Abstract
Objective
We evaluated the effect of a reduction in the systemic ratio of n-6:n-3 polyunsaturated fatty acids (PUFAs) on changes in inflammation, glucose metabolism, and the idiopathic development of knee osteoarthritis (OA) in mice. We hypothesized that a lower ratio of n-6:n-3 PUFAs would protect against OA markers in cartilage and synovium, but not bone.
Design
Male and female fat-1 transgenic mice (Fat-1), which convert dietary n-6 to n-3 PUFAs endogenously, and their wild-type (WT) littermates were fed an n-6 PUFA enriched diet for 9-14 months. The effect of gender and genotype on serum PUFAs, IL-6, TNF-α, and glucose tolerance was tested by 2-factor analysis of variance. Cortical and trabecular subchondral bone changes were documented by micro-focal computed tomography, and knee OA was assessed by semi-quantitative histomorphometry grading.
Results
The n-6:n-3 ratio was reduced 12-fold and 7-fold in male and female Fat-1 mice, respectively, compared to WT littermates. IL-6 and TNF-α levels were reduced modestly in Fat-1 mice. However, these systemic changes did not reduce osteophyte development, synovial hyperplasia, or cartilage degeneration. Also the fat-1 transgene did not alter subchondral cortical or trabecular bone morphology or bone mineral density.
Conclusions
Reducing the systemic n-6:n-3 ratio does not slow idiopathic changes in cartilage, synovium, or bone associated with early-stage knee OA in mice. The anti-inflammatory and anti-catabolic effects of n-3 PUFAs previously reported for cartilage may be more evident at later stages of disease or in post-traumatic and other inflammatory models of OA.
Keywords: Cartilage, Subchondral Bone, Inflammation, Polyunsaturated Fatty Acids, Fat-1 Transgene, Synovitis, Aging, Mouse Models
INTRODUCTION
Inflammation mediates osteoarthritis (OA) pathogenesis through a mosaic-like pattern of classical immune cell mediated cytokine signaling and activation of molecular inflammatory pathways in native cells of intra-articular joint tissues 1,2. While these inflammatory responses are most evident in post-traumatic knee OA 3,4, they are also observed in primary knee OA, suggesting that age-dependent changes in inflammatory pathways contribute to an increase in OA risk 5,6.
Recent studies suggest that chronic dietary factors can exacerbate or inhibit joint inflammation and thus may be important mediators of aging-associated knee OA. Obesity is a well established risk factor for knee OA, and several recent studies indicate that altered joint biomechanics alone are insufficient to increase OA risk with obesity 7-10. While most studies have focused on adipokines as systemic mediators of obesity-associated OA, lipids are also potent regulators of inflammation 11. In particular, the ratio of omega-6 (n-6) to omega-3 (n-3) polyunsaturated fatty acids (PUFAs) is considered one of the most important dietary mediators of inflammation 12. Arachidonic acid (AA), a major n-6 PUFA, promotes inflammation by being converted into pro-inflammatory eicosanoids, such as prostaglandins, thromboxanes, and leukotrines. In contrast, n-3 PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) inhibit inflammation and accelerate the resolution of inflammation. The anti-inflammatory effects of n-3 PUFAs occur through multiple mechanisms, including inhibition of the AA conversion into pro-inflammatory eicosanoids, synthesis of anti-inflammatory agents such as protectins and resolvins, and down-regulation of pro-inflammatory gene expression through n-3 receptor GPR120 13,14. Thus, variation in the dietary ratio of n-6:n-3 PUFAs, which is elevated in modern Western diets 15 and attributed to the increase in risk of numerous chronic diseases 16, may also contribute to differences in OA risk.
Previous studies support a role for n-6 and n-3 PUFAs in modifying OA severity. In middle-aged individuals without clinical knee OA, dietary intake of n-6 PUFAs was positively associated with the future prevalence, but not the incidence, of subchondral bone marrow lesions 17,18. In individuals who have or are at high risk for knee OA, fasting plasma AA was positively associated with synovitis, whereas patella-femoral cartilage loss was negatively associated with DHA 19. Animal and cell studies also indicate that n-3 PUFAs protect against OA. Feeding an n-3 enriched diet to OA-prone Dunkin-Hartley Guinea pigs reduced markers of OA without altering OA markers in a non-prone strain 20. In addition, mice expressing the fat-1 transgene were moderately protected from developing knee OA following transection of the medial meniscus, medial collateral ligament, and anterior crutiate ligament 21. This transgene induces endogenous conversion of n-6 to n-3 PUFAs by encoding a desaturase enzyme absent in mammals that adds a double bond into the omega-3 position of an unsaturated fatty acid. The result is a systemic reduction in the n-6:n-3 ratio 22. The protective effects of the fat-1 transgene was attributed to a reduction in inflammation, decreased protein expression of matrix metalloproteinase-13 and ADAMTS-5, and enhanced autophagy 21. In bovine cartilage explant and cell culture models, EPA and DHA inhibited the expression of pro-inflammatory and pro-catabolic genes and reduced glycosaminoglycan catabolism induced by exposure to interleukin-1 23,24. Yet not all aspects of n-3 PUFAs necessarily protect against OA. EPA and DHA levels are positively associated with bone strength and bone density 25,26, and a low ratio of n-6:n-3 fatty acids protected against ovariectomy-induced bone loss in mice 27. These pro- anabolic effects of n-3 PUFAs on bone strength and mass may promote OA by stimulating osteophyte development or subchondral bone thickening.
Our goal was to determine how a life-long reduction in the ratio of n-6:n-3 PUFA levels affects the development of idiopathic knee OA in mice. We hypothesized that a low ratio of n-6:n-3 PUFAs protects against OA markers in cartilage and synovium, but not bone. We tested this hypothesis using a transgenic mouse model containing the fat-1 transgene from C. elegans 22. By feeding mice an n-6 PUFA-enriched diet, Fat-1 mice reduce the n-6:n-3 ratio in the serum and tissues approximately 20-fold 22. Here we examined changes in serum lipids and inflammatory markers in 9-14 month-old male and female C57BL/6 mice with and without the fat-1 transgene. We determined the effect of fat-1 transgene expression on idiopathic knee OA changes in cartilage, bone, and synovium. We also conducted regression analyses to evaluate whether fat-1 expression altered associations between cartilage and bone or synovium and bone OA outcome measures.
MATERIALS AND METHODS
Animals
We obtained fat-1 transgenic breeder mice from Dr. J. X. Kang 22. Animals were bred while housed in the University of Oklahoma Health Sciences Center (OUHSC) vivarium on a 12-hour light/dark cycle under temperature-controlled conditions (20–22°C). Animals were allowed ad libitum access to water and a modified AIN-76A purified rodent diet supplemented with 10% Safflower oil by weight (#180465; Dyets, Inc., Bethlehem, PA) as previously described 28. The diet contains 59% kcal carbohydrate, 18% kcal protein, and 23% kcal fat, with an n-6:n-3 ratio of 274 28. Wild-type (C57BL/6) females were mated with male hemizygous fat-1+/0 transgenic mice maintained on a C57BL/6 background. Male and female littermates carrying either one copy of the fat-1 gene (hereafter referred to as Fat-1) or no copy of the fat-1 gene (WT) were studied. Genotyping was conducted as previously described 28. Our initial cohort of animals (cohort 1) included the following number and ages of animals: female WT mice (N=10; 59.6 ± 1.7 wks of age [58 – 62 wks], mean±SD [range]), female Fat-1 mice (N=10; 59.5 ± 1.6 wks of age [58 – 62 wks]), male WT mice (N=10; 55.4 ± 4.4 wks of age [52 – 61 wks]), and male Fat-1 mice (N=10; 57.7 ± 3.8 wks of age [51 – 61 wks]). Additional male mice were included (cohort 2) to increase the sample size for lipid, cytokine, and histologic analyses: male WT mice (N=8; 43.6 ± 4.3 wks of age [36 – 48 wks]) and male Fat-1 mice (N=7; 43.1 ± 3.9 wks of age [40 – 48 wks]). Although cohort 2 mice were younger than cohort 1, no significant age-dependent differences were observed so data were pooled for both cohorts. Following euthanasia, the hind limbs were dissected and stored in phosphate buffered saline at −80° C. All experimental procedures were conducted in accordance with a protocol approved by the OUHSC Animal Care and Use Committee.
Serum analyses
Blood was collected from anesthetized mice just before the mice were killed. The blood was allowed to clot for 60 minutes at room temperature and then centrifuged for 15 minutes at 3,500 revolutions per minute, and the serum was aliquoted for immediate storage at −80°C until analysis. Serum n-6 and n-3 PUFA analyses were performed as previously described 28. Briefly, lipid classes of serum lipid extracts were resolved, derivatized to form fatty acid methyl esters, and analyzed using gas-liquid chromatography in an Agilent 6890N gas chromatograph with flame ionization detector (GC-FID) (Agilent Technologies, Wilmington, DE). Authentic standards (NU-CHEK PREP, Elysian, MN) were used to calculate relative mole percentages for the following fatty acids: 20:4n6, 18:3n3, 20:5n3, 22:5n3, and 22:6n3. Serum concentrations of IL-6 and TNFα were investigated as prognostic markers of the effect of fat-1 transgene expression on systemic inflammation. They were measured using quantitative enzyme linked immunosorbent assays (ELISA) designed specifically for mice (eBioscience, San Diego, CA). All samples were analyzed as recommended by the protocols provided by the manufacturer. Glucose tolerance tests were conducted as previously described 29.
Micro-CT analyses
Right knee joints of mice from Cohort 1 were thawed, fixed in 10% neutral buffered formalin, and scanned using a micro-computed tomography system (vivaCT; Scanco Medical, Basserdorf, Switzerland) as described previously 30. We quantified the following parameters for the tibial epiphysis: subchondral bone thickness (mm), subchondral bone density (mg hydroxyapatite/cm3), trabecular thickness (mm), trabecular bone spacing (mm), trabecular bone volume (BV, cm3), total trabecular volume (TV, cm3), and relative trabecular bone volume (BV/TV). Bone outcome measures were evaluated separately for the medial and lateral compartments.
Histomorphometric analyses
Following fixation, intact knee joints from all animals were processed and stained for histological grading as described previously 30. OA severity scoring was conducted using a modified Mankin scoring system 31 for the following categories: changes in articular cartilage structure (score of 0-11), Safranin O staining (score of 0-8), tidemark duplication (score of 0-3) and hypertrophic chondrocytes (score of 0-2), for a maximum score of 24 per location. Scores were recorded by 3 experienced graders under blinded conditions at 4 locations within the joint—lateral femur, lateral tibia, medial femur, and medial tibia. We also quantified the severity of osteophyte formation along the anterior and posterior joint margins of the medial tibial plateau using a 0-3 histological grading scheme for each region as previously described based on a visual scale of progressive bone deposition at the peripheral osteochondral junction 32. In addition, synovial changes were determined by measuring synovial thickness (distance between synovial lining and joint capsule at the midpoint of the anterior meniscus) and quantifying synovial extension into the joint space. We quantified synovial extension as the presence (1) or absence (0) of synovial tissue outgrowth parallel to the surface of the meniscus. Extensions were evaluated along the meniscus adjacent to the femoral and tibial surfaces in both the anterior and posterior regions for a maximum score of 4. Scores were averaged for two sections within the medial and lateral compartments per animal.
Statistical analyses
All statistical analyses were conducted using either JMP 8.02 (SAS Institute, Cary, NC) or Prism 6 (GraphPad Software, La Jolla, CA). The effect of the fat-1 genotype and sex were determined by 2-factor analysis of variance, with Holm-Sidak’s multiple comparison’s post-hoc test to determine group differences. When cytokine concentrations were below the lowest levels of quantification (LLOQ), a value of one-half the LLOQ was used for statistical purposes. Statistical analyses were performed on log-transformed serum lipid and cytokine data to correct for non-normal distributions as determined by Shapiro-Wilk normality test. An F test was used to test for equality of variance between WT and Fat-1 male and female mice. Body weight and glucose AUC comparisons between genotypes within a given sex were conducted using two-tailed t-tests, and blood glucose levels during the glucose tolerance testing were analyzed by 2-way ANOVA with time as a repeated measure. For semi-quantitative histological scores, genotype and site-specific differences were determined either by Mann-Whitney U tests (cartilage) or a Kruskal-Wallis test followed by Dunn’s multiple comparisons test (osteophyte and synovial hyperplasia).
We constructed exploratory pair-wise and multivariable regression models to evaluate the effect of fat-1 transgene expression on the association between cartilage and bone or synovium and bone outcome measures. Pair-wise comparisons were conducted using Spearman’s Rho rank correlation analysis. No significant correlations were observed between any bone parameters (i.e., osteophyte score, subchondral bone thickness, or relative trabecular bone volume) and either modified Mankin OA scores or synovial thickness. Therefore, multivariable regression analyses were conducted that adjusted for fat-1 expression and sex using Generalized Linear Modeling (GLM). Models contained either the modified Mankin OA score or synovial thickness as the outcome parameter. Osteophyte score, subchondral bone thickness, or relative trabecular bone volume were the independent predictors. For both pair-wise and GLM analyses, OA scores were site-matched to the corresponding bone parameter (e.g., medial tibial plateau OA score:osteophyte score). Statistical significance was reported at the 95% confidence level for all tests (P< 0.05). All data were reported as the mean ± 95% confidence intervals (CI).
RESULTS
Serum n-6:n-3 PUFAs and cytokines
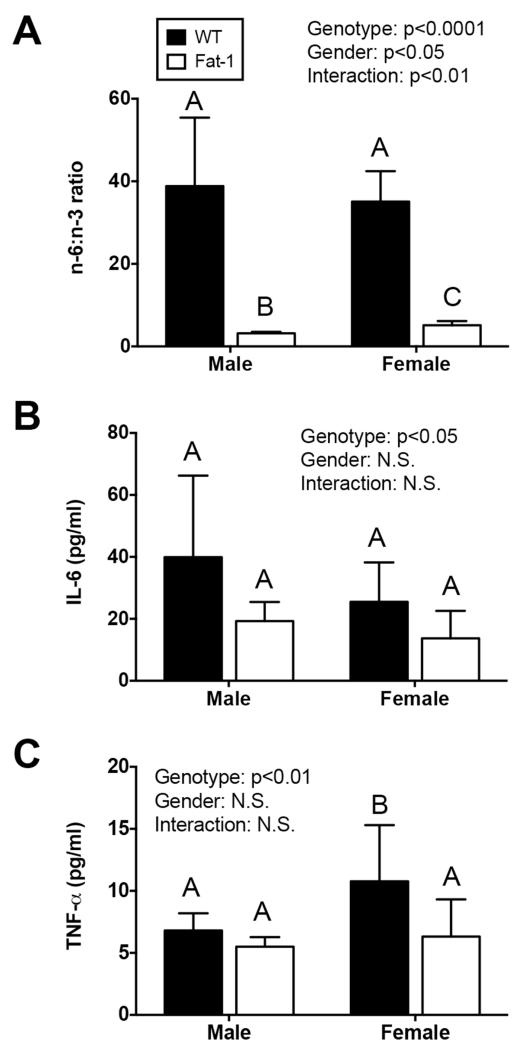
Fat-1 transgene expression significantly reduced the n-6:n-3 PUFA ratio in male and female mice. In male mice, the n-6:n-3 ratio decreased more than 12-fold, from 38.8 to 3.2 in WT versus Fat-1 mice, respectively (p<0.001; Fig. 1A). This change in PUFA ratio was due to a 14% reduction in the relative mole percentage of AA (17.6 to 15.2%) and a 9.7-fold increase in n-3 levels (0.50 to 4.87%). In female mice, the n-6:n-3 ratio decreased nearly 7-fold, from 35.1 to 5.1 in WT versus Fat-1 mice, respectively (p<0.001; Fig. 1A). This reduction in n-6:n-3 ratio was attributed to a 14% reduction in AA (22.0 to 19.0%) and a 5.8-fold increase in n-3 PUFAs (0.67 to 3.86%). Thus, while fat-1 expression dramatically reduced the n-6:n-3 ratio in serum in both sexes, the effect was greater in male mice due to a greater increase in n-3 PUFA concentrations. Along with the reduction in the n-6:n-3 PUFA ratio, fat-1 expression also reduced serum IL-6 and TNF-α levels by approximately 50% and 33%, respectively, (Fig. 1B,C). For IL-6, fat-1 expression reduced serum concentrations similarly in male and female mice (52% versus 46%; Fig. 1B). For TNF-α, fat-1 expression reduced serum concentrations to a lesser extent in male mice (19%) compared to female mice (41%; Fig. 1C). Overall, however, serum cytokine concentrations were only significantly altered by the fat-1 genotype and not gender.
Figure 1. Fat-1 transgene expression reduces n-6:n-3 serum fatty acids and systemic cytokines.
A. Fat-1 gene expression converts n-6 to n-3 fatty acids, resulting in a dramatically reduced n-6:n-3 lipid ratio in male and female mice. B. Serum IL-6 concentrations are marginally reduced in Fat-1 mice (p=0.049) independent of animal gender. C. Serum TNF-α is also reduced in Fat-1 mice (p=0.004) with the greatest differences in female mice. Samples sizes in (A) are N=5, 14, 10, and 8 for WT male, Fat-1 male, WT female, and Fat-1 female, respectively. Sample sizes in (B) and (C) are N=16, 16, 10, and 10, respectively. P-values are from a 2-Factor ANOVA; bars not sharing the same letter are statistically different from each other as determined by post-hoc analysis (Holm-Sidak’s multiple comparison test). Values are mean ± 95% CI.
Body mass and glucose tolerance
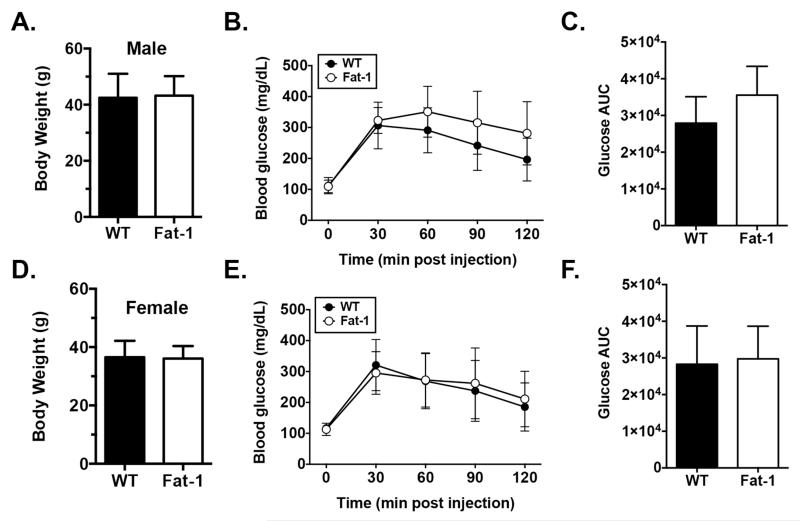
n-3 PUFAs have previously been shown to promote insulin signaling in diet-induced obese mice 13. Therefore, to investigate potential links between n-3 mediated changes in metabolism and knee OA, we tested the effect of fat-1 expression on body mass and glucose tolerance in aged mice. Male and female mice were compared separately because body weight is lower in female mice (Fig. 2A, D). In male mice, fat-1 expression did not significantly alter body mass (Fig. 2A) or glucose tolerance (Fig. 2B,C). Fat-1 expression did not alter body mass or glucose tolerance in female mice either (Fig. 2D-F). Thus, unlike with obesity 13, increased n-3 PUFAs with fat-1 expression did not improve glucose tolerance in aged mice.
Figure 2. Fat-1 transgene expression does not alter body weight or glucose tolerance in aged male (A-C) or female (D-F) mice.
Body weight (A, D) and serum blood glucose clearance following a fasted glucose tolerance test (B, E) were not significantly different between either male or female WT and Fat-1 mice (p>0.05). Both male and female WT and Fat-1 mice produced similar glucose areas under the curve (AUC) values up to 120 min post glucose injection (C, F; mg/dL/min). N=10 per genotype and sex. Values are mean ± 95% CI.
Effect of fat-1 expression on knee OA in male mice
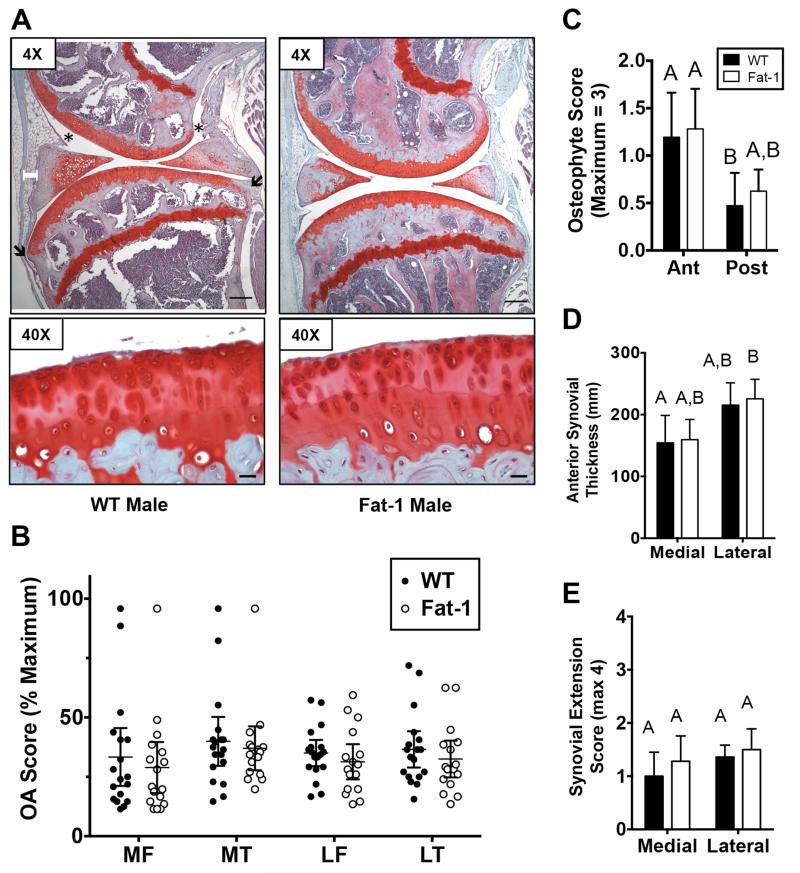
Cartilage OA changes generally ranged between 25-50% of the maximal OA scores in both WT and Fat-1 mice, although a small number of animals developed near maximal OA (Fig. 3A,B). Typical changes included cartilage loss and fibrillation in the surface zone and focal loss of Safranin-O staining in the central primary load-bearing regions (Fig. 3A). OA scores were similar between the femur and tibia in both the medial and lateral compartments of WT and Fat-1 animals (Fig. 3B). Furthermore, genotype did not alter any of the sub-component OA scores at these sites, including cartilage structural damage, tidemark duplication, Safranin-O staining loss, or hypertrophic chondrocytes. Osteophyte development was assessed in the anterior and posterior margins in the medial peripheral tibial plateau. Osteophyte development was greater in the anterior versus the posterior joint margin in WT animals (Fig. 3C). A similar trend was observed in Fat-1 mice. There was no effect of fat-1 expression on osteophyte development at either location (Fig. 3C). Finally, we assessed changes in synovial morphology by quantifying anterior thickness and the outgrowth of synovium into the joint space, parallel to the meniscus surface. While there was a trend for greater synovial thickness in the lateral compartment, joint compartment and fat-1 transgene expression did not alter synovial thickness (Fig. 3D). The occurrence of synovial extensions into the joint space was similar in both medial and lateral compartments and in both WT and Fat-1 mice (Fig. 3E).
Figure 3. Fat-1 transgene expression does not alter knee joint OA outcomes in aged male mice.
A. Representative sagittal histology sections of the medial knee joint compartment in WT and Fat-1 male mice. 4× images are from more peripheral medial sections used for osteophyte grading and 40× images are from more central load-bearing regions. Capped white bar superficial to the anterior horn of the meniscus indicates site of anterior synovial thickness measurement. Asterisks indicate examples of synovial extensions into the joint space. Black arrows indicate sites of osteophyte evaluation. Black bar = 200μm in 4× image and 20 μm in 40× image. B. Modified Mankin OA score of the medial (M) and lateral (L) tibia (T) and femur (F). Data points are mean values of multiple graded sections for individual animals. Expression of the fat-1 transgene did not alter OA scores at any site. C. Anterior (Ant) and posterior (Post) medial tibia osteophyte scores for WT and Fat-1 mice. D. Medial and lateral anterior synovial thickness in WT and Fat-1 mice. E. Medial and lateral synovial extension scores for WT and Fat-1 mice. N=18, WT; N=16, Fat-1 for all scores. Bars that do not share the same letter are statistically different from each other (p<0.05) as determined by Dunn’s multiple comparisons test. Values are mean ± 95% CI.
Effect of fat-1 expression on knee OA in female mice
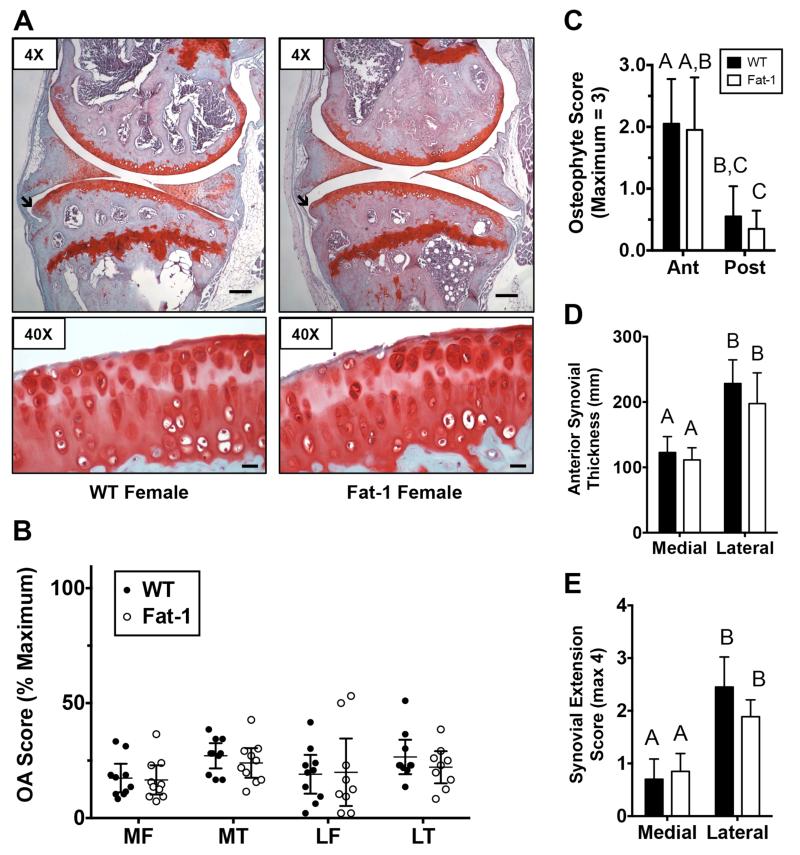
In female animals, cartilage OA changes generally ranged between 15-30% of the maximal OA scores in both WT and Fat-1 mice, with a small number of animals with OA scores around 50% maximal (Fig. 4A,B). Similar to male mice, typical changes included cartilage loss and fibrillation in the surface zone and focal loss of Safranin-O staining in the central primary load-bearing regions (Fig. 4A). Although not statistically significant, tibial OA scores tended to be greater than femoral scores in both the medial and lateral compartments of WT and Fat-1 animals (Fig. 4B). Genotype did not significantly alter the overall OA score or any of the sub-component OA scores at any of the sites with the exception of hypertrophic chondrocytes. In the medial tibia, Fat-1 mice developed fewer hypertrophic chondrocytes than WT mice (1.5 ± 0.1 versus 1.0 ± 0.1; p=0.016). As in male mice, osteophyte development was greater in the anterior versus the posterior medial peripheral joint margin, although in female mice this was true for both WT and Fat-1 mice (Fig. 4C). Similarly, though, there was no effect of fat-1 expression on osteophyte development at either location (Fig. 4C). In the synovium, the anterior thickness was 87% and 77% thicker in the lateral versus the medial compartment for WT and Fat-1 mice, respectively (Fig. 4D; p<0.001 for both genotypes). The number of synovial extensions into the joint space was also significantly greater in the lateral compartment for both genotypes (p<0.001; Fig. 4E). However, there was no effect of fat-1 expression on synovial thickness or synovial extensions in any location
Figure 4. Fat-1 transgene expression does not substantially alter knee joint OA outcomes in aged female mice.
A. Representative sagittal histology sections of the medial knee joint compartment in WT and Fat-1 female mice. 4× images are from more peripheral medial sections used for osteophyte grading and 40× images are from more central load-bearing regions. Black arrows indicate anterior osteophytes. Black bar = 200μm in 4× image and 20 μm in 40X image. B. Modified Mankin OA score of the medial (M) and lateral (L) tibia (T) and femur (F). Data points are mean values of multiple graded sections for individual animals. Expression of the fat-1 transgene did not alter OA scores at any site. C. Anterior (Ant) and posterior (Post) medial tibia osteophyte scores for WT and Fat-1 mice. D. Medial and lateral anterior synovial thickness in WT and Fat-1 mice. E. Medial and lateral synovial extension scores for WT and Fat-1 mice. N=10, WT; N=10, Fat-1. Bars that do not share the same letter are statistically different from each other (p<0.05) as determined by Dunn’s multiple comparisons test. Values are mean ± 95% CI.
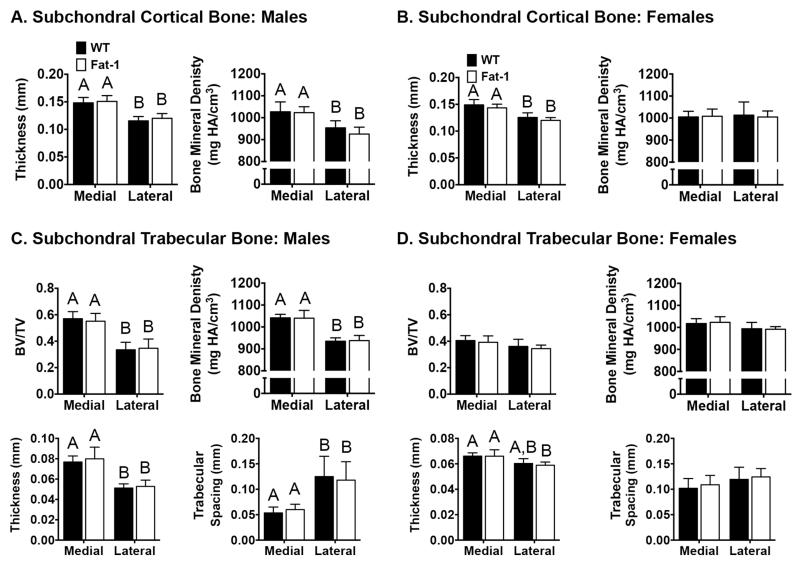
Subchondral cortical and trabecular bone
Both the morphology and density of the tibial subchondral cortical and trabecular bone were examined for male and female WT and Fat-1 mice (Fig. 5). Subchondral cortical bone thickness was significantly greater in the medial versus the lateral tibial plateau in both male (Fig. 5A) and female (Fig. 5B) animals (27% and 19%, respectively). The reduced subchondral bone thickness in the lateral compartment was associated with an 8% lower bone mineral density in male but not female mice (p<0.05; Fig. 5A). Fat-1 transgene expression did not alter any subchondral cortical bone parameters in male or female mice. Medial versus lateral differences were also observed in the trabecular bone parameters, although these differences were primarily restricted to male mice (Fig. 5C,D). For both WT and Fat-1 male mice, the lateral trabecular bone compartment, compared to the medial compartment, had a lower relative bone volume (BV/TV), lower bone mineral density, reduced trabecular bone thickness, and increased trabecular spacing (Fig. 5C). No medial-lateral differences in trabecular bone parameters were detected in female mice, except for an 11% reduction in trabecular thickness in the lateral trabecular compartment of Fat-1 mice (p<0.05; Fig. 5D).
Figure 5. Fat-1 transgene expression does not alter proximal tibial subchondral or trabecular bone morphology or density in male and female mice.
Micro-CT evaluation of the proximal tibial plateau and epiphysis was used to examine effects of fat-1 transgene expression on the bony changes related to knee OA. Medial and lateral subchondral bone thickness and bone mineral density were compared by genotype in male (A) and female (B) mice. Trabecular bone volume, density, thickness, and spacing were also compared in medial and lateral compartments in WT and Fat-1 male (C) and female (D) mice. Genotype did not alter any of the bone morphology or density outcome measures. Differences between the medial and lateral compartments were observed for several subchondral and trabecular bone measures in both male and female mice, as indicated by bars that do not share the same letter (p<0.05). Graphs without letters contain no significant group differences. Sample size: Males (N=10, WT; N=9, Fat-1); Females: (N=10 for WT and Fat-1). Values are mean ± 95% CI.
Effect of fat-1 expression on the association of OA outcome markers in cartilage, synovium, and bone
To further investigate the potential effect of a reduction in the n-6:n-3 ratio on OA-related outcomes, we conducted multivariable analyses to determine if adjusting for the Fat-1 genotype or sex altered the associations between modified Mankin OA scores and each of the following bone parameters: osteophyte score, subchondral bone thickness, and trabecular bone volume (BV/TV). We repeated these models for synovial thickness as the outcome measure. Including sex and genotype in the regression analyses did not result in significant associations between any of the bone-related parameters and the cartilage OA score or synovial thickness. In each model, though, sex had a significant effect on the relationship between bone phenotypes and cartilage OA or synovium outcome measures. Genotype, however, was not a significant parameter for any of the models (data not shown). Thus, a reduction in the n-6:n-3 ratio by expression of the fat-1 transgene did not alter the association between bone phenotypes and cartilage OA scores or synovium thickness.
DISCUSSION
We hypothesized that a low ratio of n-6:n-3 PUFAs caused by expression of the fat-1 transgene would protect against idiopathic OA development in cartilage and synovium, but not bone. However, our results do not support this hypothesis. Despite a significant, albeit moderate, reduction in the systemic inflammatory cytokines TNF-α and IL-6 in Fat-1 mice, there were no significant differences in glucose tolerance and the development of spontaneous knee OA. In fact, in nearly every OA related outcome examined, mice expressing the fat-1 transgene were nearly indistinguishable from WT mice. Moreover, when statistically controlling for differences between male and female mice, there was no effect of the fat-1 transgene on associations between cartilage and bone or synovium and bone OA-related outcomes. Thus, a life-long reduction in the n-6:n-3 ratio does not alter the development of idiopathic early to moderate stage knee OA in middle-aged mice.
These findings suggest that a systemic shift in the relative levels of n-6 and n-3 PUFAs has a minimal effect on normal joint development and remodeling. This is surprising because n-3 PUFAs are ligands for the nuclear transcription factors peroxisome proliferator activated receptor-gamma and retinoid X receptor 33, which regulate cartilage development and homeostasis 34,35. In addition, autophagy, which is implicated in aging-associated OA 36, is up-regulated in Fat-1 mice following joint injury 21. The absence of a protective effect of the fat-1 transgene on idiopathic OA is also surprising because n-3 PUFAs inhibit the activity of pro-inflammatory IKKβ/NFκB and JNK/AP1 signaling pathways 13. In chondrocytes, IKKβ/NFκB and JNK/AP1 are critical inflammatory and mechanosensitive signaling pathways involved in regulating catabolic gene expression 5,37,38. n-3 PUFAs inhibit these pathways directly through ligand-mediated activation of GPR120 and downstream inactivation of transforming growth factor-β activated kinase 1 (TAK1) 13. Chondrocytes express GPR120 39, but the physiological relevance of GPR120-mediated inactivation of TAK1 in articular chondrocytes is unknown.
With knee OA, the rate of subchondral bone remodeling increases 40 resulting in altered subchondral cortical and trabecular bone volume, morphology, and mineral content depending on the stage of disease 41. Previous studies indicate that n-3 PUFAs reduce bone absorption 42 and increase bone mass and strength 26,43, including protection against ovariectomy-induced bone loss in mice supplemented with an n-3 PUFA enriched diet 44 or in fat-1 transgene mice 27. Therefore, the absence of any changes in subchondral bone parameters in Fat-1 mice was unexpected. A reduced ratio of n-6:n-3 PUFAs may alter skeletal disease pathology in post-traumatic OA models involving higher rates of bone remodeling.
There are several limitations of the current study. First, we do not know the extent to which n-6 and n-3 PUFAs were altered in the joint tissues of Fat-1 mice. Previous studies suggest less reduction in the n-6:n-3 ratio in cartilage (−57%) 21 compared to muscle (−96%) 22 and bone (−99%) 26 based on a ratio of AA/(EPA+DPA+DHA). The beneficial effects of n-3 PUFAs using dietary supplements may achieve lower n-6:n-3 ratios. Bhattacharya and colleagues 25 observed a beneficial effect of n-3 PUFAs on reducing inflammation and osteoclastogenesis in aging mice by manipulating the n-6:n-3 ratio using corn versus fish oil supplements. The fish oil supplement group obtained a serum n-6:n-3 ratio of 0.41, which is lower than we observed in Fat-1 mice. However, dietary supplements can also alter the macronutrient composition of diets, which may contribute to differences in study outcomes. An advantage of the fat-1 transgene for mechanistic studies is that Fat-1 and WT littermate mice are fed the same diet and exposed to the same environmental conditions due to co-housing, factors which may contribute to differences in the development of idiopathic OA 45.
An additional limitation is that we did not examine older animals with more advanced OA. n-3 PUFAs inhibit inflammation and accelerate the resolution of inflammation 13,14; however, the involvement of immune cell mediated cytokine signaling in mild to moderate idiopathic OA is not well-established in C57BL/6 mice. Analysis of older animals with more severe OA or in combination with post-traumatic models of OA 21 may be required to reveal the beneficial effects of n-3 PUFAs with aging. A recent study reported that DHA supplementation reduces nociception and inflammation in an inflammatory mouse model of knee arthritis 46. Thus, OA sub-types involving more active synovial inflammation may benefit from increased levels of n-3 PUFAs as suggested in individuals that have or are at high risk for developing knee OA 19.
In summary, a life-long reduction in the ratio of n-6:n-3 PUFAs causes a modest decrease in systemic TNF-α and IL-6 but does not alter the early-stage development of idiopathic knee OA in aging Fat-1 mice; 9-14 month-old male and female Fat-1 mice develop cartilage degeneration and osteophyte formation at levels comparable to WT mice. Furthermore, subchondral cortical and trabecular bone morphology and bone mineral density are similar in Fat-1 and age-matched WT mice. Overall, these findings suggest that high levels of n-6 relative to n-3 PUFAs do not exacerbate idiopathic changes in joint tissue homeostasis that initiate the development of knee OA. The anti-inflammatory and anti-catabolic effects of n-3 PUFAs previously reported for cartilage appear to be more evident in post-traumatic and other more inflammatory models of OA.
ACKNOWLEDGMENTS
We thank Dr. J. X. Kang for providing us with Fat-1 mouse breeders. We thank AJ Dockins in the OMRF Imaging Facility and Yao Fu for their excellent assistance with sample preparation and histological grading, respectively. We also thank Melinda West for assistance with sample collection, Courtney Montgomery for assistance with data analysis, and Griffin lab members for helpful suggestions with preparing the manuscript.
ROLE OF FUNDING SOURCES
This project was supported by grants from the National Center for Research Resources (5P20RR018758-08), the National Institute of General Medical Sciences (8 P20 GM103441-08) from the National Institutes of Health to TMG; National Institutes of Health grants EY00871, EY04149, P30EY021725, and P20RR017703 to REA; NIH EY017888, Oklahoma Center for Advancement of Science and Technology (OCAST) HR10-120 and Reynolds Oklahoma Center on Aging to HM; and Japan Society for the Promotion of Science to JSPS and YK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Angela Cai and Erin Hutchison contributed to the acquisition, analysis, and interpretation of data. Joanna Hudson contributed to the acquisition and analysis of data. Yusuke Kawashima and Anil Singh contributed to the acquisition, analysis, and interpretation of data. Richard S. Brush and Robert E. Anderson contributed to the acquisition and interpretation of lipid data. Naoka Komori, Hiroyuki Matsumoto, William E. Sonntag, and Timothy M. Griffin contributed to the conception and design of the study and the acquisition, analysis, and interpretation of data. All authors have made substantial contributions to the drafting of the article or revising it critically for important intellectual content and have given final approval of the version that is submitted.
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu-Bryan R, Terkeltaub R. The growing array of innate inflammatory ignition switches in osteoarthritis. Arthritis Rheum. 2012;64(7):2055–2058. doi: 10.1002/art.34492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. Post-traumatic osteoarthritis: Improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674–1679. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23(5):471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108–113. doi: 10.1097/BOR.0b013e32835a9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karvonen-Gutierrez CA, Harlow SD, Jacobson J, Mancuso P, Jiang Y. The relationship between longitudinal serum leptin measures and measures of magnetic resonance imaging-assessed knee joint damage in a population of mid-life women. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-202685. doi:10.1136/annrheumdis-2012-202685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gierman LM, van der Ham F, Koudijs A, Wielinga PY, Kleemann R, Kooistra T, et al. Metabolic stress-induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheum. 2012;64(4):1172–1181. doi: 10.1002/art.33443. [DOI] [PubMed] [Google Scholar]
- 9.Mooney RA, Sampson ER, Lerea J, Rosier RN, Zuscik MJ. High-fat diet accelerates progression of osteoarthritis after meniscal/ligamentous injury. Arthritis Res Ther. 2011;13(6):R198. doi: 10.1186/ar3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiology of Aging & Age-related Diseases. 2012;2(0) doi: 10.3402/pba.v2i0.17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass CK, Olefsky JM. Inflammation and Lipid Signaling in the Etiology of Insulin Resistance. Cell Metabolism. 2012;15(5):635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calder PC. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie. 2009;91(6):791–795. doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142(5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eaton SB, Eaton SB, III, Sinclair AJ, Cordain L, Mann NJ. Dietary intake of long-chain polyunsaturated fatty acids during the paleolithic. In: Simopoulos AP, editor. The Return of Omega3 Fatty Acids into the Food Supply. I. Land-Based Animal Food Products and Their Health Effects. Karger Publishers; 2004. [Google Scholar]
- 16.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Davies-Tuck ML, Wluka AE, Forbes A, English DR, Giles GG, et al. Dietary fatty acid intake affects the risk of developing bone marrow lesions in healthy middle-aged adults without clinical knee osteoarthritis: a prospective cohort study. Arthritis Res Ther. 2009;11(3):R63. doi: 10.1186/ar2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wluka AE, Hodge AM, English DR, Giles GG, O’Sullivan R, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis and Cartilage. 2008;16(5):579–583. doi: 10.1016/j.joca.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Baker KR, Matthan NR, Lichtenstein AH, Niu J, Guermazi A, Roemer F, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis and Cartilage. 2012;20(5):382–387. doi: 10.1016/j.joca.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knott L, Avery NC, Hollander AP, Tarlton JF. Regulation of osteoarthritis by omega-3 (n-3) polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthritis and Cartilage. 2011;19(9):1150–1157. doi: 10.1016/j.joca.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang MJ, Wang L, Jin DD, Zhang ZM, Chen TY, Jia CH, et al. Enhancement of the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1 signalling and delays surgically induced osteoarthritis in comparison with wild-type mice. Annals of the Rheumatic Diseases. 2013 doi: 10.1136/annrheumdis-2013-203231. doi:10.1136/annrheumdis-2013-203231. [DOI] [PubMed] [Google Scholar]
- 22.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427(6974):504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 23.Wann AKT, Mistry J, Blain EJ, Michael-Titus AT, Knight MM. Eicosapentaenoic acid and docosahexaenoic acid reduce interleukin-1β-mediated cartilage degradation. Arthritis Res Ther. 2010;12(6):R207. doi: 10.1186/ar3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zainal Z, Longman AJ, Hurst S, Duggan K, Caterson B, Hughes CE, et al. Relative efficacies of omega-3 polyunsaturated fatty acids in reducing expression of key proteins in a model system for studying osteoarthritis. Osteoarthritis and Cartilage. 2009;17(7):896–905. doi: 10.1016/j.joca.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya A, Rahman M, Sun D, Fernandes G. Effect of fish oil on bone mineral density in aging C57BL/6 female mice. The Journal of Nutritional Biochemistry. 2007;18(6):372–379. doi: 10.1016/j.jnutbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Lau BYY, Ward WE, Kang JX, Ma DWL. Femur EPA and DHA are correlated with femur biomechanical strength in young fat-1 mice. The Journal of Nutritional Biochemistry. 2009;20(6):453–461. doi: 10.1016/j.jnutbio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Banu J, Bhattacharya A, Rahman M, Kang JX, Fernandes G. Endogenously produced n-3 fatty acids protect against ovariectomy induced bone loss in fat-1 transgenic mice. J Bone Miner Metab. 2010;28(6):617–626. doi: 10.1007/s00774-010-0175-2. [DOI] [PubMed] [Google Scholar]
- 28.Tanito M, Brush RS, Elliott MH, Wicker LD, Henry KR, Anderson RE. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. The Journal of Lipid Research. 2009;50(5):807–819. doi: 10.1194/jlr.M800170-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 30.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60(10):2935–2944. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: A model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 32.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis and Cartilage. 2010;18(S3):S35–S52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47(2):147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Davies MR, Ribeiro LR, Downey-Jones M, Needham MRC, Oakley C, Wardale J. Ligands for retinoic acid receptors are elevated in osteoarthritis and may contribute to pathologic processes in the osteoarthritic joint. Arthritis Rheum. 2009;60(6):1722–1732. doi: 10.1002/art.24550. [DOI] [PubMed] [Google Scholar]
- 35.Monemdjou R, Vasheghani F, Fahmi H, Perez G, Blati M, Taniguchi N, et al. Association of cartilage-specific deletion of peroxisome proliferator-activated receptor γ with abnormal endochondral ossification and impaired cartilage growth and development in a murine model. Arthritis Rheum. 2012;64(5):1551–1561. doi: 10.1002/art.33490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madhavan S, Anghelina M, Sjostrom D, Dossumbekova A, Guttridge DC, Agarwal S. Biomechanical signals suppress TAK1 activation to inhibit NF-kappaB transcriptional activation in fibrochondrocytes. The Journal of Immunology. 2007;179(9):6246–6254. doi: 10.4049/jimmunol.179.9.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chowdhury TT, Salter DM, Bader DL, Lee DA. Signal transduction pathways involving p38 MAPK, JNK, NFκB and AP-1 influences the response of chondrocytes cultured in agarose constructs to IL-1β and dynamic compression. Agents and Actions. 2008;57(7):306–313. doi: 10.1007/s00011-007-7126-y. [DOI] [PubMed] [Google Scholar]
- 39.Hissnauer TN, Baranowsky A, Pestka JM, Streichert T, Wiegandt K, Goepfert C, et al. Identification of molecular markers for articular cartilage. Osteoarthritis and Cartilage. 2010;18(12):1630–1638. doi: 10.1016/j.joca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52(8):557–563. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Botter SM, van Osch GJ, Clockaerts S, Waarsing JH, Weinans H, van Leeuwen JP. Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: an in vivo microfocal computed tomography study. Arthritis Rheum. 2011;63(9):2690–2699. doi: 10.1002/art.30307. [DOI] [PubMed] [Google Scholar]
- 42.Rahman MM, Bhattacharya A, Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J Cell Physiol. 2008;214(1):201–209. doi: 10.1002/jcp.21188. [DOI] [PubMed] [Google Scholar]
- 43.Högström M, Nordström P, Nordström A. n-3 Fatty acids are positively associated with peak bone mineral density and bone accrual in healthy men: the NO2 Study. Am J Clin Nutr. 2007;85(3):803–807. doi: 10.1093/ajcn/85.3.803. [DOI] [PubMed] [Google Scholar]
- 44.Sun D, Krishnan A, Zaman K, Lawrence R, Bhattacharya A, Fernandes G. Dietary n-3 Fatty Acids Decrease Osteoclastogenesis and Loss of Bone Mass in Ovariectomized Mice. J Bone Miner Res. 2003;18(7):1206–1216. doi: 10.1359/jbmr.2003.18.7.1206. [DOI] [PubMed] [Google Scholar]
- 45.Fang H, Beier F. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 2014 Mar 25; doi: 10.1038/nrrheum.2014.46. advanced online publication. doi:10.1038/nrrheum.2014.46. [DOI] [PubMed] [Google Scholar]
- 46.Torres-Guzman AM, Morado-Urbina CE, Alvarado-Vazquez PA, Acosta-Gonzalez RI, Chávez-Piña AE, Montiel-Ruiz RM, et al. Chronic oral or intraarticular administration of docosahexaenoic acid reduces nociception and knee edema and improves functional outcomes in a mouse model of complete Freund’s adjuvant-induced knee arthritis. Arthritis Res Ther. 2014;16(2):R64. doi: 10.1186/ar4502. [DOI] [PMC free article] [PubMed] [Google Scholar]