [PubMed] [Google Scholar]
- Bush ML, Burns SS, Oblinger J, Davletova S, Chang LS, Welling DB, Jacob A. Treatment of vestibular schwannoma cells with ErbB inhibitors. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:244–57. doi: 10.1097/MAO.0b013e31823e287f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–5. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Gu C, Chao MV. Neurotrophins in cell survival/death decisions. Advances in experimental medicine and biology. 1999;468:275–82. doi: 10.1007/978-1-4615-4685-6_22. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol. 2004;6:770–6. doi: 10.1038/ncb1152. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Xu D, Hung G, Andalibi A, Lim DJ, Luo Z, Gutmann DH, Kyriakis JM. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci U S A. 2006;103:4463–8. doi: 10.1073/pnas.0510651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao M, Casaccia-Bonnefil P, Carter B, Chittka A, Kong H, Yoon SO. Neurotrophin receptors: mediators of life and death. Brain research Brain research reviews. 1998;26:295–301. doi: 10.1016/s0165-0173(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nature reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I, Vicario A, Pediaditakis I, Gravanis A, Simi A, Ibanez CF. Genetic Dissection of Neurotrophin Signaling through the p75 Neurotrophin Receptor. Cell reports. 2012;2:1563–70. doi: 10.1016/j.celrep.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Provenzano M, Diggelmann HR, Xu N, Hansen SS, Hansen MR. The ErbB inhibitors trastuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: a preliminary study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:846–53. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewes O, Fahey MS, Tyler SJ, Watson JJ, Seok H, Catania C, Cho K, Dawbarn D, Allen SJ. Human ProNGF: biological effects and binding profiles at TrkA, P75NTR and sortilin. Journal of neurochemistry. 2008;107:1124–35. doi: 10.1111/j.1471-4159.2008.05698.x. [DOI] [PubMed] [Google Scholar]
- Costantini C, Rossi F, Formaggio E, Bernardoni R, Cecconi D, Della-Bianca V. Characterization of the signaling pathway downstream p75 neurotrophin receptor involved in beta-amyloid peptide-dependent cell death. Journal of molecular neuroscience : MN. 2005;25:141–56. doi: 10.1385/JMN:25:2:141. [DOI] [PubMed] [Google Scholar]
- Descamps S, Toillon RA, Adriaenssens E, Pawlowski V, Cool SM, Nurcombe V, Le Bourhis X, Boilly B, Peyrat JP, Hondermarck H. Nerve growth factor stimulates proliferation and survival of human breast cancer cells through two distinct signaling pathways. The Journal of biological chemistry. 2001;276:17864–70. doi: 10.1074/jbc.M010499200. [DOI] [PubMed] [Google Scholar]
- Dobrowski RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–9. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Doherty JK, Ongkeko W, Crawley B, Andalibi A, Ryan AF. ErbB and Nrg: Potential Molecular Targets for Vestibular Schwannoma Pharmacotherapy. Otol Neurotol. 2008;29:50–57. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- Evans DG. Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II] Genet Med. 2009;11:599–610. doi: 10.1097/GIM.0b013e3181ac9a27. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E, Biggerstaff J, Iacovelli J. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet. 2002;31:354–62. doi: 10.1038/ng930. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Smith DR, Schapansky J, Van Der Ploeg R, Gardiner NJ, Tweed CW, Kontos A, Freeman L, Purves-Tyson TD, Glazner GW. Activation of nuclear factor-kappaB via endogenous tumor necrosis factor alpha regulates survival of axotomized adult sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1682–90. doi: 10.1523/JNEUROSCI.3127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri CC, Bisby MA. Improved survival of injured sciatic nerve Schwann cells in mice lacking the p75 receptor. Neurosci Lett. 1999;272:191–4. doi: 10.1016/s0304-3940(99)00618-7. [DOI] [PubMed] [Google Scholar]
- Flaiz C, Chernoff J, Ammoun S, Peterson JR, Hanemann CO. PAK kinase regulates Rac GTPase and is a potential target in human schwannomas. Exp Neurol. 2009;218:137–44. doi: 10.1016/j.expneurol.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenzer JT, Pan H, Minimo L, Jr, Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23:1493–500. [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148:509–18. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JJ, Casaccia-Bonnefil P, Carter BD. Nerve growth factor activation of nuclear factor kappaB through its p75 receptor is an anti-apoptotic signal in RN22 schwannoma cells. J Biol Chem. 2000;275:7558–65. doi: 10.1074/jbc.275.11.7558. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, van der Valk M, Woodruff JM, Goutebroze L, Merel P, Berns A, Thomas G. Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev. 1999;13:978–86. doi: 10.1101/gad.13.8.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, Woodruff JM, Berns A, Thomas G. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–30. [PMC free article] [PubMed] [Google Scholar]
- Gutmann DH, Giovannini M. Mouse models of neurofibromatosis 1 and 2. Neoplasia. 2002;4:279–90. doi: 10.1038/sj.neo.7900249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nature cell biology. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Clark JJ, Gantz BJ, Goswami PC. Effects of ErbB2 signaling on the response of vestibular schwannoma cells to gamma-irradiation. Laryngoscope. 2008;118:1023–30. doi: 10.1097/MLG.0b013e318163f920. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Linthicum FH., Jr Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol Neurotol. 2004;25:155–9. doi: 10.1097/00129492-200403000-00013. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Roehm PC, Chatterjee P, Green SH. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- Harrington AW, Kim JY, Yoon SO. Activation of Rac GTPase by p75 is necessary for c-jun N-terminal kinase-mediated apoptosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:156–66. doi: 10.1523/JNEUROSCI.22-01-00156.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–83. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hilton DA, Ristic N, Hanemann CO. Activation of ERK, AKT and JNK signalling pathways in human schwannomas in situ. Histopathology. 2009;55:744–9. doi: 10.1111/j.1365-2559.2009.03440.x. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hibasami H, Yoshida T, Ogawa M, Matsumoto M, Morita A, Uchida A. Nerve growth factor signaling of p75 induces differentiation and ceramide-mediated apoptosis in Schwann cells cultured from degenerating nerves. Glia. 2001;36:245–58. doi: 10.1002/glia.1113. [DOI] [PubMed] [Google Scholar]
- Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine & growth factor reviews. 2012;23:357–65. doi: 10.1016/j.cytogfr.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Houshmandi SS, Emnett RJ, Giovannini M, Gutmann DH. The neurofibromatosis 2 protein, merlin, regulates glial cell growth in an ErbB2- and Src-dependent manner. Mol Cell Biol. 2009;29:1472–86. doi: 10.1128/MCB.01392-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS, Slattery W, Lim D. Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2. Int J Oncol. 2002;20:475–82. [PubMed] [Google Scholar]
- Jacob A, Lee TX, Neff BA, Miller S, Welling B, Chang LS. Phosphatidylinositol 3-kinase/AKT pathway activation in human vestibular schwannoma. Otol Neurotol. 2008;29:58–68. doi: 10.1097/mao.0b013e31816021f7. [DOI] [PubMed] [Google Scholar]
- James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Molecular and cellular biology. 2009;29:4250–61. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MF, Stivison E, Beauchamp R, Han S, Li H, Wallace MR, Gusella JF, Stemmer-Rachamimov AO, Ramesh V. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Molecular cancer research : MCR. 2012;10:649–59. doi: 10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, et al. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nature neuroscience. 2007;10:1449–57. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Jin H, Pan Y, He L, Zhai H, Li X, Zhao L, Sun L, Liu J, Hong L, Song J, et al. p75 neurotrophin receptor inhibits invasion and metastasis of gastric cancer. Molecular cancer research : MCR. 2007;5:423–33. doi: 10.1158/1541-7786.MCR-06-0407. [DOI] [PubMed] [Google Scholar]
- Johnston AL, Lun X, Rahn JJ, Liacini A, Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth PA, et al. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS biology. 2007;5:e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–21. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–32. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Khursigara G, Bertin J, Yano H, Moffett H, DiStefano PS, Chao MV. A prosurvival function for the p75 receptor death domain mediated via the caspase recruitment domain receptor-interacting protein 2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:5854–63. doi: 10.1523/JNEUROSCI.21-16-05854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. The Journal of biological chemistry. 1999;274:2597–600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell. 2003;12:841–9. doi: 10.1016/s1097-2765(03)00382-4. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Hazama S, Hara T, Ogura A, Kojima M. Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neuroscience letters. 2010;473:229–32. doi: 10.1016/j.neulet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Kuchler J, Hartmann W, Waha A, Koch A, Endl E, Wurst P, Kindler D, Mikeska T, Goodyer CG, Buttner R, et al. p75(NTR) induces apoptosis in medulloblastoma cells. International journal of cancer Journal international du cancer. 2011;128:1804–12. doi: 10.1002/ijc.25508. [DOI] [PubMed] [Google Scholar]
- Kuner P, Hertel C. NGF induces apoptosis in a human neuroblastoma cell line expressing the neurotrophin receptor p75NTR. Journal of neuroscience research. 1998;54:465–74. doi: 10.1002/(SICI)1097-4547(19981115)54:4<465::AID-JNR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, Lampin A, Niwa-Kawakita M, Kalamarides M, Giovannini M. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- Laskin WB, Fetsch JF, Lasota J, Miettinen M. Benign epithelioid peripheral nerve sheath tumors of the soft tissues: clinicopathologic spectrum of 33 cases. Am J Surg Pathol. 2005;29:39–51. doi: 10.1097/01.pas.0000146044.90901.4c. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–8. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lee TX, Packer MD, Huang J, Akhmametyeva EM, Kulp SK, Chen CS, Giovannini M, Jacob A, Welling DB, Chang LS. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. European journal of cancer. 2009;45:1709–20. doi: 10.1016/j.ejca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cooper J, Karajannis MA, Giancotti FG. Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO reports. 2012 doi: 10.1038/embor.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Kim H, Kim YH, Kim SW, Huh PW, Lee KH, Jeun SS, Rha HK, Kang J. Merlin suppresses the SRE-dependent transcription by inhibiting the activation of Ras-ERK pathway. Biochem Biophys Res Commun. 2003;302:238–45. doi: 10.1016/s0006-291x(03)00124-4. [DOI] [PubMed] [Google Scholar]
- Limpert AS, Carter BD. Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. The Journal of biological chemistry. 2010;285:16614–22. doi: 10.1074/jbc.M109.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linggi MS, Burke TL, Williams BB, Harrington A, Kraemer R, Hempstead BL, Yoon SO, Carter BD. Neurotrophin receptor interacting factor (NRIF) is an essential mediator of apoptotic signaling by the p75 neurotrophin receptor. J Biol Chem. 2005;280:13801–8. doi: 10.1074/jbc.M410435200. [DOI] [PubMed] [Google Scholar]
- Lopez-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Molecular and cellular biology. 2009;29:4235–49. doi: 10.1128/MCB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature reviews Neuroscience. 2005;6:603–14. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Increased activation of nuclear factor kappa B in rat lumbar dorsal root ganglion neurons following partial sciatic nerve injuries. Brain research. 1998;797:243–54. doi: 10.1016/s0006-8993(98)00380-1. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Sarmiere PD, Dewhurst S, Freeman RS. Nerve growth factor-dependent activation of NF-kappaB contributes to survival of sympathetic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:10356–65. doi: 10.1523/JNEUROSCI.18-24-10356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi R, Ioannou MS, Coughlin MD, Pagadala P, Neet KE, Clewes O, Allen SJ, Dawbarn D, Fahnestock M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. The Journal of biological chemistry. 2009;284:18424–33. doi: 10.1074/jbc.M109.007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. Journal of neuroscience research. 1997;49:681–97. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis--the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–77. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: a clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol. 2001;25:846–55. doi: 10.1097/00000478-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Morton PD, Johnstone JT, Ramos AY, Liebl DJ, Bunge MB, Bethea JR. Nuclear factor-kappaB activation in Schwann cells regulates regeneration and remyelination. Glia. 2012;60:639–50. doi: 10.1002/glia.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nature neuroscience. 2003;6:161–7. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. The Journal of biological chemistry. 2010;285:5361–8. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Dong Z, Bunting H, Whitfield J, Meier C, Marie H, Mirsky R, Jessen KR. Transforming growth factor beta (TGFbeta) mediates Schwann cell death in vitro and in vivo: examination of c-Jun activation, interactions with survival signals, and the relationship of TGFbeta-mediated death to Schwann cell differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8572–85. doi: 10.1523/JNEUROSCI.21-21-08572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petratos SBH, Shipham K, Cooper H, Bucci T, Reid K, Lopez E, Emery B, Cheema SS, Kilpatrick TJ. Schwann cell apoptosis in the postnatal axotomized sciatic nerve is mediated via NGF through the low-affinity neurotrophin receptor. J Neuropathol Exp Neurol. 2003;62:398–411. doi: 10.1093/jnen/62.4.398. [DOI] [PubMed] [Google Scholar]
- Pollock G, Pennypacker KR, Memet S, Israel A, Saporta S. Activation of NF-kappaB in the mouse spinal cord following sciatic nerve transection. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2005;165:470–7. doi: 10.1007/s00221-005-2318-6. [DOI] [PubMed] [Google Scholar]
- Provenzano MJ, Minner SA, Zander K, Clark JJ, Kane CJ, Green SH, Hansen MR. p75(NTR) expression and nuclear localization of p75(NTR) intracellular domain in spiral ganglion Schwann cells following deafness correlate with cell proliferation. Molecular and cellular neurosciences. 2011;47:306–15. doi: 10.1016/j.mcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano MJ, Xu N, Ver Meer MR, Clark JJ, Hansen MR. p75NTR and sortilin increase after facial nerve injury. The Laryngoscope. 2008;118:87–93. doi: 10.1097/MLG.0b013e31814b8d9f. [DOI] [PubMed] [Google Scholar]
- Rong R, Tang X, Gutmann DH, Ye K. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci U S A. 2004;101:18200–5. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosli C, Linthicum FH, Jr, Cureoglu S, Merchant SN. What is the site of origin of cochleovestibular schwannomas? Audiology & neuro-otology. 2012;17:121–5. doi: 10.1159/000331394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Progress in neurobiology. 2002;67:203–33. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nature medicine. 1999;5:554–9. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- Scoles DR. The merlin interacting proteins reveal multiple targets for NF2 therapy. Biochim Biophys Acta. 2008;1785:32–54. doi: 10.1016/j.bbcan.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Skeldal S, Sykes AM, Glerup S, Matusica D, Palstra N, Autio H, Boskovic Z, Madsen P, Castren E, Nykjaer A, et al. Mapping of the Interaction Site between Sortilin and the p75 Neurotrophin Receptor Reveals a Regulatory Role for the Sortilin Intracellular Domain in p75 Neurotrophin Receptor Shedding and Apoptosis. The Journal of biological chemistry. 2012;287:43798–809. doi: 10.1074/jbc.M112.374710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Ekert P, Bucci T, Syroid D, Bartlett PF, Kilpatrick TJ. Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a Bcl-2-independent pathway. J Neurosci. 1999;19:4828–38. doi: 10.1523/JNEUROSCI.19-12-04828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer-Rachamimov AO, Xu L, Gonzalez-Agosti C, Burwick JA, Pinney D, Beauchamp R, Jacoby LB, Gusella JF, Ramesh V, Louis DN. Universal absence of merlin, but not other ERM family members, in schwannomas. Am J Pathol. 1997;151:1649–54. [PMC free article] [PubMed] [Google Scholar]
- Stonecypher MS, Chaudhury AR, Byer SJ, Carroll SL. Neuregulin growth factors and their ErbB receptors form a potential signaling network for schwannoma tumorigenesis. J Neuropathol Exp Neurol. 2006;65:162–75. doi: 10.1097/01.jnen.0000199575.93794.2f. [DOI] [PubMed] [Google Scholar]
- Striedinger K, VandenBerg SR, Baia GS, McDermott MW, Gutmann DH, Lal A. The neurofibromatosis 2 tumor suppressor gene product, merlin, regulates human meningioma cell growth by signaling through YAP. Neoplasia. 2008;10:1204–12. doi: 10.1593/neo.08642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, Murray S, Cheema S, Lee KF, Lemke G, et al. Induction of postnatal Schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–8. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Martin M, Lassaletta L, San-Roman-Montero J, De Campos JM, Isla A, Gavilan J, Melendez B, Pinto GR, Burbano RR, Castresana JS, et al. Microarray analysis of gene expression in vestibular schwannomas reveals SPP1/MET signaling pathway and androgen receptor deregulation. International journal of oncology. 2013;42:848–62. doi: 10.3892/ijo.2013.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, Eldridge R, Kley N, Menon AG, Pulaski K, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Truzzi F, Marconi A, Atzei P, Panza MC, Lotti R, Dallaglio K, Tiberio R, Palazzo E, Vaschieri C, Pincelli C. p75 neurotrophin receptor mediates apoptosis in transit-amplifying cells and its overexpression restores cell death in psoriatic keratinocytes. Cell death and differentiation. 2011;18:948–58. doi: 10.1038/cdd.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truzzi F, Marconi A, Lotti R, Dallaglio K, French LE, Hempstead BL, Pincelli C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. The Journal of investigative dermatology. 2008;128:2031–40. doi: 10.1038/jid.2008.21. [DOI] [PubMed] [Google Scholar]
- Verbeke S, Meignan S, Lagadec C, Germain E, Hondermarck H, Adriaenssens E, Le Bourhis X. Overexpression of p75(NTR) increases survival of breast cancer cells through p21(waf1) Cellular signalling. 2010;22:1864–73. doi: 10.1016/j.cellsig.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nature medicine. 1999;5:412–7. doi: 10.1038/7410. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–7. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wang X, Cui M, Wang L, Chen X, Xin P. Inhibition of neurotrophin receptor p75 intramembran proteolysis by gamma-secretase inhibitor reduces medulloblastoma spinal metastasis. Biochemical and biophysical research communications. 2010;403:264–9. doi: 10.1016/j.bbrc.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Welling DB, Packer MD, Chang LS. Molecular studies of vestibular schwannomas: a review. Curr Opin Otolaryngol Head Neck Surg. 2007;15:341–346. doi: 10.1097/MOO.0b013e3282b97310. [DOI] [PubMed] [Google Scholar]
- Wickremesekera A, Hovens CM, Kaye AH. Expression of ErbB-1 and 2 in vestibular schwannomas. J Clin Neurosci. 2007;14:1199–206. doi: 10.1016/j.jocn.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Xiao GH, Chernoff J, Testa JR. NF2: the wizardry of merlin. Genes Chromosomes Cancer. 2003;38:389–99. doi: 10.1002/gcc.10282. [DOI] [PubMed] [Google Scholar]
- Yeiser EC, Rutkoski NJ, Naito A, Inoue J, Carter BD. Neurotrophin signaling through the p75 receptor is deficient in traf6-/- mice. J Neurosci. 2004;24:10521–9. doi: 10.1523/JNEUROSCI.1390-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Korade Z, Carter BD. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-kappaB promotes Schwann cell differentiation into a myelinating phenotype. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3738–46. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–81. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue WY, Clark JJ, Fernando A, Domann F, Hansen MR. Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides. Neuro-oncology. 2011;13:961–73. doi: 10.1093/neuonc/nor068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ercolano E, Ammoun S, Schmid MC, Barczyk MA, Hanemann CO. Merlin-deficient human tumors show loss of contact inhibition and activation of Wnt/beta-catenin signaling linked to the PDGFR/Src and Rac/PAK pathways. Neoplasia. 2011;13:1101–12. doi: 10.1593/neo.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Hanemann CO. Merlin, a multi-suppressor from cell membrane to the nucleus. FEBS letters. 2012;586:1403–8. doi: 10.1016/j.febslet.2012.03.016. [DOI] [PubMed] [Google Scholar]


