Abstract
Background
Fibrogenic pathways in the liver are principally regulated by hepatic stellate cells (HSC) which produce and respond to fibrotic mediators such as connective tissue growth factor (CCN2). The aim of this study was to determine whether CCN2 is shuttled between HSC in membraneous nanovesicles, or “exosomes”.
Methods
Exosomes were incubated with HSC after isolation from conditioned medium of control or CCN2-GFP-transfected primary mouse HSC or human LX-2 HSC. Some exosomes were flourescently stained with PKH26. HSC co-culture experiments were performed in the presence of GW4869 exosome inhibitor. CCN2 or CCN2-GFP were evaluated by qRT-PCR or Western blot.
Results
HSC-derived exosomes contained CCN2 or CCN2 mRNA, each of which increased in concentration during HSC activation or after transfection of HSC with CCN2-GFP. Exosomes, stained with either PKH26 or purified from CCN2-GFP-transfected cells, were taken up by activated or quiescent HSC resulting in CCN2-GFP delivery, as shown by their direct addition to recipient cells or by the GW4869-dependency of donor HSC.
Conclusions
CCN2 is packaged into secreted nano-sized exosomes which mediate its intercellular transfer between HSC. Exosomal CCN2 may amplify or fine-tune fibrogenic signaling and may, in conjunction with other exosome constituents, have utility as a noninvasive biomarker to assess hepatic fibrosis.
Keywords: CTGF; CCN; hepatic fibrosis; chronic liver disease; nanovesicle, intercellular communication
Introduction
Approximately 5.5 million American adults (i.e. 2-3% of the adult US population) suffer from chronic liver disease or cirrhosis. Together with viral hepatitis and hepatic cancer, these liver diseases collectively constitute one of the ten leading causes of death in the USA. Fibrosis is a common and debilitating pathology in many chronic liver diseases that hinders effective treatment and heightens the need for liver transplantation. A key pathophysiological event in liver injury is the activation of hepatic stellate cells (HSC), a process by which these normally quiescent cells become highly proliferative, express alpha-smooth muscle actin (α-SMA) which confers contractility and promotes would closure, and synthesize and deposit a provisional collagen matrix which promotes hepatocyte re-population 1-3. During chronic liver injury, the activated HSC phenotype persists and collagen is unrelentingly deposited into the interstitial spaces, ultimately compromising normal hepatic function 4, 5.
Recent evidence has shown that connective tissue growth factor (CCN2) tightly orchestrates fibrogenic pathways in activated HSC. CCN2 is over-expressed during liver fibrosis, with activated HSC accounting for the majority of its production 6. Further, through its interactions with cell surface binding partners, CCN2 promotes many of the differentiated functions of HSC including mitogenesis, chemotaxis, adhesion, activation and matrigenesis 6. Inhibitors of CCN2 dampen fibrogenic pathways in cultured HSC and are anti-fibrotic in models of experimental liver fibrosis in vivo 7. FG-3019, a humanized anti-CCN2 monoclonal antibody is currently in Phase 2 trials for liver fibrosis in hepatitis B patients (NCT01217632).
It has recently emerged that many cell constituents, including mRNA, microRNA (miR) and proteins, may be exported from cells within membraneous nanovesicles (approx. 50-150nm in diameter) termed “exosomes” 8. Exosomes are formed by inward budding of the limiting membranes of multivesicular bodies and are liberated extracellularly upon fusion of the multivesicular bodies with the plasma membrane. While exosomes may enter body fluids such as blood, urine or saliva, they have become recognized as conduits for possible delivery of their molecular “payload” to other cells 8. We recently showed that exosomes are in fact produced by human or mouse HSC and are responsible for intercellular shuttling of miR-214, which is a regulator of CCN2-dependent fibrogenesis in HSC 9. In this report, we show that CCN2 mRNA or protein are also present in HSC-derived exosomes and that exosomal CCN2 is intercellularly shuttled between HSC. The identification of CCN2 in exosomes suggests a novel mechanism for the extracellular transport and delivery to target cells of this important pro-fibrogenic molecule and raises the possibility that exosomal levels of CCN2 and other fibrosis-related molecules might have utility as biomarkers of fibrosis in chronic liver disease.
Materials and Methods
Isolation and characterization of exosomes from mouse or human HSC
Exosomes were isolated from conditioned medium of serum-starved passage 5-10 mouse HSC9 or the human LX-2 cell line 10, 11 and characterized by electron microscopy, expression of key markers and size-range, as described 8, 9. In some experiments, purified exosomes were either fluorescently stained with PKH26 (Sigma-Aldrich, St. Louis, MO)12 or purified from cells that had been transfected with CCN2- green fluorescent protein (GFP) or CD9-GFP plasmids 9, 12, 13.
RNA extraction, reverse transcription, and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA from cell or exosomal lysates from HSC or LX-2 cells was extracted using a microRNeasy Plus kit (Qiagen, Valencia, CA) and was reverse transcribed using a miScript II RT kit (Qiagen) according to the manufacturer’s protocols. Resulting transcripts were analyzed by qRT-PCR as previously described 9. Primers for CCN2, GFP, glyceraldyhyde-3-phosphate-dehydrogenase (GAPDH) were as previously described 9 and U6 primers were purchased from Qiagen.
Immunocytochemistry
HSC or LX-2 cells were either seeded on coverslips or in 8-well chamber slides and incubated in the presence or absence of PKH26-stained exosomes from activated HSC or LX-2 cells. After 2-24 hours, cells were fixed in 2% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked at room temperature, and incubated for 1 hour at room temperature with polyclonal chicken anti-human CCN2 14, polyclonal mouse anti-human α-SMA (Thermo Fischer Scientific, Pittsburgh, PA) or polyclonal chicken anti-green fluorescent protein (GFP; Abcam, Cambridge, MA). Slides were then washed, incubated for 1 hour at room temperature with Alexa Fluor goat anti-chicken 488 or goat anti-mouse 647 (Life Technologies, Carlsbad, CA), mounted using ProLong gold anti-fade reagent with DAPI (Life Technologies) and examined by confocal laser microscopy (LSM710, Carl Zeiss Co. Ltd, Jena, Germany).
HSC co-culture system
Two-well silicone micro-culture devices (Ibidi Inc., Verona, WI, USA) were used as described 9. One well contained control or CCN2-GFP-transfected 12, 13 donor human LX-2 cells which were cultured with 0 or 10 g/ml GW4869 (Sigma-Aldrich), an inhibitor of exosome production in various cells, including HSC 9, 15, 16. The other well contained control LX-2 cells as recipients. After 24 hours, the silicone wall separating donor cells from recipient cells was excised, the medium was replaced, and cells were allowed to directly communicate for the following 48 hours, after which donor or recipient cells were imaged for direct GFP fluorescence.
Western blot
Western blot analysis was performed on lysates of (i) exosomes isolated from conditioned medium of control or CCN2-GFP- transfected HSC; (ii) recipient HSC that were pre-treated for 2 hrs with 0. 0.5, or 1.5 g/ml cycloheximide (a dose-range that inhibits protein synthesis but is non-toxic for primary HSC17) and then incubated with the same concentrations of cycloheximide for an additional 2 or 6 hrs in the presence of exosomes from CCN2-GFP- or CD9-GFP-transfected donor HSC; or (iii) recipient LX-2 cells incubated for 2-24 hrs with exosomes from CCN2-GFP-transfected LX-2 cells . Samples were electrophoresed in polyacrylamide gels, and transferred to nitrocellulose membranes which were then blocked for 30 minutes in PBS containing 3% non-fat dry milk, and incubated overnight with rabbit anti-CCN2 (1:1000) 14, or polyclonal chicken anti-GFP (1:1000; Abcam). Membranes were incubated with goat-anti rabbit or goat-anti chicken horse radish peroxidase (1:2,000; Cell Signaling, Boston, MA or 1:3,000; Santa Cruz, Dallas, TX) for 1 hour at room temperature and developed using an Amersham ECL prime chemiluminescent detection kit (GE Life Sciences, Pittsburg, PA).
Results
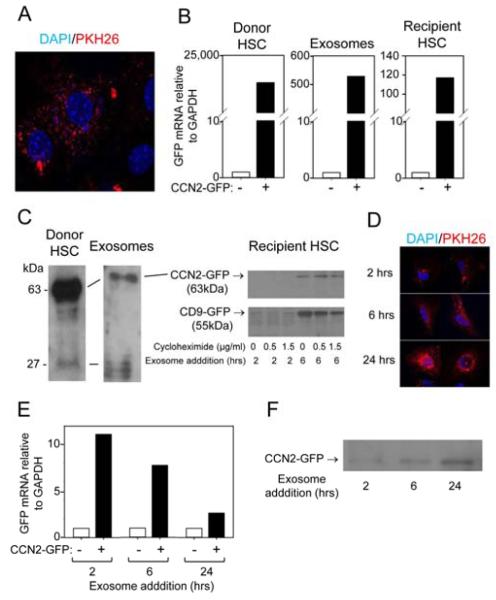
Primary mouse HSC became activated when cultured for up to 2 weeks as demonstrated by increased CCN2, collagen type 1 and α-SMA production as we have previously reported 9, 11 (data not shown). We investigated whether CCN2 was a component of the exosomes (50-150nm, CD9- and flotillin-positive, negatively-charged bi-membrane vesicles) that we recently showed to be secreted into the conditioned medium by HSC 9. As assessed by qRT-PCR, CCN2 mRNA was present in exosomes, in which its concentration progressively increased as the cells underwent progressive autonomous activation during the 14-day culture period (Figure 1A). In addition, as assessed by Western blot, exosomes also contained CCN2 protein, the levels of which increased as a function of activation status of the donor cells (Figure 1B). Exosomal CCN2 was detected as the intact full-length 36-38kDa protein on Days 3-9 and, additionally, as 16-20kDa forms on Day 9 (Figure 1B) which were likely stable proteolytic products comprising the C-terminal half of the molecule previously that retain biological activity 18, 19 (Figure 1B). Similar CCN2 proteins have been detected in HSC lysates and are increasingly produced during HSC activation 20. These various CCN2 proteins persisted in exosomes from highly activated (passage 6 (P6)) mouse HSC (Figure 1C).
Figure 1. CCN2 mRNA and protein are present in mouse HSC exosomes.
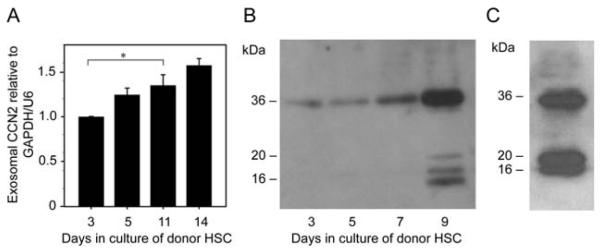
(A) Quantitative RT-PCR of CCN2 mRNA in exosomes isolated from primary mouse HSC on Day 3-14 of culture. Data are from three independent experiments and expressed as mean ± s.e.m. *P<0.05 as determined by Student’s t-test using SIGMA PLOT 11.0 software (SPSS Inc., Chicago, IL). (B) Western blot for CCN2 in exosomes isolated from 24-hour conditioned medium from individual T-75 flasks containing primary mouse HSC on days 3-9 of culture (5 g total exosomal protein/lane). (C) CCN2 Western blot of exosomes isolated from P5 mouse HSC. Data are representative of three independent determinations.
Uptake by P6 HSC of PKH26-stained exosomes isolated from other P6 HSC was documented by fluorescence microscopy (Figure 2A), confirming our previously published independent evidence for exosomal uptake by HSC that was established by exosome-dependent alterations in miR target activity in recipient HSC 9. To determine if exosomal CCN2 or CCN2 mRNA were delivered to recipient HSC, mouse primary HSC were transfected with a vector that expresses a CCN2-GFP fusion protein 13 so that exosomal CCN2 could be followed by the presence of GFP. This approach was necessary for tracking CCN2 delivery to activated HSC because their endogenous CCN2 mRNA and protein levels are already very high rendering it difficult to detect or discriminate delivery of their exosomal counterparts. GFP mRNA, as assessed by qRT-PCR, was detected in the transfected donor HSC, their secreted exosomes and in HSC recipients after 24-hr incubation with these exosomes, with appreciable diminution of the intensity of the mRNA signal across this donor-exosome-recipient axis (Figure 2B). Further, the presence of CCN2-GFP in the donor HSC, exosomes and recipient HSC was demonstrated by Western blot (Figure 2C). The accumulation of exosomal CCN2-GFP in the cells followed the same time-course as that of exosomal CD9-GFP (CD9 is a tetraspanin commonly used as a murine exosomal marker protein9), as assessed by addition of exosomes purified from CD9-GFP-transfected HSC: in both cases, the respective GFP fusion proteins were detectable in the recipient HSC after 6 hours (but not after 2 hours) of being incubated with exosomes. CCN2-GFP immunoreactivity at 6 hours was unaffected by the protein translation inhibitor, cycloheximide, suggesting it most likely represented directly-delivered exosomal CCN2-GFP as opposed to having been synthesized in the recipient cells from the delivered exosomal CCN2-GFP transcript. These experiments confirmed that increased CCN2 production by the donor cells was reflected in their increased exosomal CCN2 content, and that CCN2 protein and mRNA were exosomally transferred to other HSC.
Figure 2. Uptake of exosomal CCN2-GFP by mouse HSC or human LX-2 cells.
(A) Uptake of PKH26-labeled HSC-derived exosomes by activated HSC after 24 hours. PKH26 is in red and DAPI nuclear stain is in blue. Data are representative of five independent experiments. (B) Quantitative RT-PCR of GFP mRNA in donor HSC transfected with a CCN2-GFP fusion vector (left), exosomes isolated from CCN2-GFP-transected HSC (center) or recipient HSC after incubation with the exosomes for 24 hours (right). (C) Western blotting with anti-GFP to detect CCN2-GFP in lysates of CCN2-GFP-transfected donor HSC (left), CCN2-GFP in their secreted exosomes (center), or CCN2-GFP or CD9-GFP in lysates of recipient cells incubated with CCN2-GFP- or CD9-GFP-containing exosomes for 2 or 6 hours in the presence of absence of cylcoheximide (right). (D) Uptake by LX-2 cells of PKH26-labeled LX-2 cell-derived exosomes. Data are representative of 4 independent experiments. (E) qRT-PCR for GFP mRNA in LX-2 cells incubated for 2-24 hours with exosomes isolated from CCN2-GFP-transected LX-2 cells. (F) Western blotting with anti-GFP to detect CCN2-GFP in lysates of recipient LX-2 cells incubated for 2-24 hours with CCN2-GFP-containing LX-2 cell-derived exosomes.
To confirm that exosomal CCN2 transfer was evolutionary conserved and to validate the translational aspects of our findings, we next turned our attention to LX-2 cells, an immortalized HSC line of human origin that has been used extensively by many investigators for analysis of fibrogenic mechanisms 10. We have previously reported the isolation of exosomes from conditioned medium of LX-2 cell cultures 9 and, after staining them with PKH26 dye, they were shown to be progressively taken up by other LX-2 cells between 2 and 24 hours after addition (Figure 2D). Analysis of GFP transcript expression in recipient LX-2 cells after addition of exosomes recovered from the medium of CCN2-GFP-transfected LX-2 cells confirmed that CCN2-GFP mRNA was exosomally delivered and expressed at its the highest level (11-fold increase) within 2 hours of exosome addition (Figure 2E). Thereafter, transcript expression gradually declined but, even so it was still elevated by 3-fold after 24 hours (Figure 2E). Whether the diminished GFP mRNA signal in the recipient cells reflected its degradation by the cells due it being a “foreign” transcript or whether this is a general phenomenon for all such delivered mRNAs will require further study. Western blot analysis showed that CCN2-GFP protein levels in the cells gradually increased between 2 and 24 hours after exosome addition (Figure 2F), although the relative contribution of direct exosomal protein transfer versus translation from the delivered (albeit declining) CCN2-GFP transcript remains to be determined.
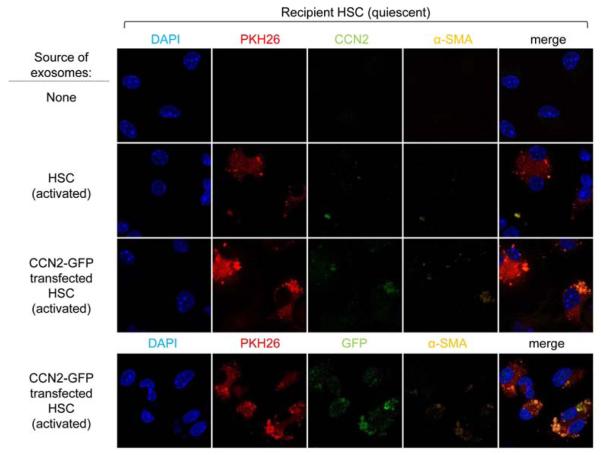
In the preceding experiments, exosomal communication was shown between donor and recipient HSC that were both in a highly activated state. We therefore next investigated whether CCN2 in exosomes from activated mouse HSC could be delivered to quiescent mouse HSC. In contrast to control Day 2 HSC, addition for 24 hours of PKH26-stained exosomes isolated from activated HSC resulted in the detection of PKH26 and weak but detectable staining for both CCN2 and α-SMA, a marker of HSC activation (Figure 3). In an alternative approach, Day 2 HSC were incubated with PKH26-stained exosomes that had been collected from CCN2-GFP-transfected activated HSC. After 24 hours, exosome uptake was confirmed by the presence of PKH26 in the recipient cells, which showed somewhat greater levels of immunofluorescence for CCN2 or α-SMA, consistent with enhanced CCN2 levels in exosomes from transfected cells (see Figure 2B). Since detection of CCN2 in recipient cells was technically challenging and might have resulted indirectly from the delivery of CCN2-inducing signals into recipient HSC by the exosomes from activated donor HSC, recipient cells were further analyzed for the presence of GFP which was detected at appreciable levels, thus confirming that CCN2-GFP was indeed exosomally transferred (Figure 3). While the possibility that increased levels of α-SMA (which appeared structurally disorganized) in recipient HSC was due to exosomal delivery of inductive signals other than CCN2 - or even direct delivery of exosomal α-SMA itself - these data nonetheless show that CCN2 was exosomally transferred from activated to quiescent HSC.
Figure 3. Exosomal delivery of CCN2-GFP to Day 2 mouse HSC.
PKH26 fluorescence and CCN2, GFP or α-SMA immunofluorescence in Day 2 HSC receiving no exosomes, exosomes from control activated HSC, or exosomes from CCN2-GFP-transfected activated HSC.
Finally, to show that CCN2 was transferred between neighboring cell in situ, rather than as a result of adding purified exosomes directly to the HSC cultures, a co-culture system was adopted using a similar approach as previously described by us 9. CCN2-GFP-transfected donor LX-2 cells were incubated in the presence or absence of the exosome inhibitor GW4869 prior to being allowed to communicate for 48 hours with control non-transfected recipient LX-2 cells. By this time, approximately 30% of donor cells were GFP-positive whether treated with GW4869 or not (Figure 4A). On the other hand, a lower frequency - but nonetheless detectable level – of GFP-positivity was detected among the recipient cells and this was reduced by ~55% by treatment of the donor cells with GW4869 (Figure 4B). Thus, CCN2 was proven to be exosomally shuttled between neighboring HSC.
Figure 4. Exosomal communication between neighboring human LX-2 cells.
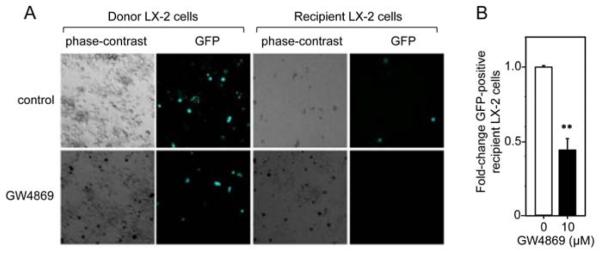
(A) Direct GFP fluorescence in donor LX-2 cells over-expressing CCN2-GFP or in co-cultured recipient LX-2 cells incubated in the presence or absence of 10 M GW4869 for 48 hours. (B) Quantification of total GFP-positive recipient cells in (A) assessed in triplicate wells from 3 independent experiments **p< 0.001.
Discussion
Activation of HSC during chronic injury triggers sustained fibrogenic pathways in the cells, a process that results in excessive collagen production and one that is highly CCN2-dependent. Although CCN2 has emerged as a possible target of anti-fibrotic therapy, the mechanisms regulating the production and action of this molecule need to be more fully understood so that rational targeting strategies can ultimately be employed. In this study, we discovered that CCN2 is packaged into exosomes produced by activated HSC and that CCN2 can be exosomally delivered to other quiescent or activated HSC. Exosomes thus allow the mRNA and protein for this important fibrogenic signaling molecule to be protected as they traverse the potentially hostile extracellular environment. Although CCN2 rapidly associates with elements of the matrix in the extracellular environment and functions matricellularly 21, 22, our identification of CCN2 in exosomes reveals a novel mechanism by which it is extracellularly transported and delivered to target cells.
The biological significance of “horizontal transfer” of CCN2 between HSC will require further study and, while it may result in the promotion of fibrogenic pathways in the recipient cells, exosome exchange or communication may serve as a feedback mechanism by which cellular surveillance and responses are fine-tuned and properly orchestrated within the context of fibrosing liver injury. Moreover, changes in recipient cells to exosomes containing increased concentrations of CCN2 (e.g. elevated α-SMA levels; see Figure 3) might alternatively reflect CCN2-mediated changes in gene transcription and translation in the donor cells that were subsequently manifested in their secreted exosomes, rather than reflecting a response of recipient cells to exosomal CCN2 itself. These various questions will be addressed in future studies. Nonetheless, functional exosomal transfer of miR-214 between HSC, with direct inhibition of miR-214 on CCN2 gene activity in the recipient cells, was recently shown by us 9. While HSC-derived exosomes thus contain CCN2 mRNA and miR-214, the relative levels of each molecule are dynamically and inversely expressed in exosomes and reflect their relative expression levels in the HSC from which they are secreted. Since autocrine or paracrine regulation has also been documented for the molecular payloads delivered by exosomes that are produced by hepatic epithelial cells (cholangiocytes, hepatocytes, hepatocarcinoma cells) 23-25, exosomes are emerging as important biological conduits for protecting and shuttling regulatory molecules that can affect a myriad of signaling events among multiple hepatic cell types. Further, CCN2 is exosomally transferred between pancreatic stellate cells12, suggesting common mechanisms of CCN2 extracellular shuttling in the liver and pancreas.
Currently, the various stages of hepatic fibrosis are difficult to accurately diagnose because of the inaccuracy and risks associated with needle biopsies as well the lack of routine laboratory tests that are 100% reliable. Moreover, while emerging imaging approaches (e.g. ultrasound-based transient elastography or magnetic resonance imaging) have gained traction for assessment of advanced fibrosis and cirrhosis 26, accurate detection of the early stages of fibrosis has remains elusive despite the potential value of such information for early intervention or evaluating efficacy of therapy. Since exosomes contain mRNAs, miRs and proteins, they hold promise as platforms for biomarker discovery, especially as their molecular payloads are likely highly contextual and reflective of the overall transcriptional and translational status of their respective producer cells. Even so, this field is in its infancy and exosomes have yet to be systematically studied in the context of fibrosing liver disease. That said, our finding that CCN2 is elevated in HSC-derived exosomes as a function of HSC activation well as our previous report that exosomal miR-214 is decreased during HSC activation and during experimental liver fibrosis in mice 9 highlights the potential value of complete “global” profiling of exosomal miRs, mRNAs and proteins with the goal of identifying a slate of predictive or diagnostic biomarkers for the various stages of fibrosis. This approach, if focused on circulating or urinary exosomes, would allow development of much-needed minimally-or non-invasive assessments of liver fibrosis that would also be highly amenable for serial determinations in the same patient to accurately assess disease progression or regression.
In conclusion, CCN2 is packaged by activated HSC into secreted nano-sized exosomes which mediate its intercellular transfer to other quiescent or activated HSC. Exosomal delivery of CCN2 may result in amplification or fine tuning of fibrogenic signaling in response to chronic liver injury. RNA and protein profiling holds promise for identifying a panel of exosomal components, possibly including CCN2, that will have utility as reliable biomarkers for noninvasive assessment of fibrosis in chronic liver disease.
Acknowledgments
Financial support: NIH grant R01 AA021276 awarded to D.R.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 9th Annual Academic Surgical Congress in San Diego, CA, February 4-6, 2014
References
- 1.Eng FJ, Friedman SL, Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiol Gastrointest Liver Physiol. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 2.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–50. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 3.Gaca MD, Zhou X, Benyon RC. Regulation of hepatic stellate cell proliferation and collagen synthesis by proteinase-activated receptors. J Hepatol. 2002;36:362–9. doi: 10.1016/s0168-8278(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Hepatic fibrosis -- overview. Toxicology. 2008;254:120–9. doi: 10.1016/j.tox.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–69. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci. 2012;17:2495–507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 7.Brigstock DR. Strategies for blocking the fibrogenic actions of connective tissue growth factor (CCN2): From pharmacological inhibition in vitro to targeted siRNA therapy in vivo. J Cell Commun Signal. 2009;3:5–18. doi: 10.1007/s12079-009-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3:15. doi: 10.3410/B3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, et al. Epigenetic regulation of connective tissue growth factor by microRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Charrier AL, Leask A, French SW, Brigstock DR. Ethanol-stimulated differentiated functions of human or mouse hepatic stellate cells are mediated by connective tissue growth factor. J Hepatol. 2011;55:399–406. doi: 10.1016/j.jhep.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell Commun Signal. 2014 Jan 26; doi: 10.1007/s12079-014-0220-3. Epub ahead of print. DOI 10.1007/s12079-014-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshijima M, Hattori T, Aoyama E, Nishida T, Yamashiro T, Takigawa M. Roles of heterotypic CCN2/CTGF-CCN3/NOV and homotypic CCN2-CCN2 interactions in expression of the differentiated phenotype of chondrocytes. FEBS J. 2012;279:3584–97. doi: 10.1111/j.1742-4658.2012.08717.x. [DOI] [PubMed] [Google Scholar]
- 14.Charrier AL, Brigstock DR. Connective tissue growth factor production by activated pancreatic stellate cells in mouse alcoholic chronic pancreatitis. Lab Invest. 2010;90:1179–88. doi: 10.1038/labinvest.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–91. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–52. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynaert H, Rombouts K, Jia Y, Urbain D, Chatterjee N, Uyama N, et al. Somatostatin at nanomolar concentration reduces collagen I and III synthesis by, but not proliferation of activated rat hepatic stellate cells. Br J Pharmacol. 2005;146:77–88. doi: 10.1038/sj.bjp.0706298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ball DK, Surveyor GA, Diehl JR, Steffen CL, Uzumcu M, Mirando MA, et al. Characterization of 16- to 20-kilodalton (kDa) connective tissue growth factors (CTGFs) and demonstration of proteolytic activity for 38-kDa CTGF in pig uterine luminal flushings. Biol Reprod. 1998;59:828–35. doi: 10.1095/biolreprod59.4.828. [DOI] [PubMed] [Google Scholar]
- 19.Steffen CL, Ball-Mirth DK, Harding PA, Bhattacharyya N, Pillai S, Brigstock DR. Characterization of cell-associated and soluble forms of connective tissue growth factor (CTGF) produced by fibroblast cells in vitro. Growth Factors. 1998;15:199–213. doi: 10.3109/08977199809002117. [DOI] [PubMed] [Google Scholar]
- 20.Williams EJ, Gaca MD, Brigstock DR, Arthur MJ, Benyon RC. Increased expression of connective tissue growth factor in fibrotic human liver and in activated hepatic stellate cells. J Hepatol. 2000;32:754–61. doi: 10.1016/s0168-8278(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–83. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 23.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol. 2010;299:G990–9. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–48. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q, Ramakrishnaiah V, Henry S, Fouraschen S, de Ruiter PE, Kwekkeboom J, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi) Gut. 2012;61:1330–9. doi: 10.1136/gutjnl-2011-300449. [DOI] [PubMed] [Google Scholar]
- 26.Sangwaiya MJ, Sherman DI, Lomas DJ, Shorvon PJ. Latest developments in the imaging of fibrotic liver disease. Acta Radiol. 2013 doi: 10.1177/0284185113510159. [DOI] [PubMed] [Google Scholar]