Abstract
Circuit reorganization after injury was studied in a cerebellar culture model. When cerebellar cultures derived from newborn mice were exposed at explantation to a preparation of cytosine arabinoside that destroyed granule cells and oligodendrocytes and compromised astrocytes, Purkinje cells surviving in greater than usual numbers were unensheathed by astrocytic processes and received twice the control number of inhibitory axosomatic synapses. Purkinje cell axon collaterals sprouted and many of their terminals formed heterotypical synapses with other Purkinje cell dendritic spines. The resulting circuit reorganization preserved inhibition in the cerebellar cortex. Following this reorganization, replacement of the missing granule cells and glia was followed by a restitution of the normal circuitry. Most of these developmental and reconstructive changes were not dependent on neuronal activity, the major exception being inhibitory synaptogenesis. The full complement of inhibitory synapses did not develop in the absence of neuronal activity, which could be mitigated by application of exogenous TrkB receptor ligands. Inhibitory synaptogenesis could also be promoted by activity-induced release of endogenous TrkB receptor ligands or by antibody activation of the TrkB receptor.
Keywords: Cerebellar cultures, Purkinje cells, Astrocytes, Collateral sprouting, Heterotypical synapses, Circuit reorganization, Activity-dependent plasticity, Inhibitory synaptogenesis, TrkB receptor
1. The Issues
The nervous systems of subhuman mammals and man have a remarkable capacity to change and reorganize after various insults resulting from disease or injury. The purpose of these changes is to preserve some functional capacity. The degree to which function can be retained or restored depends on many factors, including the stage of maturity of the affected individual, the critical location and/or magnitude of the area affected, and whether the involved nervous system cells are completely destroyed or only partially damaged. Changes can occur at the level of single cells, such as altering the type of neurotransmitter expressed by a nerve cell, to reorganization of a significant portion of the circuitry of the nervous system. Our interest was in injury-induced reorganizational changes in the central nervous system (CNS). Given the complexity of the CNS, the changes that take place to preserve function cannot be random, but must follow some rules or patterns, as had been indicated by experimental animal studies from a number of laboratories (Cotman et al., 1981; Lynch et al., 1976; Raisman and Field, 1973; Tsukahara et al., 1975). In these studies, axon collateral sprouting by neurons whose projections overlapped those of lesioned neurons were identified as a key element in circuit reorganization after injury in septal nucleus (Raisman and Field, 1973), red nucleus (Tsukahara et al., 1975) and dentate gyrus of the hippocampal formation (Cotman et al., 1981; Lynch et al., 1976) in adult animals. Synapses formed with different presynaptic elements from those originally present, but the newly formed synapses were functional. In order to obtain further definition of some of these rules, my colleagues and I undertook a series of experiments with a simplified CNS in which the injury to the system could be controlled and the subsequent reorganizational changes could be documented.
Why use a simplified CNS? The brain contains a very large number of neurons, each of which is a compartmentalized unit consisting of a cell body (soma) with multiple processes, one of which, the axon, projects electrical impulses away from the soma and the remainder, the dendrites, project electrical impulses toward the soma. Most neurons are either excitatory or inhibitory, their axon terminals, or endings, releasing chemical neurotransmitters in response to electrical impulses at specialized junctions (synapses) with a dendrite or cell body of a target cell. The released neurotransmitter either promotes or inhibits discharge of electrical impulses in the target neuron. The soma acts as an integrator of excitatory and inhibitory signals impinging on its dendrites and somatic membrane, the sum of which determines whether or not an electrical impulse is discharged down its axon. The magnitude of the complexity of the system is in the realization that a neuron may have thousands of synapses, and the number of neurons in a human brain may be on the order of 86 billion (Azevedo et al., 2009).
In addition to neurons, the central nervous system is composed of an even greater number of glia, or supporting cells. Aside from ependymal cells, which line the fluid filled cavities of the brain, there are two major glial types, oligodendroglia and astrocytes. Oligodendroglia form the myelinated sheaths that facilitate conduction of electrical impulses in axons of nerve cells. Myelin is formed by the wrapping of axons in “jelly roll” fashion by processes of oligodendroglia, followed by the extrusion of cytoplasm from the oligodendroglial processes so that the membranes of the processes become closely compacted, providing a multilayered sheath with insulation-like properties along the lengths of the axons. Astrocytes have multiple functions, including structural support for neurons, secretion of a variety of factors that promote neuron survival and growth of neuronal processes, taking up neurotransmitters and ions released into the extracellular space after neuronal discharge, serving as guides for neuronal migration and axonal pathfinding during development, and taking up debris and forming glial scars after injury. Astrocytes also have some function in compartmentalizing the nervous system and in some cases isolating neuronal membranes by ensheathing neuronal somata and dendrites, even covering the somatic and dendritic synapses. Still other functions have been attributed to astrocytes.
There is another category of glia whose origin and function differ from the previously described categories, namely the resident microglia. These cells are derived from the primitive mesodermal layer, as opposed to the ectodermal origin of other glia and neurons, and they enter into the nervous system and become widely distributed early in development. They function as macrophages, cells that become active during pathological conditions, such as after trauma, infection or loss of blood supply. Their role is to attack foreign elements in the CNS, like invasive bacteria, and to scavenge and digest neural cell debris (phagocytosis). They work in conjunction with the immune system to monitor and respond to adverse conditions in the nervous system and activate immune responses by presenting antigens (molecules that trigger immune or inflammatory reactions) to lymphocytes, immunoreactive cells of the immune system. They are an important part of the nervous system's defense mechanism, but their role in the experiments to be described is minor, and thus they will not have a prominent place in the following discussion.
In selecting a simplified central nervous system to use as a model for studies of circuit reorganization, a desirable feature was a system with a limited number of major neuronal types whose interconnections and functions were known. Thanks to the efforts of Santiago Ramón y Cajal (1960), John Eccles, Masao Ito and Janos Szentágothai (1967,1984), and Sanford Palay and Victoria Chan-Palay (1974), as well as other notable neuroanatomists and neurophysiologists, the structural and functional relationships of the rodent cerebellum have been well characterized. The cerebellar cortex contains five major neuronal types, only one of which, the Purkinje cells, projects axons to other parts of the nervous system, and this projection is primarily to the deep cerebellar nuclei, which underlie the cortex. Purkinje cell axons also emit collateral axonal branches that project to all other cortical neurons, including other Purkinje cells. Purkinje cells are inhibitory, and their neurotransmitter is gamma-aminobutyric acid (GABA). Granule cells are the only excitatory neurons in the cerebellar cortex, their neurotransmitter being glutamic acid (glutamate). Most of the excitatory inputs to the cerebellum from other areas of the nervous system (extracerebellar afferents), which are excluded in standard cerebellar cultures (see below), enter as axons called “mossy fibers.” Mossy fibers are cholinergic and synapse with the dendrites of granule cells. The granule cells relay excitatory impulses from the mossy fibers to the dendrites of all other cortical neurons via bundles of parallel axons known as “parallel fibers,” as well as to Purkinje cell dendrites and dendritic spines via their ascending fibers. The remaining three neuronal groups, the basket, stellate and Golgi cells, all inhibitory, are interneurons, with their afferents and efferents confined to the cerebellar cortex. Their presumptive neurotransmitter is GABA. Basket cells project to Purkinje cell somata and proximal dendrites, while stellate cells project their axons to more distal portions of Purkinje cell dendrites. Golgi cells give rise to complex axons that project to dendrites of granule cells. The relationships of the cerebellar cortical neurons, minus the mossy fibers, are summarized in the simplified circuit diagram in Figure 1.
Fig. 1.
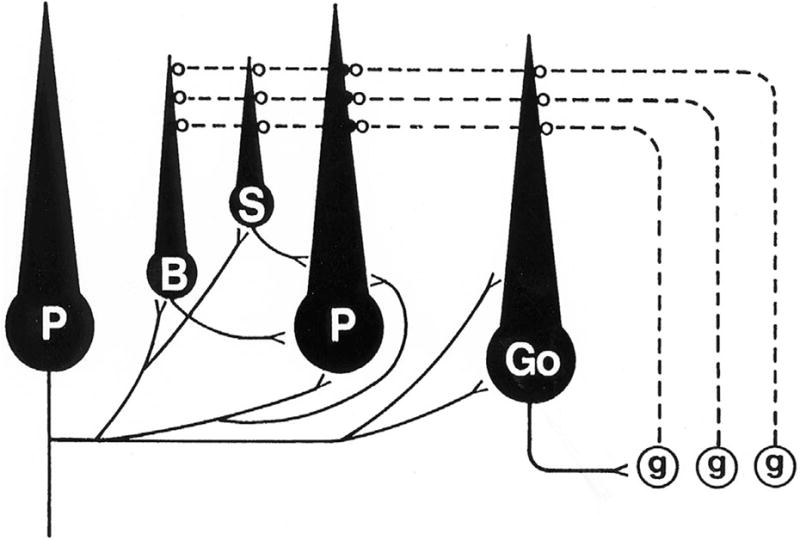
Circuit diagram of the major cerebellar cortical neurons and their projections. Absent from the diagram are the extracerebellar afferents, as would be the case in an isolated cerebellar culture. Projecting axons from Purkinje cells are shown in the Purkinje cell on the left side of the diagram, while projections to Purkinje cells from other neurons are shown in the Purkinje cell in the center. Both Purkinje cells would, of course, have both afferent and efferent projections. The only excitatory cortical neurons are the granule cells (g), and their axons, the parallel fibers (shown as dashed lines), project to the dendrites of all other cortical neurons. All other cortical neurons are inhibitory, and their axons are represented as solid lines. Purkinje cells (P) are the only neurons whose axons project from the cortex, predominantly to the deep cerebellar nuclei (not shown). Purkinje cell axon collaterals project to all other inhibitory cortical neurons, including other Purkinje cells. Basket cell axons (B) project to Purkinje cell somata and proximal dendrites, while stellate cell (S) axons inhibit more distal parts of the Purkinje cell dendritic tree. Purkinje cell somata thus receive inhibitory projections from only two cortical sources, namely basket cells and recurrent axon collaterals from other Purkinje cells. Golgi cells (Go) project their complex axons to granule cell dendrites. (From Seil, 1996, with permission).
There are other extracerebellar inputs in the intact animal. Another excitatory input is via the climbing fibers, which originate in the inferior olivary nuclei in the brain stem and project directly to Purkinje cell dendrites, where they form numerous synapses while branching to conform to the branching of the Purkinje cell dendrites. Still other inputs include catecholaminergic fibers (both norepinephrine and dopamine) from the locus coeruleus in the brain stem and serotoninergic fibers, originating from raphe nucleus neurons, also in the brain stem. All of these extracerebellar inputs are absent in the isolated cerebellum unless special efforts are made to include tissues that give rise to these projections in the culture system to be described.
The cerebellum can be studied in isolation and the system further simplified by preparing organotypic cultures derived from newborn mice and allowing them to develop structural and functional characteristics in vitro (Seil, 1979). Such cultures are prepared by removing the cerebellum and underlying brain stem en bloc from anesthetized neonatal Swiss-Webster mice and then isolating the cerebellum by cutting the cerebellar peduncles, the connections between the cerebellum and the brain stem, close to the cerebellum. After removal of the lateral cerebellar tips, the remainder is divided into 7-8 parasagittal slices (explants) and each explant is placed on a collagen coated glass coverslip with a drop of nutrient medium and incorporated into a sealed Maximow chamber and incubated at 35.5-36° C. Maximow slides are thick glass slides with a well that holds the air (oxygen) for the cultures, and when combined with an outer coverslip that covers the well, forms a Maximow chamber or assembly, originally adapted for neural cultures by Margaret Murray and Arthur Purdy Stout (Murray, 1965). The explants are fed twice weekly with fresh nutrient medium, during which the Maximow assemblies are unsealed and the cultures, attached to their original collagen coated coverslips, are transferred to clean sterile Maximow chambers. They are generally incubated in this manner for two weeks or more.
The advantage of this type of culture system is that all of the cortical cell types, including neurons and glia, that have developed by the time that the mice are born are incorporated into the explants, and the intercellular relationships that have been developed to that point, not only within the cortex, but between cortical and deep cerebellar nucleus neurons, which are included in the explants, are preserved. The mouse cerebellum is in an early stage of development at birth, but observations of cultures in the living state indicated that some development continued in vitro, including such features as myelination of axons and cortical lamination. However, the explants are not only slices of cerebellum, but, as already noted, are also deafferented with regard to most extracerebellar inputs, and it was not clear as we began our studies that these isolated cultures were capable of developing a circuitry resembling that of the mature cerebellum in vivo. The initial object then was to define the structural and functional characteristics of organotypic cerebellar cultures after development in vitro.
2. The Model
Definable cortical and subcortical regions were readily evident by light microscopy of cerebellar explants after two or more weeks in vitro (Seil, 1972; Seil and Leiman, 1977) (Figure 2). Bands of myelinated fibers, mostly Purkinje cell axons projecting from cortex to deep nucleus neurons, formed a white matter zone between cortical and subcortical areas, similar to the white matter of the cerebellum in vivo. Myelinated fibers were initially evident at 6-7 days in vitro (DIV), but most of the myelin appeared between 9-12 DIV, a schedule similar to myelination in the intact cerebellum of the same strain of mouse. Cortical lamination, or layering, which results from postnatal migration of granule cells from the cortical surface downward past the Purkinje cells, was evident in stained preparations after two weeks in culture. The migration of the granule cells in vitro was only partial, resulting in the presence of four cortical laminae rather than the characteristic three. In the intact mature mammalian cerebellum the molecular, or outer lamina of the trilaminar cortex, contains dendrites of the interneurons and the Purkinje cells, where they come into synaptic contact with perpendicularly oriented bundles of parallel fibers separated by astrocytic processes. The second cortical lamina is composed of Purkinje cells constituting a single cell layer. The innermost cortical lamina, the internal granular layer, consists of multiple layers of granule cell somata and dendrites. In the cerebellum in vitro, the outer lamina consisted of a persistent external granular layer (as during early development) containing packed granule cell somata and dendrites. The molecular layer was less well developed than in vivo, but the same relationships existed between parallel fibers and target dendrites, albeit the parallel fibers were 90° out of phase with their course in vivo because of the plane of section during preparation of the explants. As in vivo, parallel fibers in cultures appeared in bundles separated by astrocytic processes (Seil and Herndon, 1970). Purkinje cells did not become single layered in vitro, but were at least two or more cell layers thick. Possible reasons for this include the lack of complex cortical folding and expansion in culture that are characteristic of cerebellar development in the animal. The fourth and innermost lamina was an internal granule cell layer of similar thickness to the persistent external granular layer, representing the granule cells that successfully completed their downward migration before this developmental phase came to a premature halt.
Fig. 2.
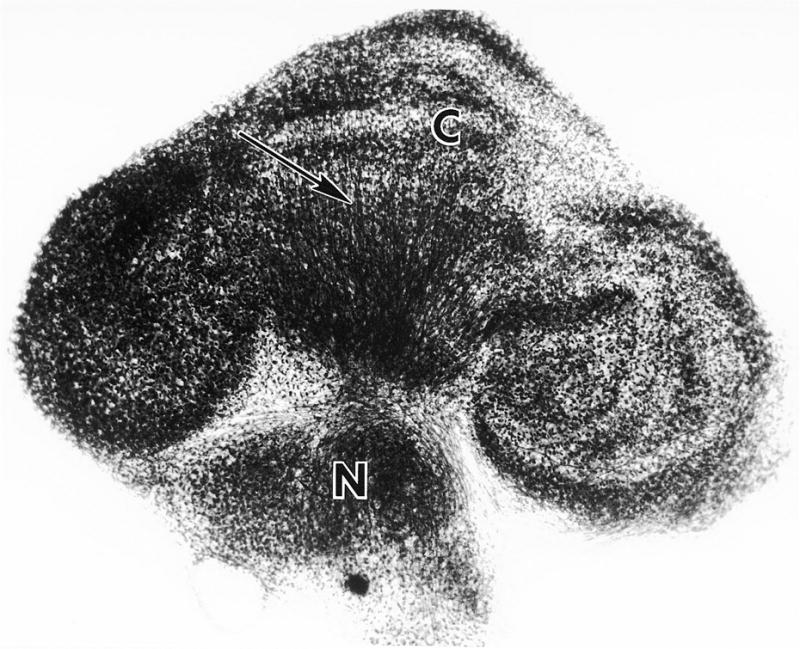
Low power view of a newborn mouse derived cerebellar culture after 23 DIV. The explant is oriented in the parasagittal plane. The cortical region (C) is readily distinguishable from an incorporated group of deep cerebellar nucleus neurons (N). Laminae are evident in regions of the cortex. Axons (arrow) of Purkinje cell origin project to the deep nucleus. The gross anatomical relationships are similar to those in the cerebellum in vivo. Whole mount preparation, Holmes silver stain, X75. From Seil and Leiman, 1977, with permission).
All of the five major cortical neuronal types present in the intact mouse cerebellum were represented in vitro (Figure 1). Granule cells, the most numerous of the cortical neurons, projected parallel fibers to all other cortical neurons. As evident by electron microscopy, typical synapses were formed with Purkinje cell dendritic spines. Such synapses were virtually absent after 5 days in culture, were apparent in small numbers by 8 DIV and were numerous by 12 DIV (Herndon et al., 1981). Granule cell dendrites were in synaptic contact with Golgi cell axon terminals in isolated cerebellar cultures without mossy fibers. If the explants were modified to include portions of vestibular or other brain stem neurons, mossy fiber terminals as well as Golgi axon terminals synapsed with granule cell dendrites, forming complex axon terminal-dendrite synaptic relationships called “glomeruli,” as occurs in vivo (Seil, 1979; Woodward et al., 1982).
Purkinje cells, the “effector” cells of the cerebellar cortex in that only their axons project from the cortex, survived well in vitro (Seil, 1972, 1979). Their axons were directed toward the deep cerebellar nuclei, in which they terminated in the proximity of dendrites of deep nucleus neurons. Each of these axons gave rise in the early part of its trajectory to a large collateral. Such recurrent axon collaterals typically made U-turns and were directed back toward the layers of Purkinje cells, where they synapsed with other Purkinje cells, as well as with basket, stellate and Golgi cells. Both axons and recurrent axon collaterals developed myelin sheaths. Purkinje cell dendrites did not achieve the complex arborization (branching) characteristic of the mature rodent cerebellum. Nevertheless, typical appearing dendritic spines developed, even on larger dendritic branches, and these were in synaptic contact with parallel fiber terminals (Seil and Herndon, 1970). Smooth portions of Purkinje cell dendrites were occasionally contacted by terminals consistent in appearance with those of stellate cells, as well as by terminals of basket cell axons and Purkinje cell recurrent axon collaterals (Blank and Seil, 1982). Contours of Purkinje cell somata were smooth and only rarely demonstrated persistent somatic spines after two weeks or more in culture. Such spines appear early in Purkinje cell development, but disappear with development in concert with astrocytic ensheathment of Purkinje cell somata. Both basket cell axon terminals and terminals of Purkinje cell recurrent axon collaterals, in approximately equal numbers, synapsed with Purkinje cell somata. The Purkinje cell somata and dendrites, including all of their synapses, were completely ensheathed by astrocytic processes. Purkinje cells are the only cerebellar cortical neurons to have complete astrocytic sheaths, the others having no or partial sheaths.
Basket, stellate and Golgi cells were more difficult to identify by light microscopy in cerebellar cultures, and therefore it was more problematic to ascertain their relative numbers. They were recognizable by electron microscopy, and typical axosomatic and axodendritic synapses were evident. As already noted, basket cell terminals were present on the somata and proximal dendrites of Purkinje cells, stellate cell terminals synapsed with smooth portions of more distal Purkinje cell dendrites, and Golgi cell axon endings were in synaptic contact with granule cell dendrites. If the cultures were modified and portions of brain stem were included with the cerebellar explants, not only were mossy fiber terminals found synapsing on granule cell dendrites, but levels of choline acetyltransferase, the enzyme that synthesizes acetylcholine, the mossy fiber excitatory neurotransmitter, were four- to six-fold higher (Woodward et al., 1982). Cerebellar cultures could also be prepared to include brain stem tissue containing locus coeruleus (Seil and Leiman, 1985) or co-cultured with fragments of brain stem containing inferior olive (Blank et al., 1983). In the case of the former, fluorescent catecholaminergic fibers were observed projecting from locus coeruleus to cortex, and in the latter case, characteristic climbing fiber synapses were apparent on Purkinje cell dendritic spines.
Functional studies further validated the cerebellar culture model. Extracellular recordings of cortical regions of cerebellar explants after two weeks in vitro revealed spontaneously occurring large spikes of Purkinje cell origin discharging in both regular and phasic patterns, the latter being more frequent (Leiman and Seil, 1973; Seil and Leiman, 1979). Phasic discharges appeared later during development in culture, and reflected synaptic interactions of Purkinje cells with inhibitory interneurons. Electrical stimulation of cortical surfaces to activate parallel fiber discharge evoked excitation-inhibition-excitation sequences similar to parallel fiber stimulation in vivo (Murphy and Sabah, 1971). Such sequences represent excitation of Purkinje cells by parallel fiber activation, followed by basket-stellate cell inhibition, followed by rebound Purkinje cell excitation. Stimulation of Purkinje cell axons in order to activate the Purkinje cells antidromically (in a direction opposite to the normal direction of transmission) resulted in transient increases in Purkinje cell discharge rates (i.e., disinhibiton) due to inhibition of the inhibitory interneurons by Purkinje cell recurrent axon collaterals (Seil et al., 1980). Similar disinhibition occurs with antidromic stimulation of Purkinje cell axons in vivo (Eccles et al., 1967). The development of functional activity in cerebellar cultures correlated with the morphological development of synapses (Herndon et al., 1981; Seil and Leiman, 1979). Occasional spontaneous cortical spikes were observed after 1 DIV. Groups of large-amplitude spikes were initially recorded at 8 DIV, and the mature pattern of phasically occurring spontaneous cortical discharges was established by 15 DIV. Single cortical spikes in response to parallel fiber stimulation were initially evident at 8 DIV, while barrages of evoked cortical spikes first appeared at 12 DIV. Inhibitory evoked responses were also initially seen at 8 DIV, and developed progressively thereafter. Sequences of excitation-inhibition-excitation in response to cortical stimulation were first noted at 12 DIV, and had assumed a mature pattern by 15 DIV. The developmental sequence in vitro paralleled that of development in vivo.
3. The Initial Experiments
Having established that our tissue culture model of the mouse cerebellum was a reasonable facsimile of the intact animal cerebellum, we were in a position to manipulate certain aspects of cerebellar development in vitro. We initially sought to characterize changes in cerebellar organization that might occur in response to destroying one group of neurons, the granule cells, early in development. Cerebellar developmental studies in other laboratories using both animals and tissue cultures revealed aberrant Purkinje cell development after exposure to antimitotic agents (Jones and Gardner, 1976; Kim, 1977; Privat and Drian, 1976; Yamano et al., 1978). Both cytosine arabinoside (Ara C) and methylazoxymethanol (MAM) destroyed or reduced the population of granule cells. In the culture studies, Purkinje cell dendritic spines without presynaptic elements or in synaptic contact with terminals of Purkinje cell recurrent axon collaterals were reported. In the case of exposure to MAM, increased numbers of myelinated Purkinje cell axons and axon collaterals were also observed. We chose to expose our cultures to Ara C (Seil et al., 1980), an inhibitor of DNA synthesis and an agent possibly having more potent destructive effects on dividing cells, which was the state of the cerebellar granule cells in the mouse at birth.
Exposure of cerebellar explants to Ara C (Sigma Chemical Company, St. Louis, MO, purchased in 1978) for the first 5 DIV, followed by subsequent maintenance in standard culture medium, resulted in the loss of granule cells and in an increased number of surviving large cortical neurons, consisting primarily of Purkinje cells. At 15 DIV, the large cortical neurons appeared closely packed and without lamination. A follow-up quantitative study affirmed a three- to four-fold increase in the number of surviving large cortical neurons (Seil, 1987). In spite of the absence of parallel fibers, Purkinje cell dendrites were studded with spines (Seil et al., 1980). The numbers of Purkinje cell axons and especially axon collaterals were markedly increased, but were not myelinated. The increase in Purkinje cell recurrent axon collaterals was out of proportion to the increase in the number of Purkinje cells, and represented a significant sprouting of recurrent axon collaterals.
With regard to the increased number of Purkinje cells, neurons are overproduced in most areas of the intact animal nervous system during development, usually by twice the final number (Oppenheim, 1985). As development proceeds, the excess neurons are pared by a process known as “programmed cell death,” in which neurons that fail to make adequate synaptic connections die by apoptosis, a sequence of events leading to cell death that is different in its histological features from necrosis, the more usual means of cell death following insult or injury. Neurons destined for programmed cell death can be “rescued” if the size of the target with which synaptic connections are made is increased, so that the neurons survive if they have available target sites (Hollyday and Hamburger, 1976). In the cerebellar culture model without extracerebellar afferents, the elimination of granule cells left a great many sites on Purkinje cell dendritic spines available for synapse formation, which was accomplished by recurrent axon collaterals from other Purkinje cells, resulting in the survival of a large excess of Purkinje cells, analogous to neuronal rescue during development (Seil, 1987, 1988).
Extracellular electrophysiological examination of Ara C exposed cultures after 15 or more DIV revealed both regular and phasic spontaneous cortical discharges, as was the case with normal control cultures (Seil et al., 1980). Also similar to control cultures was the rate of spontaneous cortical discharges (a surprising finding in the face of the vastly increased numbers of inhibitory synapses found in these preparations). Ara C treated cultures were less excitable in response to single cortical electrical shocks, but trains of stimuli evoked the same range of cortical responses as were seen in untreated control cultures. The most remarkable difference was evident upon antidromic stimulation of Purkinje cells in Ara C treated preparations, as inhibition of spontaneous cortical discharges was induced, in contrast to the disinhibition found in control explants. It appeared that Purkinje cell inhibition by the sprouted recurrent axon collaterals of other Purkinje cells, rather than basket-stellate cell inhibition, had become the dominant form of inhibition in the granule cell depleted cultures.
A subsequent study with intracellular recording demonstrated further differences between untreated control cultures and Ara C exposed preparations (Drake-Baumann and Seil, 1995). Spontaneously discharging Purkinje cells in control cultures revealed a pattern of predominantly complex spikes, with occasional simple spikes interposed. Only simple spikes were evident in Purkinje cells in comparably aged Ara C treated cultures. Complex spikes consisted of an initial large spike (fast action potential), representing a rapid membrane depolarization, followed by a prolonged lower magnitude depolarization on which one or more spike-like components were superimposed (Figure 3). Simple spikes had only the fast action potential components (Figure 3). There were also differences in membrane properties of Purkinje cells in the two preparations. Although the resting membrane potentials (the voltage difference between the inside and outside of the cells) were similar in both situations, Purkinje cells in Ara C treated cultures had a membrane change (lower input resistance) which made them less sensitive to inhibitory innervation, providing a possible explanation as to why Purkinje cells in Ara C exposed cultures discharged spontaneously at the same rate as Purkinje cells in control explants.
Fig. 3.
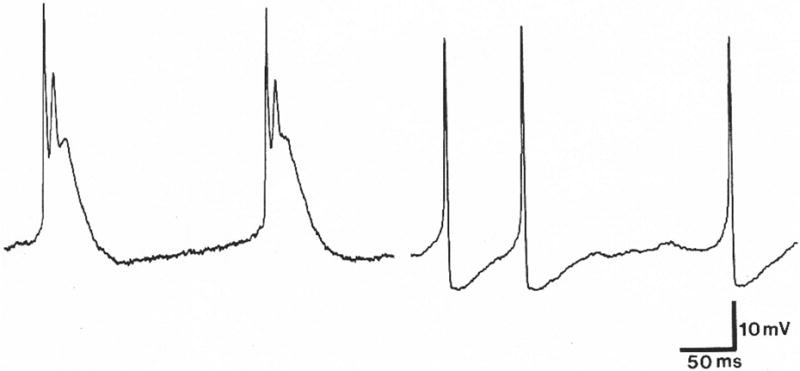
Intracellularly recorded spontaneous spike discharges from Purkinje cells in a control cerebellar culture, 14 DIV (two spikes on the left) and in a cerebellar culture, 13 DIV, which had been exposed to Sigma Ara C for the first 5 DIV (three spikes on the right). Spikes in the control Purkinje cell are complex, consisting of a large spike followed by one or more spike-like components superimposed on a prolonged lower magnitude depolarization. Only simple spikes are evident in the Purkinje cell from the Ara C treated culture. Intracellularly recorded complex spikes are characteristic of mature Purkinje cells with fully developed astrocytic sheaths, while simple spikes are seen in immature Purkinje cells prior to the development of astrocytic sheaths. Purkinje cells in Ara C treated cerebellar cultures of the same age as mature control cultures resembled immature Purkinje cells in that they had only simple spikes and lacked astrocytic sheaths. (From Drake-Baumann and Seil, 1995, with permission).
Electron microscopic examination after 15-20 DIV confirmed the almost complete absence of granule cells (Blank et al., 1982). Also absent were mature oligodendrocytes, and correspondingly there were no myelinated axons in Ara C treated cultures. This was not surprising, as oligodendrocytes were also dividing at the time of exposure to Ara C. Unexpectedly, astrocytes were reduced in treated explants and the survivors were functionally compromised in that their processes failed to appose neuronal membranes. Thus Purkinje cell somata, dendrites and dendritic spines did not have their usual astrocytic ensheathment. Unensheathed Purkinje cell somata appeared scalloped rather than rounded because of multiple abutting recurrent axon collateral terminals, some of which formed synapses with the somata. The average number of axosomatic synapses per Purkinje cell section in Ara C exposed cultures was 4.9, compared with an average of 2.2 axosomatic synapses per section in control explants, and the synapses were predominantly with terminals of recurrent axon collaterals (the numbers of Purkinje cell axosomatic synapses with basket cell and recurrent axon collateral terminals were equal in untreated control cultures). Persistent Purkinje cell somatic spines were evident in Ara C treated explants, and some of them were in synaptic contact with recurrent axon collateral terminals, while others were unattached. As noted previously, such spines are present normally in early stages of Purkinje cell development, but disappear with maturation. Their persistence on unensheathed Purkinje cell somata is another indication of interrupted maturation of Purkinje cells in Ara C treated cultures.
Recurrent axon collateral terminals also formed numerous synapses with Golgi, basket and stellate cell somata. The most dramatic finding, however, was an abundance of synapses between inhibitory recurrent axon collateral terminals and Purkinje cell dendritic spines (heterotypical synapses), sites normally occupied by excitatory parallel fiber terminals (homotypical synapses). Thus dendritic spine synapses usually formed with excitatory presynaptic elements were now in contact with inhibitory presynaptic elements, and functionally these synapses were inhibitory. This represented a complete reversal from the situation found in untreated control cultures. The anatomical changes are diagrammatically represented in Figure 4 (Standard and AraC).
Fig. 4.
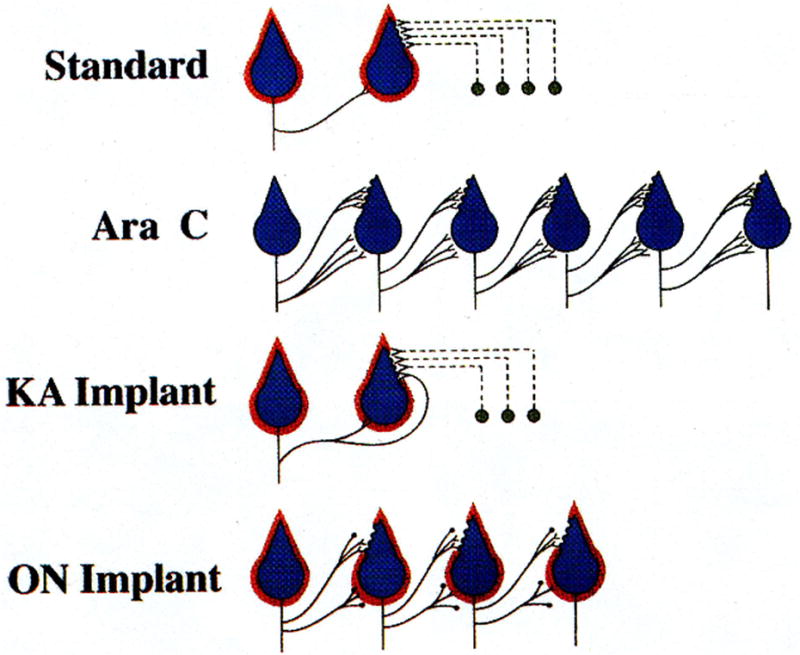
A simplified diagram showing the relationship of three cell types, Purkinje cells represented in blue, astrocytic sheaths around Purkinje cells in red, and granule cells in black in control (Standard) cultures and after exposure of cultures to Sigma cytosine arabinoside (Ara C) for the first 5 DIV to destroy granule cells and compromise glia. The solid black lines represent inhibitory Purkinje cell axons and axon collaterals and the dashed lines indicate excitatory granule cell axons (parallel fibers), which form homotypical synapses with Purkinje cell dendritic spines in control cerebellar explants. The Purkinje cells in Ara C treated cultures lack astrocytic sheaths, survive in three- to four-fold greater numbers than in standard cerebellar cultures and their axon collaterals sprout to hyperinnervate the somata of other Purkinje cells and to form heterotypical synapses with Purkinje cell dendritic spines. If kainic acid treated cerebellar cultures as a source of granule cells and glia are superimposed on Ara C exposed explants (KA Implant), much of the standard circuitry is restored. Purkinje cell numbers are reduced to approximately control levels, they aquire astrocytic sheaths and the number of axosomatic synapses is reduced, also to control levels. Granule cells integrate into the host explants and parallel fibers form homotypical synapses with Purkinje cell dendritic spines, but some heterotypical synapses persist. If optic nerve fragments as a source of glia without granule cells are superimposed on Ara C treated cerebellar cultures (ON Implant), the Purkinje cell population is decreased by an average of 27%, but the sprouted recurrent axon collaterals are not significantly reduced. Purkinje cells acquire astrocytic sheaths and their axosomatic synapses are decreased to control levels. Cortical neuropil synapses are partially reduced, even in the absence of granule cells. (From Seil, 1996, with permission).
In terms of the sequence of changes after exposure of cerebellar cultures to Ara C at the time of explantation, we found in a timed electron microscopic study that granule cell degeneration was widespread by 2 DIV, when oligodendrocyte degeneration was also beginning (Seil et al., 1991). No recognizable oligodendrocytes remained after 7 DIV, at a time when myelin was beginning to appear in control explants. Purkinje cell recurrent axon collateral sprouting began at 3 DIV in treated cultures. The sprouted terminals initially synapsed with Purkinje cell somata, somatic spines and dendritic shafts and later formed synaptic contacts with dendritic spines, at about the same stage as parallel fiber terminals synapsed with Purkinje cell dendritic spines in control cultures. This was consistent with the notion that the sequence of synapse development is a function of the maturational state of the postsynaptic components. While astrocytic ensheathment of Purkinje cell somata was well underway by 6 DIV in control cultures, glial ensheathment of Purkinje cells did not occur in Ara C exposed explants, and Purkinje cell somata were scalloped by excess impinging recurrent axon collateral terminals by 7 DIV, never attaining the smooth contours of control Purkinje cell somata. Structural reorganizational changes in Ara C treated cerebellar cultures were well established by 9 DIV and completed by 12 DIV.
The occurrence of recurrent axon collateral synapses with Purkinje cell dendritic spines, coupled with increased Purkinje cell axosomatic synapses with recurrent axon collateral terminals, was consistent with the electrophysiological results of inhibition of cortical discharges upon antidromic stimulation of Purkinje cell axons, as opposed to disinhibition. These findings reinforced the notion that in the cortical remodeling that had occurred as a consequence of granule cell destruction in isolated cerebellar explants, inhibition by recurrent axon collaterals had become the dominant mode of inhibition of Purkinje cell discharges, as contrasted with basket-stellate cell inhibition in control cultures or the intact animal cerebellum, where basket-stellate cell inhibition is the more effective mode of cortical inhibition.
In summary, exposure of cerebellar cultures to the antimitotic agent, Ara C, resulted in 1) destruction of cerebellar granule cells, 2) destruction of oligodendrocytes with associated absence of myelination and 3) reduction of the astrocyte population and functional compromise of surviving astrocytes, so that they failed to ensheath neurons and their processes. Consequent effects included 1) a three- to four-fold increased survival of large cortical neurons, 2) a profuse sprouting of Purkinje cell recurrent axon collaterals with hyperinnervation of Purkinje cell somata and formation of inhibitory heterotypical synapses with Purkinje cell dendritic spines, 3) a persistence of Purkinje cell somatic spines, 4) an absence of complex spikes in Purkinje cells, 5) a reduced sensitivity of Purkinje cells to inhibitory inputs, and 6) the inhibition of spontaneous cortical discharges upon antidromic activation of Purkinje cells. We speculated that the increase in numbers of Purkinje cells in Ara C treated cultures and the sprouting of recurrent axon collaterals were products of a vast expansion of the target field for recurrent axon collaterals in the form of available Purkinje cell dendritic spines created by the absence of parallel fibers (granule cell axons). The persistence of Purkinje cell somatic spines and the absence of complex spikes were consistent with incomplete Purkinje cell maturation, due to a number of possible factors, including a lack of interaction, trophic or otherwise, with granule cells or the absence of astrocytic ensheathment.
What kinds of changes take place in the intact animal cerebellum if granule cells are destroyed early in development? When granule cells were destroyed by treatment of ferrets with panleukopenia virus, excitatory extracerebellar afferents, primarily mossy fibers, were reported to project to sites on basket, stellate, Golgi and Purkinje cells usually occupied by parallel fiber terminals, including the Purkinje cell dendritic spines (Llinás et al., 1973). Sprouting of Purkinje recurrent axon collaterals was not described. Stimulation of the extracerebellar afferents induced cortical excitation-inhibition sequences, the excitation attributed to Purkinje cell activation and the inhibition to activation of inhibitory interneurons. In this case, excitatory terminals replaced missing excitatory terminals, even if the neurotransmitters were different (acetylcholine vs. glutamate), and sprouting of inhibitory axon collaterals was not induced. In the case of the isolated cultured cerebellum without extracerebellar afferents, inhibitory axons sprouted and inhibitory terminals replaced the missing parallel fiber terminals on Purkinje cell dendritic spines (Blank et al., 1982; Seil et al., 1980).
The development in Ara C treated cultures of inhibitory heterotypical synapses with Purkinje cell dendritic spines plus hyperinnervation of Purkinje cell somata by recurrent axon collaterals allowed retention of cortical inhibition, as indicated by the occurrence of inhibitory responses to cortical stimulation. In the control situation, where granule cells are present, parallel fiber discharges excite Purkinje cells and basket-stellate cells, which then inhibit Purkinje cells. In the absence of parallel fibers, it may be difficult to recruit sufficient numbers of basket and stellate cells to inhibit spontaneous or directly activated Purkinje cell discharges, a situation which could be compensated by the presence of a greatly expanded recurrent axon collateral projection. Without some form of inhibition in the system, Purkinje cells might discharge hyperactively or discharge continuously in a totally unchecked manner. Given the premise that remodeling of the nervous system after injury (the loss or reduction of granule cells and glia in the case of Ara C treated cerebellar cultures) is to maintain or restore neural function, the circuit reorganization that took place accomplished exactly that, with the retention of cortical inhibition in response to stimulation and with the preservation of a pattern of spontaneous cortical discharges that was like that seen in cerebellar cultures with a full complement of granule cells and glia.
4. The Corollary Experiments
The immediate question that occurred to us after noting the changes in organotypic cerebellar culture organization consequent to destruction of granule cells and oligodendrocytes and reduction and functional compromise of astrocytes was what would happen if the missing elements were restored? Would a second round of reorganizational changes occur with a return to a normal (i.e., control) structural and functional state? To address this question, we turned to another model with which we had been working, namely cerebellar cultures treated with the glutamic acid analogue, kainic acid (Seil et al., 1978, 1979). When cerebellar explants were exposed to kainic acid for the first 5 DIV, all neurons except granule cells were destroyed, while the glia were unaffected. Thus kainic acid treated explants were a perfect complement to cerebellar cultures exposed to Ara C, the former containing the components missing in the latter.
Cerebellar cultures exposed to kainic acid for the first 5 DIV and then maintained in standard nutrient medium were dissected at 9 DIV from their collagen coated coverslips and, with the use of a dissecting microscope, superimposed upon host cerebellar explants treated with Ara C for the first 5 DIV and subsequently maintained in standard medium to either 9 or 16 DIV (Seil and Blank, 1981; Seil et al., 1983). The cultures were observed in the living state for the appearance of myelin, which was initially seen associated with larger axons (such as Purkinje cell axons or recurrent axon collaterals) 3 days after superimposition of the culture pairs. Most of the myelin appeared within the 2 days thereafter, and the proportion of superimposed cultures that myelinated was 78%, which was comparable to the 80-85% seen in control cultures. Light microscopic studies of stained preparations 10 days after superimposition revealed numerous granule cell nuclei within the cortical regions of the host Ara C treated explants, the granule cells having migrated from the kainic acid exposed culture implants. Purkinje cells were reduced in numbers to control levels, presumably because of reduction of their target fields, and space was evident between Purkinje cells, some of it occupied by granule cells. The density of cortical axons was remarkably reduced, resembling that of control cultures, consistent with a reduction of both Purkinje cell axons and sprouted recurrent axon collaterals. All of these changes were evident only in superimposed cultures, and not in pairs of Ara C treated and kainic acid exposed explants placed side by side, indicating that the observed changes were not induced by diffusible factors. Moreover, in cases in which superimposition was not exact, i.e., in which cortex in host cultures was not overlain by cortex from kainic acid treated implants, the host cortex that was not overlapped did not show the changes that resulted from superimposition, and appeared indistinguishable from cortex in Ara C treated, non-superimposed cultures, suggesting that granule cells and glia migrated directly downward from the implants into the host cultures, with insignificant lateral migration.
Electron microscopic examination at 10 days after superimposition confirmed the presence of granule cells and myelinated large axons in the host explants, as well as the presence of mature differentiated oligodendrocytes (Blank and Seil, 1983; Seil and Blank, 1981). Purkinje cell somata appeared rounded, were ensheathed by astrocytic processes and had lost their somatic spines. The number of axosomatic synapses per Purkinje cell section was reduced to 2.4, almost to the control level of 2.2. Bundles of parallel fibers were present in cortical areas of superimposed explants, and these were unmyelinated, as in control cultures. Parallel fiber terminals formed synapses with most of the available Purkinje cell dendritic spines, although some heterotypical synapses between Purkinje cell dendritic spines and recurrent axon collaterals remained, as well as some unattached dendritic spines. Granule cell dendrites formed synapses with Golgi cell axon terminals, and in occasional cultures with a few mossy fibers, mossy fiber terminals also synapsed with granule cell dendrites, forming complete glomeruli, as seen in some control cerebellar cultures with incorporated fragments of brain stem. Astrocytes were abundant and well differentiated in superimposed cultures, ensheathing not only Purkinje cell somata, but also dendrites and dendritic spine synapses.
Extracellularly recorded spontaneous cortical discharge rates in superimposed cultures were similar to those in control cultures (Seil et al., 1983). However, superimposed (implanted, transplanted) cultures were less excitable than controls, requiring trains of stimuli rather than single shocks to evoke cortical activity, similar to Ara C treated cultures. The most significant functional difference between Ara C exposed explants and Ara C treated cultures superimposed with granule cells and glia was the absence of cortical inhibition upon antidromic activation of Purkinje cells in the latter. This difference was consistent with the reduction of Purkinje cell axosomatic synapses with recurrent axon collateral terminals and the reduction of inhibitory heterotypical recurrent axon collateral-Purkinje dendritic spine synapses in implanted cultures.
To summarize, implantation (transplantation) of reorganized Ara C treated cerebellar cultures at either 9 or 16 DIV with granule cells and competent glia triggered a second round of circuit reorganization that restored the structural and functional states of the cultures to those similar to controls. Granule cells migrated into cortical regions of host explants and their axons, the parallel fibers, formed synapses with Purkinje cell dendritic spines as the number of heterotypical synapses between Purkinje cell dendritic spines and recurrent axon collateral terminals was remarkably reduced. Oligodendrocytes myelinated large axons and astrocytes ensheathed Purkinje cell somata, dendrites and dendritic spine synapses. The excess numbers of Purkinje cells and sprouted recurrent axon collaterals were reduced to control levels. Antidromic stimulation of Purkinje cells no longer induced inhibition of cortical discharges. The anatomical changes are illustrated diagrammatically in Figure 4 (KA Implant).
While the results of our studies indicated that there seemed to be an ordered pattern or set of rules by which circuit reorganization took place in the central nervous system after injury or other induced changes, many more questions were raised. For example, what is the role of the individual neuronal and glial elements in bringing about restitutional changes after insertion into a reorganized neural circuit? How are synapses eliminated and what role, if any, do astrocytes have in synapse control, given the association of astrocytic ensheathment with numbers of Purkinje cell axosomatic synapses? Do parallel fiber terminals replace recurrent axon collateral terminals on existing Purkinje cell dendritic spines when heterotypical synapses are eliminated, or do they form synapses with newly developed spines? Do glia promote neuronal maturation, as in the loss of Purkinje cell somatic spines concomitant with astrocytic ensheathment, or is maturation dependent on interaction with specific presynaptic elements, such as Purkinje cells with granule cells? How are excess Purkinje cells eliminated when the control cerebellar culture circuitry is restored? What after implantation of granule cells and glia triggers the reduction of sprouted Purkinje cell recurrent axon collaterals, which are present out of proportion to increased numbers of Purkinje cells in Ara C treated cultures? What is the specific sequence of events that takes place after granule cells and glia are inserted into reorganized cerebellar explants devoid of these elements?
5. Variations on the Theme
Our first attempt to define the role of the specific elements of the tissue superimposed upon Ara C treated explants was with a preparation containing only glia without granule cells, namely fragments of 7-day-old mouse optic nerve (Meshul et al., 1987). When such optic nerve fragments were superimposed upon Ara C exposed cultures, myelin was first observed after 7 DIV, 4 days behind the schedule seen with superimposition of kainic acid treated cerebellar explants, and only 53% myelinated, as opposed to 78%. Host cultures superimposed with optic nerve also had fewer myelinated fibers than cultures superimposed with kainite exposed explants. We attributed these differences to a possible relative impedence of oligodendrocyte migration from optic nerve fragments. Sprouted recurrent axon collaterals were not appreciably reduced by light microscopic observation of stained preparations, but there was a 27% reduction of the population of Purkinje cells, though far less than the reduction occurring with the superimposition of both granule cells and glia upon host explants (diagrammatically shown in Figure 4).
There was no lack of evidence of astrocyte migration from the optic nerve fragments, as mature astrocytes were present in the host cultures by electron microscopic examination, Purkinje cells were rounded and ensheathed by astrocytic processes, and the number of Purkinje cell axosomatic synapses was reduced to control levels. As the overabundance of sprouted recurrent axon collaterals was not significantly changed, the reduction of Purkinje cell axosomatic synapses correlated with astrocytic ensheathment, not with loss of recurrent axon collaterals. These findings were consistent with results of previously reported animal studies in which loss of glial ensheathment of rat supraoptic nucleus neurons led to increased axosomatic synapse formation, followed by restoration of normal synapse numbers upon reestablishment of the astrocytic sheaths (Tweedle and Hatton, 1984). These results led us to speculate upon the role of astrocytes in the regulation of synapse density, at least in neurons with astrocytic sheaths. Cortical synapses (axodendritic and axospinous) were also reduced in host cultures with superimposed optic nerve, the reduction being on the order of one-half the number present in Ara C treated explants (Meshul et al., 1987). The mechanism of such synapse reduction was not apparent, but it also occurred in the presence of mature astrocytes and the absence of recurrent axon collateral degeneration. The synapse reduction could have relevance for the observed reduction of the Purkinje cell population, as it represented a restriction of the target field for recurrent axon collaterals.
A surprising finding in host Ara C treated cultures implanted with optic nerve fragments was the presence of clusters of Purkinje cell dendritic spines without attached presynaptic elements and without direct astrocytic apposition, although astrocytic processes were in the vicinity (Meshul and Seil, 1988). Similar groups of unattached spines had not been observed in either control or Ara C treated cerebellar explants. The occurrence of unattached spines in clusters suggested the possibility of their having arisen in local regions of dendritic branches in response to some diffusible factor, perhaps one secreted by nearby astrocytes. As astrocytes were known to secrete a variety of neuronal maintenance and growth promoting factors (Rudge et al., 1985), Ara C treated cultures were exposed to astrocyte conditioned medium, that is, a culture medium that had been collected from dissociated cell cultures of purified rat astrocytes and then concentrated prior to incorporation into the standard medium for organotypic cerebellar cultures (Seil et al., 1992b). Such a medium would be expected to contain a variety of diffusible astrocyte secreted factors. Electron microscopic examination of Ara C treated cultures after 5-6 days of exposure to astrocyte conditioned medium revealed a proliferation of Purkinje cell dendritic spines as manifested by large clusters of spines, most of which were unattached, diffusely distributed throughout the cortical regions of the explants. Subsequently, a series of known neurite promoting factors secreted by astrocytes were screened for dendritic spine inducing capability (Seil, 1998). The extracellular matrix molecule, laminin, was found to be a potent promoter of Purkinje cell dendritic spine proliferation. Dendritic branching was not affected, and dendritic spine proliferation was not induced by two laminin-derived peptides with known neurite promoting effects, indicating that the site on the laminin molecule that induced spine proliferation was different from sites that promoted outgrowth of neural processes such as axons or dendrites.
To further define the role of glia in restorative changes, we overlaid Ara C treated explants with dissociated cell suspensions of cultured purified oligodendrocytes. Myelination was the only change observed in the host cultures (Seil et al., 1989). Myelin appeared 2-5 days after application of oligodendrocytes, and 92% of the cultures myelinated. By electron microscopic examination, mature oligodendrocytes were visible in the host explants, as well as axons with normal appearing compact myelin. Also evident were spherules of compact myelin unassociated with axons. Similar formations of myelin-like membrane had been observed in cultures of dissociated oligodendrocytes, leading to speculations that the formation of myelin membranes is an intrinsic property of oligodendrocytes, and does not require induction by axons (Poduslo et al., 1982). We did not see empty spherules of myelin membranes in control cerebellar cultures, in Ara C treated explants or in Ara C exposed cultures superimposed with kainic acid treated explants or fragments of optic nerve. While formation of myelin membranes may well be an intrinsic property of oligodendrocytes, we felt that the formation of such empty myelin spherules represented a form of oligodendrocyte hyperreactivity induced by the extreme conditions of isolation imposed by dissociated cell culture, and therefore was an aberrant occurrence, and not one seen in conditions in which normal axon-glia interactions prevail. None of the other changes that occurred after superimposition of Ara C treated cultures with fragments of optic nerve were evident in host cultures overlain with purified oligodendrocytes.
To complete the analysis of the roles of glia and granule cells in restoring a circuitry similar to that of control cerebellar cultures after superimposition on Ara C treated explants, we exposed the latter to granule cells alone (Seil, 1994). Granule cells, like oligodendrocytes, can be prepared as dissociated cell cultures, but application of granule cell suspensions to Ara C treated explants resulted in too few granule cells penetrating into the host cultures to effect appreciable changes. Whereas relatively few oligodendrocytes are necessary to achieve observable axonal myelination, a great many granule cells are necessary to innervate target cells in host cultures. The granule cell suspensions could be concentrated by pelleting, and the pellets superimposed on host explants, but the pellets restricted migration into the host explants. The solution was to expose cerebellar explants to kainic acid for the first 5 DIV to destroy all neurons but granule cells, followed by exposure to Ara C for the next 4 DIV, by which time most granule cells had completed their division, to destroy or compromise the glia. The resulting explants, containing only granule cells without functional glia, could then be dissected from their collagen coated coverslips and superimposed on Ara C treated host cultures.
Myelin was not evident in host cultures superimposed with granule cells alone. By light microscopic examination of stained preparations, granule cell nuclei were visible in the host explants, but the density of sprouted recurrent axon collaterals was not appreciably reduced. Electron microscopic examination confirmed the presence of clusters of granule cells in the host cultures, along with bundles of parallel fibers. Purkinje cell somata had scalloped contours, lacked astrocytic sheaths and were hyperinnervated to the same degree as Purkinje cells in unimplanted Ara C treated cultures. Homotypical parallel fiber-Purkinje cell dendritic spine synapses were evident in cortical areas, in numbers not significantly different from Ara C treated cultures that had been superimposed with granule cells and glia (4 vs. 3.6 per 100 μm2 cortex). However, almost twice as many heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses persisted in cultures superimposed with granule cells alone than in those in which glia had been included in the implanted tissue (2.8 vs. 1.5 per 100 μm2 cortex), supporting the concept that astrocytes have a role in the elimination of heterotypical dendritic spine synapses, a concept gained from the study with optic nerve fragments, in which some reduction of heterotypical synapses on Purkinje cell dendritic spines occurred in the absence of granule cells (Meshul et al., 1987). Granule cells, when present, formed homotypical synapses with dendritic spines, accounting for some of the reduction of heterotypical synapses, but the reduction of heterotypical synapses was twice as efficient when functional astrocytes were also present (Seil, 1994).
To summarize what we learned from this set of studies, when Ara C treated cerebellar cultures were overlain with cell suspensions of purified oligodendrocytes, the only change of note was myelination of axons in host explants. When optic nerve fragments containing oligodendrocytes and astrocytes, but no granule cells, were superimposed, axons in host cultures were myelinated, the number of sprouted recurrent axon collaterals was not appreciably reduced, the Purkinje cell population decreased by 27%, Purkinje cells aquired astrocytic sheaths, the numbers of axosomatic synapses were reduced to control levels, the number of cortical synapses was halved and clusters of unattached Purkinje cell dendritic spines persisted in the absence of parallel fibers with which to form synapses. When granule cells alone were implanted, homotypical synapses were formed but no myelination occurred, sprouted Purkinje cell recurrent axon collaterals were not appreciably reduced, Purkinje cells did not acquire astrocytic sheaths, their somata remained hyperinnervated by recurrent axon collateral terminals, and twice as many heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses persisted as when glia were also included in the superimposed tissue. To place the loss of heterotypical synapses in these various conditions in perspective, we had determined from another study that the number of heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses in Ara C treated cultures was 8.3 per 100 μm2 cortex (Seil and Drake-Baumann, 1995). In the study with implantation of granule cells without glia, this number was reduced to 2.8 heterotypical synapses per 100 μm2 cortex, a loss of 66% (Seil, 1994). From the same study, comparison cultures in which host Ara C treated explants were implanted with granule cells and glia, the number of heterotypical synapses was reduced to 1.5 per 100 μm2 cortex, a loss of 82%. The effect of the presence of astrocytes was quite evident, though the mechanism by which glia reduced heterotypical synapses was not.
The relationship of glial ensheathment to the numbers of Purkinje cell axosomatic synapses was intriguing (Meshul et al., 1987), especially since a similar relationship had been observed in a different group of ensheathed neurons in animal studies (Tweedle and Hatton, 1984). The possibility of a direct stripping of excess synapses by astrocytic processes seemed likely, but had not yet been demonstrated. Other mechanisms, such as an astrocyte secreted factor, were also among the contenders.
Another point of interest from these studies related to the source of the signal for glial ensheathment. Not all neurons have astrocytic sheaths, such as the granule cells in the cerebellum, while the Purkinje cells, as noted earlier, are completely ensheathed. The glia whose processes ensheath Purkinje cells are specialized astrocytes, called “Golgi epithelial cells” by Palay and Chan-Palay (1974), and they are also the origin of the “Bergmann fibers,” radial fibers that guide migration of some cerebellar cortical neurons. Optic nerve astrocytes were totally naïve to Purkinje cells, yet they were fully capable of ensheathing Purkinje cells when placed in their vicinity, suggesting that the signal for ensheathment emanated from the neurons. The signaling molecule has not been identified, but it does not appear to be the neuronal cell adhesion molecule, N-CAM, as antibodies to N-CAM failed to inhibit Purkinje cell ensheathment (Seil and Herndon, 1991).
The finding of Purkinje cell dendritic spine proliferation after superimposition of Ara C treated cultures with glia in the absence of granule cells was unexpected (Meshul and Seil, 1988). Clusters of unattached dendritic spines had not been observed when cultures were examined by electron microscopy 10 days after superimposition with granule cells and glia (Blank and Seil, 1983). In this situation, of course, parallel fibers were available to form homotypical synapses with Purkinje cell dendritic spines. In the absence of granule cells, unattached spines persisted in Ara C treated cultures superimposed with optic nerve fragments throughout the period of observation, up to 35 DIV. That the spine proliferation was associated with the presence of astrocytes was strongly indicated by induction of spine proliferation by an astrocyte conditioned medium (Seil et al., 1992b), and subsequently by an astrocyte secreted product, laminin (Seil, 1998). It was known from animal studies that Purkinje cells could form dendritic spines autonomously, that is, presynaptic elements were not necessary for their induction (reviewed in Seil, 1997a), but this was the first link to induction of dendritic spines by an astrocyte secreted factor.
Laminin is secreted by immature astrocytes, so that it is present during development, but is downregulated as astrocytes mature (reviewed in Seil, 1998). As a developmental molecule, laminin is supportive of neurite growth and may also have a role in synapse formation. As to the possible significance of a factor that induces dendritic spine proliferation, this may be a mechanism for elaborating postsynaptic sites available for innervation. During development, astrocytes appear to guide the directional growth of axons. A parallel role might be the expansion of postsynaptic sites on target dendrites prior to the arrival of presynaptic axon terminals. In the postdevelopmental state, if the mature nervous system is injured, astrocytes proliferate, so that a mixed population of astrocytes in various stages of differentiation is present. Immature astrocytes may reassume characteristics that were downregulated in mature astrocytes, thus the same mechanisms that were available during development may be available for repair of the mature nervous system.
6. Further Variations
As we had been working with another cerebellar culture model that included portions of locus coeruleus, whose catecholaminergic axons projected to all regions of the cerebellar cortex (Seil and Leiman, 1985), we wondered if these axons would also sprout if the cultures were exposed to Ara C for the first 5 DIV. The fine catecholaminergic fibers did not stain with silver, but they were easily visualized at the light microscopic level by their histofluorescence after reaction with glyoxylic acid. Such axons were present in the outgrowth zones of the explants as well as within the cortical regions. Stimulation of locus coeruleus neurons evoked complex inhibitory extracellular cortical responses similar to those recorded after locus coeruleus stimulation in vivo. In contrast to locus coeruleus neurons in vivo, however, dopamine (DA) was the predominant catecholamine synthesized, stored and released by locus coeruleus neurons in culture, while levels of norepinephrine (NE) were very low (Woodward et al., 1987). Concentrations of dopamine-β-hydroxylase, the enzyme that converts DA to NE, were comparable to in vivo concentrations, and addition of dopamine-β-hydroxylase cofactors to the culture nutrient medium did not change the DA/NE ratio. The reason for the reversal of the DA/NE ratio in vitro was not elucidated.
Catecholaminergic axons did indeed sprout after treatment of coeruleocerebellar cultures with Ara C, and levels of DA were more than twice those of control coeruleocerebellar cultures (Seil et al., 1985). The density of histofluorescent axons was remarkably increased in cortical and subcortical regions of Ara C treated cultures, as well as in the outgrowth zones surrounding the explants. The locus coeruleus neuron somata were unaffected by exposure to Ara C. The sprouting of catecholaminergic axons was similar in its intensity to the sprouting of Purkinje cell recurrent axon collaterals after treatment with Ara C. Whether the two axonal systems responded to the same signal to sprout or to different signals was not clear. Our postulate was that recurrent axon collaterals sprouted in response to an abundance of synaptic sites made available by the absence of cerebellar granule cells in a system without competing extracerebellar afferents in the form of mossy and climbing fibers. While catecholaminergic axons project to Purkinje cells, most do not form true synapses, so that sprouting may not necessarily be induced by an increased availability of synaptic sites in the case of catecholaminergic axons. On the other hand, Purkinje cells survive in three- to four-fold greater numbers in Ara C treated cerebellar cultures, providing a larger target projection field for catecholaminergic fibers.
In an attempt to gain further insight into these possibilities, we superimposed 9 DIV cerebellar cultures that had been exposed to kainic acid for the first 5 DIV upon the cerebellar cortical portions of 9 DIV coeruleocerebellar cultures that had been treated with Ara C for the first 5 DIV (Seil and Woodward, 1988). The explants were observed in the living state for myelination and processed after 15 DIV for silver staining or glyoxylic acid reaction or quantitative cathecholamine determinations. The results of adding granule cells and glia to Ara C treated coeruleocerebellar cultures were myelination of the host explants, reduction of the Purkinje cell population and reduction of the excess sprouted Purkinje cell recurrent axon collaterals. However, catecholaminergic fibers remained hyperdense in such preparations, and tissue levels of DA and NE were not significantly different from those in Ara C treated coeruleocerebellar cultures not superimposed with granule cells and glia. These results indicated that once formed, the catecholaminergic axons were not dependent upon the continued presence of target tissue for their maintenance. This could be related to the lack of true synaptic contacts with Purkinje cells or possibly to the presence of noradrenergic receptors on astrocytes. In either case, the persistence of hyperdense catecholaminergic fibers in the presence of homotypical synapse formation after granule cell superimposition suggests a mechanism other than availability of synaptic sites for induction of sprouting in this class of axons.
As noted in Section 3, the Ara C preparation to which we had exposed cerebellar cultures in all of the above described studies had been purchased from the Sigma Chemical Company (St. Louis, MO) in 1978. Another cerebellar culture model was serendipitously presented to us when we began testing other preparations of Ara C, including others from Sigma, but especially one manufactured by Pfanstiehl Laboratories (Waukegan, IL) (Seil et al., 1992a). When cerebellar explants were treated with this preparation for the first 5 DIV, subsequently maintained in standard culture medium and then subjected to light microscopic examination, they failed to myelinate, excess numbers of closely packed large cortical neurons, primarily Purkinje cells, were present in unlaminated cortex, small nuclei characteristic of granule cells were absent or scarce and numbers of sprouted recurrent axon collaterals were greatly increased, similar to what had been observed in Sigma Ara C treated cultures. By electron microscopy, however, differences were evident, as Purkinje cell somata were rounded, had astrocytic sheaths, had a normal complement of axosomatic synapses and rarely had somatic spines. Recognizable oligodendrocytes were absent and granule cell numbers were vastly reduced, but there were abundant mature appearing astrocytes and astrocytic processes. Frequent heterotypical synapses between Purkinje cell dendritic spines and sprouted recurrent axon collateral terminals were present, many of which were ensheathed by astrocytic processes. Unattached Purkinje cell dendritic spines were also evident and sometimes appeared in small clusters. Some of the unattached spines were apposed by astrocytic processes, which rarely occurred in cultures exposed to Sigma Ara C. In essence, while Pfanstiehl Ara C destroyed oligodendrocytes and eliminated or greatly reduced granule cells, it had no significant effect on astrocytes.
Antidromic stimulation of Purkinje cells in Pfanstiehl Ara C exposed explants evoked inhibition of cortical spike discharges, similar to what was recorded in cultures exposed to Sigma Ara C and dissimilar from control cultures, in which disinhibition of cortical activity followed antidromic activation of Purkinje cells. This response indicated that, in spite of the absence of somatic hyperinnervation of Purkinje cells by recurrent axon collaterals in Pfanstiehl Ara C treated cultures, inhibition after antidromic activation of Purkinje cells was still present on the basis of the massive inhibitory innervation of Purkinje cell dendritic spines by recurrent axon collaterals.
Extracellularly recorded spontaneous cortical discharge rates were slower in Pfanstiehl Ara C treated cultures than in Sigma Ara C treated or control cultures. Intracellular recording of membrane properties of Purkinje cells indicated that the resting membrane potentials in Pfanstiehl Ara C exposed explants were comparable to those of Purkinje cells in Sigma Ara C treated and control cultures (Drake-Baumann and Seil, 1999). However, the input resistence of Purkinje cells in Pfanstiehl Ara C exposed explants was like that of control cultures, Purkinje cell somata having astrocytic sheaths in both cases, in contrast to a significantly lower input resistence in unensheathed Purkinje cells in Sigma Ara C treated cultures. While a lower input resistence may indicate a lower sensitivity to inhibition, accounting for a cortical discharge rate in Sigma Ara C treated explants similar to that of control cultures, a normal sensitivity to inhibition coupled with a large inhibitory input via recurrent axon collateral innervation of Purkinje cell dendritic spines can account for the reduced cortical discharge rates in cultures exposed to Pfanstiehl Ara C.
Purkinje cells in Pfanstiehl Ara C treated cultures had a pattern of predominantly complex spike discharges, similar to Purkinje cells in control cultures but different from Purkinje cells in Sigma Ara C treated cultures, where only simple spikes were recorded. As noted previously, complex spikes are characteristic of mature Purkinje cells, while simple spikes are seen in immature Purkinje cells. As the primary difference between Purkinje cells in Pfanstiehl and Sigma Ara C treated preparations was the presence of astrocytic sheaths in the former, and absence thereof in the latter, support was provided for the concept that astrocytic ensheathment may play a role in the development of complex spikes during Purkinje cell maturation.
This Pfanstiehl Ara C preparation, which drastically reduced or destroyed granule cells and oligodendrocytes while having no effect on astrocytes, allowed us a different look with regard to astrocyte functions. With astrocytes present and ensheathing Purkinje cells, hyperinnervation of Purkinje cell somata by abundant sprouted recurrent axon collaterals did not take place (Seil et al., 1992a), adding another claim to a role for astrocytes in the regulation of synapse numbers in specific situations. Remarkable in this regard is the presence of numerous heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses, many with astrocytic sheaths, in Pfanstiehl Ara C treated cerebellar cultures, suggesting that in this case astrocytes were at least permissive of heterotypical synapse formation. In a study from another laboratory, it was reported that in cultures of retinal ganglion cells, the presence of astrocytes was required for the formation and maintenance of synapses (Ullian et al., 2001). How can astrocytes both control and promote or permit synapse formation? One possible explanation relates to the opposing functions astrocytes have during their different maturational states, such as promotion of axonal growth by immature astrocytes (Müller et al., 1990) and inhibition of axonal growth by mature astrocytes (Eng et al., 1987). Laminin secreting astrocytes may induce the elaboration of postsynaptic membrane in the form of dendritic spines while mature astrocytes may reduce excessive synapses. Given these opposite functions, and knowing that astrocytes may co-exist in different maturational states (Hatten et al., 1991), it is conceivable that signals emanating from Purkinje cells could attract astrocytes in different maturational stages to different sites on the same cell to achieve opposite outcomes.
Equally interesting is an apparent role for astrocytes in promoting Purkinje cell maturation (reviewed in Seil, 2001a). Persistent somatic spines, some with synapses with sprouted recurrent axon collateral terminals, were evident in unensheathed Purkinje cells in Sigma Ara C treated cultures. We had shown that such spines disappeared during development in control organotypic cerebellar cultures, and also from Sigma Ara C treated cultures after superimposition of granule cells and functional glia, in both of which cases Purkinje cells acquired astrocytic sheaths. Somatic spines were rare in Pfanstiehl Ara C treated cultures, which contained both ensheathed Purkinje cells and numerous sprouted recurrent axon collaterals, suggesting that the developmentally programmed loss of Purkinje cell somatic spines was related to glial ensheathment. Similarly, complex spikes, characteristic of mature Purkinje cells, were recorded from ensheathed Purkinje cells in control and Pfanstiehl Ara C treated cultures, while only simple spikes, associated with immature Purkinje cells, were recorded from unensheathed Purkinje cells in Sigma Ara C treated cultures. The development of Purkinje cell membrane input resistence was also affected by astrocytic ensheathment, as this resistence was lower in unensheathed Purkinje cells in Sigma Ara C exposed explants than in Purkinje cells with astrocytic sheaths in control and Pfanstiehl Ara C treated cultures. The apparent Purkinje cell maturation promoting effect of glial ensheathment would appear to be a direct contact effect, but contact effects might also be mediated by secreted factors.
It turned out that the effect of the 1978 Sigma Ara C preparation on cerebellar cultures in reducing the astrocyte population and functionally compromising the survivors was unique to this preparation, and was not a property of any other Ara C preparation that we tested. The preparation had been manufactured for Sigma by a company that subsequently went out of business. Sigma analyzed a sample of this Ara C and determined that it contained 1% impurities, but the impurities were not identified. We never did discover what in this particular Ara C preparation had such a potent effect on astrocytes.
7. A Unifying Study
A serial electron microscopic study of the sequence of events that took place after superimposition of Ara C (Sigma) treated cerebellar cultures with intact granule cells and glia in the form of kainic acid exposed explants brought together much of what had been learned from preceding studies (Seil, 1997b). By 1 day after superimposition, numerous granule cells were evident in cortical regions of host cultures, having migrated from the superimposed explants. Differentiated astrocytes were also present in host cultures, and their processes were beginning to ensheath Purkinje cell somata and to separate intact or degenerating axon terminals from the somata. At this time there were occasional Purkinje cells with degenerative changes.
Remarkable changes appeared at 2 days after superimposition. Astrocytic ensheathment of Purkinje cells was extended and ensheathment of Purkinje cell dendrites was beginning. Axon terminals continued to be separated from Purkinje cell somata by interposed astrocytic processes, and the number of axosomatic synapses was reduced from a count of 4.9 per Purkinje cell section prior to superimposition to 3.9 per Purkinje cell section. Parallel fibers were evident in cortical regions, some with terminals whose structure was compatible with that of growth cones. More mature parallel fiber terminals were also present, some of which had formed early synaptic contacts with Purkinje cell dendritic spines and with smooth portions of smaller dendrites of other cortical neurons. The most remarkable finding at this stage was the onset of Purkinje cell dendritic spine proliferation, often in small and larger clusters in the vicinity of astrocytic processes. Purkinje cells with degenerative features were now more frequently encountered.
Myelin in host cultures was initially noted at 3 days post superimposition. Astrocytic ensheathment of Purkinje cell somata and dendrites continued, and astrocytic processes were seen inserting themselves between Purkinje cell somata and axon terminals, appearing to lift the terminals off the somatic membranes. The number of axosomatic synapses per Purkinje cell section was now reduced to 3.2. More parallel fiber-Purkinje cell dendritic spine synapses were present, but they were still outnumbered at this stage by dendritic spine synapses with recurrent axon collateral terminals. Purkinje cells undergoing cell death were now frequently evident. By 4 days after superimposition, Purkinje cell somata had fewer abutting axon terminals and began to lose their scalloped appearance as astrocytic ensheathment progressed. Axosomatic synapses per Purkinje cell section had now been reduced to 2.5 (compared with 2.2 in control Purkinje cell sections), a point from which little further change occurred. Glial ensheathment was extended to Purkinje cell dendritic spine synapses, including both heterotypical and recently developed homotypical synapses. Proliferation of Purkinje cell dendritic spines had accelerated and occasional synapses with presumptive parallel fiber terminals were present near clusters of proliferated spines.
Contours of Purkinje cell somata were increasingly smooth at 5 days after superimposition, and there was a notable reduction of persistent somatic spines. Proliferated unattached Purkinje cell dendritic spines remained numerous, but there were increasing numbers of parallel fiber-Purkinje cell dendritic spine synapses. Purkinje cells assumed a mature appearance by 6 days after superimposition, as their fully ensheathed somata were rounded, had smooth contours and somatic spines were infrequent. In cortical regions in which Purkinje cell dendritic spines had proliferated, increasing numbers of axospinous synapses were evident. In more developed areas of cortex, homotypical parallel fiber-Purkinje cell dendritic spine synapses now predominated, as heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses were reduced. Purkinje cells undergoing degeneration were still evident.
While there were no further changes in Purkinje cell somata, axosomatic spines or astrocytic ensheathment at 7 days after superimposition, synapse formation among clusters of proliferated Purkinje cell dendritic spines continued to increase and fewer unattached spines remained. Well-formed parallel fiber synapses were also present on smooth portions of basket, stellate and Golgi cell dendrites, as parallel fibers had fully established contacts with all of their usual targets. By 8 days post superimposition, space between mature appearing Purkinje cells in host explants had progressively increased, resembling the appearance of control cultures. Parallel fiber-Purkinje cell dendritic spine synapses were the most frequent synapses in all cortical regions, while some heterotypical synapses and occasional clusters of unattached dendritic spines persisted. The numbers of such clusters had decreased as more synapses formed in areas of Purkinje cell dendritic spine proliferation. No further changes were evident at 9 days after superimposition, except for an increased difficulty with finding residual clusters of unattached dendritic spines. A few Purkinje cells with degenerative changes were still evident. At this stage the host explants resembled the host cultures that we had previously observed at 10 days after superimposition (Blank and Seil, 1983).
A clear relationship between astrocytic ensheathment and reduction of Purkinje cell axosomatic synapses was shown in this study (Seil, 1997b), including the stripping of excess synapses by astrocytic processes interposed between the pre- and postsynaptic elements. The mechanism for reduction of heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses, while associated with the presence of astrocytic processes, was not so clear. A direct attack on heterotypical synapses similar to the stripping of axonal terminals from Purkinje cell somata was never observed. In fact, astrocytic processes ensheathed both homotypical and heterotypical synapses. The mechanism by which heterotypical synapse reduction is accomplished may be more complex, and may involve other components, such as astrocyte secreted factors, as well.
The first homotypical parallel fiber-Purkinje cell dendritic spine synapses noted were at 2 days post superimposition, in areas of cortex in which there were no clusters of proliferated spines, suggesting that these synapses were with established dendritic spines. The first clusters of proliferated spines were evident at 2 days after superimposition, and had increased in number by 4 days after superimposition, at which time some early synapses with parallel fiber terminals were present among the proliferated spines. Synapse formation within clusters of spines had accelerated by 6 days post superimposition, after which the spine clusters were progressively reduced to where they were sparsely present at 9 days post superimposition. We did not find them at all in our initial electron microscopic study, which was done 10 days after superimposition. The regional and temporal differences in appearance of homotypical synapses were consistent with parallel fiber innervation of both established and newly formed Purkinje cell dendritic spines.
Purkinje cells undergoing cell death were present throughout the post-superimposition period. The changes observed in degenerating Purkinje cells more closely fit criteria for apoptosis than necrosis, although distinction between these forms of cell death cannot always be absolute. An apoptotic form of cell death would be consistent with a programmed cell death associated with a reduction of the Purkinje cell target field. From studies with superimposition of Ara C treated cultures with fragments of optic nerve, it appeared that glia by themselves were able to induce some reduction (27%) of excess Purkinje cells, presumably by elimination of some heterotypical recurrent axon collateral-Purkinje cell dendritic spine synapses (Seil, 1987). However, excess Purkinje cells were reduced to control levels only after both granule cells and glia were superimposed, indicating that the formation of homotypical parallel fiber-Purkinje cell dendritic spine synapses, concurrent with heterotypical synapse elimination, is critical for the process of full reduction of excess Purkinje cells.
The neuron-glial interactions that we have described in various cerebellar culture conditions are summarized diagrammatically in Figure 5. In untreated control cultures, Purkinje cell somata had astrocytic sheaths which were penetrated in a few places by approximately equal numbers of basket cell or Purkinje cell recurrent axon collateral terminals to form inhibitory axosomatic synapses, and their dendritic spines were the targets of excitatory parallel fibers emanating from granule cells. On intracellular recording of spontaneous activity, Purkinje cells had predominantly complex spike discharges. In Sigma Ara C treated cultures, which lacked granule cells and functional glia, Purkinje cells were unensheathed by astrocytic processes, their somata had persistent spines and were hyperinnervated by sprouted recurrent axon collaterals, which also formed heterotypical inhibitory synapses with Purkinje cell dendritic spines. The spontaneous discharge rate was similar to that of control Purkinje cells, but the spikes were all simple and the membrane input resistance was reduced. In cultures exposed to Pfanstiehl Ara C, in which granule cells and oligodendrocytes were absent but functional astrocytes were present, Purkinje cells had astrocytic sheaths, their somatic spines had disappeared and the number of axosomatic synapses was like that of control cultures, even in the presence of numerous sprouted recurrent axon collaterals that formed heterotypical synapses with Purkinje cell dendritic spines. Purkinje cell spikes were predominantly complex, the membrane input resistance was similar to control Purkinje cells, but the discharge rate was reduced. When Sigma Ara C treated cerebellar cultures were superimposed (transplanted) with granule cells and glia, Purkinje cells acquired astrocytic sheaths, somatic spines disappeared, axosomatic synapses were reduced to control levels, sprouted recurrent axon collaterals were reduced and most heterotypical synapses with Purkinje cell dendritic spines were replaced by homotypical synapses with parallel fibers. When only glia were superimposed on Sigma Ara C treated cultures, Purkinje cells were ensheathed by astrocytic processes, somatic spines were lost and the number of axosomatic synapses was similar to that of Purkinje cells in control cultures. Sprouted recurrent axon collaterals were not significantly reduced, but there was a partial reduction of heterotypical synapses, even in the absence of granule cells, and there were clusters of unattached Purkinje cell dendritic spines (not shown). When only granule cells were superimposed, Purkinje cells did not have astrocytic sheaths and had persistent somatic spines. Sprouted recurrent axon collaterals were not appreciably reduced and continued to hyperinnervate Purkinje cell somata. Most heterotypical dendritic spine synapses were replaced by homotypical synapses with parallel fibers, but twice as many heterotypical synapses persisted as when glia were included in the implant.
Fig. 5.
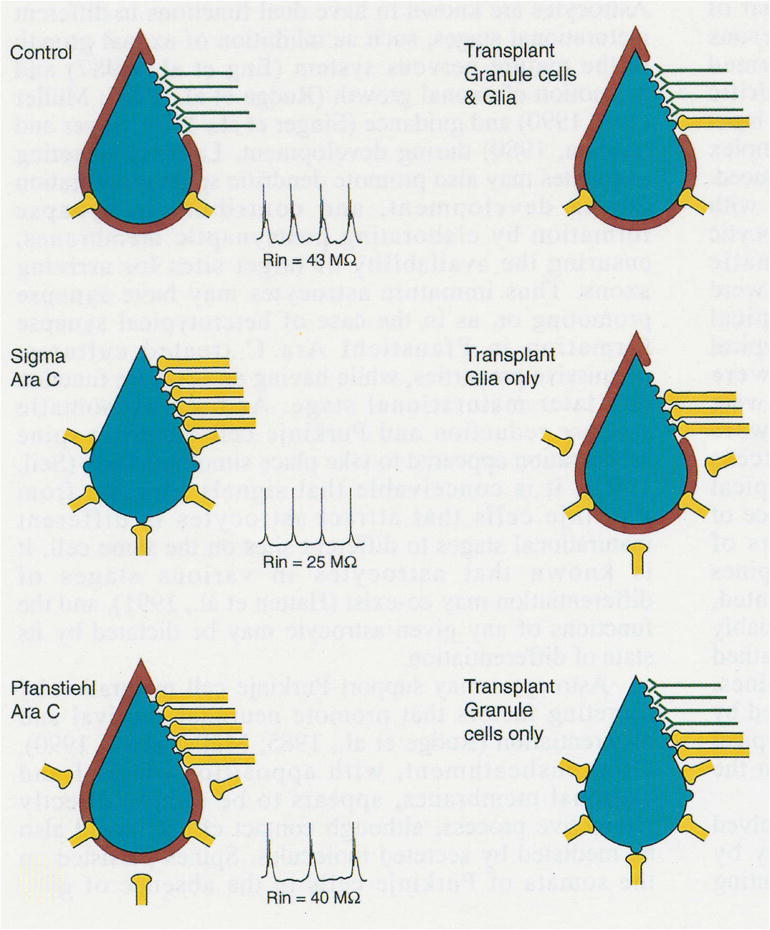
Represented diagrammatically in the left column are Purkinje cells (blue) and their astrocytic sheaths (red) in standard (Control) cultures and in cultures exposed to Sigma Ara C to destroy granule cells and compromise glia (Sigma Ara C) and to Pfanstiehl Ara C to destroy granule cells without affecting astrocytes (Pfanstiehl Ara C). Intracellularly recorded Purkinje cell spontaneous spike discharge patterns and membrane input resistances (Rin) are shown to the right of the Purkinje cells in each of the above conditions. Represented in the right column are Purkinje cells in Sigma Ara C treated cerebellar cultures implanted with granule cells and glia (Transplant Granule cells & Glia), with glia alone (Transplant Glia only) and with granule cells alone (Transplant Granule cells only). Excitatory parallel fibers and terminals are shown in black and form axospinous synapses with Purkinje cell dendritic spines. Inhibitory fibers and terminals (predominantly of recurrent axon collateral origin in cultures exposed to Ara C) are colored yellow and form synapses with Purkinje cell somata, somatic spines, proximal dendrites and heterotypical synapses with dendritic spines. Although not illustrated, axospinous synapses are also covered by astrocytic processes whenever functional astrocytes are present. Persistent Purkinje cell somatic spines are indicated when astrocytic sheaths are absent. The diagram may be used to follow the description in the text of the various changes that occur under the different experimental conditions. (From Seil, 2001a, with permission).
8. The Rules
As to the rules we had hoped to elucidate by which changes take place to preserve or restore function in an injured nervous system, the results of our studies led us to the following observations: 1) Cerebellar cultures can achieve a degree of structural and functional reorganization in response to elimination or compromise of some of their neuronal and glial components. 2) A further reorganization in the direction of structural and functional restitution can occur if the missing elements are replaced. 3) As determined from both our culture studies and animal studies by others, there is an hierarchal order of synapse formation during circuit reorganization, with axons of similar function having priority over dissimilar axons as replacements for missing presynaptic components. 4) In extreme circumstances, such as the deafferented cerebellum in vitro, terminals of opposite function may substitute for the absent presynaptic elements, and the function of the synapse is in accordance with that of the presynaptic component, i.e., a formerly excitatory synapse will become inhibitory if the new presynaptic terminal is inhibitory. 5) When missing neuronal elements are restored, appropriate synapses are formed, replacing heterotypical synapses even if the latter have become functional. 6) Competent astrocytes and oligodendrocytes may restore the normal glial milieu when inserted into an area devoid of functional glia. 7) Astrocytes have a role in the regulation of the numbers of axosomatic and axospinous synapses in some neurons, such as Purkinje cells, and direct axon terminal stripping by astrocytic processes may be one of the mechanisms of such control. 8) Astrocytes may also promote synaptogenesis by inducing the elaboration of postsynaptic membranes, such as the proliferation of dendritic spines in response to the presence of astrocyte-secreted laminin in the case of Purkinje cells. 9) Astrocytes may promote the structural and functional maturation of some neurons, such as Purkinje cells, and this effect appears to be related to astrocytic ensheathment of the neurons. 10) Survival of neurons can be enhanced in vitro as well as during development in vivo by expanding the neuronal target field. Subsequent restriction of the target field results in reduction of the neuron population.
A cautionary note in the interpretation of these studies in the context of recovery from neural injury is that the model we have used is a developmental system, and a developing nervous system has a greater capacity for reorganization and repair than one that is mature. However, a known occurrence during repair after adult neural injury is the reappearance of phenomena characteristic of development, such as the presence of undifferentiated astrocytes at sites of injury, so that strategies available to a developing nervous system may also be available for repair of a mature nervous system. The point of studying a developing system, then, is to elaborate what these strategies might be, which may also lead to discovering means of promoting the inception of such strategies during the process of neural repair and recovery in cases of CNS disease or injury in mature subjects, including human subjects.
9. A Different Form of Neuroplasticity
Another form of neural plasticity is activity-dependent plasticity, which refers to the role of neuronal activity in the development or alteration of patterns of synaptic connectivity. The presence of neuronal activity is critical for the development of some parts of the central nervous system. Ocular dominance columns fail to form in mammalian visual cortex if visual input is compromised by such maneuvers as enucleation of one eye (Wiesel and Hubel, 1963) or blockade of neurotransmission during early development (Shaw and Cynander, 1984). Auditory (Parks, 1979), olfactory (Meisami and Monsavi, 1981) and somatosensory systems (Woolsey and Wann, 1976) are similarly dependent on neural input for normal development. A variety of in vivo and in vitro studies have shown that neuronal activity is necessary for the full development and maintenance of inhibitory circuitry. A reduction of GABA immunoreactivity was found in visual cortex of animals deprived of visual input by eye enucleation (Hendry and Jones, 1986) or blockade of retinal ganglion cell discharge (Hendry and Jones, 1988). A decrease of GABA-reactive neurons in visual cortex of dark-reared rats correlated with an increase in spontaneous cortical electrical activity (Benevento et al., 1995). Increased spontaneous activity in the barrel field cortex of neonatally sensory deprived animals (Simons and Land, 1987) was consistent with a reduction of GABA-immunopositive synapses in the barrel field cortex (Michaeva and Beaulieu, 1995). Continuous exposure of cerebral neocortex cultures to activity blocking agents reduced synapse numbers (Van Huizen et al, 1985). Removal of blocking agents from continuously exposed cultures was followed by hyperactivity of electrical discharges, consistent with reduced inhibition in such preparations (Ramakers et al., 1990). Augmentation of neuronal activity also produced changes in neural circuitry, such as sprouting and synaptic reorganization in hippocampus after repeated stimulation of perforant path fibers (Sutula et al., 1988). Frequent stimulation of CNS cultures augmented synapse formation and increased synaptic efficacy (Nelson et al., 1990).
Chronic exposure of dissociated fetal rat cerebral neocortex cultures to the anti-GABA agent, picrotoxin (PTX), resulted in accelerated synaptogenesis (Van Huizen et al., 1987), reduced phasic discharges and a high incidence of very brief bursts of action potentials, suggesting that GABAergic inhibition was stronger in cultures exposed to PTX than in control cultures (Ramakers et al., 1991). As the organotypic cerebellar culture model presented a system in which GABAergic inhibition played a key role, and as structural and functional relationships in such cultures were well defined both during development and after maturation, we thought that it was an ideal system in which to explore further the effects of enhanced neuronal activity on cortical development. We continuously exposed cerebellar cultures from explantation separately to two anti-GABA agents, PTX and bicuculline (Seil et al., 1994). PTX is thought to work by uncoupling the GABAA receptor site from the chloride channel, thus preventing the latter from opening, while bicuculline competitively blocks the GABAA receptor. Acute application of either of these agents at a 10-4 M concentration to mature (13-16 DIV) cerebellar cultures during electrophysiological recording resulted in immediately evident increases in cortical discharge rates.
After continuous exposure of cerebellar cultures to 2 × 10-4 M of either PTX or bicuculline for 3 days after explantation, spontaneous cortical discharges, recorded extracellularly after transfer to a recording medium to be described below, were of low amplitude, but there were more active units and higher discharge rates than in control cultures. By 13-16 days of continuous exposure to anti-GABA agents, there was a marked overall reduction in the frequency of large amplitude cortical spikes, which are predominantly of Purkinje cell origin. Quantitatively, the discharge rates in PTX and bicuculline treated cultures were about half of those in untreated control explants. At this time, electrical stimulation of the cortical surfaces of anti-GABA agent treated cultures with stimulus trains induced prolonged inhibition of spontaneous cortical activity, the inhibitory intervals measuring more than twice those of control cultures. Moreover, cortical inhibition in response to electrical stimulation developed earlier in cultures chronically exposed to PTX or bicuculline, being consistently present by 8 DIV, compared with 12 DIV for control explants. As with the earlier studies with dissociated cerebral neocortex cultures (Ramakers et al., 1991), our results indicated that the strength of GABAergic inhibition in organotypic cerebellar cultures continuously exposed to anti-GABA agents was greater than in untreated controls.
The reason for this became apparent when we looked at the morphology of the exposed cultures (Seil et al., 1994). No differences from control explants were evident by light microscopy, including time and degree of myelination, cortical lamination, neurite density and overall architectural organization. On ultrastructural examination, however, Purkinje cells in anti-GABA agent treated cultures had twice as many axosomatic synapses as Purkinje cells in control cultures. As noted earlier, Purkinje cells average 2.2 axosomatic synapses per section in control explants, and the ratio of basket cell terminals to recurrent axon collateral terminals is 1:1. The average number of axosomatic synapses per Purkinje cell section in cerebellar cultures after 14-16 days of continuous exposure to PTX was 4.8, while the average was 4.0 in cultures chronically treated with bicuculline. In both instances, the ratio of basket cell terminals to recurrent axon collaterals was 2:1, indicating that the increase in inhibitory axosomatic synapses represented an increase predominantly of synapses with basket cell terminals, suggesting a sprouting primarily of basket cell axons in response to increased neuronal activity induced by anti-GABA agents early in development.
Evident in cultures exposed to PTX and bicuculline was an increased number of synapses on persistent Purkinje cell somatic spines. These spines generally disappear with maturation, but do tend to persist if they have formed synapses. The accelerated appearance of cortical inhibition in treated cultures is consistent with the formation of synapses at a time when more synaptic spines were still available to form synapses with inhibitory presynaptic terminals. Also notable was the presence of intact astrocytic sheaths around the somata of Purkinje cells in cultures continuously exposed to anti-GABA agents. The hyperinnervation of Purkinje cell somata by inhibitory axon terminals in such cultures occurred in the presence of functional astrocytes, indicating that the usual synapse regulatory properties of the astroglia were overridden. As the signal for astrocytic ensheathment appears to emanate from the Purkinje cells, these cells would also be a likely source for the override signal.
In essence, agents that increased neuronal activity early during development provoked changes in cerebellar cultures that resulted in strengthened inhibition and reduced neuronal activity. These changes include inhibitory hyperinnervation of Purkinje cell somata in the presence of intact astrocytes and the early development of cortical inhibition. As neurons must have some mechanism for adjusting their overall activity, it is likely that these changes occur in response to Purkinje cell hyperactivity, and represent either an exaggerated attempt at homeostasis or a response to protect the cells from such possibilities as excitotoxic injury or death. In any case, a rise in neuronal activity during development induced an increase in inhibitory synaptogenesis.
The following question then was what would happen if neuronal activity was continuously blocked in cerebellar cultures after explantation. To completely silence Purkinje cells, we exposed them to nutrient medium containing a combination of 10-8 M tetrodotoxin (TTX) to block somatic Na+ spikes and 11.1 mM Mg2+ to block Ca2+ dependent dendritic spikes (Seil and Drake-Baumann, 1994). When control cultures were transferred after 13-16 DIV to our standard electrophysiological recording medium, which consisted of Simms' X-7 balanced salt solution (BSS) containing 1.1 mM Mg2+ additionally buffered with 1.5 × 10-2 M HEPES, they were immediately spontaeously active, showing the usual complement and rate of extracellularly recorded spikes. However, cultures that had been exposed to the activity blocking agents were completely silent for at least 10 minutes after transfer to the recording medium. Then slow spontaneous discharges began to appear in cortical regions of the explants, after which the spikes steadily increased in frequency until the cultures were discharging hyperactively 30-40 minutes after transfer, sometimes with bursts of rapid spikes, similar to activity described in previous studies of cultures released from activity blockade (Ramakers et al., 1990). High cortical discharge rates were maintained for the duration of the recording sessions, up to 2 hours.
No structural changes were evident by light microscopy in cultures continuously exposed to activity blocking agents (Seil and Drake-Baumann, 1994). Myelination proceeded at the usual time and rate, not requiring the presence of neuronal activity, and overall architecture and cortical-subcortical relationships were unaffected. Purkinje cells and granule cells both appeared to survive well, and Purkinje cell dendritic development was indistinguishable from that in control cultures. In particular, there was no Purkinje cell dendritic elongation with minimal branching, as had been reported in dissociated Purkinje cell cultures exposed to activity blocking agents (Schilling et al., 1991). On ultrastructural examination, Purkinje cells in exposed cultures had half the number of inhibitory axosomatic synapses as Purkinje cells in control explants (Seil and Drake-Baumann, 1994). Synapses with basket cell terminals and recurrent axon collateral terminals were equally affected, their numbers being the same. Astrocytic sheaths around the somata of Purkinje cells were intact. Astrocytic ensheathment proceeded in the absence of neuronal activity, indicating that the signal for glial ensheathment is not activity-dependent. Synapses with dendritic spines and smooth portions of dendrites in the cortical neuropil were also counted. Axospinous synapses are with excitatory parallel fiber terminals in cerebellar cultures, which have essentially no climbing fibers. Axodendritic synapses in the cerebellar cortical neuropil are with a mixture of excitatory parallel fiber and granule cell ascending axon terminals and inhibitory terminals from Purkinje cell recurrent axon collaterals and inhibitory interneurons (Eccles et al., 1967; Palay and Chan-Palay, 1974). Counts of cortical axospinous synapses in cultures exposed to activity blocking agents were the same as in control cultures, indicating that these excitatory synapses are not dependent on neuronal activity for development, consistent with findings from earlier studies with organotypic spinal cord and cerebral neocortex cultures (Crain et al., 1968; Model et al., 1971). Less than half as many cortical axodendritic synapses were present in cultures exposed to activity blocking agents as in control explants (Seil and Drake-Baumann, 1994). The development of proportionally fewer axosomatic and axodendritic synapses than axospinous synapses in activity blocked cultures is indicative of an incomplete development of inhibitory circuitry. It appears that the full complement of inhibitory synapses does not develop when there is no activity to inhibit. Incomplete inhibitory synaptogenesis is consistent with the observed cortical hyperactivity after release from activity blockade.
Taken together, the results of our studies indicated that continuously exposing cerebellar cultures during development to agents that increase neuronal activity promoted formation of excess inhibitory synapses, with correlative reduction of spontaneous cortical activity and prolongation of inhibitory responses. In contrast, exposure to agents that block neuronal activity during development led to reduced inhibitory synaptogenesis and sustained cortical hyperactivity following release from activity blockade. These studies support the notion that neuronal activity is critical for the development of adequate inhibitory transmission (Corner and Ramakers, 1992). Excitatory synapses, on the other hand, appear to develop fully in the absence of neuronal activity.
10. Neuronal Activity and Circuit Reorganization
Having observed the dramatic effects of neuronal activity, or the absence thereof, on the development of cerebellar cultures, we were now interested in possible effects on the models of neural circuit reorganization that occurred after destruction of granule cells and compromise of glia, followed by restoration of the circuitry after implantation of granule cells and functional glia, as described in Sections 3 and 4. We exposed cerebellar explants for the first 5 DIV to Ara C alone or to a combination of Ara C and PTX (Seil and Drake-Baumann, 1996a). A third group of cultures was explanted with standard nutrient medium and maintained in this medium until electrophysiological recording or fixation. Cultures exposed only to Ara C were subsequently maintained in standard nutrient medium, while cultures exposed to Ara C and PTX were thereafter continuously maintained in medium with incorporated PTX.
Spontaneous cortical discharge rates were similar in all three culture groups after 13-16 DIV. Antidromic stimulation of Purkinje cell axons in untreated control cultures induced a transient increase in cortical discharges, while similar stimulation of Purkinje cell axons in Ara C treated cultures maintained in standard nutrient medium evoked a pronounced inhibition of cortical spikes, as noted previously. This latter response had been attributed to the massive projection of sprouted inhibitory recurrent axon collaterals to the somata and dendritic spines of Purkinje cells. Antidromic stimulation of Purkinje cell axons in Ara C treated cultures maintained in medium with PTX also resulted in a pronounced inhibition of cortical discharges.
No differences were evident at the light microscopic level between Ara C treated cultures and Ara C treated cultures continuously exposed to PTX. Large cortical neurons in increased numbers and without lamination were present in both groups. Both groups also demonstrated the same degree of increase in cortical neurites, previously shown to be due to Purkinje cell recurrent axon collateral sprouting (Seil et al., 1980). By electron microscopic examination, rounded, well ensheathed Purkinje cell somata were present in untreated control cultures, while Purkinje cell somata in both Ara C treated groups were practically devoid of astrocytic sheaths and were scalloped by multiple abutting inhibitory axon terminals (Seil and Drake-Baumann, 1996a). The average number of Purkinje cell axosomatic synapses was almost identical in the two groups, and was more than twice the control value. Counts of axospinous and axodendritic synapses in the cortical neuropil of the two Ara C treated culture groups were also the same.
In essence, increased neuronal activity did not appear to affect the circuit reorganization of cerebellar cultures induced by an early elimination of granule cells. As the critical feature of such reorganization was development of an abundance of inhibitory synapses, and since the full development of inhibitory circuitry is dependent on the presence of neuronal activity, it is conceivable that the PTX-induced increased neuronal activity had no effect because inhibitory synaptogenesis was already at an optimal level in Ara C treated cultures.
Changes were evident, however, when we did the corollary experiment, namely maintaining Ara C treated explants in medium with activity blocking agents, TTX and elevated levels of Mg 2+ , to silence both somatic and dendritic Purkinje cell spikes (Seil and Drake-Baumann, 1995). Granule cell depleted cultures maintained in standard nutrient medium were immediately spontaneously active when recorded from extracellularly following transfer to a recording medium after 13-16 DIV. Similar cultures maintained in medium with activity blocking agents were silent for 12-20 minutes after transfer to the recording medium. By 30-40 minutes after transfer, sustained hyperactivity of cortical discharges was evident, and was maintained for the duration of the recording sessions.
No differences were observed by light microscopy between the two Ara C treated groups. Ultrastructurally, Purkinje cells in both groups lacked astrocytic sheaths and their somata were scalloped by abutting axons and hyperinnervated by inhibitory axon terminals. Quantitatively, Ara C treated cultures maintained in standard nutrient medium had a mean number of 4.9 axosomatic synapses per Purkinje cell section, whereas the mean number of axosomatic synapses per Purkinje cell section in Ara C treated cultures continuously exposed to activity blocking agents was 3.9, a statistically significant reduction, although almost double the average for untreated control cultures. A more significant difference was found on counting axospinous synapses in the cortical neuropil, where granule cell depleted cultures maintained in standard medium had a mean number of 8.3 axospinous synapses per 100 μm2 field while Ara C treated explants chronically exposed to acivity blocking agents had a mean of 5.7 axospinous synapses per 100 μm2 field. The difference in the number of axodendritic synapses was not statistically significant between the two groups.
What is critical here is that the axospinous synapses in Ara C treated cultures were inhibitory, since they were heterologous synapses with terminals from sprouted Purkinje cell recurrent axon collaterals, as opposed to homologous excitatory axospinous synapses with parallel fiber terminals in untreated control cultures. Excitatory axospinous synapses in cultures with a full complement of granule cells were not reduced after exposure to activity blocking agents (Seil and Drake-Baumann, 1994). The decided reduction of inhibitory axospinous synapses in Ara C treated cultures exposed to activity blocking agents, plus the moderate reduction of axosomatic synapses, are consistent with the spontaneous cortical hyperactivity such cultures display after release from activity blockade (Seil and Drake-Baumann, 1995). These results again support the notion that neuronal activity is necessary for the full development of inhibitory circuitry, even if the circuitry has been reorganized as a consequence of depleting one of the neuronal elements.
The next step then was to determine the effects of increased or blocked neuronal activity on the changes induced by implantation of Ara C treated cultures at 9 DIV with cerebellar cultures exposed to kainic acid. As described in Section 4, addition of granule cells and intact glia to reorganized Ara C treated explants resulted in restoration of the normal morphology and circuitry of cerebellar cultures. Thus cultures exposed to kainic acid for the first 5 DIV followed by maintenance in standard nutrient medium were superimposed at 9 DIV upon explants treated with Ara C for the first 5 DIV and then maintained in standard medium to 9 DIV (Seil and Drake-Baumann, 1996b). One such group was maintained in nutrient medium with added PTX after implantation, another in medium with added TTX and elevated Mg2+, while a third group continued to be maintained in standard nutrient medium.
After 14-16 DIV, control implanted cultures and cultures exposed to PTX after implantation were spontaneously active immediately upon transfer to a recording medium, and the cortical discharges remained stable thereafter. Implanted cultures maintained in medium with activity blocking agents showed the familiar pattern of initial silence after transfer to the recording medium, followed by the appearance of cortical spikes 15-20 minutes later and progression to persistent hyperactive discharges by 30 minutes after transfer. Antidromic activation of Purkinje cells did not inhibit the spontaneous cortical discharges in any of the implanted culture groups, contrary to the cortical inhibition following antidromic stimulation of Purkinje cells in unimplanted Ara C treated cultures.
Observation of implanted cultures by light microscopy revealed no significant differences among the three groups. Myelination was evident in a comparable proportion of cultures at 6 days after implantation. Granule cell nuclei were visible and cortical neurite density was reduced in all groups. Neither myelination nor reduction of excess cortical neurites (sprouted Purkinje cell recurrent axon collaterals) appeared to be activity-dependent. In electron micrographs of Purkinje cell sections, astrocytic sheaths were present around rounded somata in all groups, and the numbers of axosomatic synapses in the three conditions were similarly reduced. However, the number of axodendritic synapses (mix of inhibitory and excitatory) in the cortical neuropil was significantly reduced (mean of 2.9 per 100 μm 2) in cultures maintained in medium with activity blocking agents after implantation compared with implanted cultures exposed to PTX (mean of 5.0 per 100 μm 2) or maintained in standard medium (mean of 4.1 per 100 μm 2). The number of axodendritic synapses in PTX treated implanted cultures appeared greater than in control implanted cultures, but this difference did not attain statistical significance. The mean number of axospinous synapses in the cortical neuropil was somewhat greater in activity blocked implanted cultures than in the other groups, but the difference became significant when the synapses were classified as homotypical (excitatory, newly formed with parallel fiber terminals) or heterotypical (inhibitory, with residual recurrent axon collateral terminals). The numbers of homotypical synapses were similar in all three groups. There were, however, almost twice as many heterotypical axospinous synapses in the activity blocked implanted cultures as in the other two groups, suggesting that elimination of heterotypical axospinous synapses was to some degree activity-dependent. A similar persistence of increased numbers of heterotypical axospinous synapses was seen when Ara C treated cultures were implanted with granule cells in the absence of functional glia (Seil, 1994). Possibly both intact astrocytes and neuronal activity have a role in the reduction of heterotypical axospinous synapses. It is notable that homotypical synapses in the cortical neuropil still outnumbered heterotypical synapses by a ratio of 1.8:1 in activity blocked implanted cultures, compared with a ratio of 3:1 in the other implanted culture groups (Seil and Drake-Baumann, 1996b).
In summary, a number of changes induced by implantation of Ara C treated cultures with granule cells and functional glia were unaffected by an increase or absence of neuronal activity (Seil, 1996). These include (1) loss of inhibition of spontaneous cortical discharges in response to antidromic activation of Purkinje cells, (2) myelination, (3) reduction of excess sprouted Purkinje cell recurrent axon collaterals, (4) astrocytic ensheathment of Purkinje cells, (5) reduction of excess Purkinje cell axosomatic synapses and (6) formation of homotypical excitatory parallel fiber-Purkinje cell dendritic spine synapses. Changes that were induced by an absence of neuronal activity include (1) hyperacivity of cortical discharges after release from activity blockade, (2) reduced formation of axodendritic synapses and (3) persistence of a greater number of inhibitory heterotypical axospinous synapses than in the PTX treated and control implanted groups. The observed cortical hyperactivity after recovery from activity blockade is most likely a function of reduced axodendritic inhibitory synaptogenesis. The loss of inhibition of cortical spontaneous activity following antidromic activation of Purkinje cells is likely due to the replacement of the majority of cortical inhibitory heterotypical axospinous synapses with excitatory homotypical axospinous synapses with parallel fiber terminals, combined with the reduction of inhibitory Purkinje cell axosomatic synapses.
11. Neurotrophins and Activity-Dependent Plasticity
On the basis of evidence derived from animal studies, it seemed that neurotrophins had essential roles in activity-dependent neuroplastic changes in the CNS (reviewed in Thoenen, 1995; Shatz, 1997). Members of the neurotrophin family include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4; also known as NT-4/5). The Trk family of receptor tyrosine kinases with which they bind with high affinity are, respectively, TrkA, TrkB, TrkC and TrkB (reviewed in Bothwell, 1995; Lewin and Barde, 1996; Lindsay, 1994). Neurotrohins appear to be synthesized and released in an activity-dependent manner (Thoenen, 1995).
In a signal study, it was reported that application of TTX to dissociated visual cortex cultures containing GABAergic interneurons and target pyramidal cells reduced the percentage of GABA-immunopositive neurons without affecting neuronal survival (Rutherford et al., 1997). Similar results were obtained by exposing the cultures continuously to the “universal” Trk receptor inhibitor, K252a. Correlative electrophysiological studies showed that GABA-mediated inhibition onto pyramidal neurons was decreased and pyramidal cell discharge rates were increased. All of the effects of activity blockade were prevented by simultaneous exposure of the cultures to BDNF, but not to NGF or NT-3 (NT-4 was not tested). The results of this study suggested to the authors that activity regulates cortical inhibition by regulation of BDNF.
As synapses were not morphologically evaluated in this study, we determined to examine the effects of added neurotrophins on inhibitory synaptogenesis in activity blocked organotypic cerebellar cultures. Since TrkA, the high affinity receptor for NGF, had been reported to be only transiently expressed on Purkinje cells during development (Ernfors et al., 1992), we did not examine the effects of exogenous NGF application, but focused our efforts on the other three members of the neurotrophin family, namely BDNF, NT-3 and NT-4 (Seil, 1999). Each of these neurotrophins was applied separately in a concentration of 25 ng/ml nutrient medium, along with activity blocking agents, TTX and elevated levels of Mg2+, at explantation and continuously thereafter until fixation at 15 DIV for ultrastructural analysis. Cultures maintained continuously in standard nutrient medium and in nutrient medium with activity blocking agents but without neurotrophins were set up for comparison.
Counts of Purkinje cell axosomatic synapses in cultures exposed to activity blocking agents plus BDNF or activity blocking agents plus NT-4 were the same as those in untreated control cultures, while equivalent counts in cultures maintained in medium with activity blocking agents plus NT-3 were reduced to the same level as cultures exposed to activity blocking agents without neurotrophins, which was half of the number in untreated controls. Thus exogenous application of TrkB receptor ligands promoted development of inhibitory Purkinje cell axosomatic synapses in the absence of neuronal activity, while this effect was not evident with application of the TrkC receptor ligand, NT-3, to cerebellar cultures during activity blockade. These results supported a role for TrkB receptor ligands in activity-dependent inhibitory synaptogenesis.
When cerebellar cultures were exposed to exogenously applied neurotrophins in the absence of activity altering agents at the same concentrations (25 ng/ml nutrient medium) as were used during activity blockade, no changes were evident in the numbers of Purkinje cell axosomatic synapses (Seil and Drake-Baumann, 2000). However, when the concentration of BDNF was increased to 100 ng/ml medium, there was a 50-60% increase in Purkinje cell axosomatic synapses (unpublished observations). A greater concentration of BDNF was required to increase the number of Purkinje cell axosomatic synapses above untreated control levels at baseline activity than was needed to mitigate the effects of activity blockade.
Subsequently we examined the electrophysiological consequences of exogenous application of neurotrophins during activity blockade and the role of endogenous neurotrophins in inhibitory synaptogenesis (Seil and Drake-Baumann, 2000). All activity blocked cultures, with or without neurotrophins, were electrically silent during the first 10 minutes following transfer to a recording medium after 15 DIV, while control cultures maintained in standard nutrient medium were immediately spontaneously active. After recovery from activity blockade, cultures treated with BDNF or NT-4 had spontaneous cortical discharge rates comparable to those of untreated control explants, while cultures treated with NT-3 were hyperactive, with twice the number of cortical discharges, like those of activity blocked cultures without neurotrophins. These results were consistent with the morphological findings.
To investigate the role of endogenous neurotrophins in the development of inhibitory synapses in cerebellar cultures, we added antibodies to BDNF and NT-4 in combination (50 μg/ml each) to the nutrient medium at explantation and during subsequent maintenance. After 15 DIV, only half the number of Purkinje cell axosomatic synapses was present in antibody treated cultures as in control explants. The reduction in the development of inhibitory axosomatic synapses was of the same magnitude as activity blockade over the same time period. Antibody to BDNF alone only partially reduced Purkinje cell axosomatic synapse formation, suggesting that both TrkB receptor ligands contributed to inhibitory synaptogenesis in cultures with control levels of neuronal activity.
We subsequently applied the same combination and concentration of antibodies to cerebellar cultures simultaneously exposed to PTX (10-4 M) to increase neuronal activity, which would presumably increase the release of BDNF and NT-4. This concentration of PTX, in the absence of antibodies to neurotrophins, resulted in a 50% increase in the number of Purkinje cell axosomatic synapses after 15 DIV, compared to an over 100% increase when the concentration was 2 × 10-4 M (Seil et al., 1994). When antibodies to BDNF and NT-4 were included in the nutrient medium along with PTX, the number of Purkinje cell axosomatic synapses was similar to that in untreated control cultures, indicating that the effect of increased neuronal activity on inhibitory synaptogenesis had been mitigated (Seil and Drake-Baumann, 2000). When the concentration of antibodies was doubled, the number of axosomatic synapses was half that in untreated controls, equivalent to the results of antibody application to cultures with control levels of neuronal activity, suggesting that the lower dose of antibodies did not bind all of the endogenous BDNF and NT-4 released by the PTX-induced increased neuronal activity.
The results of these studies indicated a role specifically for TrkB receptor ligands in activity-dependent inhibitory synaptogenesis, a role not shared by the TrkC receptor ligand, NT-3, and raised the possibility that signaling for activity-dependent inhibitory synaptogenesis might be via the TrkB receptor. It had been shown that the TrkA receptor could be activated by binding with specific antibody, and that antibody activation of the receptor produced biological effects that mimicked those of the specific receptor ligand (Clary et al., 1994). For example, the effects of monocular enucleation in rats were prevented by cortical infusion of anti-TrkA antibody, similar to infusion of NGF (Pizzorusso et al., 1999). Following this model, cerebellar cultures were exposed from explantation to 15 DIV to an antibody to the extracellular domain of TrkB (1 μg/ml nutrient medium) or to an antibody that bound the extracellular domain of TrkC (1 μg/ml medium) and examined ultrastructurally for Purkinje cell axosomatic synapse development (Seil, 2001b). The number of Purkinje cell axosomatic synapses in cultures maintained in antibody to the extracellular domain of TrkC was no different than that in untreated control cultures. The number of axosomatic synapses in cultures exposed to antibody to the extracellular domain of Trk B was approximately 60% greater than the average for untreated control explants. This increase was similar to that obtained by maintaining otherwise untreated cerebellar cultures in large doses of BDNF (100 ng/ml nutrient medium). Doubling the concentration of the anti-TrkB extracellular domain antibody did not result in additional TrkB receptor activation, as the number of Purkinje cell axosomatic synapses was not increased further, suggesting that TrkB receptor binding by antibody was saturated. The results of this study support the concept that signaling for activity-dependent inhibitory synaptogenesis is via the TrkB receptor.
12. Summary of Neuronal Activity and Inhibitory Synaptogenesis
The experimental morphological data pertinent to Purkinje cell axosomatic synapse formation under baseline states of neuronal activity, under activity blockade, after exposure to agents that increase neuronal activity, and the effects of exogenously applied and endogenous neurotrophins and TrkB receptor activation are summarized in Table 1 and presented diagrammatically in Figure 6 (Seil, 2003). The three vertical columns in Figure 6 represent the different neuronal activity states, beginning with baseline activity on the left. Purkinje cell somata in this column are represented as blue circles and are from cultures not exposed to activity altering agents. Purkinje cells continuously exposed to TTX and elevated levels of Mg2+ to block neuronal activity are shown as green circles in the middle column and the red circles in the right column indicate the somata of Purkinje cells chronically treated with PTX to increase neuronal activity during development. Inhibitory axon terminals forming synapses with Purkinje cell somata are represented as small yellow circles abutting the somata. The numbers of axosomatic synapses were rounded up from mean figures for each experimental situation, as derived from Table 1, and these figures appear in parentheses to the left of the circles. The asterisk in parentheses in the left column indicates unpublished data from my laboratory.
Table 1.
Quantitative data on Purkinje cell axosomatic synapses from control cerebellar cultures, cultures continuously exposed to picrotoxin (PTX) and bicuculline to increase neuronal activity (Seil et al., 1994), to elevated levels of Mg2+ and tetrodotoxin (Mg2+/TTX) to block neuronal activity (Seil and Drake-Baumann, 1994) to neuronal activity blocking agents plus neurotrophins BDNF, NT-3 and NT-4 (Seil, 1999; Seil and Drake-Baumann, 2000), to antibodies to BDNF and NT-4 (αBDNF/αNT-4) to block endogenous neurotrophins (Seil and Drake-Baumann, 2000), to PTX combined with αBDNF/αNT-4 (Seil and Drake-Baumann, 2000) and to specific antibodies to the extracellular domains of the TrkB (αTrkB) and TrkC (αTrkC) receptors (Seil, 2001b). SEM: standard error of the mean. (From Seil, 2003, with permission).
| Cultures | No. of Cell Profiles | No. of Synapse Profiles | Mean Ratio of Synapse to Cell Profiles ± sem |
|---|---|---|---|
| Control | 78 | 168 | 2.15±0.15 |
| PTX(2×10-4 M) | 58 | 279 | 4.81±0 35 |
| Bicuculline | 79 | 318 | 4.03±0.22 |
| Control | 23 | 44 | 1.91±0 34 |
| Mg2+/TTX | 45 | 43 | 0.96±0.15 |
| Control | 103 | 228 | 2.21±0.13 |
| Mg2+/TTX | 102 | 127 | 1.25±0.10 |
| Mg2+/TTX/BDNF | 102 | 235 | 2.30±0.14 |
| Control | 100 | 200 | 2.00±0.14 |
| Mg2+/TTX | 101 | 104 | 1.03±0.11 |
| Mg2+/TTX/NT-3 | 103 | 107 | 1.04±0.11 |
| Control | 100 | 215 | 2.15±0.13 |
| Mg2+/TTX | 100 | 121 | 1.21±0.11 |
| Mg2+/TTX/NT-4 | 104 | 209 | 2.01±0.12 |
| Control | 93 | 241 | 2.59±0.18 |
| αBDNF/αNT-4 (50 μg/ml each) | 92 | 124 | 1.35±0.11 |
| Control | 80 | 183 | 2.29±0.15 |
| PTX(10-4M) | 80 | 277 | 3.46±0.24 |
| PTX/αBDNF/αNT-4 (α: 50 μg/ml each) | 82 | 204 | 2.49±0.17 |
| PTX/αBDNF/αNT-4 (α: 100 μg/ml each) | 80 | 112 | 1.40±0.15 |
| Control | 77 | 157 | 2.04±0.14 |
| αTrkB | 80 | 261 | 3.26±0.19 |
| αTrkC | 77 | 158 | 2.05±0.17 |
Fig. 6.
A diagrammatic representation of the data from Table 1. Purkinje cell somata are shown as large circles colored blue, green or red, and axon terminals that form axosomatic synapses are depicted as small yellow circles at the periphery of the somata. The numbers of these terminals are rounded up from the means (in parentheses to the left of each Purkinje cell representation) derived from the data in Table 1, except for the asterisk under BDNF in the first column, as these data are from unpublished work by the author. In the left column, in which the Purkinje cell soma representations are colored blue, none of the cerebellar cultures were exposed to activity altering agents. Under these conditions, addition of BDNF or specific antibody activation of the Trk B receptor increased Purkinje cell axosomatic synapse formation, while blocking endogenous neurotrophins with a combination of antibodies to BDNF and NT-4 reduced Purkinje cell axosomatic synapse formation to about half of the control value. In the middle column, in which the Purkinje cell soma representations are colored green, the cultures were continuously exposed to elevated levels of Mg2+ and tetrodotoxin (Mg/TTX) to block neuronal activity, which reduces axosomatic synapse formation by about one-half. Addition of BDNF or NT-4 maintained the control number of axosomatic synapses, while NT-3 had no effect. In the right column, in which the Purkinje cell soma representations are colored red, cultures were continuously maintained in picrotoxin (PTX) to increase neuronal activity. At a concentration of 2×10-4 M PTX, axosomatic synapse formation was increased by over 100% (PTX 2X), while halving the concentration of PTX resulted in a 50% increase above control levels. The effect was mitigated by blocking endogenously released neurotrophins with antibody to BDNF and NT-4, and further reduced by doubling the antibody concentrations. Additional details are given in the text. (From Seil, 2003, with permission).
In the left column it can be seen that both addition of BDNF (at a 100 ng/ml medium concentration) and specific antibody activation of the TrkB receptor to saturation result in a 50-60% increase in Purkinje cell axosomatic synapse formation. Blocking endogenous neurotrophins with a combination of antibodies to BDNF and NT-4 reduced Purkinje cell axosomatic synapse formation to approximately half the control value, similar to activity blockade, the latter shown at the top of the middle column. The addition of TrkB receptor ligands BDNF or NT-4 to activity blocked cultures maintained the formation of control levels of Purkinje cell axosomatic synapses, a property not shared by the TrkC receptor ligand NT-3, as shown in the middle column. Looking at the right column, Purkinje cell axosomatic synapse formation was increased by over 100% by maintenance of cultures in 2×10 -4 M PTX, while halving the concentration of PTX resulted in approximately half as great an increase of axosomatic synapses. The effect of the presumptive release of elevated levels of endogenous BDNF and NT-4 by increased neuronal activity was mitigated by blocking with antibodies to BDNF and NT-4 and further reduced by a doubling of the antibody concentrations (Seil and Drake-Baumann, 2000).
There appears to be a limit to the degree to which inhibitory synaptogenesis can be increased at baseline neuronal activity, as shown in the left column, the limit being at approximately 60% with either addition of exogenous BDNF or with specific antibody activation of the TrkB receptor. However, with increased neuronal activity, such as attained with cultures exposed from explantation to 2×10-4 M PTX, as shown in the right column, inhibitory synaptogenesis can be increased over 100%. This suggests an effect of neuronal activity beyond the release of neurotrophins, such as augmentation of the sensitivity of TrkB receptors to activation by specific ligands. Thus the activity state of the neuron may not only govern the release of neurotrophins, but may also define the neurotrophic effect.
Conclusions that can be drawn from this series of studies are that 1) neuronal activity is necessary for the full development or reconstruction of inhibitory circuitry, 2) TrkB receptor ligands have a role in inhibitory synaptogenesis and 3) signaling for inhibitory synaptogenesis is via the TrkB receptor. Neuronal activity does not appear to be a requirement for excitatory synaptogenesis, as the full complement of parallel fiber-Purkinje cell dendritic spine synapses developed in cerebellar cultures in the absence of neuronal activity. Also not dependent on neuronal activity are 1) increased survival of Purkinje cells in the absence of granule cells and the subsequent reduction of Purkinje cells after the reintroduction of granule cells, 2) plastic changes such as recurrent axon collateral sprouting or reduction and 3) neuron-glia interactions such as myelination, astrocytic ensheathment of Purkinje cells and stripping of excess Purkinje cell axosomatic synapses. An exception is the reduction of excess heterotypical axospinous synapses upon reintroduction of granule cells and glia to cultures depleted of these elements. Astrocytes appear to be involved in heterotypical axospinous synapse reduction, but by some other mechanism than direct stripping, as with axosomatic synapses, and this mechanism appears to be at least partially activity-dependent.
What we have shown with these experiments is that there are at least three ways of promoting inhibitory synaptogenesis, including 1) addition of exogenous TrkB receptor ligands, 2) increasing release of endogenous TrkB receptor ligands by activity and 3) activating the TrkB receptor by binding with antibody. As to the possible clinical significance of these studies, the necessity of neuronal activity for the development or restoration of inhibitory circuitry may be critical for recovery of function after such injurious processes as stroke or CNS trauma, in which the balance of excitatory and inhibitory elements may be disrupted. Activity is known to promote functional recovery in both experimental (Nudo et al., 1996) and clinical (Dimyan and Cohen, 2011) conditions. The finding that the effects of activity appear to be mediated via TrkB receptor ligands may have implications with regard to therapy for CNS injury. Recent animal studies have shown that infusion of BDNF or measures that enhance BDNF after appropriate intervals following induction of experimental stroke improve functional recovery (Clarkson et al, 2011; Ploughman et al., 2009; Schäbitz et al., 2004). Further developments may extend such therapeutic possibilities to human cases of stroke and trauma, including spinal cord injury, some forms of epilepsy, degenerative disorders and still other neurological diseases.
Elimination of granule cells and glia induced cerebellar circuit reorganization.
Replacement of the missing elements resulted in restitution of the normal circuitry.
Most of the reconstructive changes were not dependent on neuronal activity.
Inhibitory synapses did not develop fully in the absence of neuronal activity.
Inhibitory synaptogenesis could be promoted by activation of TrkB receptors.
Acknowledgments
This review was not intended as a review of the literature, but as a cohesive presentation of studies related to the subject of neuroplasticity performed in my laboratory over a span of more than 30 years. The references are primarily to our own work, with reference to the work of others to add perspective to our studies. To enhance readability for a more general audience, I have limited the number of micrographs and electrophysiological traces and emphasized diagrammatic illustrations, even to the exclusion of electron micrographs, and refer those interested in the more technical details to the published papers. The work was done with a large number of collaborators, and their names appear as co-authors in the references to the original papers, some with recurring frequency. I would especially like to acknowledge my late colleagues, Arnold L. Leiman for his electrophysiological studies and Nathan K. Blank for his electron microscopic contributions. Both played critical roles in the design and performance of this work. I am also grateful to Robert M. Herndon (electron microscopy) and Rosemarie Drake-Baumann (electrophysiology) for their participation in these studies, along with the many other investigators who were involved, as well as a host of technical contributors. I am indebted to the United States Department of Veterans Affairs and to the National Institutes of Health for research support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Bakkum BW, Cohen RS. Gamma-aminobutyric acid and somastatin immunoreactivity in the visual cortex of normal and dark reared rats. Brain Res. 1995;689:172–182. doi: 10.1016/0006-8993(95)00553-3. [DOI] [PubMed] [Google Scholar]
- Blank NK, Seil FJ. Mature Purkinje cells in cerebellar tissue cultures. J Comp Neurol. 1982;208:169–176. doi: 10.1002/cne.902080206. [DOI] [PubMed] [Google Scholar]
- Blank NK, Seil FJ. Reorganization in granuloprival cerebellar cultures after transplantation of granule cells and glia. II. Ultrastructural studies. J Comp Neurol. 1983;214:267–278. doi: 10.1002/cne.902140305. [DOI] [PubMed] [Google Scholar]
- Blank NK, Seil FJ, Herndon RM. An ultrastructural study of cortical remodeling in cytosine arabinoside induced granuloprival cerebellum in tissue culture. Neuroscience. 1982;7:1509–1531. doi: 10.1016/0306-4522(82)90261-5. [DOI] [PubMed] [Google Scholar]
- Blank NK, Seil FJ, Leiman AL. Climbing fibers in mouse cerebellum co-cultured with inferior olive. Brain Res. 1983;271:135–140. doi: 10.1016/0006-8993(83)91373-2. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Ann Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011;31:3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–563. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Nieto-Sampedro M, Harris EW. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981;61:684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Corner MA, Ramakers GJA. Spontaneous firing as an epigenetic factor in brain development – physiological consequences of chronic tetrodotoxin and picrotoxin exposure to cultured rat neocortex neurons. Develop Brain Res. 1992;65:57–64. doi: 10.1016/0165-3806(92)90008-k. [DOI] [PubMed] [Google Scholar]
- Crain SM, Bornstein MB, Peterson ER. Maturation of cultured embryonic tissue during chronic exposure to agents which prevent bioelectric activity. Brain Res. 1968;8:363–372. doi: 10.1016/0006-8993(68)90055-3. [DOI] [PubMed] [Google Scholar]
- Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nature Rev Neurol. 2011;7:76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake-Baumann R, Seil FJ. Electrophysiological differences between Purkinje cells in organotypic and granuloprival cerebellar cultures. Neuroscience. 1995;69:467–476. doi: 10.1016/0306-4522(95)00263-i. [DOI] [PubMed] [Google Scholar]
- Drake-Baumann R, Seil FJ. Influence of functional glia on the electrophysiology of Purkinje cells in organotypic cerebellar cultures. Neuroscience. 1999;88:507–519. doi: 10.1016/s0306-4522(98)00229-2. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentágothai J. The Cerebellum as a Neuronal Machine. Springer; New York: 1967. [Google Scholar]
- Eng LF, Reier PJ, Houle JD. Astrocyte activation and fibrous gliosis: glial fibrillary acidic protein immunostaining of astrocytes following intraspinal cord grafting of fetal CNS tissue. In: Seil FJ, Herbert E, Carlson BM, editors. Neural Regeneration in Brain Research. Vol. 71. Amsterdam: Elsevier; 1987. pp. 439–455. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Liem RKH, Shelanski ML, Mason CA. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- Hendry SAC, Jones EG. Reduction in number of immunostained GABAergic neurons in deprived-eye dominance columns of monkey area 17. Nature. 1986;320:750–753. doi: 10.1038/320750a0. [DOI] [PubMed] [Google Scholar]
- Hendry SAC, Jones EG. Activity-dependent regulation of GABA expression in the visual cortex of adult monkeys. Neuron. 1988;1:701–712. doi: 10.1016/0896-6273(88)90169-9. [DOI] [PubMed] [Google Scholar]
- Herndon RM, Seil FJ, Seidman C. Synaptogenesis in mouse cerebellum: a comparative in vivo and tissue culture study. Neurosci. 1981;6:2587–2598. doi: 10.1016/0306-4522(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170:311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. Raven Press; New York: 1984. [Google Scholar]
- Jones MZ, Gardner E. The pathogenesis of methtlazoxymethanol-induced lesions in the postnatal mouse cerebellum. J Neuropathol Exp Neurol. 1976;35:413–444. doi: 10.1097/00005072-197607000-00004. [DOI] [PubMed] [Google Scholar]
- Kim SU. Weaver cerebellum and cytosine arabinoside-treated normal mouse cerebellum: a tissue culture study of unattached Purkinje cell dendritic spines. J Neuropathol Exp Neurol. 1977;36:610. [Google Scholar]
- Leiman AL, Seil FJ. Spontaneous and evoked bioelectric activity in organized cerebellar tissue cultures. Exp Neurol. 1973;40:748–759. doi: 10.1016/0014-4886(73)90109-x. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Ann Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lindsay RM. Neurotrophins and receptors. In: Seil FJ, editor. Neural Regeneration Progress in Brain Research. Vol. 103. Amsterdam: Elsevier; 1994. pp. 3–14. [PubMed] [Google Scholar]
- Llinás R, Hillman DE, Precht W. Neuronal circuit reorganization in mammalian agranular cerebellar cortex. J Neurobiol. 1973;4:69–94. doi: 10.1002/neu.480040106. [DOI] [PubMed] [Google Scholar]
- Lynch G, Gall C, Rose G, Cotman CW. Changes in the distribution of the dentate gyrus associational system following unilateral or bilateral entorhinal lesion in the adult rat. Brain Res. 1976;110:57–71. doi: 10.1016/0006-8993(76)90208-0. [DOI] [PubMed] [Google Scholar]
- Meisami E, Monsavi R. Lasting effects of early olfactory deprivation on the growth, DNA, RNA and protein content, and Na-K-ATPase and AchE activity of the olfactory bulb. Develop Brain Res. 1981;2:217–229. doi: 10.1016/0165-3806(81)90033-x. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Seil FJ. Transplanted astrocytes reduce synaptic density in the neuropil of cerebellar cultures. Brain Res. 1988;441:23–32. doi: 10.1016/0006-8993(88)91379-0. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Seil FJ, Herndon RM. Astrocytes play a role in regulation of synaptic density. Brain Res. 1987;402:139–145. doi: 10.1016/0006-8993(87)91056-0. [DOI] [PubMed] [Google Scholar]
- Michaeva KD, Beaulieu C. An anatomical substrate for experience dependent plasticity of the rat barrel field cortex. Proc Natl Acad Sci USA. 1995;92:11834–11838. doi: 10.1073/pnas.92.25.11834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model PG, Bornstein MB, Crain SM, Pappas GD. An electron microscopic study of the development of synapses in cultured fetal mouse cerebrum continuously exposed to xylocaine. J Cell Biol. 1971;49:362–371. doi: 10.1083/jcb.49.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller HW, Matthiesen HP, Schmalenbach C. Astroglial factors supporting neurite growth and long-term neuronal survival. In: Seil FJ, editor. Advances in Neural Regeneration Research Neurology and Neurobiology. Vol. 60. New York: Wiley-Liss; 1990. pp. 147–159. [Google Scholar]
- Murphy JT, Sabah NH. Cerebellar Purkinje cell responses to afferent inputs. I. Climbing fiber activation. Brain Res. 1971;25:449–467. doi: 10.1016/0006-8993(71)90454-9. [DOI] [PubMed] [Google Scholar]
- Murray MR. Nervous Tissues in Vitro. In: Willmer EN, editor. Cells and Tissues in Culture Methods, Biology and Physiology. Vol. 2. New York: Academic Press; 1965. pp. 373–455. [Google Scholar]
- Nelson PG, Fields RD, Yu C, Neale EA. Mechanisms involved with activity-dependent synapse formation in mammalian central nervous system cultures. J Neurobiol. 1990;21:138–156. doi: 10.1002/neu.480210110. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milikan GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Naturally occurring cell death during neural development. Trends Neurosci. 1985;8:487–493. [Google Scholar]
- Palay SL, Chan Palay V. Cerebellar Cortex. Springer; New York: 1974. [Google Scholar]
- Parks TN. Afferent influences on the development of the brainstem auditory nuclei of the chicken: otocyst ablation. J Comp Neurol. 1979;183:665–678. doi: 10.1002/cne.901830313. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Berardi N, Rossi FM, Viegi A, Venstrom K, Reichardt LF, Maffei L. Trk A activation in the rat visual cortex by antirat trkA IgG prevents the effect of monocular deprivation. Eur J Neurosci. 1999;11:204–212. doi: 10.1046/j.1460-9568.1999.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- Poduslo SE, Miller K, Wolinsky JS. The production of a membrane by purified oligodendroglia maintained in culture. Exp Cell Res. 1982;137:203–215. doi: 10.1016/0014-4827(82)90021-0. [DOI] [PubMed] [Google Scholar]
- Privat A, Drian MJ. Postnatal maturation of rat Purkinje cells cultivated in the absence of two afferent systems: an ultrastructural study. J Comp Neurol. 1976;166:201–243. doi: 10.1002/cne.901660207. [DOI] [PubMed] [Google Scholar]
- Raisman G, Field PM. A quantitative investigation of the development of collateral reinnervation after partial deafferentation of the septal nuclei. Brain Res. 1973;71:1–16. doi: 10.1016/0006-8993(73)90729-4. [DOI] [PubMed] [Google Scholar]
- Ramakers GJA, Corner MA, Habets AMMC. Development in the absence of spontaneous bioelectric activity results in increased stereotype burst firing in cultures of dissociated cerebral cortex. Exp Brain Res. 1990;79:157–166. doi: 10.1007/BF00228885. [DOI] [PubMed] [Google Scholar]
- Ramakers GJA, Corner MA, Habets AMMC. Abnormalities in the spontaneous firing patterns of cultured rat neocortical neurons after chronic exposure to picrotoxin during development in vitro. Brain Res Bull. 1991;26:429–432. doi: 10.1016/0361-9230(91)90018-f. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. In: Studies on Vertebrate Neurogenesis. Guth L, translator. Thomas; Springfield, Ill: 1960. [Google Scholar]
- Rudge JS, Manthorpe M, Varon S. The output of neuronotrophic and neurite-promoting agents from rat brain astroglial cells: a microculture method for screening potential regulatory molecules. Develop Brain Res. 1985;19:161–172. doi: 10.1016/0165-3806(85)90188-9. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäbitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiesling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- Schilling K, Dickinson MH, Connor JA, Morgan JI. Electrical activity in cerebellar cultures determines Purkinje cell dendritic growth patterns. Neuron. 1991;7:891–902. doi: 10.1016/0896-6273(91)90335-w. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Neuronal groups and fiber patterns in cerebellar tissue cultures. Brain Res. 1972;42:33–51. doi: 10.1016/0006-8993(72)90040-6. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Cerebellum in Tissue Culture. In: Schneider DM, editor. Reviews of Neuroscience. Vol. 4. New York: Raven Press; 1979. pp. 105–177. [Google Scholar]
- Seil FJ. Enhanced Purkinje cell survival in granuloprival cerebellar cultures. Develop Brain Res. 1987;35:312–316. doi: 10.1016/0165-3806(87)90057-5. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Neuronal rescue in cerebellar cultures. In: Gorio A, Perez Polo JR, de Vellis J, Haber B, editors. Cellular and Molecular Aspects of Neural Development and Regeneration, NATO ASI Series. H22. New York: Springer; 1988. pp. 429–438. [Google Scholar]
- Seil FJ. Persistence of heterotypical synapses in transplanted cerebellar cultures in the absence of functional glia. Int J Develop Neurosci. 1994;12:411–421. doi: 10.1016/0736-5748(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Neural plasticity in cerebellar cultures. Prog Neurobiol. 1996;50:533–556. doi: 10.1016/s0301-0082(96)00044-5. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Cerebellar culture models of dendritic spine proliferation after transplantation of glia. J Neural Transplant Plast. 1997a;6:1–10. doi: 10.1155/NP.1997.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seil FJ. Serial changes in granuloprival cerebellar cultures after transplantation with granule cells and glia: A timed ultrastructural study. Neuroscience. 1997b;77:695–711. doi: 10.1016/s0306-4522(96)00546-5. [DOI] [PubMed] [Google Scholar]
- Seil FJ. The extracellular matrix molecule, laminin, induces Purkinje cell dendritic spine proliferation in granule cell depleted cerebellar cultures. Brain Res. 1998;795:112–120. doi: 10.1016/s0006-8993(98)00265-0. [DOI] [PubMed] [Google Scholar]
- Seil FJ. BDNF and NT-4, but not NT-3, promote development of inhibitory synapses in the absence of neuronal activity. Brain Res. 1999;818:561–564. doi: 10.1016/s0006-8993(98)01304-3. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Interactions between cerebellar Purkinje cells and their associated astrocytes. Histol Histopathol. 2001a;16:955–968. doi: 10.14670/HH-16.955. [DOI] [PubMed] [Google Scholar]
- Seil FJ. Signaling for activity-dependent inhibitory synaptogenesis via the TrkB receptor. Exp Neurol. 2001b;171:422–424. doi: 10.1006/exnr.2001.7741. [DOI] [PubMed] [Google Scholar]
- Seil FJ. TrkB receptor signaling and activity-dependent inhibitory synaptogenesis. Histol Histopathol. 2003;18:635–646. doi: 10.14670/HH-18.635. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Blank NK. Myelination of central nervous system axons in tissue culture by transplanted oligodendrocytes. Science. 1981;212:1407–1408. doi: 10.1126/science.7233230. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Blank NK, Leiman AL. Toxic effects of kainic acid on mouse cerebellum in tissue culture. Brain Res. 1979;161:253–265. doi: 10.1016/0006-8993(79)90067-2. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. Reduced cortical inhibitory synaptogenesis in organotypic cerebellar cultures developing in the absence of neuronal activity. J Comp Neurol. 1994;342:366–377. doi: 10.1002/cne.903420305. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. Circuit reorganization in granuloprival cerebellar cultures in the absence of neuronal activity. J Comp Neurol. 1995;356:552–562. doi: 10.1002/cne.903560406. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. Circuit reorganization in Ara C treated cerebellar cultures chronically exposed to picrotoxin. Int J Develop Neurosci. 1996a;14:45–54. doi: 10.1016/0736-5748(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. Activity-dependent changes in “transplanted” cerebellar cultures. Exp Neurol. 1996b;138:327–337. doi: 10.1006/exnr.1996.0071. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J Neurosci. 2000;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R, Herndon RM, Leiman AL. Cytosine arabinoside effects in mouse cerebellar cultures in the presence of astrocytes. Neuroscience. 1992a;51:149–158. doi: 10.1016/0306-4522(92)90479-l. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R, Leiman AL, Herndon RM, Tiekotter KL. Morphological correlates of altered neuronal activity in organotypic cultures chronically exposed to anti-GABA agents. Develop Brain Res. 1994;77:123–132. doi: 10.1016/0165-3806(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Eckenstein FP, Reier PJ. Induction of dendritic spine proliferation by an astrocyte secreted factor. Exp Neurol. 1992b;117:85–89. doi: 10.1016/0014-4886(92)90114-6. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Herndon RM. Cerebellar granule cells in vitro: A light and electron microscopic study. J Cell Biol. 1970;45:212–220. doi: 10.1083/jcb.45.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seil FJ, Herndon RM. Myelination and glial ensheatment of Purkinje cells in cerebellar cultures are not inhibited by antibodies to the neural cell adhesion molecule, NCAM. Int J Develop Neurosci. 1991;9:587–596. doi: 10.1016/0736-5748(91)90020-m. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Herndon RM, Tiekotter KL, Blank NK. Reorganization of organotypic cultures of mouse cerebellum exposed to cytosine arabinoside: A timed ultrastructural study. J Comp Neurol. 1991;313:193–212. doi: 10.1002/cne.903130202. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Johnson ML, Saneto RP, Herndon RM, Mass MK. Myelination of axons within Ara C treated mouse cerebellar explants by cultured rat oligodendrocytes. Brain Res. 1989;503:111–117. doi: 10.1016/0006-8993(89)91710-1. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Leiman AL. Spontaneous versus driven activity in intracerebellar nuclei: A tissue culture study. Exp Neurol. 1977;54:110–127. doi: 10.1016/0014-4886(77)90239-4. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Leiman AL. Development of spontaneous and evoked electrical activity of cerebellum in tissue culture. Exp Neurol. 1979;64:61–75. doi: 10.1016/0014-4886(79)90005-0. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Leiman AL. Cerebellum plus locus coeruleus in tissue culture: I. Catecholamine histofluorescence and extracellular electrophysiology. Develop Brain Res. 1985;17:165–170. doi: 10.1016/0165-3806(85)90140-3. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Leiman AL, Blank NK. Reorganization in granuloprival cerebellar cultures after transplantation of granule cells and glia. I. Light microscopic and electrophysiological studies. J Comp Neurol. 1983;214:258–266. doi: 10.1002/cne.902140304. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Leiman AL, Woodward WR. Cytosine arabinoside effects on developing cerebellum in tissue culture. Brain Res. 1980;186:393–408. doi: 10.1016/0006-8993(80)90984-1. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Tiekotter KL, Woodward WR. Catecholamine fiber sprouting in granuloprival coeruleo-cerebellar cultures. Develop Brain Res. 1985;21:251–255. doi: 10.1016/0165-3806(85)90213-5. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Woodward WR. Differential effects of granule cell transplantation on two sprouted axonal systems in granuloprival coeruleocerebellar cultures. Develop Brain Res. 1988;43:153–157. doi: 10.1016/0165-3806(88)90161-7. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Woodward WR, Blank NK, Leiman AL. Evidence against chronic depolarization as a mechanism of kainic acid toxicity in mouse cerebellar cultures. Brain Res. 1978;159:431–435. doi: 10.1016/0006-8993(78)90553-x. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Neurotrophins and visual system plasticity. In: Cowan WM, Jessell TM, Zipursky SL, editors. Molecular and Cellular Approaches to Neural Development. New York: Oxford; 1997. pp. 509–524. [Google Scholar]
- Shaw C, Cynander M. Disruption of cortical activity prevents ocular dominance changes in monocularly deprived kittens. Nature. 1984;308:731–734. doi: 10.1038/308731a0. [DOI] [PubMed] [Google Scholar]
- Simons DJ, Land PW. Early experience of tactile stimulation influences organization of somatic sensory cortex. Nature. 1987;326:694–697. doi: 10.1038/326694a0. [DOI] [PubMed] [Google Scholar]
- Sutula T, Xiao-Xian H, Cabazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tsukahara N, Hultborn H, Murakami F, Fujito Y. Electrophysiological study of formation of new synapses and collateral sprouting in red nucleus neurons after partial denervation. J Neurophysiol. 1975;38:1359–1372. doi: 10.1152/jn.1975.38.6.1359. [DOI] [PubMed] [Google Scholar]
- Tweedle CD, Hatton GI. Synapse formation and disappearance in adult rat supraoptic nucleus during different hydration states. Brain Res. 1984;309:373–376. doi: 10.1016/0006-8993(84)90607-3. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Saperstein SK, Christoperson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Van Huizen F, Romijn HJ, Habets AMMC. Synaptogenesis in rat cerebral cortex cultures is affected during chronic blockade of spontaneous bioelectric activity by tetrodotoxin. Develop Brain Res. 1985;19:67–80. doi: 10.1016/0165-3806(85)90232-9. [DOI] [PubMed] [Google Scholar]
- Van Huizen F, Romijn HJ, van den Hoof P, Habets AMMC. Picrotoxin induced disinhibition of spontaneous bioelectric activity accelerates synaptogenesis in rat cerebral cortex cultures. Exp Neurol. 1987;97:280–288. doi: 10.1016/0014-4886(87)90089-6. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Woodward WR, Blank NK, Seil FJ. Choline acetyl transferase activity in mouse cerebellar cultures. Brain Res. 1982;241:323–327. doi: 10.1016/0006-8993(82)91070-8. [DOI] [PubMed] [Google Scholar]
- Woodward WR, Seil FJ, Hammerstad JP. Cerebellum plus locus coeruleus in tissue culture: II. Development and metabolism of catecholamines. J Neurosci Res. 1987;17:184–188. doi: 10.1002/jnr.490170214. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Yamano T, Shimada M, Nakao K, Nakamura T, Wakaizumi S, Kusunoki T. Maturation of Putkinje cells in mouse cerebellum after neonatal administration of cytosine arabinoside. Acta Neuropathol (Berlin) 1978;44:41–45. doi: 10.1007/BF00691637. [DOI] [PubMed] [Google Scholar]


