Abstract
Backgrounds
The t(11;14)(q13;q32) is seen in 15–20% patients with multiple myeloma (MM). In general, it is not associated with worse outcome. We studied the impact of t(11;14)(q13;q32) on the outcome of patients with MM who received high-dose chemotherapy followed by an autologous hematopoietic stem cell transplantation (auto-HCT).
Method
Eligible patients underwent high-dose chemotherapy followed by auto-HCT at the M.D. Anderson Cancer Center between February 2000 and August 2010, and had conventional cytogenetic (CC) or fluorescent in situ hybridization (FISH) results available prior to auto-HCT. The cohort is divided into three groups of patients: (1) normal (diploid by CC, and negative by FISH); (2) t(11;14)(q13;q32) by CC or FISH; and (3) high-risk (HR) abnormalities by CC or FISH. The primary objective was to compare the outcome of patients with t(11;14)(q13;q32) to patients with diploid or HR markers by CC or FISH studies.
Results
CC or FISH studies were available for 993 patients in the 3 groups described above. Eight-hundred and sixty-nine patients had normal, 27 patients had t(11;14)(q13;q32) and 97 patients HR markers by CC or FISH studies. Of the 27 patients with t(11;14)(q13;q32), 18 had isolated t(11;14)(q13;q32) while 9 patients had concurrent HR abnormalities. We compared the outcome of patients with t(11;14)(q13;q32) to patients with normal or HR markers. Median follow up in surviving patients was 37 months. 3-year PFS for normal, t(11;14)(q13;q32) and HR groups were 47%, 27% and 13%, respectively (p=<0.00001). 3-year OS for normal, t(11;14)(q13;q32) and HR groups were 83%, 63% and 34%, respectively (p=<0.00001). On multivariate analyses, t(11;14)(q13;q32) and HR abnormalities by CC or FISH, and relapsed disease at auto-HCT were associated with shorter PFS, while t(11;14)(q13;q32) and HR abnormalities by CC or FISH, β2 microglobulin of >3.5 and relapsed disease at auto-HCT were associated with shorter OS.
Conclusions
Patients with t(11;14)(q13;q32) had worse outcome than patients with normal CC or FISH, but better than patients with HR markers by CC or FISH studies.
Introduction
Multiple myeloma (MM) accounts for approximately one percent of all cancers and approximately 10 percent of hematologic malignancies in the United States [1, 2]. Routine conventional cytogenetic (CC) analyses and interphase fluorescence in situ hybridization (FISH) are helpful in stratifying patients with MM into high or standard-risk disease categories [3–5]. Deletion of 13q or monosomy 13 and hypoploidy by CC, and t(4;14)(p16.3;q32), t(14;16)(q32;q23), t(14;20)(q32;q11.2) and del17p13 by CC or FISH are considered HR abnormalities [5–8]. Approximately 15% of patients with symptomatic myeloma have HR disease and their median overall survival despite optimal therapy is about 2–3 years [7, 8]. Recent trials incorporating bortezomib as part of induction regimen have shown significant improvement in the outcome of patients with t(4;14), which is now considered an intermediate-risk abnormality by some investigators [9–11]. In the HOVON study, use of bortezomib before and after an auto-HCT was associated with a longer PFS and OS in a subset of patients with del 17p [10]. Based on these preliminary results, bortezomib-based induction and maintenance is now the preferred therapy for patients with HR myeloma.
The t(11;14)(q13;q32) abnormality involving IgH and CCND1-XT genes can be seen in approximately 15 to 20% of patients with newly diagnosed MM [12–14]. Previous studies have shown that t(11;14)(q13;q32) is associated with non-secretory myeloma, IgM or IgE monoclonal protein [14,15], lymphoplasmacytic or small mature plasma cell morphology, and CD20 expression [15–19]. In general, the presence of t(11;14)(q13;q32) is not associated with a worse outcome in MM [13, 20–22]. However, there are limited data about the prognostic significance of t(11;14)(q13;q32) in the context of autologous stem cell transplant (auto-HCT) [21]. In this study, we report the impact of t(11;14)(q13;q32) on the outcome of patients with MM who underwent auto-HCT at our institution, and compared their outcome to patients with diploid CC or negative FISH, and patients with HR markers by CC or FISH.
Materials and Methods
Patients
We performed a retrospective chart review on patients with symptomatic MM who underwent high-dose chemotherapy followed by auto-HCT at the M.D. Anderson Cancer Center between February 2000 and August 2010. Patients who had CC or FISH results available at any point before auto-HCT were eligible for the study. The t(11;14)(q13;q32) was defined as an abnormal signal pattern in at least 2 metaphases by CC or IgH/CCND1-XT rearrangement using 7% as a cutoff by interphase FISH. HR cytogenetic abnormalities were defined as del(13q)/−13 or hypoploidy by CC studies only, or t(4;14)(p16.3;q32), t(14;16)(q32;q23), t(14;20)(q32;q11.2) or del(17p13) by CC or FISH studies [5–7]. Normal was defined as diploid by CC and negative FISH studies at every time point before auto-HCT.
Response and Outcome
Response criteria were as defined by the International Myeloma Working Group (IMWG) uniform response criteria [23]. PFS was defined as the time from the day of auto-HCT to the date of disease progression or death [24], whereas OS was defined as the time from the day of auto-HCT to the date of death from any cause.
Statistical Analysis
The primary objective of the study was to compare the rate of progression free survival (PFS) and overall survival (OS) in patients with t(11;14)(q13;q32) to those with normal or HR CC or FISH studies. PFS was defined as the time from transplant to either disease progression or death, and OS as the time form transplant to death of any cause. Patients who were alive at the time of analysis were censored on the last date of the last follow-up. Actuarial estimates of PFS and OS were calculated by the Kaplan-Meier method [25]. Cox’s proportional hazards regression analysis was used to evaluate the impact of a number of risk factors, including t(11;14)(q13;q32), on the rate of PFS and OS. Factors considered included age, sex, year of auto-HCT, interval between diagnosis and auto-HCT, CC or FISH abnormalities, β2 microglobulin level at diagnosis and at auto-HCT, disease status at auto-HCT, response to induction, prior autologous transplant, and prior use of novel agents. Statistical significance was defined at the 0.05 level and factors significant in univariate analysis were considered in multivariate analyses. Statistical analyses were performed using STATA 9.0.
Results
Patient Characteristics
We identified 993 patients with MM and CC and FISH as described above, who underwent auto-HCT between February 2000 and August 2010. Patient characteristics are summarized in Table 1. Among 993 patients, 869 patients had normal CC or FISH results, 27 patients had t(11;14)(q13;q32) and 97 patients had HR abnormalities by CC or FISH. Patients in 3 groups were fairly evenly matched. Overall, 15% patients had relapsed disease at auto-HCT, with 13%, 37% and 7%, in normal, HR and t(11;14)(q13;q32) groups.
Table 1.
Patient Characteristics
| Overall | Normal | High Risk | t=(11;14) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N=993 | N=869 | N=97 | N=27 | N vs. HR | N vs. t(11;14) | |||||
| Sex -no. (%) | ||||||||||
| M | 564 | (57) | 494 | (57) | 55 | (57) | 15 | (56) | 0.9 | 0.9 |
| F | 429 | (43) | 375 | (43) | 42 | (43) | 12 | (44) | ||
| Age - yr | ||||||||||
| Median | 58 | 57 | 58 | 59 | ||||||
| (Range) | (29–80) | (29–80) | (31–75) | (44–72) | ||||||
| Age>65 - no. (%) | ||||||||||
| <=65 | 820 | (83) | 721 | (83) | 77 | (79) | 22 | (81) | 0.4 | 0.5 |
| >65 | 173 | (17) | 148 | (17) | 20 | (21) | 5 | (19) | ||
| Year of Auto-HCT - no. (%) | ||||||||||
| <=2005 | 421 | (42) | 374 | (43) | 41 | (42) | 6 | (22) | 0.9 | 0.02 |
| >2005 | 572 | (58) | 495 | (57) | 56 | (58) | 21 | (78) | ||
| Diagnosis to Auto-HCT - (months) | ||||||||||
| Median | 7.8 | 7.9 | 7.7 | 6.6 | ||||||
| (Range) | (1.7–262.8) | (1.7–262.8) | (2.1–65.7) | (3.3–60.5) | ||||||
| <=6 m | 330 | (33) | 287 | (33) | 34 | (35) | 9 | (33) | 0.7 | 0.6 |
| >6 m | 662 | (67) | 581 | (67) | 63 | (65) | 18 | (67) | ||
| Unknown | 1 | 1 | ||||||||
| Prior Auto-HCT - no. (%) | ||||||||||
| 0 | 950 | (96) | 832 | (96) | 92 | (95) | 26 | (96) | 0.4 | 0.7 |
| 1 | 41 | (4) | 35 | (4) | 5 | (5) | 1 | (4) | ||
| 2 | 1 | (0) | 1 | (0) | 0 | (0) | 0 | (0) | ||
| Unknown | 1 | 1 | ||||||||
| B2 M at diagnosis- no. (%) | ||||||||||
| Median | 3.3 | 3.07 | 4.9 | 5.1 | ||||||
| (Range) | (0.5–40) | (0.5–40) | (2–33.1) | (1.4–21.2) | ||||||
| <=3.5 | 373 | (56) | 345 | (59) | 22 | (34) | 6 | (27) | ||
| 3.5–5.5 | 130 | (19) | 109 | (19) | 15 | (23) | 6 | (27) | ||
| >5.5 | 167 | (25) | 129 | (22) | 28 | (43) | 10 | (45) | ||
| Unknown | 323 | 286 | 32 | 5 | ||||||
| B2 M at Auto-HCT- no. (%) | ||||||||||
| Median | 2.6 | 2.6 | 3 | 2.7 | ||||||
| (Range) | (1.2–40) | (1.2–40) | (1.6–23.2) | (1.5–6.9) | ||||||
| <=3.5 | 688 | (75) | 606 | (76) | 62 | (67) | 20 | (80) | 0.05 | 0.4 |
| 3.5–5.5 | 141 | (15) | 124 | (16) | 14 | (15) | 3 | (12) | ||
| >5.5 | 86 | (9) | 67 | (8) | 17 | (18) | 2 | (8) | ||
| Unknown | 78 | 72 | 4 | 2 | ||||||
| Disease Status at Auto-HCT - no. (%) | ||||||||||
| Relapse | 148 | (15) | 110 | (13) | 36 | (37) | 2 | (7) | <0.001 | 0.3 |
| Other | 845 | (85) | 759 | (87) | 61 | (63) | 25 | (93) | ||
| Response prior to Auto-HCT - no. (%) | ||||||||||
| CR/VGPR/PR | 805 | (81) | 711 | (82) | 69 | (71) | 25 | (93) | ||
| MR/SD/PD/NR | 181 | (18) | 151 | (17) | 28 | (29) | 2 | (7) | 0.007 | 0.1 |
| NE | 7 | (1) | 7 | (1) | 0 | (0) | 0 | (0) | ||
| Stem Cell Source- no. (%) | ||||||||||
| BM | 3 | (0) | 2 | (0) | 0 | (0) | 1 | (4) | 0.8 | 0.09 |
| PB | 990 | (100) | 867 | (100) | 97 | (100) | 26 | (96) | ||
| Melphalan Alone- no. (%) | ||||||||||
| Yes | 824 | (83) | 720 | (83) | 80 | (82) | 24 | (89) | 0.9 | 0.3 |
| No | 169 | (17) | 149 | (17) | 17 | (18) | 3 | (11) | ||
| Novel Agent - no. (%) | ||||||||||
| Yes | 668 | (67) | 573 | (66) | 74 | (76) | 21 | (78) | 0.04 | 0.2 |
| No | 325 | (33) | 296 | (34) | 23 | (24) | 6 | (22) | ||
In 27 patients with t(11;14)(q13;q32), CC studies were available for all 27 patients, while FISH studies were performed on 19 patients only. In 19 patients with IgH/CCND1-XT rearrangement, t(11;14)(q13;q32) by CC was detected in only 13 (68%) patients, reinforcing the greater sensitivity of FISH studies in detecting this abnormality. Overall, 21 of 27 patients had t(11;14)(q13;q32) on CC studies. Out of these 21 patients with this abnormality, 5 (24%) patients had a hyperdiploid clone, 9 (43%) a hypodiploid and 7 (33%) a pseudodiploid clone. Eighteen of 27(67%) patients with t(11;14)(q13;q32) had either isolated t(11;14)(q13;q32) abnormality or associated with other non-HR abnormalities. Nine patients (33%) had at least one HR abnormality: t(11;14)(q13;q32) plus del(13q) in 7 patients and t(11;14)(q13;q32) plus del(13q) and del(17p13) in 2 patients (7%). Bone marrow flow cytometric analyses showed CD20 expression on plasma cells in 3 (11%) patients, and absence of CD20 expression on plasma cells in 24 (89%) patients with t(11;14)(q13;q32).
Eighty-nine (92%) of the 97 patients with HR abnormalities had del(13q) alone or in combination with other HR abnormalities, 4 (4%) patients had del(17p13) alone and 4 (4%) had t(4;14)(p16.3;q32), t(14;16)(q32;q23) or t(14;20)(q32;q11.2).
Induction Therapy and Preparative Regimen
Disease status and responses to prior induction therapy are summarized in Table 1. Novel agents including thalidomide, lenalidomide or bortezomib were used for induction or salvage before auto-HCT in 573 (66%) patients with normal CC or FISH, 21 (78%) patients with t(11;14)(q13;q32) and 74 (76%) patients with HR CC or FISH (Table 1). Melphalan alone was used as preparative regimen for 720 (83%) in normal, 24 (89%) in t(11;14)(q13;q32) and 80 (82%) in HR patients.
Survival
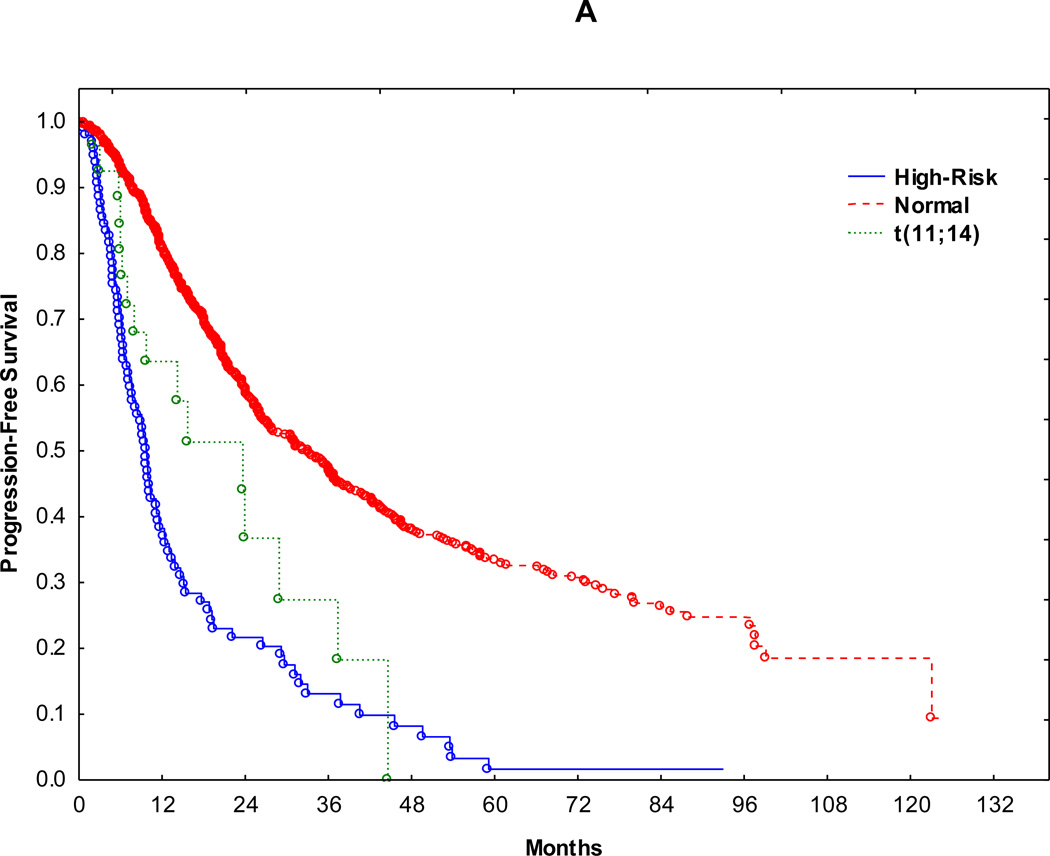
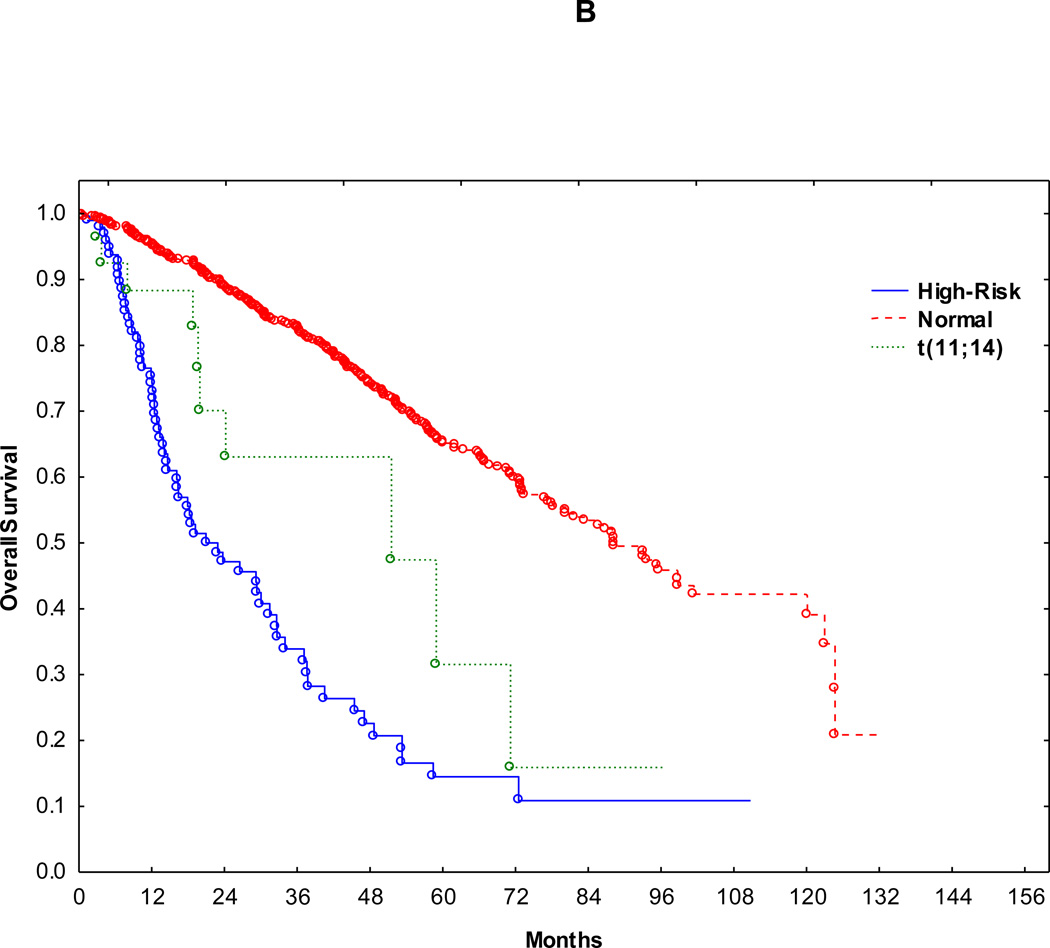
Median follow up in surviving patients was 37 months (range 1 to 130 months). Accordingly, outcomes were compared within 3 years following transplant. Median PFS for normal, t(11;14)(q13;q32) and HR groups were 33 months, 23 and 9.7 months, respectively (Figure 1A). 3-year PFS for normal, t(11;14)(q13;q32) and HR groups were 47%, 27% and 13% (normal vs. t(11;14)(q13;q32): p=0.02, t(11;14)(q13;q32) vs. HR: p= 0.05), respectively (Fig. 1A). Median OS for normal, t(11;14)(q13;q32) and HR groups were 87, 51 and 21 months, respectively (Fig. 1B). 3-year OS for normal, t(11;14)(q13;q32) and HR groups were 82%, 63% and 34% (normal vs. t(11;14)(q13;q32): p=0.01, t(11;14)(q13;q32) vs. HR: p= 0.04), respectively (Fig. 1B). Median PFS and OS showed similar trends when patients with relapsed disease at auto-HCT were excluded from the survival anlyses (p <0.0001 for both PFS and OS).
Figure 1.
On univariate analyses, t(11;14)(q13;q32), HR abnormalities by CC or FISH, β2 microglobulin of >3.5 either at diagnosis or at auto-HCT, relapsed disease at auto-HCT, <PR at auto-HCT and induction therapy without novel agents were associated with significantly shorter PFS and OS (Table 2). Because of the large proportion of unknown values of β2 microglobulin at diagnosis, this variable was not considered in multivariate analysis despite reaching statistical significance on univariate analysis. On multivariate analyses, t(11;14)(q13;q32) and HR abnormalities by CC or FISH and relapsed disease at auto-HCT were independently associated with shorter PFS. On multivariate analyses for OS, t(11;14)(q13;q32) and HR abnormalities by CC or FISH, β2 microglobulin of >3.5 and relapsed disease at auto-HCT were independently associated with shorter OS (Tables 3 and 4).
Table 2.
Univariate Analysis
| Median f/up in alive (n=670) | Overall | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes at 3 yrs | N=993 | died | HR | 95% CI | p | prg/died | HR | 95% CI | p |
| Normal | 869 | 124 | Ref. | 388 | Ref. | ||||
| High-Risk | 97 | 53 | 5.96 | (4.3–8.3) | <0.001 | 78 | 3.45 | (2.7–4.4) | <0.001 |
| t(11; 14) | 27 | 7 | 2.7 | (1.2–5.7) | 0.01 | 14 | 1.9 | (1.1–3.2) | 0.02 |
| Sex | |||||||||
| M | 564 | 109 | 276 | ||||||
| F | 429 | 75 | 0.9 | (0.7–1.2) | 0.6 | 204 | 1.02 | (0.85–1.2) | 0.8 |
| Age>65 | |||||||||
| <= 65 | 820 | 152 | 399 | ||||||
| > 65 | 173 | 32 | 1.1 | (0.8–1.6) | 0.6 | 81 | 1.05 | (0.8–1.3) | 0.7 |
| Year of Auto-HCT | |||||||||
| <=2005 | 421 | 89 | 241 | ||||||
| > 2005 | 572 | 95 | 1.2 | (0.9–1.6) | 0.2 | 239 | 0.99 | (0.8–1.2) | 0.9 |
| Months DX to Auto-HCT | |||||||||
| <= 6 m | 330 | 47 | 153 | ||||||
| > 6 m | 662 | 136 | 1.5 | (1.1–2.1) | 0.02 | 326 | 1.1 | (0.9–1.4) | 0.2 |
| Unknown | 1 | ||||||||
| Prior Auto-HCT | |||||||||
| 0 | 950 | 174 | 0.7 | (0.4–1.4) | 0.3 | 460 | 1.1 | (0.7–1.7) | 0.8 |
| 1 | 41 | 9 | 18 | ||||||
| 2 | 1 | 1 | 1 | ||||||
| Unknown | 1 | ||||||||
| B2 M at Auto-HCT | |||||||||
| <=3.5 | 688 | 102 | |||||||
| 3.5–5.5 | 141 | 33 | 1.6 | (1.1–2.4) | 0.01 | 320 | 1.2 | (0.9–1.5) | 0.2 |
| >5.5 | 86 | 31 | 2.8 | (1.9–4.2) | <0.001 | 72 | 1.4 | (1.04–1.9) | 0.02 |
| Unknown | 78 | ||||||||
| <=3.5 vs. >3.5 | 0.5 | (0.4–0.7) | <0.001 | 0.8 | (0.6–0.9) | 0.035 | |||
| >5.5 vs. <=5.5 | 1.4 | (1.02–1.9) | 0.03 | ||||||
| Disease Status at Transplant | |||||||||
| Relapse | 148 | 57 | 3.2 | (2.3–4.4) | <0.001 | 107 | 2.5 | (2.0–3.11) | <0.001 |
| Other | 845 | 127 | 373 | ||||||
| Response prior | |||||||||
| CR/VGPR/PR | 805 | 127 | 373 | ||||||
| MR/SD/PD/NR | 181 | 52 | 1.8 | (1.3–2.5) | <0.001 | 102 | 1.3 | (1.06–1.6) | 0.01 |
| NE | 7 | 5 | excld | 5 | excld | ||||
| Melphalan Alone | |||||||||
| Yes | 824 | 143 | 0.85 | (0.6–1.2) | 0.4 | 377 | 0.8 | (0.7–1.01) | 0.07 |
| No | 169 | 41 | 103 | ||||||
Table 3.
Multivariate Analysis for PFS
| Overall | PFS MV | |||
|---|---|---|---|---|
| N=993 | HR | 95% CI | p | |
| Normal | 869 | |||
| High-Risk | 97 | 2.9 | 2.2 | <0.001 |
| t(11; 14) | 27 | 2 | 1.2 | 0.01 |
| Disease Status at Transplant | ||||
| Relapse | 148 | 1.96 | 1.5–2.5 | <0.001 |
| Other | 845 | |||
Table 4.
Multivariate Analysis for OS
| Overall | OS MV | |||
|---|---|---|---|---|
| N=993 | HR | 95% CI | p | |
| Normal | 869 | |||
| High-Risk | 97 | 4.6 | 3.2 | <0.001 |
| t(11; 14) | 27 | 3.1 | 1.4 | 0.004 |
| B2 M at SE | ||||
| <=3.5 vs. >3.5 | 0.6 | 0.4–0.8 | 0.001 | |
| >5.5 vs. <=5.5 | ||||
| Disease Status at Transplant | ||||
| Relapse | 148 | 2.2 | 1.5–3.1 | <0.001 |
| Other | 845 | |||
Discussion
This retrospective analysis shows that myeloma patients carrying t(11;14)(q13;q32) by CC or FISH had intermediate PFS and OS when compared to patients with normal or HR CC or FISH studies before auto-HCT. This translocation is frequently seen in myeloma patients; however there are conflicting data about its clinical implications. Several studies have studied the impact of t(11;14)(q13;q32) on the outcome of patients with MM [4–7,12–21].
Fonseca et al. reported on 336 patients with newly diagnosed myeloma that were enrolled into the Eastern Cooperative Oncology Group (ECOG) trial, E9486. They identified 53 (16%) patients with t(11;14)(q13;q32) detected by FISH. Patients with the t(11;14)(q13;q32) appeared to have better survival and response to treatment, although this did not reach statistical significance. The patients in this study did not receive auto-HCT and novel agents [4–7, 13].
Dewald et al. compared the clinical efficacy of metaphase CC and FISH with interphase FISH in 154 patients with newly diagnosed myeloma. They found increased detection of chromosomal abnormalities with interphase FISH (86%) vs. metaphase CC or FISH (40%). In this study, presence of t(11;14)(q13;q32) by CC was associated with intermediate outcome in terms of OS between normal CC and HR CC. However, detection of t(11;14)(q13;q32) by interphase FISH was not associated with an adverse outcome [20].
Moreau et al. reported the impact of recurrent 14q32 translocations on the outcome of 168 newly diagnosed patients with multiple myeloma enrolled between January 1995 and December 2000 with the median follow-up of 27 months. All patients received at least one auto-HCT while 10 patients received allogeneic HCT. They identified 26 patients (15.5%) with t(11;14)(q13;q32) by interphase FISH. Patients with t(11;14)(q13;q32) had longer survival compared to those without this abnormality [21].
Gertz et al. evaluated the clinical implications of t(11;14)(q13;q32) in myeloma patients treated with high-dose therapy. No survival difference was found in patients with and without t(11;14)(q13;32) [26]. In another report, 1064 patients with newly diagnosed myeloma enrolled into the Intergroupe Francophone du Myélome (IFM) 99 trials were screened for chromosomal aberrations frequently seen in MM: t(11;14)(q13;q32) was seen in 21% of patients. Majority of the patients received auto-HCT while 65 underwent allogeneic HCT. The presence of t(11;14)(q13;q32) did not adversely impact the survival [27].
In our study, patients with t(11;14)(q13;q32) had an intermediate outcome between patients with normal or HR CC or FISH abnormalities. We also compared patients with t(11;14)(q13;q32) only (18/27) and t(11;14)(q13;q32) plus other HR abnormalities (9/27) to patients with normal or HR CC or FISH studies in a multivariate model. When analyzed separately, both t(11;14)(q13;q32) alone and t(11;14)(q13;q32) with other HR abnormalities were predictive of significantly shorter PFS, while t(11;14)(q13;q32) only showed a trend strong trend towards a shorter OS.
These results are consistent with earlier observations that patients with any CC abnormality, regardless of risk category, had worse outcome than patients with normal karyotype [5, 20, 28]. This may be a function of higher proliferation in the cells where a karyotypic abnormality is detected [21]. Furthermore, the relatively worse outcome for patients with t(11;14)(q13;q32) in our study could be due to the inclusion of a number of patients with relapsed disease at auto-HCT (15%) or with concurrent HR abnormalities in 9/27 (33%) patients. Using gene expression profiling, Nair et al. categorized patients with t(11;14)(q13;q32) into two subsets [29]. The subset with CD20 expression was associated with a durable remission while the subset lacking CD20 expression had shorter duration of remission. Since almost 90% of patients with t(11;14)(q13;q32) in our study lacked CD20 expression, it may have been partly responsible for their worse outcome.
Other significant prognostic markers were a high β2 microglobulin level at auto-HCT and patients with relapsed disease at auto-HCT. Both of these are known risk factors for worse outcome for myeloma [30–33]. High β2 microglobulin level either at diagnosis or at auto-HCT has been shown to be associated with worse outcome in several prior studies and retrospective analyses. Similarly, it is well known that patients transplanted after a relapse have significantly shortened PFS and OS after an auto-HCT.
There are several potential limitations to consider in interpreting the results of our study. They included the retrospective nature of the study, heterogeneous patient population in terms of disease status and non-transplant therapy, relatively small number of patients with t(11;14)(q13;q32), and concurrent HR CC or FISH abnormalities in about a third of patients with t(11;14)(q13;q32).
In summary, our study showed that patients with t(11;14)(q13;q32) had intermediate outcome compared to patients with normal and HR abnormalities by CC or FISH studies. This finding may be used to stratify patients into risk categories and may predict the outcome.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: a clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dispenzieri A, Rajkumar SV, Gertz MA, et al. Treatment of newly diagnosed multiple myeloma based on Mayo Stratification of Myeloma and Risk-adapted Therapy (mSMART): consensus statement. Mayo Clin Proc. 2007;82:323–341. doi: 10.4065/82.3.323. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64:1546–1558. doi: 10.1158/0008-5472.can-03-2876. [DOI] [PubMed] [Google Scholar]
- 5.Munshi NC, Anderson KC, Berqsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart AK, Bergsagel PL, Greipp PR, et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia. 2007;21:529–534. doi: 10.1038/sj.leu.2404516. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV. Multiple myeloma, 2012 update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:78–88. doi: 10.1002/ajh.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 10.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 11.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) J Clin Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 12.Avet-Loiseau H, Li JY, Facon T, et al. High incidence of translocations t(11;14)(q13;q32) and t(4;14)(p16;q32) in patients with plasma cell malignancies. Cancer Res. 1998;58:5640–5645. [PubMed] [Google Scholar]
- 13.Fonseca R, Blood EA, Oken MM, et al. Myeloma and the t(11;14)(q13;q32); evidence for a biologically defined unique subset of patients. Blood. 2002;99:3735–3741. doi: 10.1182/blood.v99.10.3735. [DOI] [PubMed] [Google Scholar]
- 14.Königsberg R, Zojer N, Ackermann J, et al. Predictive role of interphase cytogenetics for survival of patients with multiple myeloma. J Clin Oncol. 2000;18:804–812. doi: 10.1200/JCO.2000.18.4.804. [DOI] [PubMed] [Google Scholar]
- 15.Avet-Loiseau H, Garand R, Lodé L, et al. Translocation t(11;14)(q13;q32) is the hallmark of IgM, IgE, and nonsecretory multiple myeloma variants. Blood. 2003;101:1570–1571. doi: 10.1182/blood-2002-08-2436. [DOI] [PubMed] [Google Scholar]
- 16.Feyler S, O'Connor SJ, Rawstron AC, et al. IgM myeloma: a rare entity characterized by a CD20-CD56-CD117- immunophenotype and the t(11;14) Br J Haematol. 2008;140:547–551. doi: 10.1111/j.1365-2141.2007.06969.x. [DOI] [PubMed] [Google Scholar]
- 17.Garand R, Avet-Loiseau H, Accard F, Moreau P, Harousseau JL, Bataille R. t(11;14) and t(4;14) translocations correlated with mature lymphoplasmacytoid and immature morphology, respectively, in multiple myeloma. Leukemia. 2003;17:2032–2035. doi: 10.1038/sj.leu.2403091. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer JD, Hanson CA, Fonseca R, Greipp PR, Dewald GW, Kurtin PJ. The (11;14)(q13;q32) translocation in multiple myeloma. A morphologic and immunohistochemical study. Am J Clin Pathol. 2000;113:831–837. doi: 10.1309/4W8E-8F4K-BHUP-UBE7. [DOI] [PubMed] [Google Scholar]
- 19.Robillard N, Avet-Loiseau H, Garand R, et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 2003;102:1070–1071. doi: 10.1182/blood-2002-11-3333. [DOI] [PubMed] [Google Scholar]
- 20.Dewald GW, Therneau T, Larson D, et al. Relationship of patient survival and chromosome anomalies detected in metaphase and/or interphase cells at diagnosis of myeloma. Blood. 2005;106:3553–3558. doi: 10.1182/blood-2005-05-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau P, Facon T, Leleu X, et al. Recurrent 14q32 translocations determine the prognosis of multiple myeloma, especially in patients receiving intensive chemotherapy. Blood. 2002;100:1579–1583. doi: 10.1182/blood-2002-03-0749. [DOI] [PubMed] [Google Scholar]
- 22.Oken MM, Leong T, Lenhard Jr RE, et al. The addition of interferon or high dose cyclophosphamide to standard chemotherapy in the treatment of patients with multiple myeloma: phase III Eastern Cooperative Oncology Group Clinical Trial EST 9486. Cancer. 1999;86:957–968. [PubMed] [Google Scholar]
- 23.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 24.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Meler P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn. 1958;53:457–481. [Google Scholar]
- 26.Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and -17p13 in myeloma patients treated with high-dose therapy. Blood. 2005;106:2837–2840. doi: 10.1182/blood-2005-04-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed S, Lin H, Baladandayuthapani V, et al. Impact of Non High-Risk Chromosomal Abnormalities on the Outcome of Autologous Hematopoietic Stem Cell Transplantation in Multiple Myeloma [abstract] Blood. 2011 Nov;118:333. (ASH Annual Meeting Abstracts) [Google Scholar]
- 29.Nair B, van Rhee F, Shauqhnessy JD, et al. Superior results of Total Therapy 3 (2003-33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006-66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qazilbash MH, Saliba RM, Hosing C, et al. Autologous stem cell transplantation is safe and feasible in elderly patients with multiple myeloma. Bone Marrow Transplant. 2007;39:279–283. doi: 10.1038/sj.bmt.1705580. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Khan H, Thall PF, et al. A randomized phase 2 trial of a preparative regimen of bortezomib, high-dose melphalan, arsenic trioxide, and ascorbic acid. Cancer. 2012;118:2507–2515. doi: 10.1002/cncr.26517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greipp PR, Miguel JS, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 33.O'Shea D, Giles C, Terpos E, et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transplant. 2006;37:731–737. doi: 10.1038/sj.bmt.1705307. [DOI] [PubMed] [Google Scholar]