Abstract
Myocardial infarction is the leading cause of death worldwide and phase I clinical trials utilizing cardiac progenitor cells (CPCs) have shown promising outcomes. Notch1 signaling plays a critical role in cardiac development and in the survival, cardiogenic lineage commitment, and differentiation of cardiac stem/progenitor cells. In this study, we functionalized self-assembling peptide (SAP) hydrogels with a peptide mimic of the Notch1 ligand Jagged1 (RJ) to evaluate the therapeutic benefit of CPC delivery in the hydrogels in a rat model of myocardial infarction. The behavior of CPCs cultured in the 3D hydrogels in vitro including gene expression, proliferation, and growth factor production was evaluated. Interestingly, we observed Notch1 activation to be dependent on hydrogel polymer density/stiffness with synergistic increase in presence of RJ. Our results show that RJ mediated Notch1 activation depending on hydrogel concentration differentially regulated cardiogenic gene expression, proliferation, and growth factor production in CPCs in vitro. In rats subjected to experimental myocardial infarction, improvement in acute retention and cardiac function was observed following cell therapy in RJ hydrogels compared to unmodified or scrambled peptide containing hydrogels. This study demonstrates the potential therapeutic benefit of functionalizing SAP hydrogels with RJ for CPC based cardiac repair.
1. Introduction
Heart failure is the leading cause of death worldwide and myocardial infarction (MI) is the predominant cause [1]. While heart transplantation is the most viable treatment, limited availability of donor hearts, complications from immunosuppression and possibility of graft failure have necessitated the search for treatment alternatives. New emphasis has been placed on regenerative approaches to treat MI including delivery of growth factors and/or stem cells in natural scaffolds such as gelatin and alginate or artificial self-assembling peptide scaffolds [2, 3]. Evidence for myocyte renewal in human hearts, existence of cardiac progenitor cells (CPCs) within post natal heart niches, promising outcomes in phase I trials and the inherent ability of CPCs to differentiate into cardiovascular cell types have established CPCs as a clinically relevant cell source for cardiac therapy [4–7]. However, extensive cell death and poor survival of transplanted cells in the infarcted heart limits functional improvement following cell based therapies.
The reparative capacity of various CPCs is dependent on the myocardial environment, activation of specific signaling pathways, and sustained retention in the infarcted heart. While VEGF, IGF-1 and Notch signaling promote CPC mediated cardiac repair, Wnt activation exerts anti-proliferative effects [8–11]. Studies have demonstrated the effect of incorporating such signals into natural or synthetic scaffolds to promote cardiac regeneration [12, 13]. The self-assembling peptide (SAP) used in this study (RARADADA)2 has hydrophilic and hydrophobic residues with alternating charges that allows for self-assembly into nanofiber (5–10 nm diameter) hydrogels at physiologic pH and osmolarity [14]. These hydrogels can also be precisely modified with spatially and temporally defined regenerative cues to direct stem cells responses [15, 16].
The Notch pathway is universally conserved across the animal kingdom and is fundamental to cell to cell communication and cell fate determination. Deletion of Notch, the ligands or the downstream effectors is embryonic lethal due to cardiovascular defects suggesting the importance of Notch signaling in early cardiac development [17]. Furthermore, Notch signaling has been shown to precede cardiac regeneration in zebrafish [18]. As Notch receptors and their ligands are presented on the surface of a signal sending and signal receiving cell respectively, activation requires physical contact between the two cells. Ligand-binding catalyzes the proteolytic cleavage of the Notch extracellular domain, and subsequent γ-secretase mediated release of the Notch intracellular domain, a potent transcription factor that regulates gene expression in several cell types [19]. The most widely accepted model of Notch activation proposes that external force generated by ligand endocytosis on binding to the Notch1 receptor is required for Notch1 activation in the signal receiving cell [20]. This mechanical model is supported by structural studies of the Notch receptor, as well as the observation that soluble ligand blocks activation, in contrast to surface immobilized ligand that potently activates the pathway. Accordingly, most in vitro approaches to Notch activation involve immobilizing Notch ligands, such as Jagged1 in 2D. However, given the limitations in 2D cell culture, and the need to develop more physiologically relevant Notch activation platforms that are chemically and physically well defined, we developed a new approach to trigger the Notch pathway by using a 3D hydrogel that is functionalized with a peptide mimic of the Notch1 ligand Jagged1 (RJ). RJ has been shown to promote Notch1 activation in different cell types [21, 22]. Therefore, we investigated the effect of RJ hydrogel-mediated Notch1 activation on CPC based cardiac regeneration through in vitro and in vivo studies on cardiogenic gene expression, growth factor production and cardiac repair.
2. Materials and Methods
2.1 Preparation and characterization of the hydrogel
The self-assembling peptide RADA16-II (H2N-RARADADARARADADA-OH) was generated with a 7 glycine linker followed by the Notch ligand Jagged-1 (H2N-CDDYYYGFGCNKFCRPR-OH) to create RJ (Fig. S1B) or a scrambled sequence (H2N-RCGPDCFDNYGRYKYCF-OH) to create RS. Hydrogels with varying % concentration from 1–3% w/v RADA were combined with RJ or RS in a ratio of 1:10 as in [12]. To determine the Young’s modulus of the hydrogels, using an MFP-3D-BIO atomic force microscope (Asylum Research; Santa Barbara, CA), samples were probed under fluid conditions using 295mM sucrose solution made in water. A 15 μm bead tipped-silicon nitride cantilever (Bruker, Camarillo, CA) was used. Cantilever spring constants were measured prior to sample analysis using the thermal fluctuation method, with nominal values of 20–30 mN/m. The force-indentation curve was obtained for each measurement and then analyzed with a Hertzian model for a spherical tip (Wavemetrics, IgorPro) from which the Young’s modulus was calculated. The sample Poisson’s ratio was assumed as 0.5, and the deflection set point was 10 nN. For determination of swelling ratio, the hydrogels were created in a 100 μL total volume and allowed to swell in 295mM sucrose solution. After 24h, the wet weight of the gels was measured. The gels were then lyophilized and the dry weight noted. Swelling ratio was calculated as the (wet weight − dry weight)/wet weight.
2.2 Media and Cell culture
The isolation and culture of rat cardiac progenitor cells (CPCs) is described in [23]. Mouse embryonic stem cells (mESCs) were cultured in in high glucose DMEM supplemented with 10% FBS, 1% non-essential amino acids, 1% L-glutamine, 0.1 mM β-mercaptoethanol, 1% penicillin/streptomycin and 2,000 U/mL mouse LIF (Millipore) on feeder layers of mitotically inactivated STO cells, a mouse embryonic fibroblast line (ATCC). Two days after embryoid bodies (EBs) were initiated by suspending the cells at 107 cells/mL in 10 mL of media, floating EBs were enzymatically dissociated by treatment with Accutase (e-Bioscience) and were further cultured in hydrogel.
The CHO cells with Notch1 responsive YFP expression were cultured in Alpha MEM containing 10% FBS, 1X Penicillin-Sterptomycin, L-Glutamine, Zeocin (400 μg/mL), Blasticidin (10 μg/mL) and Geneticin (600 μg/mL). The rat cardiac endothelial cells were cultured in low glucose DMEM media (GIBCO) supplemented with 10% FBS, 50 μg/mL endothelial cell growth supplement (Sigma E2759) and 1% 100X MEM Non-essential amino acids solution (GIBCO). The primary rat cardiomyocytes were isolated from rat hearts and cultured as described in [24]. For 3D cell culture studies, the cells were cultured for 48h in the hydrogels (1R, 1RS, 1RJ, 2R, 2RS, 2RJ; 100μL total volume) in Transwell cell culture insets.
2.3 Gene expression
RNA was isolated from cells in the hydrogels using Trizol (Invitrogen) according to the manufacturer’s instructions. The concentration and purity of the RNA was determined by A260/A280nm reading (BioTek). cDNA was synthesized with 2 μg RNA, random hexamers, oligo dT and dNTP as described in [25]. Real time PCR was performed for the genes Flt1, vWF, Tagln, Acta2, nkx2–5, Mef2c, Gata4, and Hey1 using Power SYBR Green (Step One Plus, Life Technologies). Primer sequences are provided in Supplemental Table 1. Changes in gene expression between CPCs cultured for 48h in 1R and 2R hydrogels were analyzed using the Qiagen Rat Signal Transduction pathway finder PCR array (Cat# PARN014Z). The Ct values were normalized to the housekeeping gene Rplp1 (ribosomal protein large subunit 1) and fold change calculated by 2^(−ΔΔCt). A fold change of 5 was chosen as the threshold for the analysis.
2.4 Measurement of Notch activation
CHO cells with Notch1-responsive YFP expression were cultured in 1–3% hydrogels containing empty, scrambled or Jagged1 peptide (R, RS or RJ) for 48h. YFP expression was quantified by measurement of fluorescent intensity on a plate reader (BioTek Synergy 2). In CPCs, the mRNA expression of Hey1, a downstream target of Notch1 was measured by qPCR to indicate Notch1 activation.
2.5 Detection of growth factors by ELISA
CPCs were cultured in the different hydrogels (1R, 1RS, 1RJ, 2R, 2RS, 2RJ) for 48h. The conditioned media was screened for secreted growth factors using a custom array (Signosis, USA) according to the manufacturer’s directions. Growth factors of interest were confirmed by specific ELISAs (SCF, PDGF; Signosis). All immunosorbent measures were analyzed based on a standard curve and normalized to initial cell number.
2.6 In vitro tube formation
The rat cardiac endothelial cells were cultured in 1:1 CPC conditioned media and low serum endothelial growth media for 24h. The cells were then cultured on Geltrex (Life Tech) for 8h and stained with calcein. The tube formation was imaged using a fluorescent microscope (Olympus) and tube length quantified using ImageJ.
2.7 In vitro cell migration
Cell Tracker Orange CMRA (Life Technologies C34551) labeled CPCs were seeded in the top well of the Boyden chamber (Corning #3422) at a density of 100000 cells/well and allowed to adhere to the wells. Conditioned media obtained from CPCs cultured in 1R, 1RJ, 2R or 2RJ hydrogels was added to the bottom chamber. After 12h, the extent of cell migration was quantified by measuring CMRA fluorescence on a plate reader (BioTek Synergy 2). A standard curve of CMRA fluorescence to cell number was used to calculate number of migrated cells normalized to initial seeded cell number.
2.8 Flow cytometry
The CPCs were characterized for surface expression of cKit (H-300, Santa Cruz), cardiac transcription factors nkx2–5 and gata4 (Santa Cruz) and Notch1 Extracellular Domain (N1ECD, clone 8G10, Millipore) using FACSCalibur flow cytometer (Becton Dickinson). Data was analyzed using FlowJo version 7.6.
2.9 In vivo imaging
Rats were subjected to 30minutes of Ischemia-Reperfusion (IR). CPCs were labeled with DiR as per the manufacturer’s instructions (Life Technologies). One million DiR-labeled CPCs in the different hydrogels (2R, 2RS and 2RJ) were injected in 3 areas in the border zone surrounding the infarct (n=5/group). The rats were imaged on days 0, 1, 2, 3, 4, 7, 10, 14 and 21. The fluorescent intensity in the rat hearts due to the DiR-labeled CPCs was quantified as % retention (100% on day 0) over time to account for variation in fluorescent intensity between rats.
2.10 Myocardial infarction and cell injection
All animal experiments were performed with the approval of the Institutional Animal Care and Use Committee of Emory University. Myocardial infarction was performed in female adult Sprague Dawley rats (Charles River Laboratories) in a randomized double-blinded manner. Briefly, the rats were anaesthetized (1–3% isoflurane), intubated and heat exposed by separation of ribs. The left anterior descending (LAD) coronary artery was ligated for 30 minutes. During reperfusion, for groups receiving CPCs in hydrogels, 40μL of one of the following was injected intramyocardially into 3 areas in the border zone surrounding the infarct through a 31Gauge Ultra-Fine short syringe (BD Biosciences). Cardiac function was evaluated 21 days after treatment by echocardiography (Acuson Sequoia 512 with a 14 MHz transducer) and invasive pressure-volume hemodynamics (Millar Instruments) to assess the functional effects of each cell therapy. All functional evaluations were conducted and analyzed by investigators blinded to the animal’s treatment group. The rats were euthanized and the hearts were excised for histological analysis. The hearts were fixed in 4% paraformaldehyde, dehydrated in ethanol, embedded in paraffin, and sectioned at 5 μm thickness.
2.11 Picosirius Red staining
The tissue sections were dewaxed in Histoclear followed by a series of washes in ethanol and stained with pico-sirius red solution for 1 hour (Sigma). The sections were washed in acidified water and ethanol and mounted with resinous medium (Histomount). Images of the entire heart section were taken at 2.5x magnification on a bright field microscope (Olympus) and tiled together using Adobe Photoshop. The % fibrosis was quantified using Image J as the ratio of fibrotic tissue (stained red) to total tissue.
2.12 Statistics
Data are represented as mean±SEM. All data analyzed by Student’s t-test, One-way or Two-way ANOVA followed by the appropriate post test (GraphPad Prism5). A p value <0.05 was considered significant.
3. Results
3.1 Characterization of hydrogels
Mechanical testing of hydrogel formulations by atomic force microscopy showed that addition of either scrambled (RS) or Jagged1 (RJ) peptide to the SAP did not alter the Young’s modulus in comparison with unmodified hydrogels at both 1 and 2% w/v. The average Young’s modulus for 1% and 2% hydrogels was 500 Pa and 1800 Pa respectively (Fig. 1A). The 1% hydrogels had a significantly higher swelling ratio than the corresponding 2% hydrogels indicating a more loosely linked peptide network at lower polymer density with no significant effect in network structure on addition of either RS or RJ peptide (n=3, p<0.05, Fig. 1B).
Figure 1. Mechanical characterization of hydrogels.

(A) Atomic Force Microscopy measurements of Young’s modulus of hydrogels. The average Young’s modulus of the 1% hydrogels (1R, 1RS and 1RJ) is 500Pa and the 2% hydrogels (2R, 2RS, 2RJ) is 1800Pa. *** p<0.001, Student’s t-test. (B) Swelling ratio of the hydrogels determined at 24h following gellation. All the 1% hydrogels had a significantly higher swelling ratio than the corresponding 2% hydrogels. n=3, * p<0.05, Student’s t-test.
3.2 Notch1 activation by hydrogel
CHO cells with Notch1-responsive YFP expression were cultured in media containing 20 or 40 μM R, RS or RJ for 48h. A significant increase in YFP expression was observed on treatment with RJ at 40 μM compared to all other treatment groups (p<0.05, n=3, Fig. 2A). These cells were also cultured in 3D in 1–3% w/v hydrogels composed of R, RS or RJ for 48h. A significant increase in YFP expression was observed with increasing hydrogel concentration in absence of any ligand (n=7, p<0.01, open bars, Fig. 2B). Interestingly, presence of RJ in 2% hydrogels resulted in maximum Notch1 activation (p<0.05, middle black bar, Fig. 2B). Further increase to 3% w/v did not promote Notch1 activation.
Figure 2. Notch1 activation in 3D is dependent on hydrogel concentration.<.

br>(A) CHO cells with Notch1 responsive YFP expression were cultured in 2D with media containing R, RS or RJ at 20 or 40μM for 48h. A significant increase in Notch1 activation was observed on treatment with 40μM RJ, n=3, * p<0.05, one-way ANOVA. (B) CHO cells were cultured in 3D in 1–3% w/v hydrogels containing R, RS or RJ for 48h. A significant increase in Notch1 activation was observed on increasing hydrogel concentration(open bars) and presence of RJ at 2% had the highest levels of Notch1 activation (middle black bar), n=7, * p<0.05, two-way ANOVA.
3.3 In vitro gene expression
CPCs were clonally expanded and characterized as in [23] to be >96% Notch1+, >90% c-Kit+, nkx2–5+ (nk2 homeobox 5) and gata4+ (GATA binding protein 4) (Fig. S1A). CPCs were cultured in 1 or 2% (w/v) SAP hydrogels (1R, 1RS, 1RJ or 2R, 2RS, 2RJ) for 48h. Culture in 1RJ hydrogels resulted in a significant increase in Hey1 (hairy/enhancer-of-split related with YRPW motif 1), a downstream target of Notch1 signaling (p<0.05, n=4; Fig. 3A). A significant increase in expression of the endothelial genes Flt1 (fms-related tyrosine kinase 1, p<0.05, n=5) and vWF (von Willebrand factor, p<0.05, n=8; Fig. 3B and C), and the smooth muscle genes Tagln (Transgelin, p<0.05, n=7) and Acta2 (sm α-actin, p<0.05, n=7; Fig. 3D and E) was observed with no change in expression of the cardiac genes, nkx2–5, Mef2c and Gata4 (n=4, Fig. S2A–C).
Figure 3. Culture of CPCs in 1% hydrogels with RJ activates Notch1 signaling and promotes endothelial and smooth muscle gene expression.
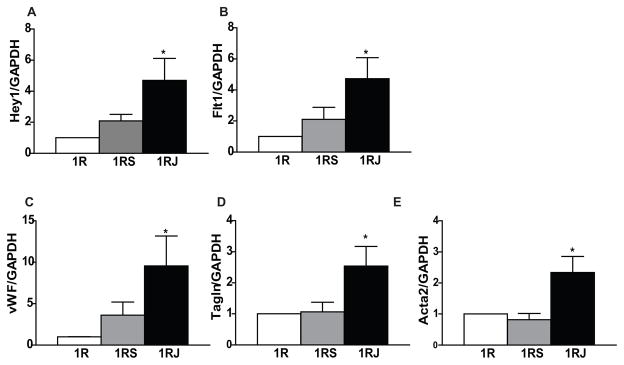
CPCs were cultured in 1% hydrogels (R, RS, RJ) for 48h and expression of (A) Notch1 downstream target, Hey1, (B) VEGF receptor1 Flt1, (C) vWF, (D) Tagln, and (E) Acta2 was measured by qPCR. n=4–8, * p<0.05 vs 1R.
Culture of CPCs in 2RJ hydrogels increased expression of Hey1 (p<0.05, n=4, Fig. 4A) and promoted expression of the cardiac genes nkx2–5 (p<0.001, n=5), mef2c (p<0.05, n=8) and gata4 (p<0.05, n=6; Fig. 4B–D) with no changes in expression of the endothelial or smooth muscle genes (n=5–7, Fig. S3 A–D). No significant differences were observed in gene expression between CPCs in control and scrambled hydrogels.
Figure 4. Culture of CPCs in 2% hydrogels with RJ activates Notch1 signaling and promotes cardiac gene expression.
CPCs were cultured in 2% hydrogels (R, RS, RJ) for 48h and expression of (A) Notch1 downstream target, Hey1, (B) nkx2–5, (C) MEF2C, and (D) GATA4 was measured by qPCR. n=4–8, * p<0.05, *** p<0.001 vs 2R.
To determine if gene expression changes in response to hydrogel-mediated Notch1 activation were conserved between stem/progenitor cells, day 2 embryoid bodies (EBs) from mouse embryonic stem cells were cultured in low concentration (1%) hydrogels for 48h. A significant increase in expression of endothelial and smooth muscle genes such as VEGF receptor KDR (Kinase insert domain receptor, p<0.05), PECAM1 (platelet/endothelial cell adhesion molecule 1, p<0.001) and Myh11 (smooth muscle myosin heavy chain, p<0.01) was observed in cells cultured in 1RJ hydrogels (n=3, Fig. 5 A–C).
Figure 5. Culture of mouse embryoid bodies in 1% hydrogels with RJ promotes endothelial and smooth muscle gene expression.

Mouse EBs were cultured in 1% hydrogels (R, RS, RJ) for 1 week and expression of (A) KDR, (B) PECAM1, and (C) Myh11 was measured by qPCR. n=3, * p<0.05, ** p<0.01 and *** p<0.001 vs 1R.
3.4 In vitro gene array
Expression levels of several signal transduction pathway targets in CPCs cultured in 1R or 2R hydrogels were analyzed by qPCR array. CPCs in 1R hydrogels had higher expression of proinflammatory genes such as TNFα, TRAIL and IFNγ, MMP7 and CyclinD2 compared to CPCs in 2R hydrogels. The downstream targets of Notch1- Hes5 and Heyl were also increased indicative of Notch1 activation (Fig. 6A).
Figure 6. Hydrogel concentration differentially regulates gene expression in CPCs.
(A) Higher pro-inflammatory gene expression in CPCs cultured in 1R hydrogels compared to 2R. (B) Increased cardioprotective gene expression in CPCs cultured in 2R hydrogels compared to 1R. The pie charts indicate the number of genes and known associated function.
Culture of CPCs in higher concentration hydrogel (2R) resulted in increase of the Notch1 target Hes1, the Notch1 ligand Jagged1 and γ-secretase interacting gene Herpud1 (Fig. 6B). Higher expression of i) antioxidant genes - glutathione reductase (Gsr), NAD(P)H dehydrogenase quinone 1 (Nqo1), Sequestosome 1(Sqstm1) and carbonic anhydrase 9 (Car), ii) PPAR targets - Glut1, Fatty acid transporter member 4 (Slc27a4) and long chain acyl-CoA synthetases- members 4 and 5(Acsl4, Acsl5) iii) Bone morphogenetic protein 2 (BMP2), iv) Wnt ligands- Wnt5A and Wnt1 inducible signaling pathway protein 1 (Wisp1), v) TGFβ targets -Cyclin-dependent kinase inhibitor 1B (Cdkn1b) and epithelial membrane protein 1 (Emp1), vi) NFκB target macrophage colony stimulating factor 1 (M-CSF) and vii) JAK/STAT inhibitor- Suppressor of cytokine signaling 3 (Socs3) and JAK/STAT target myeloid cell leukemia sequence 1 (Mcl1) was detected. The fold changes in gene expression are summarized in Table S2.
3.5 Paracrine effects of RJ hydrogels
Conditioned media (CdM) was obtained from CPCs cultured for 48h in 1R, 1RS, 1RJ, 2R, 2RS and 2RJ hydrogels. No change in levels of VEGF, GM-CSF, IP10, bFGF and IGF1 were observed between CPCs in 1R and 1RJ hydrogels (n=6, p>0.05, ELISA array). However, culture in 1RJ hydrogels increased expression of SCF, IL6 (p<0.05) and PDGF (p=0.08, n=6, Fig S4).
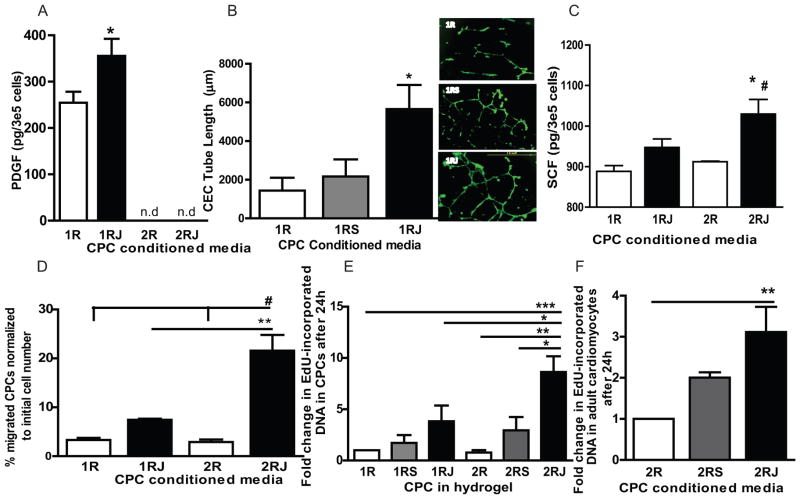
CPCs cultured in 1RJ hydrogels had significantly increased platelet-derived growth factor-BB (PDGF) compared to 1R gels with levels below the limit of detection in CPCs in 2R and 2RJ hydrogels (p<0.05, Fig. 7A, ELISA). Culture of rat cardiac endothelial cells (CECs) in 1RJ CdM led to more tubes and interconnections with a significant increase in tube length compared to 1R and 1RS CdM (n=3, p<0.05, representative images in right inset, Fig. 7B). ELISA for stem cell factor (SCF) in CdM showed a significant increase in 2RJ CdM (n≥5, p<0.05 vs 2R, p<0.001 vs 1R, Fig. 7C). SCF binds to c-Kit on CPCs and is a chemoattractant for progenitor cell migration [26]. CPC migration in a transwell Boyden chamber was significantly higher towards CdM from CPCs in 2RJ hydrogels compared to other groups (n=3, p<0.001 vs 1R and 2R, Fig. 7D). As Notch1 signaling promotes cell proliferation, CPCs were cultured in 1R, 1RS, 1RJ, 2R, 2RS and 2RJ hydrogels for 24h and cell proliferation was quantified as extent of EdU incorporated DNA in cells by click-iT EdU assay. CPCs cultured in 2RJ hydrogel had a significant increase in EdU incorporated DNA indicative of proliferation compared to all other groups (n=3–5, p<0.05 vs 2RS and 1RJ; p<0.01 vs 2R; p<0.001 vs 1R, Fig. 7E). A significant increase in EdU incorporation was observed in primary adult cardiomyocytes cultured in 2RJ CdM indicative of proliferation (n=5, p<0.01 vs 2R, Fig. 7F). A similar result was observed in H9C2 myoblasts (Fig. S5, n=4, p<0.05 vs 2RS, p<0.01 vs 2R).
Figure 7. Hydrogel concentration dependent paracrine effects of Notch1 activated CPCs.<.
br>(A) Higher PDGF levels in conditioned media (CdM) from CPCs in 1RJ compared to 1R hydrogels. * p<0.05, n=6, Student’s t-test, n.d not detectable. (B) Quantification of rat cardiac endothelial cell (CEC) tube length. A 3-fold increase in tube length is observed in CECs cultured in CdM from CPCs cultured in 1RJ hydrogels. n=3, * p<0.05 vs 1R. Representative images of CEC tube formation in CdM from CPCs in 1R (top), 1RS (middle) and 1RJ (bottom). Scale bar 1mm. (C) Higher SCF levels in CdM from CPCs in 2RJ compared to all other hydrogels. * p<0.05 vs 2R, # p<0.001 vs 1R, n≥5, One-way ANOVA. (D) Quantification of CPC migration. Higher % of migrated CPCs was observed in CPCs cultured in CdM from CPCs in 2RJ hydrogels. ** p<0.01 vs 1RJ, # p<0.001 vs 1R and 2R, n=3, One-way ANOVA. (E) Increased EdU-incorporated DNA in CPCs cultured in 2RJ compared to all other hydrogels. * p<0.05, ** p<0.01 and *** p<0.001, n=3–5, One-way ANOVA. (F) Increased EdU-incorporated DNA in rat primary cardiomyocytes cultured in 2RJ compared to all other hydrogels. ** p<0.01, n=5, One-way ANOVA.
3.6 In vivo cardiac retention
A rat model of myocardial infarction was used to determine the effect of CPC delivery in hydrogels. To analyze the extent of myocardial cell retention, DiR-labeled CPCs were injected in 2R, 2RS or 2RJ hydrogels in 3 areas around the infarct border zone. Time course fluorescent in vivo imaging of rats as a readout of cardiac retention showed that CPCs in 2RJ hydrogels had significantly higher % retention compared to CPCs in 2R and 2RS hydrogels until day 7 (Fig. 8, n=5 per group, p<0.05 vs 2R at days 1,2,3 and 4; p<0.05 vs 2RS at days 4 and 7, two-way ANOVA). Representative images of each treatment group are shown in Fig S6.
Figure 8. Improved myocardial retention of CPCs in 2RJ hydrogels.<.
br>Rats with intramyocardial injection of DiR labeled CPCs were imaged on days 0,1,2,3,4,7,10,14 and 21. Increased acute retention of implanted CPCs is observed in 2RJ hydrogels compared to CPCs in 2R and 2RS. * p<0.05 vs 2R and ^ p<0.05 vs 2RS, n=5 per group, Two-way ANOVA.
3.7 In vivo cardiac function
The functional consequences of CPC implantation in 2R, 2RS or 2RJ hydrogels was investigated in rats subjected to 30 minutes of ischemic/reperfusion (IR). CPCs in 2R, 2RS, or 2RJ hydrogels were injected intramyocardially in 3 infarct border zone areas. Cardiac function was evaluated on day 21 by invasive pressure-volume hemodynamic measurements. IR significantly decreased ejection fraction of the hearts when compared to sham operated rats and treatment with CPCs in 2RJ hydrogels significantly improved ejection function comparable to sham operated rats (Fig. 9A). No improvement in function was observed in rats treated with CPCs in empty (2R) or scrambled (2RS) hydrogels indicating the importance of 2RJ containing hydrogels in CPC mediated functional improvement following infarction. Treatment with CPCs in 2RJ hydrogels showed a trend for improvement in the cardiac contractility indicator ±dP/dt and significant improvement in end systolic volume (Fig. 9B and D). Among other parameters of ventricular function, the significant decrease in stroke work, stroke volume and cardiac output following IR was reversed in rats treated with CPCs in 2RJ hydrogels (Fig. 9C, Fig. S7A and B). No change in end diastolic volume was observed (Fig. 9D). These results are summarized in Table S3. Picosirius Red staining of heart sections demonstrated a significant increase in cardiac fibrosis in untreated infarcted hearts. In comparison, treatment with CPCs in 2RJ hydrogel resulted in a significant decrease in fibrosis (p<0.05, n≥5, Fig. 9F). Representative images of Pico-Sirius Red stained heart sections are shown in Fig. S8.
Figure 9. CPCs in 2RJ hydrogel improve cardiac function and decrease fibrosis following MI.
(A) Pressure Volume Hemodynamic measurements and Pico-Sirius Red staining indicating (A) ejection fraction (EF%), (B) dP/dT, (C) Stroke Work, (D) End Systolic Volume (ESV), (E) End Diastolic Volume (EDV), (F) % fibrosis in rats treated with CPCs in 2RJ hydrogels. (G) Representative Pico-Sirius Red stained heart sections. * p<0.05, **p<0.01, # p<0.001, n=10–12 for A–E, n≥5 for F, One-way ANOVA and Tukey’s posttest. Scale bar 1mm. 3
4. Discussion
Cardiac progenitor cells (CPCs) can be easily obtained from autologous sources and have shown promising outcomes in Phase I trials in patients with cardiovascular disease. However, lack of both myocardial cell retention and specific soluble cues impedes successful regeneration. Thus, hydrogels that mimic cardiac-tissue could provide appropriate mechanical and chemical cues to augment CPC function. Stem cell function is regulated by mechanical stimuli such as substrate stiffness, rigidity, shear stress, stretch and topography [27]. Substrate stiffness depending on correlation with native tissue stiffness has been shown to regulate lineage specification of adult stem cells into lineages, endothelial differentiation of cardiac precursor cells and human pluripotent stem cell derived cardiomyocyte contraction [28–30]. These studies elucidate control of stem cell fate and function through mechanical properties of culture environment. Here, we demonstrate that hydrogel-stiffness and ligand density activate Notch1 signaling and regulate CPC gene expression and function in vivo. These effects could also be due to changes in other physical properties such as the pore size of the hydrogel system.
Self-assembling peptide (SAP) hydrogels have been used to deliver growth factors [15], cytokines [31] and cardiac stem cells [32] to the infarcted heart. Moreover, hydrogel stiffness and biofactor concentration can be tuned to provide well-regulated mechanical and biochemical properties. SAP hydrogels also recruit host endothelial cells and cardiomyocytes into the myocardium and promote neovascularization [33]. Delivery of CPCs in IGF1 containing SAP improved cardiac function in infarcted rats [12]. As RADA16-II used in this study has no known cell adhesion motifs, physical entrapment of implanted cells within the hydrogel and the cell synthesized extracellular matrix are potential explanations for cell retention [14]. The ease of synthesis, low immunogenicity [31], and ability to incorporate bioactive motifs (such as RJ) within SAP hydrogels provides a platform for future studies.
Development of biomaterials in which signaling pathways critical for CPC function such as Notch1 can be incorporated is of great importance as CPCs are endogenously present in niches of defined composition [34, 35] and exert reparative effects depending on environmental cues following injury, aging or disease [4, 36]. The current work demonstrates that in CHO cells with Notch-responsive YFP expression, increase in hydrogel concentration (1–3% w/v) in absence of ligand augmented Notch1 activation indicating that physical properties of the hydrogel dependent on concentration such as stiffness and hydrogel porosity can affect Notch1 activation in 3D. However, high concentration hydrogels could lead to stiffness increases that saturate notch1 signaling. Further, comparison of gene expression changes between CPCs in hydrogels of increasing concentration in absence of ligand (1R and 2R hydrogels) indicates increased activation of Notch responsive genes and certain other signal transduction mediators. These results could also support the observed increase in YFP expression in CHO cells on increasing hydrogel concentration in absence of the ligand. Recent studies have demonstrated reliance of Notch activation on ligand-mediated pulling force exerted on the notch extracellular domain [20]. Such mechanotransduction-mediated notch activation has been attempted here using RJ containing hydrogels of varying stiffness. In vitro 3D culture of CPCs in RJ containing 1 or 2% hydrogels increased expression of Hey1, a downstream target of Notch1. Interestingly, culture on lower stiffness 1RJ hydrogels resulted in increased endothelial and smooth muscle gene expression. However, higher stiffness 2RJ hydrogels promoted cardiac gene expression indicating that the synergistic effect of hydrogel stiffness and Notch1 activation on cardiogenic gene expression could pre-commit CPCs towards the endothelial/smooth muscle or cardiac lineage respectively before in vivo delivery. This is critical as the fibrotic infarct environment is less compliant and delivery of CPCs in 2RJ hydrogel provides an environment amenable to remodeling and repair [28, 37]. While determination of the extent of Notch activation by Western Blot for the Notch1 Intracellular Domain (NICD) would yield useful information, the difficulty in isolating protein from cells cultured in more than 1mg of peptide hydrogel led to use of qPCR as the primary method for detection of Notch activation.
To determine potential cell signaling pathways apart from Notch1 that are regulated by increasing concentration of the hydrogel (1R vs 2R), a qPCR array was performed. CPCs cultured in lower concentration hydrogel (1R) had increased expression of proinflammatory genes such as TNFα, TRAIL and IFNγ. Increased Heyl and Hes5 support the observed increase in its downstream target smooth muscle genes [38]. The increase in TNFα along with higher levels of PDGF in CdM from 1RJ hydrogels could support the increased endothelial gene expression in CPCs in 1% hydrogels as a recent study indicates that TNFα secreted by macrophages mediates endothelial differentiation of mouse cardiac progenitor cells [39]. MMP7 is increased in CPCs in 1R hydrogels and fibronectin which has been shown to be essential for reparative function of CPCs following infarction is a myocardial target of MMP7 [35, 40]. Hence, increased MMP7 could degrade the CPC environment. The proinflammatory environment makes the low concentration hydrogels unfavorable for progenitor cell delivery in vivo.
Culture of CPCs in higher concentration 2R hydrogel increased expression of Hes1 and its downstream target Jagged1 [41]. Hence, increasing hydrogel concentration promotes ligand expression that could sustain Notch1 activation. Increase in Herpud1 which is known to interact with presenilins, a component of the γ-secretase complex could also enhance Notch1 activation [42]. Moreover, increased expression of long chain acyl-CoA synthetases, BMP2 and TGFβ related genes that are known to exert beneficial effects on cardiomyogenesis combined with the cardioprotective effects of antioxidant genes, M-CSF and Wnt5A may improve function of CPCs delivered in 2R hydrogels and provides the basis for in vivo utilization [39, 43–46]. Presence of RJ could further enhance CPC survival, promote cardiac gene expression, and function.
Paracrine signaling is pivotal in stem cell therapy as soluble factors released by transplanted cells can augment cardiac repair [47]. We observed secretion of PDGF and SCF in CdM from CPCs in 1RJ and 2RJ hydrogels respectively. While the role of PDGF in promoting angiogenesis and SCF in regulating CPC migration is well known [26, 48], SCF has also been shown to promote endothelial cell tube formation [49]. The CdM from CPCs in 1RJ hydrogels promoted cardiac endothelial cell tube formation indicating paracrine angiogenic benefits. Matsuura et al have attributed the beneficial effects of CPC transplantation in a mouse model of MI to secreted factor- mediated angiogenesis [50]. Proteomic analysis of cardiosphere-derived cell secretome has also identified key factors that are differentially secreted compared to cardiomyocytes [51]. High levels of SCF in CdM from CPCs in 2RJ hydrogels promoted CPC migration towards the CdM. Interestingly, mouse embryoid bodies cultured in 1RJ hydrogels had increased endothelial and smooth muscle gene expression similar to CPCs indicating that 3D Notch1 activation results in a conserved response of stiffness-dependent gene expression. As Notch1 signaling has been shown to regulate stem cell proliferation, we examined proliferative response of CPC and cardiomyocytes. Increased EdU incorporated DNA in CPCs in 2RJ hydrogels and in adult cardiomyocytes cultured in 2RJ CdM was observed. These results suggest that differential soluble factor secretion by CPCs cultured in RJ hydrogels is stiffness dependent and these exert paracrine benefits on cardiomyocytes, endothelial cells and CPCs.
Next we examined if beneficial effects observed in vitro translated to improvements in cardiac retention and function in a rat model of myocardial infarction (MI). Delivery of CPCs in 2RJ hydrogels led to sustained retention of implanted CPCs (~80%) in the myocardium for 1 week. Such high level of acute retention is significant as Terrovitis et al. report less than 20% retention of transplanted cardiac derived stem cells 1 week after administration in a rat model of MI [52]. As low myocardial retention of implanted stem cells impedes cardiac regeneration and repair, delivery in 2RJ hydrogels provides an environment for successful CPC engraftment. Pressure-volume hemodynamic assessment of metrics indicative of functional improvement such as ejection fraction, stroke work and end systolic volume indicated preferential improvement in rats treated with CPCs in 2RJ hydrogels.
5. Conclusions
We have shown that Notch1 activation is dependent on physical properties of the hydrogel, which differentially regulates cardiogenic gene expression in CPCs, and that delivery of CPCs in 2RJ hydrogels improves acute myocardial retention and hemodynamic function after MI. Intramyocardial injection of CPCs in 2RJ hydrogels could be an efficient and robust cell delivery strategy for myocardial repair. These studies need to be performed in large animal models of MI to be predictive of effects in humans. As cKit+ CPCs can be easily isolated from cardiac biopsies even from patients with advanced heart failure [53], the improvement in cardiac function, contractility and retention in the CPC in 2RJ group could translate into positive clinical outcomes in patients.
Supplementary Material
Acknowledgments
This publication has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C to MED. This work was also supported by funding from the National Institutes of Health grant DP3DK094346 to YY and an American Heart Association Predoctoral fellowship 11PRE7840078 to AVB.
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–13. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oikonomopoulos A, Sereti KI, Conyers F, Bauer M, Liao A, Guan J, et al. Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ Res. 2011;109:1363–74. doi: 10.1161/CIRCRESAHA.111.250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang J, Wang J, Kong X, Yang J, Guo L, Zheng F, et al. Vascular endothelial growth factor promotes cardiac stem cell migration via the PI3K/Akt pathway. Exp Cell Res. 2009;315:3521–31. doi: 10.1016/j.yexcr.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–86. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, et al. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:15529–34. doi: 10.1073/pnas.0808357105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–87. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salimath AS, Phelps EA, Boopathy AV, Che PL, Brown M, Garcia AJ, et al. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PloS one. 2012;7:e50980. doi: 10.1371/journal.pone.0050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segers VF, Lee RT. Local delivery of proteins and the use of self-assembling peptides. Drug Discov Today. 2007;12:561–8. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Lin YD, Luo CY, Hu YN, Yeh ML, Hsueh YC, Chang MY, et al. Instructive nanofiber scaffolds with VEGF create a microenvironment for arteriogenesis and cardiac repair. Sci Transl Med. 2012;4:146ra09. doi: 10.1126/scitranslmed.3003841. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Wang X, Wang X, Ren H, He J, Qiao L, et al. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 2013;9:6798–805. doi: 10.1016/j.actbio.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes & Dev. 2004;18:901–11. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, et al. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci U S A. 2003;100 (Suppl 1):11889–95. doi: 10.1073/pnas.1834204100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 20.Meloty-Kapella L, Shergill B, Kuon J, Botvinick E, Weinmaster G. Notch ligand endocytosis generates mechanical pulling force dependent on dynamin, epsins, and actin. Dev Cell. 2012;22:1299–312. doi: 10.1016/j.devcel.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickoloff BJ, Qin JZ, Chaturvedi V, Denning MF, Bonish B, Miele L. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–55. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 22.Weijzen S, Velders MP, Elmishad AG, Bacon PE, Panella JR, Nickoloff BJ, et al. The Notch ligand Jagged-1 is able to induce maturation of monocyte-derived human dendritic cells. J Immunol. 2002;169:4273–8. doi: 10.4049/jimmunol.169.8.4273. [DOI] [PubMed] [Google Scholar]
- 23.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8:4357–64. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Gu C, Cabigas EB, Pendergrass KD, Brown ME, Luo Y, et al. Functionalized dendrimer-based delivery of angiotensin type 1 receptor siRNA for preserving cardiac function following infarction. Biomaterials. 2013;34:3729–36. doi: 10.1016/j.biomaterials.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boopathy AV, Pendergrass KD, Che PL, Yoon YS, Davis ME. Oxidative stress-induced Notch1 signaling promotes cardiogenic gene expression in mesenchymal stem cells. Stem Cell Res Ther. 2013;4:43. doi: 10.1186/scrt190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuang D, Zhao X, Xiao G, Ni J, Feng Y, Wu R, et al. Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol. 2008;103:265–73. doi: 10.1007/s00395-007-0690-z. [DOI] [PubMed] [Google Scholar]
- 27.Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, et al. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol (Camb) 2012;4:1008–18. doi: 10.1039/c2ib20080e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kshitiz, Hubbi ME, Ahn EH, Downey J, Afzal J, Kim DH, et al. Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Sci Signal. 2012;5:ra41. doi: 10.1126/scisignal.2003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gelain F, Unsworth LD, Zhang S. Slow and sustained release of active cytokines from self-assembling peptide scaffolds. J Control Release. 2010;145:231–9. doi: 10.1016/j.jconrel.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Tokunaga M, Liu ML, Nagai T, Iwanaga K, Matsuura K, Takahashi T, et al. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. J Mol Cell Cardiol. 2010;49:972–83. doi: 10.1016/j.yjmcc.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, et al. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A. 2006;103:9226–31. doi: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstandin MH, Toko H, Gastelum GM, Quijada P, De La Torre A, Quintana M, et al. Fibronectin is essential for reparative cardiac progenitor cell response after myocardial infarction. Circulation research. 2013;113:115–25. doi: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogorek B, et al. c-kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ Res. 2014;114:41–55. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol-Heart C. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 38.Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–81. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- 39.Wong MM, Chen Y, Margariti A, Winkler B, Campagnolo P, Potter C, et al. Macrophages control vascular stem/progenitor cell plasticity through tumor necrosis factor-alpha-mediated nuclear factor-kappaB activation. Arterioscler Thromb Vasc Biol. 2014;34:635–43. doi: 10.1161/ATVBAHA.113.302568. [DOI] [PubMed] [Google Scholar]
- 40.Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala K, et al. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. J Proteome Res. 2010;9:2649–57. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi T, Mizuno H, Imayoshi I, Furusawa C, Shirahige K, Kageyama R. The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes & Dev. 2009;23:1870–5. doi: 10.1101/gad.1823109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhanot U, Kohntop R, Hasel C, Moller P. Evidence of Notch pathway activation in the ectatic ducts of chronic pancreatitis. J Pathol. 2008;214:312–9. doi: 10.1002/path.2293. [DOI] [PubMed] [Google Scholar]
- 43.Koyanagi M, Iwasaki M, Haendeler J, Leitges M, Zeiher AM, Dimmeler S. Wnt5a increases cardiac gene expressions of cultured human circulating progenitor cells via a PKC delta activation. PloS one. 2009;4:e5765. doi: 10.1371/journal.pone.0005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyares RL, Stein C, Renisch B, Anderson JL, Hammerschmidt M, Farber SA. Long-chain Acyl-CoA synthetase 4A regulates Smad activity and dorsoventral patterning in the zebrafish embryo. Dev Cell. 2013;27:635–47. doi: 10.1016/j.devcel.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okazaki T, Ebihara S, Asada M, Yamanda S, Saijo Y, Shiraishi Y, et al. Macrophage colony-stimulating factor improves cardiac function after ischemic injury by inducing vascular endothelial growth factor production and survival of cardiomyocytes. Am J Pathol. 2007;171:1093–103. doi: 10.2353/ajpath.2007.061191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlange T, Andree B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000;91:259–70. doi: 10.1016/s0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- 47.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J Cell Biol. 1994;125:917–28. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c-kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–7. doi: 10.1074/jbc.M311643200. [DOI] [PubMed] [Google Scholar]
- 50.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–17. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stastna M, Chimenti I, Marban E, Van Eyk JE. Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics. 2010;10:245–53. doi: 10.1002/pmic.200900515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–94. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D’Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, et al. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res. 2011;108:857–61. doi: 10.1161/CIRCRESAHA.111.241380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.