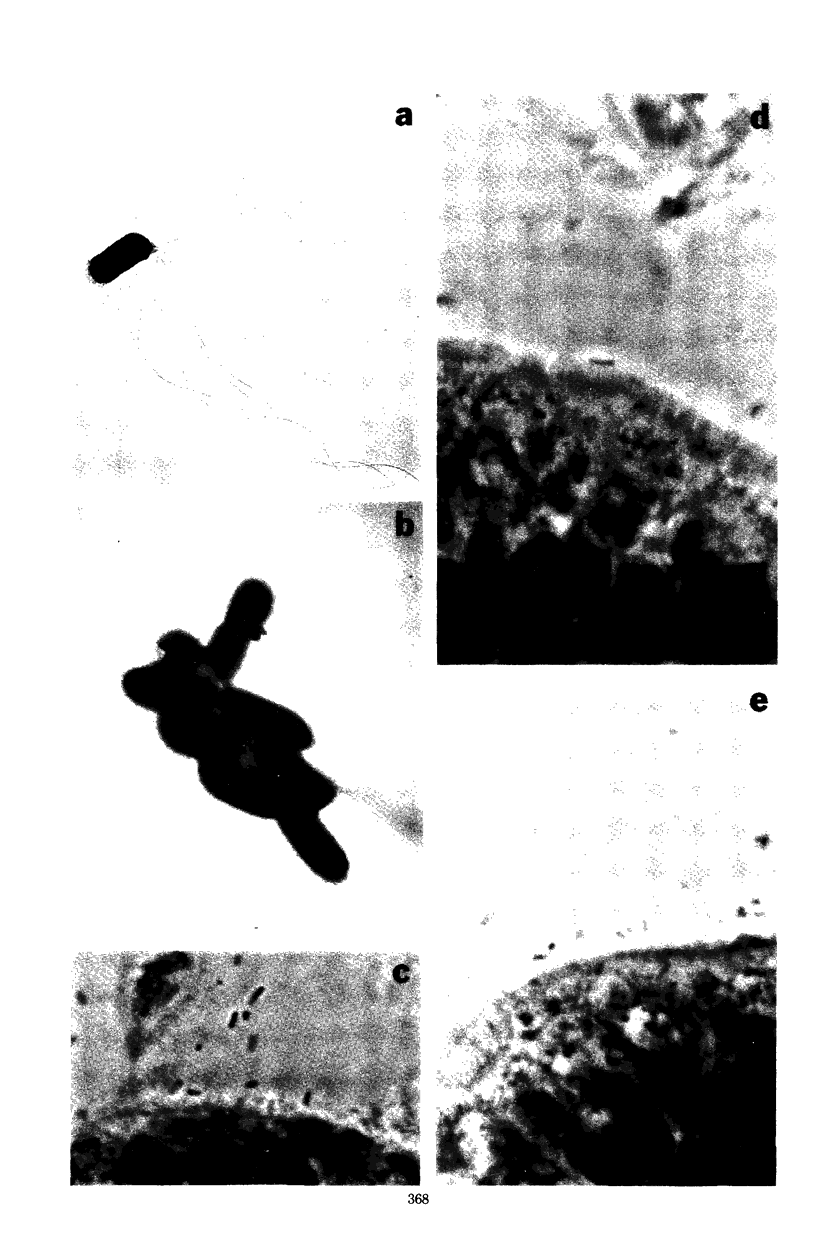
Abstract
A wild-type strain of Salmonella typhimurium and three mutant rough colonial variants of the wild type were compared for their ability to become associated with and invade the ileal mucosa of germfree and specific-pathogen-free mice. The rough-mutant strains differed from the wild type in having incomplete lipopolysaccharides lacking one or more sugars in the polysaccharide moiety. The wild-type and mutant strains also differed one from the other in the types of appendages (flagella, pili) on their surfaces. Depending upon the dosage of bacteria given, all mutant strains as well as the wild type could associate with and invade the intestinal mucosa of infected gnotobiotic mice. If the infecting dosage was high enough, at least two of the mutant strains and the wild type invade the intestinal mucosa of the specific-pathogen-free animals. O antigen, flagella, or pili do not appear to be essential for the association of S. typhimurium with the mucosal surface of the mouse ileum. O antigen on the bacterial cell surface may be important, but not essential, for invasion of the ileal mucosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Bishop J. E. Effect of the normal microbial flora on the resistance of the small intestine to infection. J Bacteriol. 1966 Dec;92(6):1604–1608. doi: 10.1128/jb.92.6.1604-1608.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Gordon H. A., Pesti L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 1971 Dec;35(4):390–429. doi: 10.1128/br.35.4.390-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Saito K. Chemical components in the cell wall of Salmonella typhimurium affecting its virulence and immunogenicity in mice. Nature. 1969 Jun 14;222(5198):1085–1086. doi: 10.1038/2221085a0. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Froholm L. O., Bovre K. Fimbriation and colony type of Moraxella bovis in relation to conjunctival colonization and development of keratoconjunctivitis in cattle. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):911–918. doi: 10.1111/j.0365-5563.1973.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R. J. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962 Jun 1;115:1149–1160. doi: 10.1084/jem.115.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., McAllister J. S., Davis C. P. Anaerobic bacteria on the mucosal epithelium of the murine large bowel. Infect Immun. 1971 Oct;4(4):492–502. doi: 10.1128/iai.4.4.492-502.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]