Abstract
The biological actions of the endocannabinoids anandamide and 2-arachidonoyl glycerol (2-AG) are terminated by enzymatic hydrolysis of these lipids via fatty acid amide hydrolase (FAAH ) and monoacylglycerol lipase (MAGL), respectively. While several selective FAAH inhibitors have been developed and characterized in vitro and in vivo, none of the initial MAGL blockers have shown adequate potency and specificity for in vivo applications. More recently, a selective MAGL inhibitor, JZL184, has been shown to produce a long-lasting elevation of brain 2-AG, as well as cannabinoid-like behavioral responses in mice. However, its effectiveness in rats remains controversial. Indeed, although JZL184 can elicit behavioral responses that are mediated, at least in part, via activation of cannabinoid CB1 receptors, several reports indicate that this compound does not alter 2-AG levels in this species. In this study we compared the behavioral and neurochemical effects of JZL 184 with those of the dual FAAH/MAGL inhibitor JZL195, and showed that systemic administration of the former can selectively elevate brain 2-AG in rats and produce motor suppression through a CB1-independent mechanism. These findings indicate that, despite its lower potency against rat MAGL, JZL184 can be used to enhance 2-AG transmission and elicit behavioral responses in rodents.
Keywords: anandamide, 2-arachidonoyl glycerol, Cannabinoid, CB1, gas chromatography/mass spectrometry, motor activity
Introduction
The endocannabinoids are neuromodulatory signaling molecules implicated in a large range of biological processes including cognition, analgesia, stress, anxiety, neuroprotection, and motor coordination, to name a few (Di Marzo, 2008; Giuffrida et al., 1999; Hohmann et al., 2005; Marsicano et al., 2002; Piomelli, 2008). The most studied endocannabinoids, N-arachidonoyl ethanolamine (anandamide, AEA) and 2-arachidonoyl glycerol (2-AG), are primarily synthesized on demand following an increase of intracellular calcium or neuronal depolarization (Piomelli, 2005). Endocannabinoids activate two G-protein coupled cannabinoid receptors (CB1 and CB2), but they can also target other non-CB1/CB2 receptors, showing a complex pharmacological profile (Di Marzo and De Petrocellis, 2010; O’Sullivan, 2007).
The biological actions of AEA and 2-AG are terminated by a two-step process including their reuptake into neuronal and glial cells followed by intracellular hydrolysis. While the existence of an active reuptake remains controversial (Fegley et al., 2004; Fu et al., 2012; McFarland et al., 2004), the catabolic enzymes responsible for AEA and 2-AG degradation – fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively – have been extensively characterized (Cravatt et al., 1996; Dinh et al., 2002). In addition to AEA, FAAH hydrolyzes monoacyl glycerols in vitro (Goparaju et al., 1998) and possibly participates in the inactivation of 2-AG (Di Marzo et al., 1998) together with the serine hydrolases ABHD6 and ABHD12 (Marrs et al., 2010; Savinainen et al., 2012). Nevertheless, several lines of evidence indicate that MAGL is the primary enzyme responsible for 2-AG degradation (Blankman et al., 2007; Dinh et al., 2004; Long et al., 2009a).
These findings have led to the development of selective inhibitors that are used as pharmacological tools to manipulate AEA and 2-AG signaling independently and to study their possible interactions (King et al., 2007; Saario et al., 2005). None of the initial MAGL inhibitors, however, showed adequate potency and specificity for in vivo applications (Long et al., 2009c). More recently, Long et al. developed a dual FAAH/MAGL inhibitor, named JZL195 (Long et al., 2009c), and a selective MAGL blocker, JZL184, which caused a rapid and long-lasting elevation of 2-AG in mouse brain without affecting AEA content (Long et al., 2009a). The same lab also developed new MAGL inhibitors - i.e. KML29 (Chang et al., 2012) and MJN110 (Niphakis et al., 2013) - showing enhanced activity and selectivity as compared to those of JZL184.
Microdialysis studies carried out in rat nucleus accumbens (NAc) have shown that JZL195 elevates both AEA and 2-AG, whereas JZL184 has no effect on endocannabinoid output (Wiskerke et al., 2012). In agreement with its known lower activity against rat MAGL (Long et al., 2009b), other groups have shown unchanged 2-AG levels in rat CNS following systemic or local administration of JZL184 (Kerr et al., 2013; Woodhams et al., 2012). Despite the lack of neurochemical effects, JZL-184 produces behavioral effects mediated via activation of CB1 receptors (Long et al., 2009a; Woodhams et al., 2012). Thus, these observations indicate that JZL184 effects in rats might be dose-dependent and/or area-specific, leading to 2-AG elevation only in those brain regions where this lipid is produced on demand.
To address this question, we compared the effects of JZL195 and JZL184 in rats, focusing on locomotor activity – a behavior typically affected by cannabinoids – and on endocannabinoid transmission in cortical-, striatal- and hippocampal-like brain areas.
Materials and methods
Drugs
JZL184 and JZL195 were a gift from Dr. J. Long (Scripps Research Institute, San Diego); SR141716A (synthesized at the Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD) was a gift from Dr. McMahon at the University of Texas Health Science Center at San Antonio (UTHSCSA); Tween-80 and polyethylene glycol (PEG) were purchased from Sigma/RBI (St Louis, MO), AM251 was from Tocris (Ellisville, MO); methanol, chloroform, water, hexane from Honewell/Burdick & Jackson (Muskegon, MI); [2H5]-2-arachidonoyl glycerol (2-AG-d5) from Cayman Chemicals (Ann Arbor, MI); BSTFA from Supelco (Bellefonte, PA) and saline solution from Hospira, Inc. (Lake Forest, IL). [2H4]-anandamide (AEA-d4) and [2H4]-oleylethanolamide (OEA-d4) were synthesized in the lab as previously described (Hardison et al., 2006).
Animals
All experiments were carried out according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the IACUC of UTHSCSA. Male Wistar rats (200-225g; Charles River Laboratories, USA) were housed at 22±1°C, under a 12-h light/dark cycle with food and water available ad libitum. Upon arrival, animals were habituated to the housing conditions for 1 week before the experimental testing, which was carried out during the light period of the cycle.
Drug treatments and behavioral tests
Animals were transferred into the experimental room in their own home cages and then placed in the ActiMot Activity Measuring System (version 6.07, TSE Systems GmbH, Bad Homburg, Germany) 1 hour after receiving an acute intraperitoneal (i.p.) injection of vehicle (Tween-80/PEG/saline, 10/10/80, 1 ml/kg), or either JZL195 or JZL184 (5, 15 and 30 mg/kg, respectively) to measure their horizontal (distance travelled) and vertical (number of rearings) motor activity in a novel environment for 60 min. Dose ranges were selected based on previous pilot experiments (data not shown). Rearing was detected automatically by measuring the number of beam breakings on the Z axis. To investigate the pharmacological mechanisms underlying JZL195- and JZL184-induced effects on motor activity, a second cohort of rats received an acute injection of vehicle (controls) or the CB1 antagonists AM251 (1 mg/kg, i.p.) or SR141716A (1 mg/kg, i.p.) immediately before administration of an effective dose of JZL195 (15 mg/kg, i.p.) or JZL184 (30 mg/kg, i.p.). Animals were not habituated to the experimental room containing the Actimot System and were placed into the System 1 hour after drug administration to record their horizontal and vertical locomotor activity as described above. All experiments were carried out in the morning.
Endocannabinoid measurements
Animals were anaesthetized with halothane (by inhalation) immediately after the behavioral assessment (2 h after drug injection) and their brains were rapidly collected, frozen in 2-methylbutane (−45°C) and stored at −80°C until use. Frozen brains were placed on a stainless-steel mould (Roboz; Rockville, USA) and sliced into 1-mm coronal sections using razor blades, from which the nucleus accumbens (NAc), hippocampus (Hip), prefrontal cortex (PFC) and caudateputamen (CP) were dissected out (5-20 mg of wet tissue). Tissue samples were spiked with 50 pmol of [2H4]-anandamide (AEA-d4), [2H4]-oleylethanolamide (OEA-d4) and [2H5]-2-arachidonoylglycerol (2-AG-d5) (internal standards) and processed for gas chromatography/mass spectrometry (GC/MS), as previously described (Hardison et al., 2006). Lipids were extracted by adding methanol/chloroform/water (1:2:1, v/v/v), and the chloroform layer was further purified by solid phase extraction using C18 Bond Elut cartridges (100 mg; Varian, USA). Endocannabinoid-containing fractions were dried under a stream nitrogen, derivatized in BSTFA (30 μl) for 30 min at room temperature and analyzed with a TraceDSQ system (Thermo Electron; San Jose, CA) equipped with an Rtx-5MS column (15 m × 0.25 mm; Restek; Bellefonte, PA) using a previously published isotope dilution assay (Hardison et al., 2006).
The following ions were used for selected ion monitoring (SIM) analyses: AEA, m/z 420.3 ([M + H]+ ); AEA-d4, m/z 424.3 ([M + H]+); 2-AG, m/z 433.3 ([M + H − 18]+); 2-AG-d5, m/z 438.3 ([M + H −18]+); OEA, m/z 398.0 ([M + H]+); OEA-d4, m/z 402.0 ([M + H]+). Peak areas were obtained by manual integration using the DSQ Xcalibur software. AEA, OEA and 2-AG concentrations were reported as mean ± S.D.
Statistical analyses
The time course of the JZL compounds was analyzed by two-way ANOVA with Treatment (4 levels) as between subject factor and Time (10 min intervals) as repeated measures (for JZL195 and JZL184), followed by the Newman-Keuls test for post-hoc comparisons.
Other behavioral and neurochemical data were analyzed by one-way ANOVA with Treatment (4 levels) as independent factor for each inhibitor, followed by the Dunnett test for post-hoc comparisons when required. The experiments with the CB1 antagonists were analyzed first by two-way ANOVA with Drug (vehicle, AM251, SR141716A) and Treatment (vehicle, JZL195, JZL184) as between-subject factors, and subsequently by three-way ANOVA with Drug (vehicle, AM251, SR141716A), and Treatment (vehicle, JZL195, JZL184) as between-subject factors, and Time (10 min intervals) as repeated measures. Both analyses were followed by the Newman-Keuls test for post-hoc comparisons. The level of significance was set at p<0.05.
Results
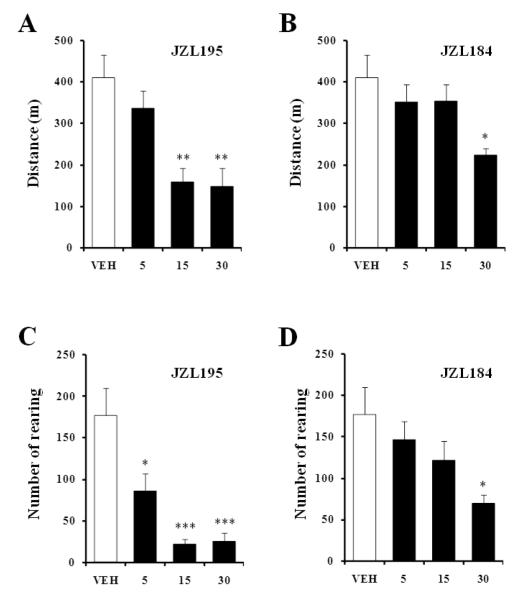
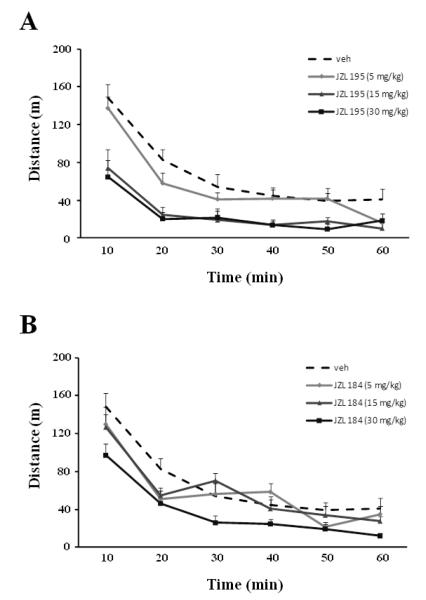
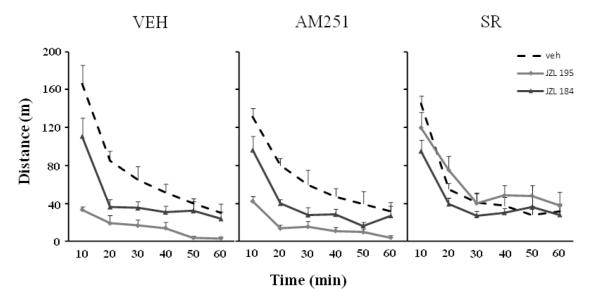
Systemic administration of JZL195 or JZL184 reduced horizontal (Fig. 1A, F3,22=8.18, p<0.001; Fig. 1B, F3,21=3.46, p<0.05, respectively) and vertical (Fig. 1C, F3,20=11.17, p<0.001; Fig. 1D, F3,22=3.39, p<0.05, respectively) motor activity dose-dependently, and increased time spent in immobility (data not shown). In contrast to JZL195, which decreased motor activity at all doses tested, JZL184 was effective only at the highest dose (30 mg/kg, p<0.05). The motor effects of both JZL compounds are also presented as time course over a 60 min period (Fig. 2).
Fig. 1.
Dose response of the effects of systemic (i.p.) administration of JZL195 (A, C) and JZL184 (B, D) on horizontal (travelled distance) and vertical (number of rearings) activity in a novel environment. Values are expressed as mean ± S.E.M. (n=6-7/group). VEH, vehicle (empty bars); escalating doses of drugs (filled bars). * p<0.05, ** p<0.01, and *** p<0.001 compared to vehicle control.
Fig. 2.
Time course of the effects of systemic (i.p.) administration of JZL195 (A) and JZL184 (B) on horizontal (travelled distance) activity in the same experimental groups used in figure 1. Values are expressed as mean ± S.E.M. (n=6-7/group). Treatment effects: F3,22=8.18, p<0.001 (for JZL 195); F3,21=3.46, p<0.05 (for JZL184).
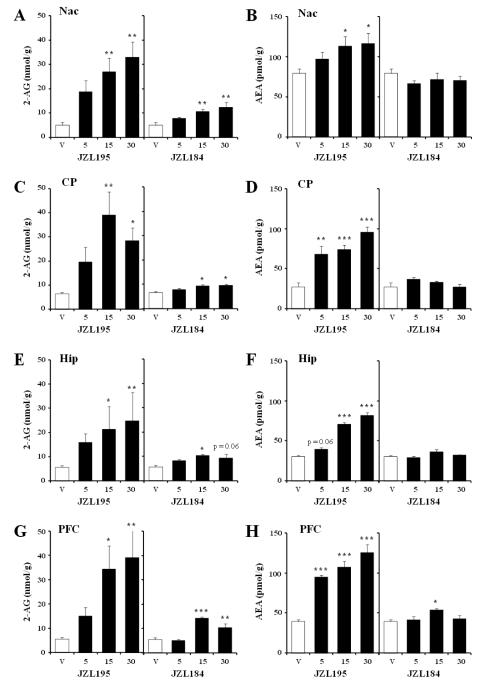
Immediately after completion of the behavioral test (1 h after drug injection), animals were sacrificed to assess the ability of JZL195 and JZL184 to elevate AEA and 2-AG in nucleus accumbens (NAc), caudate-putamen (CP), hippocampus (Hip), and prefrontal cortex (PFC). As a control for inhibitory activity of the JZL compounds at FAAH, we also measured the FAAH substrate N-oleyl ethanolamine (OEA) in the same brain areas.
JZL195 induced a significant increase (4.5-7 fold) of 2-AG levels at the doses of 15 and 30 mg/kg in all the regions tested (Fig. 3A,C,E,G: F3,21=5.47, p<0.01; F3,17=5.76, p<0.01; F3,19=5.10, p<0.01; F3,21=4.79, p<0.05, for NAc, CP, Hip and PFC, respectively). In agreement with its ability to also block FAAH activity, JZL195 increased AEA levels at the doses of 15 and 30 mg/kg in all brain areas, whereas the dose of 5 mg/kg was active in the CP and PFC only (Fig. 3B,D,F,H: F3,21=3.05, p=0.051; F3,19=17.20, p<0.001; F3,21=91.54, p<0.001; F3,19=33.33, p<0.001; for NAc, CP, Hip and PFC, respectively). JZL195 also elevated OEA in all brain areas investigated (Table 1; F3,19=8.53, p<0.001; F3,19=9.67, p<0.001; F3,20=5.46, p<0.01; F3,18=8.97, p<0.001; for NAc, CP, Hip and PFC, respectively).
Fig. 3.
Effects of JZL195 and JZL184 on 2-AG and AEA levels in Nac (A,B), CP (C,D), Hip (E,F) and PFC (G,H). Values are expressed as mean ± S.E.M. The doses are reported below the bars (n=5-7/group). V, vehicle (empty bars); escalating doses of drugs (filled bars). * p<0.05, ** p<0.01, and *** p<0.001 compared to vehicle control.
Table 1.
Effects of JZL195 and JZL184 on OEA levels.
| vehicle | JZL195 | JZL184 | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 5 mg/kg | 15 mg/kg | 30 mg/kg | 5 mg/kg | 15 mg/kg | 30 mg/kg | ||
| NAc | 102.2 ± 3.4 | 271.9 ± 22.3* | 231.4 ± 16.6* | 220.1 ± 28.3* | 93.9 ± 4.7 | 88.1 ± 4.2 | 89.1 ± 9.9 |
| CPu | 177.0 ± 8.9 | 354.1 ± 53.0* | 334.0 ± 18.4* | 371.5 ± 18.7* | 162.7 ± 5.8 | 179.6 ± 5.5 | 172.1 ± 4.9 |
| Hip | 141.2 ± 17.1 | 242.4 ± 26.9* | 278.7 ± 27.2* | 235.8 ± 17.0* | 118.2 ± 5.6 | 136.7 ± 11.3 | 163.8 ± 11.5 |
| mPFC | 232.3 ± 9.5 | 314.7 ± 23.4* | 253.9 ± 13.8 | 341.0 ± 16.7* | 230.8 ± 18.6 | 236.2 ± 32.5 | 233.8 ± 11.9 |
Each value represents the mean ± SEM of OEA levels (pmol/g).
p<0.05 compared to vehicle.
Abbreviations: NAc, nucleus accumbens; CPu, caudate putamen; Hip, hippocampus; mPFC, medial prefrontal cortex.
JZL184 produced approximately a 2-fold increase of 2-AG at the highest doses in all areas with the exception of the Hip, where only the dose of 15 mg/kg was effective (Fig. 3A,C,E,G: F3,19=5.86, p<0.01; F3,19=4.42, p<0.05; F3,21=3.61, p<0.05; F3,20=19.24, p<0.001; for NAc, CP, Hip and PFC, respectively). Unlike JZL195, JZL184 had no effect on either AEA (Fig. 3B, D, F, H: F3,21=0.82, NS;, F3,19=1.66, NS; F3,19=1.91, NS; for NAc, CP, and Hip, respectively) or OEA levels (Table 1; F3,18=1.24, NS, F3,21=1.19; NS, F3,19=2.75, NS; F3,19=0.01, NS; for NAc, CP, Hip and PFC, respectively), with the exception of the PFC where the dose of 15mg/kg elevated AEA (F3,19=3.10, p=0.051).
To investigate possible relationships between brain endocannabinoid levels and motor behavior, we performed correlations on the data described above in each experimental group. In JZL195-treated animals, 2-AG levels in Nac, CP, Hip and PFC were inversely correlated to motor activity (r2= 0.19, p=0.027; r2=0.18; p=0.056; r2=0.39, p=0.0014; r2=0.18, p=0.037, respectively). Similarly, a significant inverse correlation between travelled distance and AEA levels was observed in CP (r2=0.33; P=0.0044), Hip (r2=0.50, p=0.0001) and PFC (r2=0.34, p=0.0033), but not in Nac (r2=0.01, p=0.58).
On the other hand, in JZL184-treated animals, locomotor activity was not correlated with 2-AG levels in any brain area, with the exception of the CP, where we found a significant inverse correlation (r2=0.23; P=0.02). No significant correlations were observed between OEA levels and motor activity following administration of either JZL compounds, with the exception of a significant inverse correlation between CP levels and travelled distance in JZL195-treated rats (r2=0.24; P=0.017).
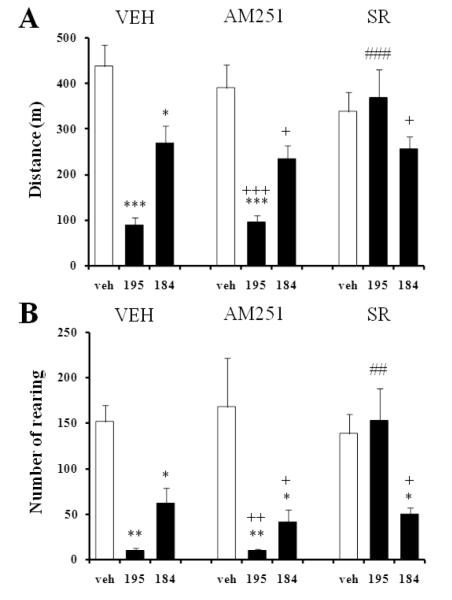
Given the lack of correlation between 2-AG levels and the motor effects of JZL184, we conducted an independent pharmacological study to assess whether the suppression of locomotor activity observed with both JZL compounds was CB1 dependent. Pretreatment with an effective dose of the selective CB1 antagonist AM251 (1 mg/kg, i.p.) did not affect the horizontal (Fig. 4A) or vertical (4B) JZL195-induced hypomotility, whereas administration of the CB1 antagonist SR141716A (1 mg/kg, i.p.) completely reversed the JZL195 effect (Fig. 4A,B). By contrast, neither AM251 nor SR141716A antagonized the motor effects of JZL184 given at its highest dose (30 mg/kg, i.p.). Figure 5 illustrates the effects of both CB1 antagonists on the hypomotility induced by the JZL compounds over a 60 min period.
Fig. 4.
AM251 and SR141716A (SR) differentially affect horizontal (A) and vertical (B) hypomotility induced by JZL195 (15 mg/kg) and JZL184 (30 mg/kg) (filled bars). V, vehicle (for AM251/SR); VEH, vehicle (for JZL, empty bars). Values are expressed as mean ± S.E.M. (n=7/group). * p<0.05, ** p<0.01, and *** p<0.001 compared to vehicle control (same pretreatment); ## p<0.01, ### p<0.001 compared to vehicle pretreated-group (same treatment); + p<0.05, ++ p<0.01, and +++ p<0.001 compared to vehicle-vehicle control.
Fig. 5.
Time course of the effects of AM251 and SR141716A (SR) on horizontal hypomotility induced by JZL195 (15 mg/kg) and JZL184 (30 mg/kg). Values are expressed as mean ± S.E.M. (n=7/group). VEH, vehicle for CB1 antagonists; veh, vehicle for JZL compounds. Interaction between Drug and Treatment: F4,54=7,27, p<0.001.
Discussion
In this study we compared the behavioral and neurochemical effects of the endocannabinoid inactivation inhibitors JZL 195 and JZL 184 in rats.
Both compounds produced hypolocomotion dose-dependently, although the effect of JZL184 was observed only at the highest dose tested. JZL195 produced a pronounced 2-AG elevation at all doses, and increased brain AEA and OEA levels due to its inhibitory activity at FAAH. On the other hand, JZL184 elevated 2-AG levels only, although with lower potency than JZL195 and without affecting the other two N-acyl ethanolamines. Although we cannot rule out possible endocannabinoid enhancing effects induced by either JZL compounds in different brain areas than those considered in this study (PFC, Nac/CP and Hip), these regions were selected as representative examples of cortical-, striatal- and hippocampal-like brain areas, respectively, with the intent to obtain a more comprehensive idea of the global effects of these drugs in the rather than focusing only on areas traditionally involved in motor behaviors (Nac/CP).
The enhancing effects of JZL195 on brain 2-AG and AEA are consistent with previous reports showing the ability of this drug to elevate both endocannabinoids in mouse brain (Long et al., 2009a) and rat dialysates from Nac (Wiskerke et al., 2012), and to produce cannabinoid-like behavioral effects (Long et al., 2009c). On the other hand, while JZL184 has been shown to selectively increase 2-AG in mice (Long et al., 2009a), no conclusive findings have been obtained in rats. Indeed, a systemic dose of 10 mg/kg can produce a near-complete blockade of MAGL activity in mice, resulting in a 7-fold elevation in brain homogenates (Long et al., 2009a) and in a 3-fold increase of depolarization-induced interstitial 2-AG (Wiskerke et al., 2012). By contrast, JZL184 failed to alter 2-AG levels in Wistar rats even under depolarizing conditions (Wiskerke et al., 2012). Nevertheless, other groups have reported a two-fold increase of 2-AG in rat VTA after i.v. administration (Oleson et al., 2012), as well as behavioral responses, such as anxiolytic-like effects (Sciolino et al., 2011) and anti-inflammatory properties in vivo (Kerr et al., 2013). In agreement with these studies, we found that JZL184 produced a significant inhibition of motor activity at the dose of 30 mg/kg and, more importantly, it elevated 2-AG at the highest doses (15 and 30 mg/kg), whereas it had no effect at 5mg/kg. These observations indicate that JZL184 has lower potency in rats than mice, which is in line with the 10-fold reduced activity against rat MAGL reported in vitro (Long et al., 2009b). Thus, the lack of JZL184 effect reported by Wiskerke and coworkers in rats may be attributed to the low dose (10 mg/kg vs 30 mg/kg) or the different vehicle (ethanol/emulphor/saline vs Tween-80/PEG/saline) used in their study. Interestingly, the effectiveness of JZL184 in rats also appears to be strain-dependent. Indeed, while we did not observe motor effects with low doses of JZL184 (5-15 mg/kg) in Wistar rats, recent reports have shown behavioral effects at similar low doses (8-10 mg/kg) in Sprague-Dawley rats (Sciolino et al., 2011). Nevertheless, our observations suggest that newer MAGL inhibitors, such as KML29 and MJN110, which have recently shown excellent in vivo activity and selectivity in rats, negligible cross reactivity with FAAH and compatibility with acute and chronic dosing in rodents (Chang et al., 2012; Niphakis et al., 2013), may be better options than JZL184 when conducting pharmacological studies in this species. In this regard, strong antihyperalgesic activity has been observed in vivo in a rat model of diabetic neuropathy following MJN110 systemic administration (Niphakis et al., 2013).
To assess the selectivity of JZL184 for MAGL versus FAAH, in addition to AEA we tested the possible actions of this drug on brain OEA, another well-known substrate of FAAH. As in the study of Long et al. (2009), in which mouse OEA levels were increased only by the highest dose of JZL184 (40 mg/kg) possibly due to a partial inhibition of FAAH activity (Long et al., 2009a), we did not observe elevation of this N-acylethanolamine in rats, confirming the selectivity of this drug for brain MAGL.
Our data build on previous experiments showing similar, as well as conflicting, pharmacological effects of FAAH and MAGL inhibitors when tested in behavioral paradigms affected by cannabinoid drugs. In rodents, direct activation of cannabinoid CB1 receptors induces distinct behavioral responses collectively referred to as the “tetrad test” (hypomotility, analgesia, hypothermia and catalepsy) (Wiley and Martin, 2002). Interestingly, enhancement of AEA tone via administration of FAAH inhibitors has been shown to produce analgesia in several pain models {Long, 2009 #4230; Russo, 2007 #4233; Kinsey, 2009 #4231, but not other typical cannabinoid-like effects (Lichtman et al., 2004; Stewart and McMahon, 2011). Administration of the dual inhibitor JZL195 in mice exhibited broad activity in the tetrad test, as well as THC-like responses in the drug discrimination test, which were both mediated via CB1 receptors (Long et al., 2009c). In agreement with these studies, JZL195 administration reduced locomotion in rats, which was inversely correlated with 2-AG and AEA levels in all the brain areas tested and dependent on CB1 receptor activation. The observation that only the CB1 antagonist SR141716A – but not AM251 - blocked the JZL195 effect is in line with previous studies carried out in rats showing lack of effect of AM251 in reversing the anxiogenic response induced by the cannabinoid agonist WIN-55,212 (Haller et al., 2007). This phenomenon might be attributed to the ability of AM251 to inhibit CB1-induced responses that are predominantly dependent on GABAergic, rather than glutamatergic, transmission (Haller et al., 2007; Pistis et al., 2004).
On the other hand, injection of JZL184 at its highest dose (30 mg/kg) produced a significant suppression of locomotor activity that was not reversed by either SR141716A, or AM251, even though both antagonists were given at doses known to produce behavioral effects in rats (Seillier et al., 2013). In addition, we found no correlations between locomotor activity and 2-AG levels in all the brain areas examined, with the exception of the CP. These observations confirm previous study carried out by Long et al. (2009) in mice showing that JZL184 induced 2-AG elevation and hypomotility (Long et al., 2009a). In their work, however, these researchers were not able to determine the contribution of CB1 receptors to JZL184-induced hypolocomotion, as SR141716A alone also caused motor inhibition at the dose tested (3 mg/kg, i.p.), a phenomenon that we also observed in our rats following administration of a similar dose of rimonabant (data not shown). In agreement with these studies, Lichtman et al. showed that systemic injection of 2-AG in mice produces hypolocomotion and behavioral effects indicative of cannabinoid-like activity in a CB1-independent fashion (Lichtman et al., 2002). In addition, previous experiments carried out with the putative MAGL inhibitor N-arachidonyl maleimide (NAM) showed that this drug enhanced 2-AG-induced tetrad effects, which were only partially reversed by SR141716A (Burston et al., 2008). Although these observations indicate that JZL184-mediated motor responses may occur via 2-AG-mediated stimulation of non-CB1 receptors, JZL184 failed to inhibit locomotor activity in CB1 knockout mice (Long et al., 2009a), suggesting that the motor suppressive effects of 2-AG do require the concomitant stimulation of CB1, at least in this species.
In conclusion, our study shows that despite its reduced potency for rat MAGL in vitro, JZL184 selectively elevates brain 2-AG in vivo and suppresses locomotor activity in rats. However, the neurochemical and behavioral effects of JZL184 are less pronounced than those induced by the dual MAGL/FAAH inhibitor JZL195 in the same species. In addition, the hypolocomotive effect of JZL184 is not correlated to 2-AG changes and appears to be mediated by a CB1-independent mechanism. Thus, further studies are necessary to identify the molecular mechanisms underlying JZL184-induced hypolocomotion and the contribution of 2-AG to this response.
Highlights.
- We studied the pharmacological effects of two MAGL inhibitors in rats
- JZL184 elevated 2-AG and produced hypomotility through a CB1-independent mechanism
- JZL195 had enhanced effects on brain 2-AG and motor behavior as compared to those of JZL184
Acknowledgement
This work was supported by NARSAD and NIMH RO1MH91130-01 (to AG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burston JJ, Sim-Selley LJ, Harloe JP, Mahadevan A, Razdan RK, Selley DE, Wiley JL. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J Pharmacol Exp Ther. 2008;327:546–553. doi: 10.1124/jpet.108.141382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JW, Niphakis MJ, Lum KM, Cognetta AB, Wang C, Matthews ML, Niessen S, Buczynski MW, Parsons LH, Cravatt BF. Highly selective inhibitors of monoacylglycerol lipase bearing a reactive group that is bioisosteric with endocannabinoid substrates. Chem Biol. 2012;19:579–588. doi: 10.1016/j.chembiol.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Sugiura T, Melck D, De Petrocellis L. The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal- and basophil-like cells: connections with anandamide. Biochem J. 1998;331(Pt 1):15–19. doi: 10.1042/bj3310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17:1430–1449. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Goparaju SK, Ueda N, Yamaguchi H, Yamamoto S. Anandamide amidohydrolase reacting with 2-arachidonoylglycerol, another cannabinoid receptor ligand. FEBS Lett. 1998;422:69–73. doi: 10.1016/s0014-5793(97)01603-7. [DOI] [PubMed] [Google Scholar]
- Haller J, Matyas F, Soproni K, Varga B, Barsy B, Nemeth B, Mikics E, Freund TF, Hajos N. Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci. 2007;25:2445–2456. doi: 10.1111/j.1460-9568.2007.05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison S, Weintraub ST, Giuffrida A. Quantification of endocannabinoids in rat biological samples by GC/MS: technical and theoretical considerations. Prostaglandins & Lip. Med. 2006;81:106–112. doi: 10.1016/j.prostaglandins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Kerr DM, Harhen B, Okine BN, Egan LJ, Finn DP, Roche M. The monoacylglycerol lipase inhibitor JZL184 attenuates LPS-induced increases in cytokine expression in the rat frontal cortex and plasma: differential mechanisms of action. Br J Pharmacol. 2013;169:808–819. doi: 10.1111/j.1476-5381.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AR, Duranti A, Tontini A, Rivara S, Rosengarth A, Clapper JR, Astarita G, Geaga JA, Luecke H, Mor M, Tarzia G, Piomelli D. URB602 inhibits monoacylglycerol lipase and selectively blocks 2-arachidonoylglycerol degradation in intact brain slices. Chem Biol. 2007;14:1357–1365. doi: 10.1016/j.chembiol.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Hawkins EG, Griffin G, Cravatt BF. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Cravatt BF. Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol. 2009b;16:744–753. doi: 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009c;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Moller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McFarland MJ, Porter AC, Rakhshan FR, Rawat DS, Gibbs RA, Barker EL. A role for caveolae/lipid rafts in the uptake and recycling of the endogenous cannabinoid anandamide. J Biol Chem. 2004;279:41991–41997. doi: 10.1074/jbc.M407250200. [DOI] [PubMed] [Google Scholar]
- Niphakis MJ, Cognetta AB, Chang JW, Buczynski MW, Parsons LH, Byrne F, Burston JJ, Chapman V, Cravatt BF. Evaluation of NHS carbamates as a potent and selective class of endocannabinoid hydrolase inhibitors. ACS Chem Neurosci. 2013;4:1322–1332. doi: 10.1021/cn400116z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, Lichtman AH, Cheer JF. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–373. doi: 10.1016/j.neuron.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The element of surprise. Nat Med. 2008;14:720–721. doi: 10.1038/nm0708-720. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Gessa GL, Muntoni AL. Cannabinoids modulate neuronal firing in the rat basolateral amygdala: evidence for CB1- and non-CB1-mediated actions. Neuropharmacology. 2004;46:115–125. doi: 10.1016/j.neuropharm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Jarvinen T, Niemi R. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chem Biol. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Savinainen JR, Saario SM, Laitinen JT, The serine hydrolases MAGL. ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors. Acta Physiol (Oxf) 2012;204:267–276. doi: 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Zhou W, Hohmann AG. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res. 2011;64:226–234. doi: 10.1016/j.phrs.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Martinez A, Giuffrida A. Phencyclidine-induced social withdrawal results from deficient stimulation of cannabinoid CB1 receptors. Neuropsychopharmacology. 2013;38:1816–1824. doi: 10.1038/npp.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. The fatty acid amide hydrolase inhibitor URB 597: interactions with anandamide in rhesus monkeys. Br J Pharmacol. 2011;164:655–666. doi: 10.1111/j.1476-5381.2011.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids. 2002;121:57–63. doi: 10.1016/s0009-3084(02)00146-9. [DOI] [PubMed] [Google Scholar]
- Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH. Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci. 2012;3:407–417. doi: 10.1021/cn300036b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Wong A, Barrett DA, Bennett AJ, Chapman V, Alexander SP. Spinal administration of the monoacylglycerol lipase inhibitor JZL184 produces robust inhibitory effects on nociceptive processing and the development of central sensitization in the rat. Br J Pharmacol. 2012;167:1609–1619. doi: 10.1111/j.1476-5381.2012.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]