Abstract
Purpose
Intravesical bacillus Calmette-Guérin is used to decrease recurrence rates of nonmuscle invasive urothelial carcinoma. Irritative urinary symptoms are a common side effect of treatment and frequently limit treatment tolerance. While anticholinergic medications may be used for symptom prophylaxis, to our knowledge they have not been evaluated in a randomized controlled trial.
Materials and Methods
A total of 50 bacillus Calmette-Guérin naïve patients were randomized to 10 mg extended release oxybutynin daily or placebo starting the day before 6 weekly bacillus Calmette-Guérin treatments. A questionnaire assessing urinary symptoms (frequency, burning on urination, urgency, bladder pain, hematuria), systemic symptoms (flu-like symptoms, fever, arthralgia) and medication side effects (constipation, blurred vision, dry mouth) was recorded daily throughout the therapeutic course. A linear mixed repeated measures model tested the differences between each point and baseline score.
Results
The treatment group had a greater increase in urinary frequency and burning on urination compared to placebo (p = 0.004 and p = 0.04, respectively). There were no significant differences between groups for other urinary symptoms, which increased in severity after bacillus Calmette-Guérin but concomitantly returned to baseline in both groups. The treatment group experienced increases in fever, flu-like symptoms, dry mouth and constipation compared to placebo (p <0.0001, p = 0.0008, p = 0.045 and p = 0.001, respectively). There were otherwise no significant differences in nonurinary symptoms or medication adverse reactions.
Conclusions
Oxybutynin increased urinary frequency and burning on urination compared to placebo in patients receiving intravesical bacillus Calmette-Guérin treatment. Our results do not support the routine use of oxybutynin as prophylaxis against urinary symptoms during bacillus Calmette-Guérin therapy.
Keywords: carcinoma, transitional cell, urinary bladder neoplasms, BCG vaccine, cholinergic antagonists, comparative effectiveness research
IN 2012 in the United States bladder cancer had an estimated incidence of 73,500 new cases and accounted for nearly 15,000 deaths.1 Approximately 70% of incident cases are nonmuscle invasive, invading no deeper than the lamina propria.2 In the 1970s intravesical BCG, a live attenuated mycobacterium strain, emerged as an immunotherapeutic agent for the treatment of NMIBC.3,4 It has since become a first line treatment option for NMIBC because of a reduction in disease recurrence and progression.5–7
Despite its efficacy, the side effects of BCG frequently limit a patient’s ability to tolerate a full treatment course.8–10 A 2003 study from the European Organisation for Research and Treatment of Cancer reported that 75% of patients had local side effects and 39% had systemic side effects from intravesical BCG.11 Importantly a quarter of patients delayed treatment secondary to side effects (18.3% local, 6.2% systemic) and 20.3% stopped treatment altogether as a result of local side effects and/or systemic side effects. Symptomatic treatment of BCG induced lower urinary tract symptoms may include the use of anticholinergic medications.12–14
Oxybutynin chloride is a tertiary amine with a direct antispasmodic effect on smooth muscle, and anticholinergic, analgesic and local anesthetic effects. Oxybutynin chloride extended release is approved for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency.15 To the best of our knowledge the efficacy of this treatment for urinary symptoms during BCG therapy is entirely anecdotal and has not been studied in a systematic method. Through a randomized controlled trial we evaluated the effectiveness of an anticholinergic prophylaxis on urinary symptoms during induction BCG treatment.
MATERIALS AND METHODS
After obtaining institutional review board approval we initiated a prospective, randomized, placebo controlled, double-blind trial to determine if oxybutynin ER improved urinary symptoms during induction with intravesical BCG immunotherapy.
Patient Eligibility
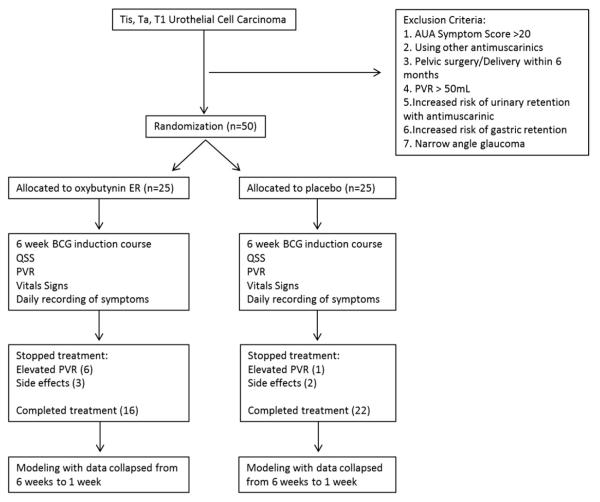
A total of 50 BCG naïve patients were enrolled in the study. Study inclusion criteria were patients older than age 18 years with pathologically demonstrated NMIBC (CIS, Ta or T1). Patients were excluded from study for an AUA symptom score greater than 20, the use of medications for overactive bladder, pelvic surgery within the previous 6 months, a PVR greater than 50 ml or other medical conditions that would be adversely affected by anticholinergics (fig. 1).
Figure 1.
Outline of study design
Treatment and Randomization
As participants were enrolled in the study they were randomly assigned an identification number corresponding to a course of medication. Of the 50 patients 25 received active medication with 10 mg oxybutynin ER and 25 received placebo medication. Patients were instructed to take 1 tablet daily, beginning the night before the first intravesical treatment and continuing throughout the 6 weeks of treatment. Patient and health care professionals were blinded to group assignment.
Outcome Assessment
A quantitative symptom score questionnaire was completed by the patients before treatment to establish baseline symptoms and daily during the 6 weeks of treatment. This questionnaire was designed to evaluate 5 urinary symptoms (frequency, burning with urination, urinary urgency, bladder pain or spasm and hematuria), 3 nonurinary symptoms (fever, flu-like symptoms, joint ache) and 3 anticholinergic adverse drug reactions (constipation, blurry vision, dry mouth). Most symptoms were scored on a 0 to 3-point scale, corresponding to none/mild/moderate/severe. Frequency was scaled as voiding more than every 3 hours, every 2 to 3 hours, every 1 to 2 hours and at intervals of less than 1 hour. Hematuria was scaled as none, pink-red urine, red with clots and very red with many clots. Fever was divided into none, temperature less than 100.5, 100.5 to 102.5 and greater than 102.5F. If patients had a PVR greater than 50 ml, the test was repeated. If PVR was still greater than 50 ml on second attempt, the treatment course was terminated.
Statistical Methods
Each of the 8 symptoms and the 3 adverse drug reactions were analyzed individually. Eight points (morning before treatment, evening after treatment, days 1 to 7) in each of 6 weeklong cycles were recorded for patients completing the full treatment course. The 6 weeks of treatment data were collapsed during the length of a 1-week cycle as there was little weekly variation in symptoms and stronger modeling of each symptom could be performed. Thus, the score for each symptom on EAT is the averaged score from 6 evenings after treatment for each of the 6 weeks. A linear mixed repeated measures model was used to test the differences between each point and patient baseline score as reported on MBT with the QSS. Patient urinary symptoms were evaluated as a change compared to pretreatment values. Specifically a decrease in score with time represented a return to baseline (pretreatment) levels rather than an overall decrease in a particular symptom or adverse event. This approach controlled for inter-patient variability (as patient baseline values would have substantial variability) and provided an adjustment for differing starting levels of each symptom.
The model predictors were the study group (treated vs placebo) and time of treatment (EAT to PD 6). The Fisher exact and Wilcoxon rank sum tests were used to compare patient characteristics by treatment. For rare events (fever, flu-like symptoms, constipation) p <0.05 was considered significant. SAS® 9.0–9.2 was used for all statistical analyses.
RESULTS
Placebo and treatment groups were similar in baseline characteristics (see table). Completion of the entire 6-week course was statistically equivalent in the 2 groups (treatment group 16 of 25 vs placebo group 22 of 25, p = 0.10).
Pretreatment patient demographics
| Placebo | Oxybutynin | p Value | |
|---|---|---|---|
| No. gender: | |||
| M | 18 | 23 | 0.14 |
| F | 7 | 2 | |
| Age | 66.9 | 68.6 | 0.27 |
| No. race (Caucasian: African-American) | 21:4 | 23:2 | 0.67 |
| Body mass index (kg/m2) | 28.2 | 30.2 | 0.36 |
| AUA symptom score | 8.52 | 7.29 | 0.41 |
| % Tobacco use | 80 | 88 | 0.80 |
Urinary Symptoms
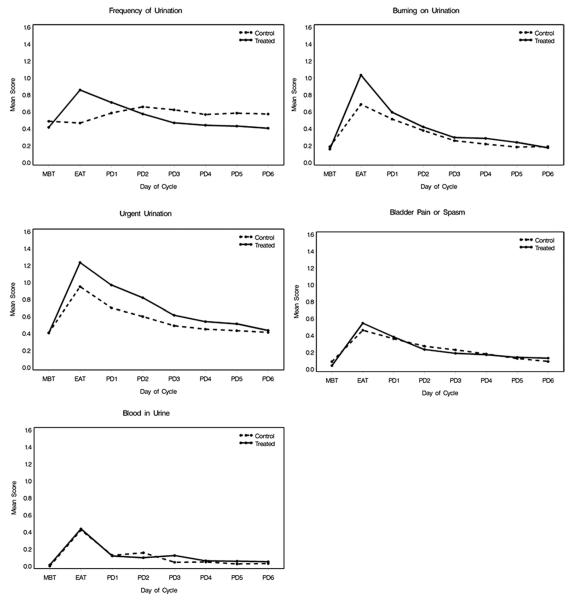
The treatment group had a greater increase in urinary frequency scores vs baseline on the first evening after treatment compared to the placebo group (p = 0.004, fig. 2). In the control group urinary frequency scores increased gradually over baseline from the evening after treatment through PD 2. After day 2 the increase in urinary frequency plateaued and began to return to baseline. In the treatment group urinary frequency scores peaked on the evening after treatment and gradually returned to the baseline level. There was a significant difference between the time courses of these groups (p = 0.003).
Figure 2.
Urinary symptom profiles
The increase in burning on urination on the evening after treatment was greater in the treated group compared to placebo (p = 0.04), with a statistically significant difference in the trend in the 2 groups with time (p = 0.01). There were no significant differences in the symptom profiles in the treatment vs placebo groups for urinary urgency (p = 0.49), bladder pain or spasm (p = 0.65), or hematuria (p = 0.97). For each of these symptoms the scores increased over baseline on the evening after treatment and then returned to baseline in both study groups.
Systemic Symptoms
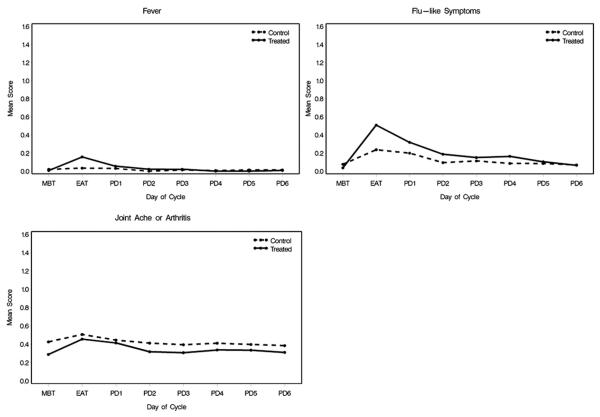
Fever (any severity score greater than 0) was more common in the treatment group than in the placebo group (p <0.0001, fig. 3). Likewise, flu-like symptoms were more common in patients receiving oxybutynin (p = 0.0008). There was no changes in arthralgia between the 2 study groups (p = 0.32).
Figure 3.
Nonurinary symptom profiles
Adverse Reactions to Oxybutynin
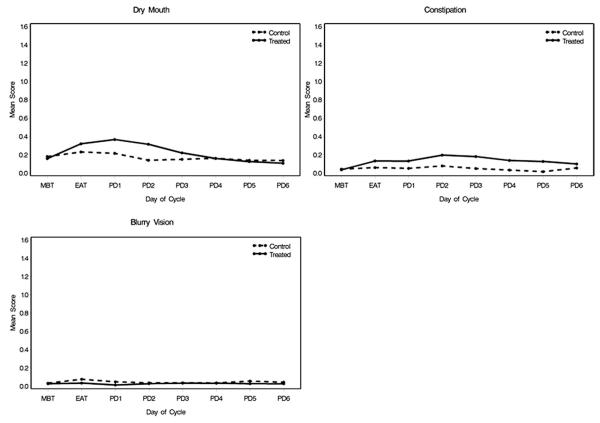
There was an increase in dry mouth symptoms in the treatment group during a treatment cycle compared to the control group (p = 0.045, fig. 4). Constipation (any severity score greater than 0) was more common in the treatment group than in the placebo group (p = 0.001). Blurred vision symptoms rarely occurred throughout the treatment course and could not be modeled statistically.
Figure 4.
Medication adverse reactions
DISCUSSION
In this randomized, placebo controlled, double-blind study we evaluated the effectiveness of a long-acting anticholinergic in reducing urinary symptoms associated with intravesical BCG therapy. Oxybutynin ER did not improve urinary symptoms associated with intravesical BCG.
We paradoxically found that patients receiving oxybutynin ER experienced increased urinary frequency and burning with urination compared to placebo. This may have contributed to the fact that fewer patients in the oxybutynin arm completed BCG treatment. These unanticipated results may be a result of anticholinergic medications causing an element of incomplete bladder emptying and allowing an increased BCG dwell time. In turn, increased urothelial exposure would create a more pronounced immunological response. This theory is supported by the increased likelihood of a fever and flu-like symptoms immediately after treatment. Increases in dry mouth and constipation in the treatment group, known side effects of anticholinergics, suggest that patients were adhering to the treatment regimen.
The lower urinary tract side effects of intravesical BCG, although incompletely studied, may be due to local irritation from inflammation and comparable to a chemical cystitis rather than induction of uninhibited bladder contractions and, thus, may not benefit from anticholinergic therapy. Oxybutynin is also known to have a local anesthetic effect on the bladder, but this affect appears to be inadequate to ameliorate BCG induced urinary symptoms.
This trial provides level 1 evidence against the prophylactic use of anticholinergic therapy during BCG intravesical treatment. Despite the widespread use of anticholinergics to ameliorate symptoms from BCG, there are no other reported trials of the effects on BCG related symptoms. The other options for the management of BCG induced symptoms include BCG dose reduction, antibiotics, steroid therapy or treatment cessation. However, these approaches have limited evidence and are also based largely on anecdotal experience.16
Our study also provided detailed insight into the day-to-day severity and duration of symptoms during induction intravesical BCG treatment. No prior study has examined in detail the side effects of BCG and no validated questionnaire existed. The questionnaire we created was based on the clinical experience of patients receiving intravesical treatment and the possible side effects of anticholinergics. We found that most urinary symptoms peaked on EAT then slowly improved toward baseline during the subsequent week. Clinically these findings are relevant for physicians when counseling patients regarding expectations of symptom severity and duration during a 6-week course of BCG.
This study has some limitations. The small population size may make differences between the study groups potentially undetectable due to an underpowered sample size. However, given that the results favor the placebo arm, it appears unlikely that a larger study would demonstrate that treatment improved outcomes with oxybutynin. We initially planned on a larger study but when the initial analysis after 50 patients showed no benefit, the study was terminated. In addition, the use of a non-validated questionnaire that only included a 0 to 3-point grading system for severity was a limitation. Unfortunately no validated questionnaire exists for this population and, therefore, our study required the creation of a questionnaire. Our study design started the night before treatment and did not include a run-in period of treatment. However, plasma concentrations of oxybutynin ER increased for 4 to 6 hours after the initial dose, with steady state levels reached by day 3 of treatment.17 By following the patients for 6 weeks during induction BCG, we minimized the effect of initial dosing and allowed assessment of a patient baseline just before treatment. Additionally, the doses of BCG and oxybutynin ER were standardized without any adjustments based on urinary symptoms. Thus, our study did not evaluate dose escalation, but the lack of any positive effect at initial starting doses is evidence against improvement at larger doses.
CONCLUSIONS
Patients receiving daily oxybutynin experienced no improvement in urinary symptoms compared to placebo and, to the contrary, experienced worsening urinary frequency and burning with urination. These patients also experienced fever and flu-like symptoms more commonly. Based on the results of our randomized controlled study we would not recommend the prophylactic use of oxybutynin to decrease urinary symptoms during induction intravesical BCG treatment.
ACKNOWLEDGMENTS
The active medication and placebo were provided by Alza Pharmaceuticals.
Abbreviations and Acronyms
- AUA
American Urological Association
- BCG
bacillus Calmette-Guérin
- EAT
evening after treatment
- ER
extended release
- MBT
morning before treatment
- NMIBC
nonmuscle invasive bladder cancer
- PD
posttreatment day
- PVR
post-void residual
- QSS
quantitative symptom score
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 4.AUA Guideline for the Management of Non-muscle Invasive Bladder Cancer [Accessed October 15, 2012]; Available at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/bladcan07/chapter1.pdf.
- 5.Babjuk M, Oosterlinck W, Sylvester R, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008;54:303. doi: 10.1016/j.eururo.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 6.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guérin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 7.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Lamm DL. Complications of bacillus Calmette-Guerin immunotherapy. Urol Clin North Am. 1992;19:565. [PubMed] [Google Scholar]
- 9.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ translational cell carcinoma of the bladder: a randomized Southwest Oncology Group study. J Urol. 2000;163:1124. [PubMed] [Google Scholar]
- 10.Lamm DL. Efficacy and safety of bacille Calmette-Guérin immunotherapy in superficial bladder cancer. Clin Infect Dis. 2000;31:586. doi: 10.1086/314064. [DOI] [PubMed] [Google Scholar]
- 11.van der Meijden AP, Sylvester RJ, Oosterlinck W, et al. Maintenance bacillus Calmette-Guerin for Ta, T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group phase III trial. Eur Urol. 2003;44:429. doi: 10.1016/s0302-2838(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 12.Kova MP, Simon MA, Soloway MS. Complications of intravesical therapy for urothelial cancer of the bladder. J Urol. 2006;175:2004. doi: 10.1016/S0022-5347(06)00264-3. [DOI] [PubMed] [Google Scholar]
- 13.Uchida A, Yonou H, Hayashi E, et al. Intravesical instillation of bacille Calmette-Guérin for super-ficial bladder cancer: cost-effectiveness analysis. Urology. 2007;69:275. doi: 10.1016/j.urology.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Lamm DL, Blumenstein BA, Crawford ED, et al. Randomized intergroup comparison of bacillus Calmette-Guerin immunotherapy and mitomycin C chemotherapy prophylaxis in superficial transitional cell carcinoma of the bladder a Southwest Oncology Group study. Urol Oncol. 1995;1:119. doi: 10.1016/1078-1439(95)00041-f. [DOI] [PubMed] [Google Scholar]
- 15.Anderson R, Mobley D, Blank B, et al. Once a day controlled release versus immediate release oxybutynin chloride in the treatment of urge urinary incontinence. J Urol. 1999;161:1809. [PubMed] [Google Scholar]
- 16.Gontero P, Bohle A, Malmstrom PU, et al. The role of bacillus Calmette-Guérin in the treatment of non-musical-invasive bladder cancer. Eur Urol. 2010;57:410. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed October 15, 2012];FDA approved drug products. Available at www.accessdata.fda.gov/drugsatfda_docs/label/2008/017577s034,018211s017,020897s018lbl.pdf.