Abstract
Neuropathic pain is currently an insufficiently treated clinical condition. There remains a critical need for efficacious therapies without severe side effects to treat the uniquely persistent and tonic pain of neuropathy. Inhibitors of the soluble epoxide hydrolase (sEH) enzyme which stabilize endogenous epoxy fatty acids have demonstrated antihyperalgesia in clinical chronic inflammatory pain and modeled neuropathic pain. Recently, the conditioned place preference (CPP) assay has been used to evaluate the tonic nature of neuropathy in several animal models. The current experiments use the CPP assay alongside withdrawal thresholds to investigate the antihyperalgesic efficacy of sEH inhibitors in a murine model of diabetic neuropathy. Here, the sEH inhibitor t-TUCB at 10 mg/kg induced a robust place preference in diabetic neuropathic mice representative of pain relief. Importantly, this effect was absent both in control mice and in sEH knockout mice at the same dose indicating t-TUCB is not positively reinforcing or rewarding. When compared to gabapentin, t-TUCB elicited a similar degree of withdrawal threshold improvement without the same degree of spontaneous locomotion decline in neuropathic mice. Overall, these experiments show that inhibiting the sEH attenuates chronic pain and offers an alternative to current side-effect limited therapies to meet this clinical need.
Introduction
Neuropathy is a debilitating condition currently with no adequate therapy. Despite decades of research investigating alternatives to treat chronic pain, few improvements have been made and it remains a largely unmet clinical need. This need is becoming urgent as the diabetic population increases worldwide and neuropathy occurs in one of every two patients 8, 12. The pain of diabetic neuropathy is due to nerve damage that progresses to central sensitization of the nervous system characterized by ectopic and idiopathic firing of nerves 21. This pain is felt both as hyperalgesia, a hypersensitivity to painful stimuli, and allodynia, a painful response to innocuous stimulation.
The arachidonic acid (ARA) cascade has been exploited for decades to alter pain sensation and inflammation. Several classes of enzymes, including the cyclooxygenase, lipoxygenase, and cytochrome P450 epoxygenase (P450), metabolize the parent polyunsaturated fatty acids into bioactive lipid metabolites. The most well-known metabolites are the prostaglandins formed by cyclooxygenase enzymes and in particular, prostaglandin E2 (PGE2) a sensitizing and directly acting algogen. Recently the importance of epoxy fatty acids as signaling mediators and their functional significance in nociception has received attention. The epoxy fatty acids formed by P450 enzymes are chemically stable, though are rapidly degraded by the soluble epoxide hydrolase (sEH) enzyme. Small molecule inhibitors of sEH have been used to stabilize and elevate levels of these natural molecules in vivo, allowing observation of their antinociceptive activity. Inhibiting the sEH has been shown to be antihyperalgesic in models of inflammatory pain15, 16. These experiments used a quantitative metabolomic profile to show this antihyperalgesia was correlated to changes in the epoxy fatty acid substrate to corresponding diol product ratios after sEH inhibition. Additionally, application of exogenous epoxyeicosatrienoic acids (EETs) formed from ARA has been shown to block pain in rodents 15. Subsequently the epoxides of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have also demonstrated antihyperalgesia in modeled pain 26. Because epoxy fatty acid metabolites of all three classes have been shown to be substrates of the sEH inhibiting this enzyme is a uniquely suited strategy for eliciting antihyperalgesia. Recently, sEH inhibition also successfully treated a clinical case of severe chronic neuropathic pain in equine laminitis 13. Here, we test the antihyperalgesic efficacy of sEH inhibitors in a model of chronic pain, specifically diabetic neuropathy.
The von Frey assay is a traditional measure of allodynia using a narrow filament to probe for increased sensitivity to innocuous mechanical stimulation. However, clinical descriptions of diabetic neuropathy often include a tonic, persistent pain that is not stimulus evoked1, 3. While pin prick assays are still used clinically, these assays measure the response to an acutely applied stimulus, and thus do not represent the tonic nature of neuropathy 4, 25. Consequently there are limitations to using only withdrawal threshold assays as measures in modeled neuropathic pain 32. Recently the conditioned place preference (CPP) paradigm has been used to address these limitations when investigating neuropathic pain 10, 17, 28. The CPP uses a non-evoked and drug-free testing paradigm to assess pain. It has therefore been suggested that the CPP assay allows for a better assessment of tonic pain 10, 17, 33. An added advantage of the CPP assay in testing analgesics is its ability to assess both the negative (relief of a pain status) and positive (rewarding) reinforcing effects of compounds associated with environmental cues 5, 32, 36.
Here, we employed the CPP assay to determine the effects of sEH inhibition on diabetic neuropathy. We then used the CPP assay to test for reward or positive reinforcement associated with the small molecule sEH inhibitor in both wild type and sEH null mice.
Materials and Methods
Animals
All procedures and animal care adhered to the guidelines of the National Institutes of Health guide for the care and use of Laboratory animals and were performed in accordance with the protocols approved by the Animal Use and Care Committee (IACUC) of the University of California, Davis. Great care was taken to minimize suffering of the animals and to reduce the number of animals used. Experiments on wild type mice used groups of male C57BL/6 mice (20–22 grams) purchased from Charles River Laboratories. Experiments on sEH knockout mice used mice on a 129X1/SvJ _C57BL/6 background, backcrossed over ten generations with targeted disruption of the Ephx2 gene and maintained at the facilities of the University of California, Davis 30. Both wild type and sEH null mice were housed under standard conditions (25°C) in a fixed 12-h light/dark cycle with ad libitum food and water.
To induce diabetes, wild type mice were injected with 150 mg/kg i.p. streptozocin 9. After one week the mice were assessed for a decrease in hindpaw mechanical withdrawal thresholds indicating allodynia and tested for their blood glucose levels via tail vein blood.
For quantification of the sEH inhibitor, whole blood was collected per Liu et al. 23. Briefly, 10μL whole blood was taken via the tail vein with a pipette tips rinsed with 7.5% EDTA(K2) and added to 50μL deionized water before and 15, 30, 60 and 90 minutes after dosing with the inhibitor. Samples were taken from eight mice per group and the mean plasma concentration ± standard error of the mean (S.E.M.) is reported.
Chemicals
The sEH inhibitor t-TUCB: trans-4-[4-(3-trifluoromethoxyphenyl-l-ureido)-cyclohexyloxy]-benzoic acid (also referred to as UC1728) used for the experiments was synthesized and characterized in house as previously described 14. The sEH inhibitor t-TUCB has a mass of 438.3 g/mole, a 1.6 cLogP and 212.2 °C melting point. It is soluble in water at 5 μg/ml but up to 10 mg/ml in polyethylene glycol (PEG400). As a high melting crystal it dissolves slowly in water and thus is first dissolved in an organic co-solvent. t-TUCB is a potent low nanomolar inhibitor in vitro on murine recombinant sEH enzyme determined with an α-cyanocarbonate substrate in a fluorescent assay 22, 38. Doses of t-TUCB were formulated in PEG400 for the experiments and injected subcutaneously. Morphine sulfate and gabapentin purchased from Fisher Scientific, USA were diluted in saline and injected subcutaneously for morphine and intraperitoneally for gabapentin.
Nociceptive and Motor Skill Bioassays
A conditioned place preference apparatus was based on the method of Carr et al. with modifications 6. The apparatus is a 30×16×20 cm rectangular acrylic box with distinct visual patterns and a floor with tactilely distinct sides of equal size. The patterned visual cues avoided light and dark solid visual environments that influence conditioning given the basal preference of rodents for dark conditions. Mice were first habituated to the box for 30 minute sessions at the same time of day on 2 consecutive days. For the pre-conditioning measurement mice were placed into the apparatus with access to both chambers and observed for 30 minutes. Preconditioning was followed by 3 conditioning days where the vehicle was counterbalanced daily with the compounds each for 30 minute intervals. Briefly, the mice received an injection of vehicle and were immediately placed in the box isolated to one chamber in the morning. At least four hours after the vehicle, the same mouse was injected with sEH inhibitor or drug and immediately isolated to the counterbalanced chamber. On the next day after this, mice were tested for preference by being placed in the box with free access to both chambers and observed drug free for 30 minutes. The preconditioning day and test day were videoed and analyzed with custom software that tracked the mouse and calculated the time spent in either chamber of the box. Measurements were calculated as test minus preconditioning time in the drug paired chamber. There was a slight bias in the baseline of all mice tested and therefore mice were conditioned with vehicle to their preferred chamber and compounds to the non-preferred chamber. Increased time spent in a chamber (e.g. increased time in the drug-paired, non-preferred chamber) indicated preference for that chamber. The PEG400 vehicle was tested in a separate group of mice following the same procedure for test compounds. The vehicle control group received PEG400 counterbalanced to the non-preferred chamber at least 4 hrs after sham injection and placement in the preferred chamber. Several preliminary tests to refine the experimental conditions showed no difference in PEG400 vehicle or saline injections compared to naïve mice.
For the von Frey assay an electronic von Frey aesthesiometer (IITC, Woodland Hills, CA) fitted with a 0–90 gram probe arm was used to quantify allodynia in the diabetic mice. Mice were placed in clear acrylic chambers on a steel mesh floor. The hind paw of the mouse was probed through the mesh with a rigid tip probe connected to an electronic readout pressure meter displaying the grams of force required to elicit a hind paw withdrawal. The withdrawal thresholds were measured 3 times per mouse at 1 minute intervals for each point. The scores are reported with the diabetic baseline normalized to 100% and subsequent scores as the percent of diabetic baseline (time point/diabetic baseline ×100). The graph depicts the mean and S.E.M. for a group of mice tested on the same day under the same conditions. The mean mechanical withdrawal thresholds for the groups tested for the time course with vehicle, t-TUCB and gabapentin declined from an average pre-diabetic baseline of 8.0± 0.2 to 3.4± 0.2 grams of force as the diabetic baseline. Diabetic mice tested in the CPP paradigm were assessed for allodynia before the beginning of the assay. The mean mechanical withdrawal thresholds for these mice decreased from an average 8.3± 0.2 to 3.0± 0.3 grams of force to elicit a hindpaw withdrawal indicating allodynia. Mechanical withdrawal thresholds for non-diabetic control mice do not decrease and remain at their baseline levels without significant deviation (8.5± 0.3 to 8.8± 0.4 grams of force) over the same time period.
For observation of spontaneous locomotion, mice were assessed in an open-field arena (40×40×30 cm) of a 16-square grid clean floor with slight modifications from Luria et al. 24. The open field assay was tested for 2 minutes once before injection as the diabetic baseline (BL), and then at 30 min and 60 minutes post injection. Mice were tested for 2 minutes and returned to their home cage for each interval. The open field assay was scored manually with the score a combination of vertical rears and lines crossed completely with both hind paws. Scores are reported as the percent of diabetic baseline values (normalized to 100%) on the day of treatment averaged per group ± S.E.M.
Statistics
Data were analyzed using SigmaPlot 11.0 for windows (Systat Software Inc., San Jose, CA). The applied statistical methods are reported in the results section with p values ≤ 0.05 considered significant.
Results
Dose Response of t-TUCB in the CPP assay
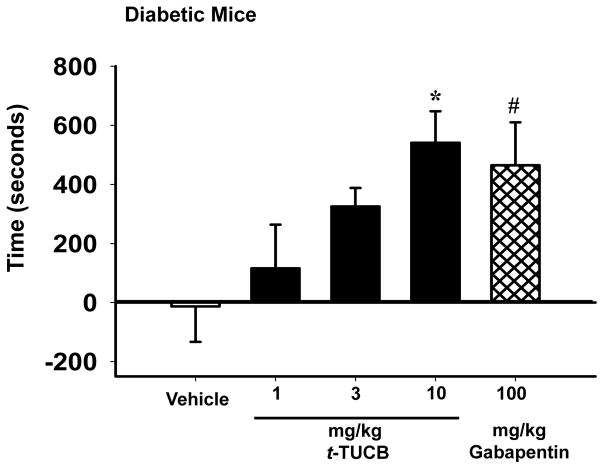
Prior to the CPP assay allodynia was confirmed in the diabetic mice. The average was a 63 % decrease in mechanical withdrawal thresholds compared to pre-diabetic baselines. We then tested t-TUCB in these mice to assess the negative reinforcing effects of sEH inhibition in the CPP assay. The effects were measured and calculated as the amount of time spent in the drug paired chamber at the post conditioning test minus the preconditioning time in the drug paired chamber. Increased time in the drug-paired chamber indicated preference for that chamber. Diabetic C57/B6 male mice showed no place preference for the vehicle-paired chamber in the CPP assay (Fig 1). The dose range of 1, 3, and 10 mg/kg/day t-TUCB exhibited a clear dose response relationship in this assay. The 1 mg/kg/day dose of t-TUCB showed no statistically significant change for time spent in the drug-paired chamber (Fig 1). An intermediate dose of 3 mg/kg/day resulted in an increased conditioned place preference that was significant when compared to vehicle controls (T-test, t(10) = 2.493, p = 0.032, n=6). However, when the entire dose range was analyzed for the effect of dose on CPP acquisition with a One Way ANOVA, 10 mg/kg/day t-TUCB remained the only statistically significant dose compared to vehicle controls. At this dose, t-TUCB induced a robust place preference for the drug-paired chamber in neuropathic mice indicating antihyperalgesia (All t-TUCB doses, One Way Analysis of Variance, Holm-Sidak method, F(3,20) = 5.141, p= 0.008 vs. vehicle, n=5–7). Gabapentin, a positive control for diabetic neuropathy, produced an expected place preference in the diabetic mice (T-test, t(12) = 2.307, p = 0.040 vs. vehicle, n=6–8 Fig 1).
Figure 1.
Repeated administration of t-TUCB relieves diabetic neuropathy in the CPP assay. Increasing the dose of t-TUCB results in a clear dose response relationship in the CPP assay depicted as the change in time (test-preconditioning) spent in the drug paired chamber. The 10 mg/kg/day dose of t-TUCB significantly increases the conditioned place preference for the drug paired chamber (* p= 0.008). The positive control gabapentin (100 mg/kg/day) induces a drug-paired chamber preference compared to the vehicle (# p= 0.040). However, the 10 mg/kg/day dose of t-TUCB outperformed the positive control gabapentin at 10 fold lower dose.
Effects of t-TUCB in withdrawal threshold and open field assays
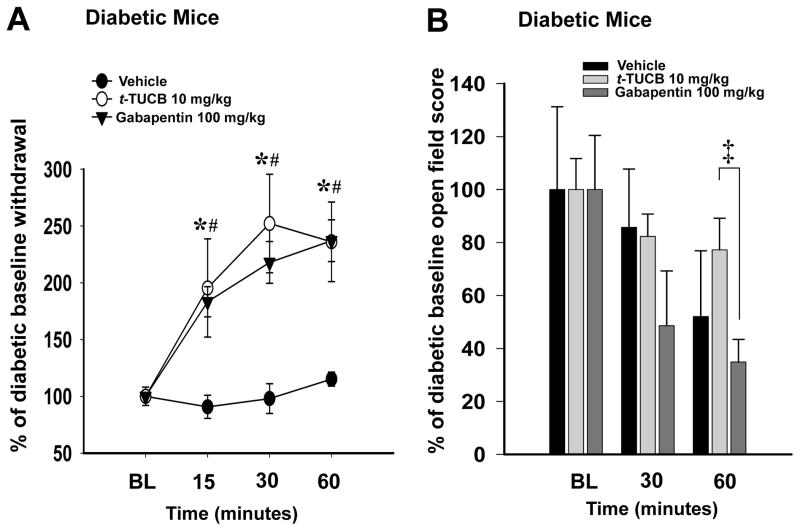
Separate groups of diabetic mice were subsequently treated with a single administration of t-TUCB (10 mg/kg) or gabapentin (100 mg/kg) or vehicle and tested in the von Frey assay. The diabetic mice treated with t-TUCB and gabapentin significantly increased their withdrawal thresholds at each point in the time course while the PEG400 vehicle had no effect (Two Way Repeated Measures Analysis of Variance, Holm-Sidak method, (main effects: time) F(2,34) = 14.034, p ≤ 0.001, n=6–8, the interaction was not significant F(4,34) = 2.031, p=0.112 Fig 2A). Post hoc analysis showed both the t-TUCB and gabapentin treatments were also significant compared to the vehicle (main effects: treatment) F(2,34) = 8.147, p = 0.003 Fig 2A). However, there was no significant difference between the analgesia mediated by t-TUCB and gabapentin over the time course (p = 0.647). To investigate if the withdrawal responses were related to changes in sedation or motor skill the mice were also tested in the open field assay (Fig 2B). The open field scores of all treatment groups tended to decline over time possibly associated with habituation to the open field apparatus. However, statistical analysis showed no significant changes (Two Way Repeated Measures Analysis of Variance, Holm-Sidak method, main effects: treatment F(2,17) = 2.550, p ≤ 0.108, n=4–10, time F(1,17) = 3.549, p =0.007, n=4–10 the interaction was not significant F(2,17) = 0.781, p=0.112 Fig 2B). When compared directly at the 60 minute time point, the spontaneous motor activity of gabapentin treated mice was significantly reduced compared to t-TUCB (T-test, t(14)= 2.417, p = 0.030, n=6–10).
Figure 2.
A single dose of t-TUCB relieves pain similar to gabapentin. (A) A single 10 mg/kg dose of t-TUCB increases mechanical withdrawal threshold scores from painful diabetic baselines (BL) normalized to 100% (* p ≤ 0.001). A single dose of gabapentin 100 mg/kg also increases mechanical withdrawal thresholds (# p ≤ 0.001). Gabapentin analgesia is not significantly increased compared to t-TUCB (p= 0.647). (B) The analgesia mediated by a single dose of gabapentin also correlates with a decrease in open field activity at 60 minutes post administration compared to t-TUCB (‡ p = 0.030).
Effects of t-TUCB in controls and blood concentration assessment
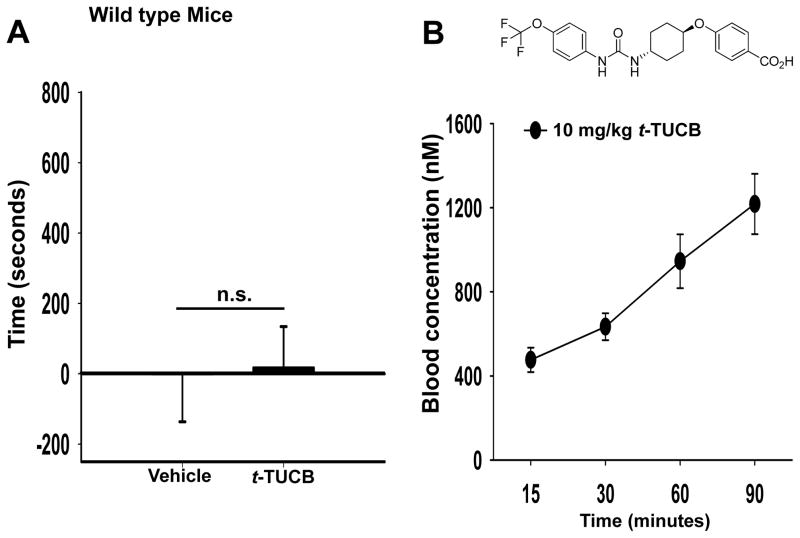
We tested t-TUCB in control animals absent of pain to assess potential positive reinforcing effects of sEH inhibition. In non-diabetic C57/B6 male mice the PEG400 vehicle had no effect on place preference (Fig 3A). Notably, the sEH inhibitor t-TUCB at 10 mg/kg/day also did not induce a place preference in non-diabetic control mice (T-test, t(9) = 0.0896, p = 0.931 vs. vehicle, n=4–7 Fig 3A). These results indicate that t-TUCB is devoid of a positively reinforcing effect. To confirm the presence of t-TUCB in the absence of a place preference we administered 10 mg/kg of t-TUCB in control mice and determined the blood concentration to correlate to the nociceptive assay results. The graph depicts the mean ± S.E.M. for a group of male C57/B6 control mice, n=8 Fig 3B. A single 10 mg/kg subcutaneous dose resulted in a blood concentration of more than 40X–100X of the reported murine IC50 of 11nM 22. Additionally, the half-life of t-TUCB at a lower dose of 3 mg/kg in mice was reported to be a minimum of 3.1 hours which extends longer than the conditioning phase of the CPP assay. At these doses there is also an increase in epoxide to diol ratio indicating significant target engagement 22.
Figure 3.
Repeated administration of t-TUCB is not active in absence of pain state. (A) In control mice the vehicle does not significantly alter place preference. Similarly in non-diabetic controls, t-TUCB at 10 mg/kg/day for 3 days does not induce a preference for the drug-paired chamber (n.s. p = 0.931). (B) Although no place preference is induced the blood concentration of t-TUCB is well above the IC50 for the duration of the CPP assay. A single 10 mg/kg administration of the inhibitor results in blood concentration 40X the IC50 after 15 minutes and continues to increase to over 100X the IC50 by 90 minutes.
Effects of t-TUCB on sEH knockout mice in the CPP assay
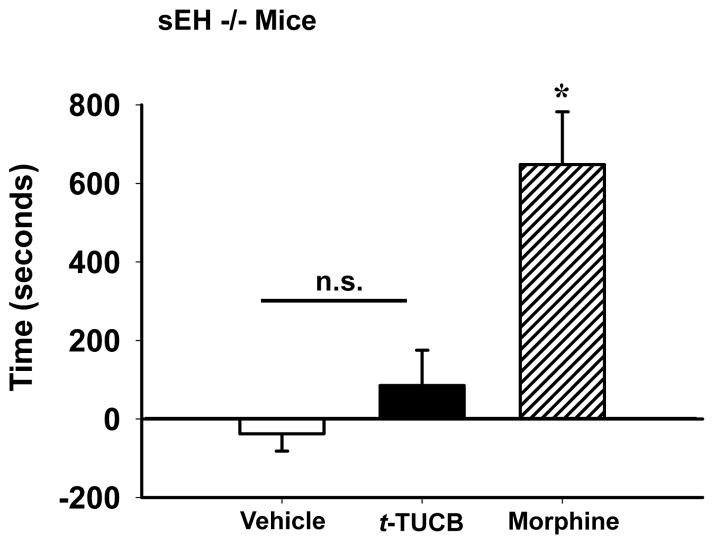
We then used sEH knockout mice to address the possibility that the sEH inhibitor could have a positively reinforcing effect unrelated to the inhibition of the sEH enzyme. As expected, there was no effect of the PEG400 vehicle on place preference in the sEH knockout mice (Fig 4). When administration of t-TUCB at 10 mg/kg/day for 3 days was tested per the post hoc test there was no significant increase compared to vehicle in the time spent in the t-TUCB-paired chamber. Morphine was also tested in sEH knockout mice to ensure their capacity to respond to a positive control and induced a robust place preference (One Way Analysis of Variance, Holm-Sidak method, F(2,18) = 10.988, p ≤ 0.001, n=6–8 Fig 4). The lack of induced place preference in sEH knockout mice is consistent with results in non-diabetic wild type controls indicating there are no positive reinforcing effects of t-TUCB.
Figure 4.
t-TUCB does not induce conditioned place preference in sEH knockout mice. At 10 mg/kg/day in sEH knockout mice t-TUCB does not significantly increase time spent in the drug-paired chamber (test-preconditioning) compared to vehicle. However, morphine showed a characteristic conditioned place preference in the sEH null mice. Morphine at 10 mg/kg/day significantly increased time spent in the drug paired chamber compared to both t-TUCB and vehicle (* p ≤ 0.001).
Discussion
This study demonstrates that inhibiting the soluble epoxide hydrolase enzyme can attenuate chronic pain in a model of murine diabetic neuropathy. The sEH inhibitor t-TUCB exhibits a clear dose response relationship and equals the effects of gabapentin, a first line therapy for this condition. Additionally, t-TUCB does not induce place preference in control or sEH knockout mice indicating there are no rewarding side effects of inhibition of the sEH enzyme or the small molecule inhibitor itself.
Withdrawal thresholds and open field assays
The use of withdrawal threshold assays is a standard protocol for determining pain like behavior in rodent models. We used the von Frey assay to verify allodynia in the diabetic mice for this study which assesses sensitivity to innocuous mechanical stimulation. We also tested the sEH inhibitors with this reflex withdrawal assay to confirm the CPP results. In these studies administration of the sEH inhibitor to neuropathic mice significantly improved their mechanical withdrawal thresholds. We compared 10 mg/kg t-TUCB to 100 mg/kg gabapentin, both of which effectively increased neuropathic withdrawal thresholds. The side effects of gabapentin including sedation and loss of motor activity have been previously established in mice. It is possible that the motor skill impairment may also affect reflex withdrawals. Here, we used 100 mg/kg gabapentin which was reported as a minimally effective dose in murine neuropathic pain models 18, 20. However, a slightly lower dose of 90 mg/kg has been shown to reduce both rotorod performance and spontaneous motor activity in mice 11. In contrast to these effects of gabapentin, 10 mg/kg t-TUCB treated mice previously displayed no observable effects on sedation or loss of spontaneous motor activity 19. The results of the current experiments using t-TUCB in the open field assay show a trend, though not significant, of increasing spontaneous motor activity with t-TUCB treatment over time. It is possible that the unexpected trend of t-TUCB induced locomotor increases could be due to anxiolytic effects. An important distinction regarding the open field assay emphasized by Prut et al. is that given the gregarious nature of rodents and their fear of open spaces the drug effect measure is on the behavioral change to a stressful event rather than on increasing exploration 29. Interestingly, the opioid analgesic morphine also has a paradoxical effect of increasing spontaneous locomotion at moderate to high doses in rodents 31. There is an indication that some of the antinociceptive activity of EETs and other epoxy fatty acids may be mediated through the endogenous opioid system 7, 34. While it is possible that the epoxy fatty acids could be acting through the opioid system, sEH inhibition lacks other characteristic opioid effects such as positive reinforcement in the CPP assay seen here with morphine or hypoalgesia in the von Frey assay 38. The spontaneous movement associated with t-TUCB administration could be associated with pain relief and a change from the basal pain-like behavior in diabetic animals. While the basis for the different result in spontaneous movement is unknown, it is evident that t-TUCB does not share the side effect of sedation with gabapentin.
CPP negative reinforcement
The CPP is an alternative for assessing the on-going tonic pain of neuropathy by using non-evoked conditions to measure pain relief 33. This is compared to reflex withdrawal tests that use acutely applied stimulus to measure response. The CPP results in these experiments have revealed that sEH inhibition relieves diabetic neuropathic pain in mice in a dose dependent manner. There was a positive dose response relationship although 1 mg/kg t-TUCB did not induce a CPP place preference. However, the 10 mg/kg/day dose of t-TUCB was effective in reducing pain related behavior evidenced by a robust drug-paired chamber preference in neuropathic mice. It was surprising that only the high dose of t-TUCB was statistically significant because sEH inhibitors typically have biological outcomes at much lower doses than those used in these experiments 23, 37. However, previous use of t-TUCB in the rat also demonstrated 10 mg/kg to be the minimum significantly effective dose in the diabetic neuropathy model compared to lower doses which were effective on inflammatory pain38. The plasma concentrations of t-TUCB in mice measured in this study do not reach a maximum within 30 minutes which is equivalent to the duration of the conditioning in the CPP assay. However, at 10 mg/kg, a single administration of t-TUCB reached maximal efficacy by 30 minutes in diabetic mice in the von Frey assay and a single 10 mg/kg dose reached over 60x the murine IC50 in control mice within this time period. The difference in effective dose compared to other outcomes could also be due to the requirement of conditioning in the CPP assay. As described by Bardo et al. the necessity for temporal contiguity to achieve a place preference was accounted for in this study by immediately placing the mice in the drug paired chamber after injection 2. The onset of the drug effect, in this case alleviating pain, was demonstrated by both increased withdrawal thresholds in the von Frey assay and induced place preference in the CPP assay within 30 minutes of administration. The positive control for these experiments gabapentin is a first line therapy to treat human diabetic neuropathy. Gabapentin has been found minimally effective after nerve ligation induced neuropathy at 100mg/kg 18, 20. Therefore, a dose of 100 mg/kg gabapentin was chosen for the positive control. Gabapentin induced a statistically significant preference for the drug-paired chamber in diabetic mice. The lack of effect in control mice administered 100mg/kg gabapentin has been previously observed 28. The previous studies differed in the model and assay parameters they employed compared to the current experiments. To our knowledge the experiments here are the first to examine diabetic neuropathy in the CPP assay. While the 100mg/kg dose of gabapentin induced a place preference in diabetic neuropathic mice it was outperformed by a 10 fold lower dose of the sEH inhibitor. The determination of a minimal effective dose of gabapentin in this model was not the object of these experiments. However, previously a matching dose of 10 mg/kg of gabapentin was ineffective against streptozocin induced neuropathy in mice using a tail flick assay 35. Furthermore, these observations in mice are consistent with a recently reported equine study where a sEH inhibitor reversed the severe inflammatory and neuropathic pain associated with terminal laminitis 13. In this case the patient failed to respond to the standard of care steroid, NSAID, and gabapentin treatment. The complete recovery of the thoroughbred suggests that the efficacy of sEH inhibition reported in mice in the CPP assay extends beyond rodent models.
CPP positive reinforcement
The conditioned place preference paradigm was originally designed for investigating drug reinforcement and has been used to test the rewarding quality of several addictive drugs 5, 27, 36. This is relevant to pain research because opioids as the most potent class of analgesics have reward and addiction side effects that limit their use. Given the potent antihyperalgesia mediated by use of sEH inhibitors, the CPP paradigm was used to also investigate possible positive reinforcement (reward) upon t-TUCB administration. Importantly, there was no effect of the 10 mg/kg/day dose in non-diabetic control mice in the CPP assay. This is despite the plasma levels in control mice demonstrating a concentration 40x of the murine IC50 within 15 minutes after a subcutaneous injection of the inhibitor. Thus, there were no observable rewarding effects mediated by sEH inhibition in control mice. This result in controls, while remarkable, was expected because the sEH inhibitors have demonstrated a lack activity in the absence of a painful state, even at high dose 16, 38. It is also essential to note that the controls treated with 10 mg/kg t-TUCB also did not show an aversion to the drug-paired chamber. An aversion to the drug-paired chamber may indicate negative physiologic or sensory effects related to the treatment 25, 36. Overall, the outcome indicates the sEH inhibitor is not active in the absence of a painful state and does not have effects that induce aversion in the CPP assay. The results in the sEH knockout mice further support the absence of positive reinforcement related to t-TUCB. The repeated administration of the inhibitor at the same 10 mg/kg dose in sEH null mice did not produce a place preference. Therefore, there are also no observed rewarding effects of the small molecule inhibitor independent of sEH inhibition. When morphine was tested in the sEH null mice as a positive control for reward there was a robust place preference response. Thus, the inhibitor did not demonstrate positive reinforcement in mice lacking the target enzyme and additionally these sEH null mice respond appropriately to reward conditioning. This is the first test of potential off target rewarding effects of a sEH inhibitor, though these inhibitors were previously known to have no hypoalgesic effects in control animals. Given the lack of positive reinforcing effects and high efficacy against neuropathy, we believe inhibiting the sEH enzyme has potential as a novel strategy in treating chronic pain.
Supplementary Material
Perspective.
These experiments demonstrate antihyperalgesia in a murine chronic pain model mediated by inhibiting the soluble epoxide hydrolase (sEH) enzyme. The results of this study indicate that inhibiting the sEH is a promising alternative for blocking chronic pain.
Acknowledgments
We thank Drs. Carstens and Akiyama of UC Davis for their assistance with the conditioned place preference assay. We thank Bo Wang for developing the tracking software used to measure the assay results and Edward Hackett for technical assistance.
Research Funding: This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Grant R01 ES002710 (to B.D.H.), NIEHS Superfund Research Program P42 ES004699, NIH/NIAMS R21AR062866 and Grants NIEHS T32ES007059 and NIH 5T32DC008072-05 (to K.W.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Foundation.
Footnotes
Conflict of interest: The University of California holds patents on the sEH inhibitors used in this study as well as their use to treat inflammation, inflammatory pain, and neuropathic pain. BD Hammock and B Inceoglu are co-founders of Eicosis L.L.C., a startup company advancing sEH inhibitors into the clinic. K Wagner and J Yang have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backonja MM, Stacey B. Neuropathic pain symptoms relative to overall pain rating. The journal of pain: official journal of the American Pain Society. 2004;5:491–497. doi: 10.1016/j.jpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn-Munro G, Erichsen HK. Antiepileptics and the treatment of neuropathic pain: evidence from animal models. Current pharmaceutical design. 2005;11:2961–2976. doi: 10.2174/1381612054865000. [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 5.Carr GD, Fibiger HC, Phillips AG. Conditioned place preference as a measure of drug reward. In: Liebman JMC, SJ, editors. The Neuropharmacological Basis of Reward. Clarendon Press; Oxford: 1989. pp. 264–319. [Google Scholar]
- 6.Carr GD, White NM. Conditioned place preference from intra-accumbens but not intra-caudate amphetamine injections. Life Sciences. 1983;33:2551–2557. doi: 10.1016/0024-3205(83)90165-0. [DOI] [PubMed] [Google Scholar]
- 7.Conroy JL, Fang C, Gu J, Zeitlin SO, Yang W, Yang J, VanAlstine MA, Nalwalk JW, Albrecht PJ, Mazurkiewicz JE, Snyder-Keller A, Shan Z, Zhang S-Z, Wentland MP, Behr M, Knapp BI, Bidlack JM, Zuiderveld OP, Leurs R, Ding X, Hough LB. Opioids activate brain analgesic circuits through cytochrome P450/epoxygenase signaling. Nat Neurosci. 2010;13:284–286. doi: 10.1038/nn.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 9.Davidson E, Coppey L, Lu B, Arballo V, Calcutt NA, Gerard C, Yorek M. The roles of streptozotocin neurotoxicity and neutral endopeptidase in murine experimental diabetic neuropathy. Experimental diabetes research. 2009;2009:Article ID 431980. doi: 10.1155/2009/431980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU. HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology. 2011;60:626–632. doi: 10.1016/j.neuropharm.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guedes AGP, Morisseau C, Sole A, Soares JHN, Ulu A, Dong H, Hammock BD. Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis. Vet Anaesth Analg. 2013;40:440–448. doi: 10.1111/vaa.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–2319. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105:18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiso T, Watabiki T, Tsukamoto M, Okabe M, Kagami M, Nishimura K, Aoki T, Matsuoka N. Pharmacological characterization and gene expression profiling of an L5/L6 spinal nerve ligation model for neuropathic pain in mice. Neuroscience. 2008;153:492–500. doi: 10.1016/j.neuroscience.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 19.Kundu S, Roome T, Bhattacharjee A, Carnevale KA, Yakubenko VP, Zhang R, Hwang SH, Hammock BD, Cathcart MK. Metabolic products of soluble epoxide hydrolase are essential for monocyte chemotaxis to MCP-1 in vitro and in vivo. Journal of lipid research. 2013;54:436–447. doi: 10.1194/jlr.M031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kusunose N, Koyanagi S, Hamamura K, Matsunaga N, Yoshida M, Uchida T, Tsuda M, Inoue K, Ohdo S. Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Molecular pain. 2010;6:83. doi: 10.1186/1744-8069-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu JY, Lin YP, Qiu H, Morisseau C, Rose TE, Hwang SH, Chiamvimonvat N, Hammock BD. Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2013;48:619–627. doi: 10.1016/j.ejps.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu JY, Tsai HJ, Hwang SH, Jones PD, Morisseau C, Hammock BD. Pharmacokinetic optimization of four soluble epoxide hydrolase inhibitors for use in a murine model of inflammation. Br J Pharmacol. 2009;156:284–296. doi: 10.1111/j.1476-5381.2008.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luria A, Morisseau C, Tsai HJ, Yang J, Inceoglu B, De Taeye B, Watkins SM, Wiest MM, German JB, Hammock BD. Alteration in plasma testosterone levels in male mice lacking soluble epoxide hydrolase. Am J Physiol Endocrinol Metab. 2009;297:E375–383. doi: 10.1152/ajpendo.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 26.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mucha RF, van der Kooy D, O’Shaughnessy M, Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. 1982;243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- 28.Park HJ, Stokes JA, Pirie E, Skahen J, Shtaerman Y, Yaksh TL. Persistent hyperalgesia in the cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesthesia and analgesia. 2013;116:224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. European Journal of Pharmacology. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 30.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–40510. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 31.Stevens KE, Mickley GA, McDermott LJ. Brain areas involved in production of morphine-induced locomotor hyperactivity of the C57B1/6J mouse. Pharmacology, biochemistry, and behavior. 1986;24:1739–1747. doi: 10.1016/0091-3057(86)90514-9. [DOI] [PubMed] [Google Scholar]
- 32.Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 33.Sufka KJ. Translational challenges and analgesic screening assays. Pain. 2011;152:1942–1943. doi: 10.1016/j.pain.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther. 2008;326:614–622. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomić MA, Vučković SM, Stepanović-Petrović RM, Micov AM, Ugrešić ND, Prostran MŠ, Bošković B. Analysis of the antinociceptive interactions in two-drug combinations of gabapentin, oxcarbazepine and amitriptyline in streptozotocin-induced diabetic mice. European Journal of Pharmacology. 2010;628:75–82. doi: 10.1016/j.ejphar.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- 37.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, Inceoglu B, Fiehn O, Hammock BD, Weiss RH. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in apolipoprotein e-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–323. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner K, Inceoglu B, Dong H, Yang J, Hwang SH, Jones P, Morisseau C, Hammock BD. Comparative efficacy of 3 soluble epoxide hydrolase inhibitors in rat neuropathic and inflammatory pain models. European Journal of Pharmacology. 2013;700:93–101. doi: 10.1016/j.ejphar.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.