Abstract
In many infections, especially those that are chronic such as Herpes Simplex Virus-1 (HSV-1), the outcome may be influenced by the activity of one or more types of regulatory T cells (Tregs). Some infections can cause Treg expansion, but how viruses might promote preferential Treg expansion is has been unclear. In this report, we demonstrate a possible mechanism by which HSV (Herpes Simplex virus-1) infection could act to signal and expands the Treg population. We show that CD4+ FoxP3+ Tregs up- regulate HVEM (herpes virus entry mediator), which is a binding site for major viral glycoprotein HSVgD, following HSV infection, which is a binding site for major viral glycoprotein HSVgD. Recombinant HSVgD enhanced the proliferation of CD4+ FoxP3+ Tregs cells in vitro. Furthermore, compared to wild type (WT), HVEM deficient mice (HVEM−/−) generated a weaker Treg responses represented by significantly diminished ratios of CD4+FoxP3+/CD4+FoxP3− cells along with diminished proportions of FoxP3+ Tregs cells co-expressing Treg activation markers and a reduced MFI of FoxP3 expression on CD4+ T cells. Consistent with defective Treg responses, HVEM−/− animals were more susceptible to HSV-1 induced ocular immunopathology, with more severe lesions in HVEM−/− animals. Our results indicate that HVEM regulates Treg responses, and its modulation could represent a useful approach to control HSV induced corneal immunopathology
Keywords: HSV-1, TREGS, HSK, HVEM, gD
1. Introduction
The magnitude of a T cell mediated immune response to an acute viral infection may be influenced by the activity of one or more types of regulatory T cells (Tregs)[1], particularly those that express FoxP3. Whereas the magnitude of protective immunity can be limited by Tregs [1], this can be beneficial when the response is immunopathological [2]. Variation in Treg responses among individuals may contribute to differential infection outcomes, such as in hepatitis [3]. Moreover, It is not clear if Tregs need to be pathogen-specific in order to regulate a response [3, 4]. In the case of HSV, several observations have indicated that antigen specificity is not needed but it remains unclear how non-specific Tregs can become expanded and activated [1, 2]. Here we report that HSV can expand Tregs by engaging its gD glycoprotein with the entry molecule HVEM, abundantly expressed on resting CD4+ FoxP3+ Tregs.
It has been shown that HSV infection is largely unchanged in HVEM deficient mice [5] it remains to be determined whether binding of gD to HVEM has immunomodulatory effects and how it might benefit the virus in vivo. The functional network of TNF receptor superfamily members such as HVEM is composed of complex cross-talk between multiple ligands and multiple receptors, which regulate pleiotropic functions in the immune system [6]. gD binding to HVEM was shown to be immunomodulatory, as recombinant soluble gD or fibroblasts expressing gD alone inhibited T cell proliferation. In another study, interaction of HSVgD with HVEM alters innate events in the murine immune response to infection [7]. HVEM−/− mice showed increased T cell proliferation and cytokine production in response to antigen specific challenge [8]. HVEM is also involved in NFkB dependent protection against apoptosis by HSV-1 gD.[9] T cells from scurfy mice, which have truncated form of FoxP3 [10] lacked HVEM expression even after activation and over expression of HVEM enhances Treg suppressive function [10] suggesting a role of HVEM in Treg suppressive function.
Our studies demonstrate that CD4+ FoxP3+ regulatory T cells expand and up regulate HVEM following HSV-1 infection. Additionally HSV-1gD was detectable in the draining popliteal lymph nodes (PLN) following footpad infection. Recombinant HSV-1 gD promoted the proliferative response of sorted FoxP3+ cells in vitro and consistent with this HVEM−/− animals generated weaker Treg responses represented by significantly diminished ratios of CD4+FoxP3+/CD4+FoxP3− cells along with diminished proportions of FoxP3+ cells co-expressing the Treg activation markers, a reduced MFI of FoxP3 expression on CD4 T cells and a significantly diminished proliferation following HSV-1 infection. Additionally, HVEM−/− animals were more susceptible to HSV induced corneal immunopathology and exhibited more severe lesions compared to wild type mice. Our data points towards a role of gD /HVEM interaction in promoting Treg responses.
2. Materials and Methods
2.1 Mice and Virus
Female C57BL/6 (B6) mice were purchased from Harlan Sprague-Dawley. GFP-Foxp3 knock in mice were a kind gift from Dr. M. Oukka (Brigham and Women’s Hospital, Harvard Medical School). HVEM knockout mice (HVEM−/−) mice [11] were obtained from Dr. Carl Ware at Sanford Burnham medical research institute, La Jolla, California and were bred and maintained in the animal facility at the University of Tennessee. The animals were housed in American Association of Laboratory Animal Care approved facilities at the University of Tennessee, Knoxville. All investigations followed guidelines of the Institutional Animal Care and Use Committee. HSV-1 strain KOS and HSV-1 strain RE were grown in vero cells obtained from American Type Culture Collection (Manassas, VA). The viruses were titrated, and stored in aliquots at −80°C until use.
2.2 Foot pad infection
Mice were injected subcutaneously in each hind footpad (FP) with 2×105 PFU HSV-1 Kos in 30 μl of phosphate buffered saline (PBS). Mice were sacrificed at various times post infection and the draining PLN were isolated for analysis.
2.3 Corneal HSV-1 infection and clinical observations
Corneal infections of 6–10 week old C57BL/6 mice were conducted under deep anesthesia (tribromoethanol). Mice were scarified on their corneas with 27 gauge needles and a 3 μl drop containing the required viral dose (104 PFU of HSV-1 RE) was applied to the eye. The eyes were examined on different time points post infection with a slit lamp biomicroscope (KOWA), and the clinical severity of keratitis and angiogenesis of individually scored mice was recorded. The scoring system was as described previously [12].
2.4 Antibodies and Reagents
For flow cytometry antibodies purchased from BD biosciences were PerCP-CD4, FITC and PE anti-FoxP3, APC–anti-CD25 (PC61); CD62 ligand (CD62L), CD44, CD69, CD103. PE-anti HVEM was purchased from eBioscience. Recombinant HSV-1gD (secreted form of Herpes Simplex Virus Type 1 Glycoprotein gD Synthesized by Baculovirus-Infected Insect Cells) [13] and anti- HVEM antibody were kindly provided by Dr. Gary Cohen and Dr. Roselyn Eisenberg, University of Pennsylvania, Philadelphia. Recombinant human IL-2 was obtained from Peprotech. Complete RPMI 1640 was used for in vitro cultures. All samples were collected on a FACS Calibur and data were analyzed using Flowjo software.
2.5 Surface and Bromodeoxyuridine Staining
Surface staining was performed as described previously [14] [15]. For bromodeoxyuridine (BrdU) staining, the BrdU kit from BD Biosciences was used according to the manufacturer’s instructions. Briefly, HSV-1 infected mice received i.p. (intra peritoneal) injection of BrdU (0.8 mg/ml) 1 day before termination of mice. Draining PLN cells were stained first with anti-CD4 and FoxP3 and subsequently with labeled anti-BrdU mAb. Samples were acquired on a FACS calibur and samples were analyzed with flowjo software.
2.6 Western blotting
For the detection of HSV-1gD, draining popliteal lymph nodes were dissected at the indicated time points in 300 μl of RIPA buffer containing protease inhibitor cocktail (aprotinin, PMSF and sodium orthovanadate), cell debris was removed by centrifugation and the samples stored at −80°C till used for SDS-PAGE. In brief, after 2 hours of blocking with 3% nonfat milk in TBS, membranes were incubated with 1:1000 dilution of rabbit polyclonal anti HSV-1gD antibody (Santa Cruz biotechnology) and membranes were then incubated for 1 hr with secondary antibody coupled to horse raddish peroxidase. Specific bands were detected with ImmobilionTM western; Millipore). Membranes were stripped and then reprobed to detect B actin antibody.
2.7 Cell purification and in-vitro cell culture
CD4+ T cells were purified from Foxp3-GFP knock-in animals using a CD4 T cell isolation kit, and 1×106 cells were cultured with the indicated concentrations of recombinant HSV-1gD. In some cultures anti-HVEM antibody (10μg/mL) was added to the cultures. In additional experiments FoxP3+ cells were sorted from the enriched CD4T cell population based on the GFP expression. CD4+ FoxP3+ Tregs were incubated with recombinant HSV-1gD. 48 hours post incubation Tregs in the culture were analyzed by flow cytometry for the expression of surface activation markers.
2.8 Thymidine incorporation assay
Splenocytes from the naïve GFP-FoxP3 mice were enriched for CD4+ T cells using CD4 T cell isolation kit according to the manufacturer’s protocol (Miltenyi Biotec, Auburn, CA). Tregs were sorted from this enriched CD4+ T cell population based on GFP expression. Sorted CD4+FoxP3+ Treg populations were stimulated with anti-CD3 and recombinant HSV-1gD at the indicated concentrations. The cells were plated at a density of 5x 105 in 96-well round-bottom tissue culture plates in a total volume of 200 μl of RPMI medium (Gibco). Sixteen hours before harvest, [3H] thymidine (1mCi/well; 1Ci=37GBq) was added. The cells were harvested onto a UniFilter (PerkinElmer). [3H] thymidine incorporation was measured with a scintillation counter, and the results were expressed as mean cpm from triplicate cultures.
2.9 Quantitation of HSV-1 in Foot Pad Tissues
The quantitation of HSV-1 in foot pad (FP) tissue was determined as reported by Jennings et al.[9]. Briefly, the mice were sacrificed at the indicated time post infection (p.i.), the FP surface was cleaned with 70% isopropyl alcohol, and the tissues were removed with a scalpel. The tissues were stored in virus diluent (PBS supplemented with 0.6 mM CaCl2, 0.5 mM MgCl2/H2O, 20 mg Phenol red, and 50g gentamicin sulfate per ml) at 80° C. Tissues were disrupted by homogenization in 1 ml ground glass grinders (Wheaton) and centrifuged, and the supernatant was used to assay viral titration on vero cells. Finally, plaques were visualized with crystal violet stain.
2.10 Statistical analysis
using either one-way ANOVA (Dunnett’s post hoc test) or two way ANOVA (Bonferroni test). Values of p≤0.001 (***), p≤0.01 (**), and p≤ 0.05 (*) were considered significant. Results are expressed as means ± SD.
3. Results
3.1 Infection with HSV-1 induces FoxP3+ CD4+ regulatory T cell expansion
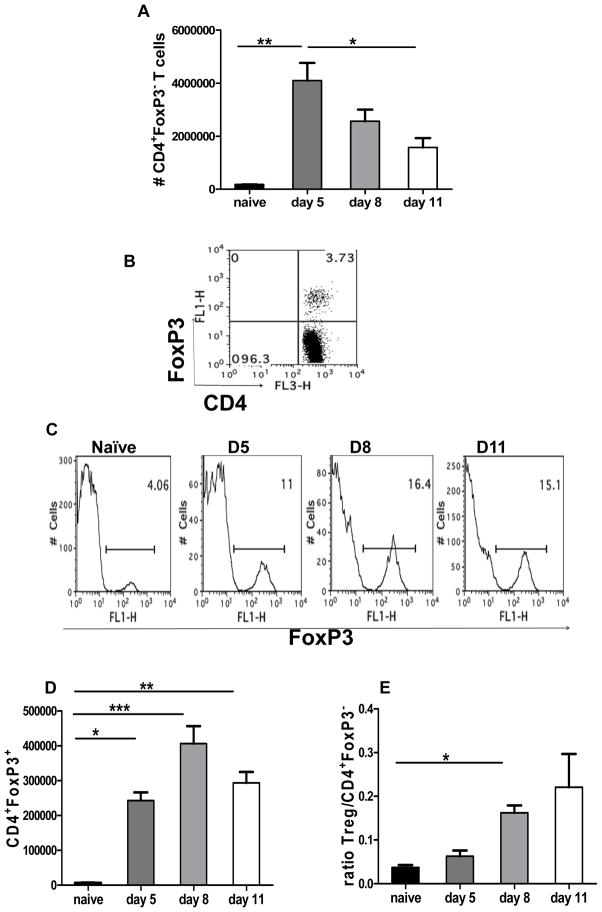
To follow changes in CD4+ FoxP3+ Treg responses after HSV infection, FoxP3-GFP knock in mice were used and analysis were performed in draining PLN populations. The infection changed the absolute numbers of CD4+ T cells enumerated. Within the total CD4+ T cell population, CD4+Foxp3− T cells expanded and reached a peak around day 5pi (post infection) (Fig. 1A), which coincides with the peak effector response [16] during acute HSV-1 infection in the hind foot pads. This was followed by a steady decline in the number of PLN CD4+ Foxp3− T cells by day 11 pi (Fig. 1A). In contrast, CD4+ FoxP3+ Tregs expanded as early as day 5 pi, but reached a peak around day 8pi i.e. after the peak effector response (Fig. 1B, C, and D). In addition, the numbers of CD4+FoxP3+ cells were relatively stable resulting in a higher ratio of CD4+FoxP3+ T cells compared to CD4+ FoxP3− T cell populations at the later time points measured (Fig. 1D, and E).
Figure 1. HSV-1 infection results in the expansion of CD4+FoxP3+ regulatory T cells.
FoxP3-GFP knock in mice were infected with 2×105 PFU of HSV kos in a 30 μl drop in the footpad. The kinetics of FoxP3+ Tregs was measured at indicated time points in the draining PLN following HSV-I infection of the footpad. (A) Bar graphs depicting absolute numbers of CD4+FoxP3− T cells. (B Representative FACS plot showing Treg frequency in a naïve animal. (C) Representative histograms showing frequencies of CD4+FoxP3+ Tregs at indicated time points post HSV infection. (D) CD4+FoxP3+ T cells in the draining PLN of naïve and HSV infected mice at indicated time points post infection. (E) Ratios of CD4+FoxP3+/ CD4+FoxP3− T cells at indicated time points are shown. Data are representative of three independent experiments with 4–5 mice per group in each experiment. Error bars represent SEM. The level of significance was determined using one way ANOVA with Bonferroni post hoc settings.
3.2 HVEM expression is up-regulated on regulatory T Cells following HSV-1 infection
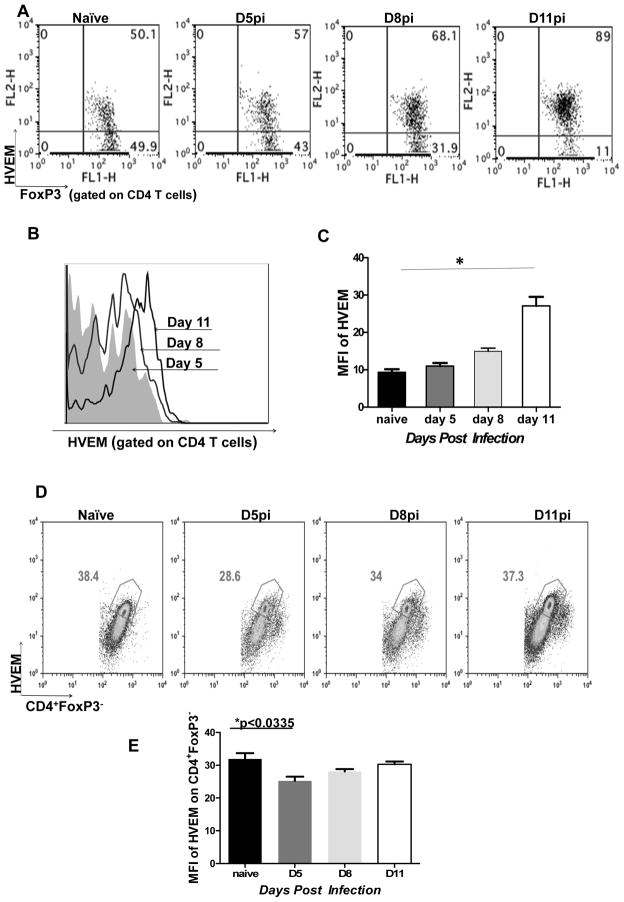
We next examined the expression of HVEM on regulatory T cells as this receptor was previously shown to be associated with immune regulation and suppressive functions of Tregs [10]. Our results showed that around 40–50% of CD4+FoxP3+ T cells isolated from the PLN of naive mice expressed HVEM (Fig. 2A). Notably, the proportion of CD4+FoxP3+ Tregs expressing HVEM increased at different time points in HSV-1 infected mice, with almost 80–90% of Tregs expressing the receptor in the PLN at 11 days pi (Fig. 2A). In addition, analysis of the MFI of HVEM on Tregs from day 11 pi showed significant up regulation of the receptor in infected animals. (Fig. 2B and C). In contrast to the FoxP3+ cells, around 35–40% of CD4+ FoxP3− T cells isolated from PLN of naïve mice expressed HVEM (Fig. 2D) and the proportion of HVEM- expressing CD4+FoxP3− remained similar following HSV infection. However, the MFI of HVEM expression on CD4+ FoxP3− cells was significantly reduced at day 5 pi and at later time points was equivalent to the MFI of HVEM expression on naïve FoxP3− cells (Fig. 2E).
Figure 2. HVEM expression is up-regulated on regulatory T Cells following HSV-1 infection.
Mice were infected with 2×105 PFU of HSV-1 kos and the kinetics of HVEM expression by FoxP3+ Tregs was measured at indicated time points in the draining popliteal lymph nodes (PLN) by flow cytometry. (A) Representative FACS plots depicting HVEM expression by FoxP3+ Tregs. Numbers in the quadrants indicate percent of each subset. (B) HVEM expression by CD4+FoxP3+ Tregs at indicated time points post infection is shown by histograms. (C) Bar graphs shows the MFI of HVEM expression on FoxP3+T cells. (D) Representative FACS plots depicting HVEM expression by CD4+FoxP3− T cells. Numbers in the quadrants indicate percent of each subset. (E) Bar graphs show the MFI of HVEM expression on CD4+FoxP3− cells. Data are representative of three independent experiments. Error bars represent SEM. The level of significance was determined using one way ANOVA with Bonferroni post hoc settings and student’s t test.
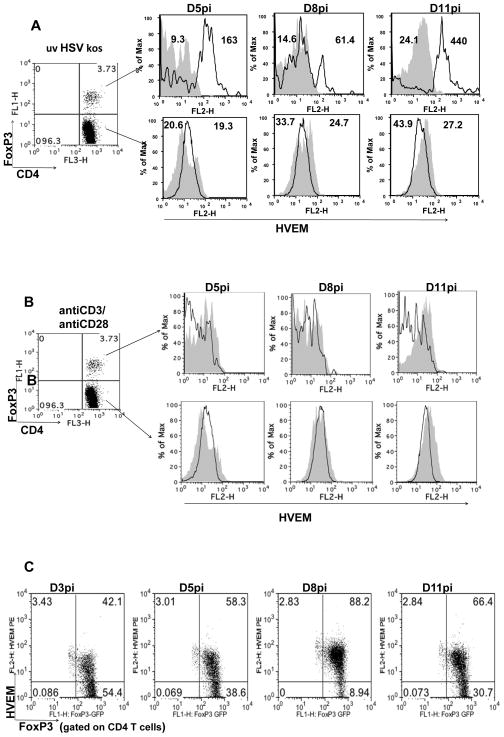
In some experiments, draining lymph node (DLN) cells from infected FoxP3-GFP knock in mice were obtained at indicated time points post infection and were stimulated in vitro with either UV inactivated HSV-1 or anti-CD3/anti-CD28 for 72 hours. HVEM expression was analyzed on CD4+ FoxP3+ and CD4+ FoxP3− cells by flow cytometry. Our results demonstrated that HVEM expression was further up-regulated on FoxP3+ CD4+ T cells (Fig. 3A upper panel) upon stimulation with UV inactivated HSV-1, but not on CD4+ FoxP3− cells (Fig. 3A lower panel). The highest MFI of HVEM expression after UV-inactivated HSV stimulation was observed when the cells were obtained after day 6 pi (Fig. 3A). Stimulation with anti-CD3/ anti-CD28 did not result in altered HVEM expression levels on either the FoxP3+ or FoxP3− CD4+ T cells (Fig. 3B).
Figure 3. Further up regulation of HVEM on FoxP3+ Tregs upon in-vitro re-stimulation of primed cells with UV inactivated HSV kos.
FoxP3-GFP knock in mice were infected with 2×105 PFU of HSV kos in a 30 ul drop in the footpad. Draining PLN cells were isolated at indicated time points p.i and stimulated with either UV inactivated HSV kos or anti-CD3 (1 mg/ml) anti-CD28 (0.5 mg/ml) for 72 hours. (A) The cells were then analyzed flow cytometrically for HVEM expression on CD4+FoxP3+ (upper panel) and CD4+FoxP3− (lower panel) cells. Histograms depicting HVEM expression by un-stimulated (shaded area) and UV inactivated HSV kos stimulated (black line). (B) FACS analysis for HVEM expression on CD4+FoxP3+ (upper panel) and CD4+FoxP3− (lower panel) cells. Histograms depicting HVEM expression by un-stimulated (shaded area) and anti-CD3 anti-CD28 stimulated (black line). (C) HVEM expression on CD4+FoxP3+ cells following foot pad immunization of FoxP3GFP knock in animals with UV inactivated HSV kos is shown.
The expression of HVEM on Tregs in draining PLN populations after foot pad infection with UV inactivated HSV was also measured. As shown in Fig. 3C, around 50–58% FoxP3+ cells were HVEM positive at day 5 pi, rising to 80–90% of the cells at 8 days pi. This was followed by a gradual decrease in HVEM expression on FoxP3+ CD4+ T cells by day 11 pi These results show that HVEM expression is up-regulated in mice immunized with UV inactivated HSV-1, suggesting a direct role for a viral component in the up regulation of the HVEM receptor.
3.3 The Viral ligand (glycoprotein D) of HVEM is expressed in the draining lymph nodes following HSV-1 infection
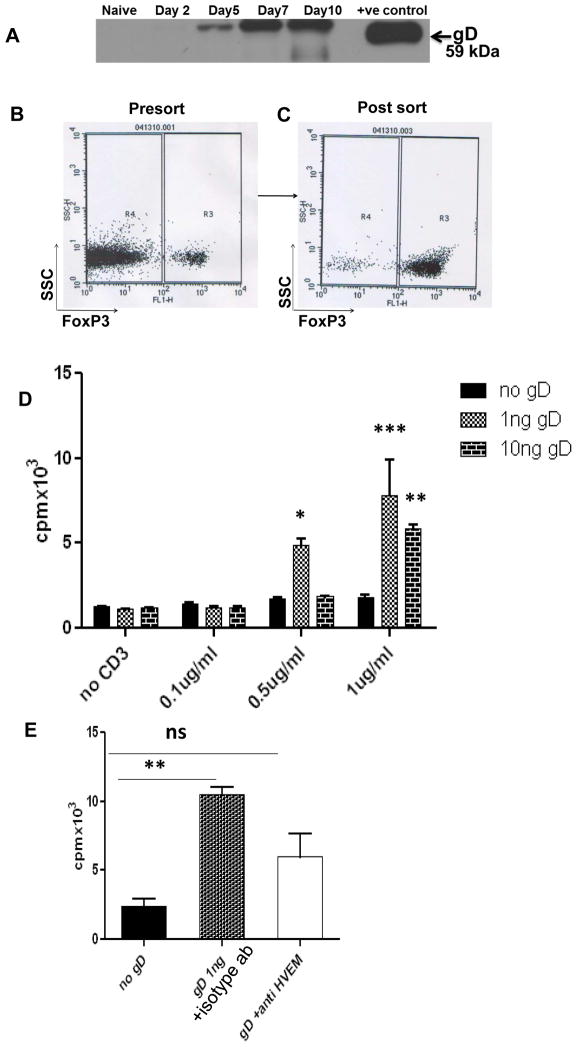
Previous studies have shown that gD interacts with HVEM and promotes virus entry [17] and is expressed on the surface of infected T cells. To explore the mechanism that might be responsible for triggering Treg expansion, we hypothesized that possibly HSV itself could trigger Treg expansion. Therefore, experiments were performed to detect if gD is detectable in the DLN of mice infected with HSV-1. Western blot analysis was performed on the draining PLN samples obtained from naive and HSV infected animals at day 48, and 72 hours pi. The results showed that PLN homogenates from naive mice completely lacked gD expression and negligible amounts of gD were detectable at day 2 p.i (Fig. 4A). However gD was detectable in the PLN samples at 72 hours and later, post HSV-1 infection (Fig. 4A).
Figure 4. HSV-1gD can help to expand Tregs.
(A) Expression of HSV-gD in the PLN homogenates obtained at various time points post infection is shown. CD4 T cells were enriched from the splenocytes of naïve mice using miltenyi biotech kit and FoxP3+ cells were sorted from enriched CD4 T cell population based on GFP expression. Representative FACS plots showing presort (B) and post sort (C) population. (D) CD4+FoxP3+ Treg populations from naive WT mice were stimulated with anti-CD3 alone, anti-CD3 plus recombinant HSV-1gD, or anti-CD3 plus anti-CD28 at the indicated concentrations. The proliferation was determined by [3H] thymidine incorporation. Tregs proliferated significantly at relatively high concentrations of anti-CD3 with HSV-1gD compared with anti-CD3 alone (p value). (E) CD4+FoxP3+ Treg populations from naive WT mice were stimulated with anti-CD3 (1 ug/ml), anti-CD3 (1 ug/ml) plus HSV-1gD (1 ng) and isotype control antibody, or anti-CD3 plus HSV-1gD (1 ng) and soluble anti-HVEM Ab (100 ug/ml) as indicated. The proliferation was determined by [3H] thymidine incorporation. Error bars represent SEM. The level of significance was determined using student’s t test.
3.4 Recombinant HSV-1 gD expands CD4+ FoxP3+ T cells
Given our observations that Tregs expand following HSV-1 infection, that HVEM is preferentially up-regulated by regulatory T cells and that detectable levels of HSV-1 gD were present in the DLN, we hypothesized that the interaction of HVEM with its known viral ligand gD could be of functional significance. To address this question, we enriched CD4+ T cells (Fig. 4B) from FoxP3-GFP mice and subsequently sorted FoxP3+ cells (Fig. 4C) from this enriched population based on GFP expression. Sorted FoxP3+ cells (2×105 cells) were stimulated with different concentrations of anti-CD3 alone or anti-CD3 plus recombinant HSV-1 gD in vitro. In the presence of gD, Tregs showed proliferation when relatively higher but suboptimal concentrations of anti-CD3 was used (Fig. 4D). This expansion of FoxP3+ cells was reduced when anti-HVEM antibody was added to the culture suggesting an effect that is mediated by gD binding with HVEM (Fig. 4E). Our results indicate that in addition to being an entry receptor for HSV, HVEM binding with HSV-1 gD can trigger proliferation of CD4+ FoxP3+ T cells. Additionally incubation of Tregs with gD also resulted in increased expression of CD69 (Fig 1S panel A) and CD25 (Fig 1S panel B) on sorted Tregs.
3.5 HVEM knockout mice generate weaker Treg responses
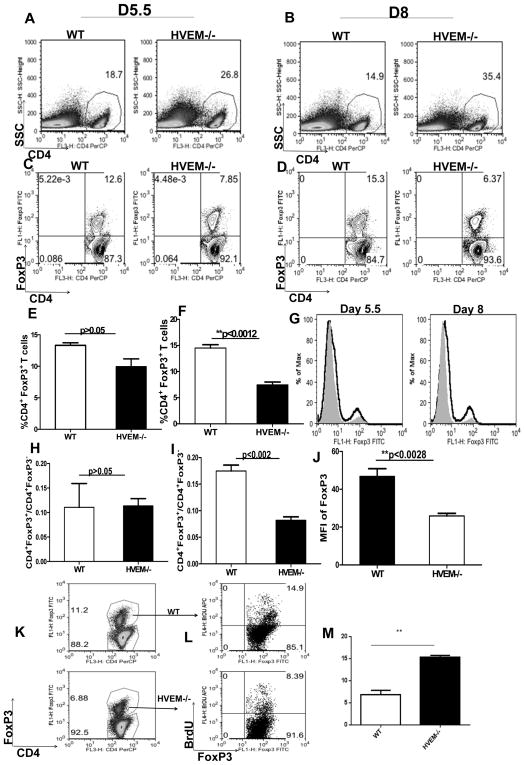
Our in-vivo and in-vitro observations showed that HSV gD is expressed in the draining PLN nodes following HSV-1 infection and that gD-HVEM interaction may result in the expansion of CD4+FoxP3+ regulatory T cells. Therefore to provide in-vivo evidence for the role of HVEM in Treg activation and expansion, WT and HVEM−/− mice were infected with 2×105 HSV-1 in the footpad. At the time points examined the frequencies of CD4+ T cells were significantly higher (p<0.05) in HVEM−/− mice as compared with WT animals both at day 5.5 (Fig. 5A) and day 8 pi (Fig. 5B). These results are consistent with previous findings, which showed hyper proliferative CD4+ T cell responses in HVEM−/− mice [8]. However when compared with WT mice the frequencies of CD4+ Foxp3+ T cells were lower in HVEM−/− mice at day 5.5 (Fig. 5C and E) and these differences became highly significant at day 8 pi (Fig. 5D and F). In addition, when the representation (ratio of absolute numbers) of CD4+ FoxP3+ among the CD4+FoxP3− T cells was compared, in HVEM−/− mice there was significantly lower (p<0.002) Treg to Teff ratios at day 8 pi (Fig. 5I); however, the ratios did not reach the level of significance at day 5.5 pi (Fig. 5H). In addition, the MFI data showed that FoxP3 expression was significantly reduced in CD4 T cells obtained from HVEM−/− mice as compared to WT mice (Fig. 5G and J).
Figure 5. Diminished representations of CD4+FoxP3+ regulatory T cells in HVEM−/− mice.
CD4+ and CD4+FoxP3+ T cell responses were compared among age and gender matched HSV-1 infected WT and HVEM−/− animals at indicated time points post infection. Representative FACS plots show CD4+ T cells from WT and HVEM−/− animals in the draining PLN at day 5.5 (A) and day 8 (B) following HSV-1 kos infection in the footpad. Representative FACS plots and bar graphs showing CD4+FoxP3+ T cells from WT and HVEM−/− animals in the draining PLN at day 5.5 (C and E) and day 8 (D and F) following HSV-1kos infection in the footpad. Bar graphs represent the % CD4+FoxP3+T cells in WT and HVEM−/− animals at day 5.5 (E) and day 8 (F) post infection. (G) FACS analysis of CD4 T cell FoxP3 level from WT (black line) or HVEM−/− (shaded area) mice in draining PLN at indicated time points post HSV infection in the foot pad is shown. Ratios of absolute numbers of CD4+FoxP3+ cells to CD4+FoxP3− T cells at day 5.5 (H) and day 8 (I) p.i. is shown. (J) MFI of FoxP3 expression in WT and HVEM−/− animals at day 8 p.i is shown. HSV-1 infected mice were pulsed with BrdU 12 hours before sacrifice. At day 8 p.i. (K and L) draining popliteal lymphoid populations were analyzed by FACS for evidence of BrdU incorporation. Data are representative of three independent experiments with 5 mice per group in each experiment. (M) Bar graphs show the statistics of the data from 5L. Error bars represent SEM. The level of significance was determined using student’s t test.
We next performed BrdU incorporation assay to determine whether the lower frequencies of CD4+Foxp3+ T cells observed in HVEM−/− mice was due to their reduced proliferative capacities in-vivo in the absence of HVEM. Our results showed decreased proliferation of CD4+FoxP3+ T cells in HVEM−/− mice as compared to intact WT mice (Fig. 5K and L).
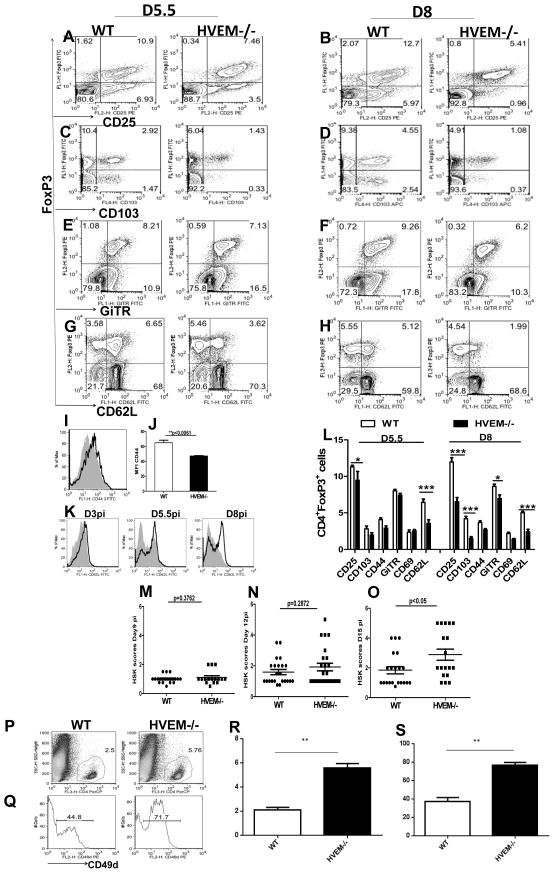
Not only the ratio of the absolute numbers of CD4+FoxP3+ T cells to CD4+ FoxP3− was reduced in the HVEM−/− mice but also the proportion of FoxP3+ cells expressing activation markers was also reduced in HVEM−/− mice. These included CD25 (Fig. 6A and B), CD103 (Fig. 6C and D), GiTR (Fig. 6E and F), and CD62L (Fig. 6G, H and K), CD44 (Fig. 6I and J), all of which were significantly diminished on Tregs at both day 5.5 and day 8 pi in the HVEM −/− mice compared to the intact WT animals. A summary of these results is represented in in (Fig. 6L). Taken together, these data from HVEM−/− mice show that HVEM is required for the activation and expansion of FoxP3+ regulatory T cells after an HSV-1 infection.
Figure 6. Co expression of FoxP3+ with the activation markers in WT and HVEM−/− animals.
Gated CD4+T cells from draining PLN of WT and HVEM−/− mice were analyzed at indicated time points by flow cytometry for co expression of the Treg surface markers CD25 (A and B), CD103 (C and D), GiTR (E and F), CD62L with the Treg associated transcription factor FoxP3 (G and H). (I) Histograms showing CD44 expression on gated CD4+FoxP3+ cells in WT (black line) or HVEM−/− (shaded area). (J) MFI of CD44 expression on Tregs in draining PLN at day 8 post HSV infection in the foot pad is shown. (K) Histograms showing CD62L expression on gated CD4+FoxP3+ cells from WT (black line) or HVEM−/− (shaded area) mice in draining PLN at different time points post HSV infection in the foot pad is shown. (L) Percentage of CD4+ FoxP3+ cells that are positive for Treg markers in WT and HVEM−/− animals in draining PLN at indicated time points is shown. Error bars represent SEM. The level of significance was determined using two way ANOVA. WT and HVEM−/− mice were infected via corneal scarification with 1×104 PFU of HSV-RE and mice were scored daily for development of SK. HSK scores in WT and HVEM−/− animals as measured at day 9 (M), day 12 (N) and day 15 (O) post HSV-1 infection. The data are cumulative of three independent experiments. A single-cell suspension of the infected corneas was prepared from 6-pooled corneas at day 15 post HSV infection from WT and HVEM−/− animals. The cells were labeled for CD4 (P) and CD49d (Q). (R) Bar graphs show the statistics of the data from 5P for CD4 T cells and (S) for CD49d from 5Q. The numbers on the dot plots indicate the percentages of the cells expressing the particular markers in WT and HVEM−/− animals.
Consistent with this HVEM knockout animals mounted better immune response (Fig 2S) compared to the WT animals. HVEM deficient animals showed higher virus specific CD8T cells (A). Higher proportions of tetramer positive cells were CD44hi and CD62Llo in HVEM−/− animals (Fig 2S: B, C, D and E). In addition, higher proportions (Fig 2S–F) and numbers (Fig 2S–G) of IFNγ positive cells along with reduced viral loads (Fig 2S–H) were also observed in HVEM deficient animals..
3.6 HVEM knockout mice develop enhanced clinical signs of ocular HSV-1 infection
Previous studies demonstrated that Tregs play a modulatory role in the outcome of herpes stromal keratitis (SK) [18]. Hence, we suspected that animals impaired in Treg induction would show more severe disease. To test this, WT and HVEM−/− mice were infected via corneal scarification with 1×104 PFU of HSV-RE and mice were scored daily for development of SK as mentioned in materials and methods. As shown in Fig. 6M–O, comparisons of SK severity, HVEM−/− and WT animals showed differences although not at all time points. Both groups of mice developed SK at comparable levels until day 12 pi (Fig. 6M and N), but by day 15 pi (Fig. 6O), HVEM−/− animals showed significantly higher SK scores compared to WT animals. Furthermore there was a significantly increased CD4+ T cell infiltration into the corneas of HVEM−/− animals (Fig. 6P). Another effect observed was the change in the phenotype of CD4+ T cells in the corneas (Fig. 6Q). Our data showed that 44.0% of CD4+ T cells in the corneas of WT were positive for CD49d, while around 72% of CD4+ T cells entering the corneas of HVEM−/− animals were positive for CD49d. CD49d is a molecule involved in the migration of cells to the eye [19] and Tregs have been shown to modulate CD49d expression in secondary lymphoid organs. These observations suggest that the weaker Treg responses in HVEM−/− animals result in up regulation of CD49d on CD4+T cells which in turn results in increased migration and infiltration of CD4+T cells in the cornea and consequent enhanced pathology.
4. Discussion
In a previous report, we have shown that HSV infection activates and expands CD4+CD25+ regulatory T cells [1]. In the current study we describe a mechanism by which HSV-1 infection can act to signal and expand the Treg population. We show that CD4+ FoxP3+ regulatory T cells up-regulate HVEM following HSV-1 infection and consistent with this, primed cells stimulated with UV inactivated HSV kos resulted in further up regulation of HVEM preferentially on FoxP3+ Tregs. Furthermore recombinant HSV-1 gD, a viral ligand of HVEM, in presence of IL-2 promoted the proliferation of sorted CD4+FoxP3+ cells, an effect that was blocked by anti-HVEM antibody. In support of this, HVEM−/− mice generated weaker Treg responses represented by significantly diminished ratios of CD4+ FoxP3+/CD4+ FoxP3− cells along with diminished proportions of FoxP3+ cells co-expressing the Treg activation markers, a reduced MFI of FoxP3 expression on CD4 T cells and a significantly diminished proliferation following HSV-1 infection. These data collectively suggests a role for gD/HVEM interaction in promoting Treg responses following HSV-1 infection. Additionally, in a mouse model of the blinding immuno-inflammatory disease, HSK, our results showed that lesions were more severe in HVEM−/− mice with higher susceptibility to HSV infection and increased corneal CD4 T cell infiltration. Hyperproliferative CD4 T cell responses have also been shown in a model of ConA-mediated T cell-dependent autoimmune hepatitis [8]. Such observations suggest that HVEM could be added to the arsenal of Treg effector molecules relevant to therapy in inflammatory conditions.
Several TNFR family members have co-stimulatory effects on T cells and are also implicated in control of Treg function. In the present study, we report yet another TNFR family member, HVEM, that is preferentially up-regulated on Tregs and down-regulated on CD4+Foxp3− (at least at early time points pi) following HSV-1 infection consistent with previous reports indicating that HVEM is up regulated on Tregs and down regulated on T effectors upon activation [10]. Interestingly our data points towards the maximum Treg numbers and HVEM up regulation after the peak of effector responses. Various studies have established a role for HVEM in Treg function. In one study, HVEM knockout Tregs had decreased suppressive activity and over expression of HVEM in WT Tregs increased their suppressive function [10]. Additionally, T cells from scurfy mice were shown to lack HVEM expression even after activation, suggesting the involvement of FoxP3 in the regulation of HVEM expression [10]. Our data indicate that the upregulation of HVEM on Tregs could result from ligand interactions with the viral protein gD. Detection of HSVgD in the PLN was unexpected but it may be argued that since infected T cells have been shown to express gD, they may be the source of this protein at this site. However we did not formally investigate whether T cells in the PLN were the primary source of gD, and thus cannot exclude other possibilities.
Other studies suggest that HSV-1 gD can modulate the activities of T cells via HVEM during the viral replication stages of binding, entry, or egress [20–23]. It has been shown that HVEM is involved in NFkB dependent protection against apoptosis by HSV-1 gD [24]. One consequence of this interaction may be NFkB activation in Tregs, but such effects were not formally investigated in this study. Soluble gD has also been shown to inhibit T cell proliferation [25]. It is conceivable that gD binding to HVEM might be able to deliver strong negative signals, the outcome of which is manifested as a defect in effector T cell proliferation [25]. Alternatively our observation might suggest that gD binding to HVEM on Tregs result in Treg activation and expansion which in turn inhibits effector T cell proliferation. These possibilities are not mutually exclusive since the same type of receptor ligand interaction might have different outcomes depending on the cell type, as engagement of HVEM on T cells by its natural ligands may augment (in the case of LIGHT) or attenuate (in the case of BTLA or CD160) immune responses [26].
Further support for the immune-modulatory role of gD HVEM interaction came from the studies using HVEM knockout mice. Tregs from naïve HVEM knockout mice had reduced frequencies and FoxP3 expression levels compared to the WT animals. However, more interesting was the observation that following HSV infection the expression levels of FoxP3 increased on WT Tregs but was decreased on the HVEM−/− Tregs. Additionally significantly diminished ratios of CD4+ FoxP3+Tcells to CD4+FoxP3− cells were observed in the HVEM−/− mice with the differences being more evident at day 8 p.i. (After the peak of effector responses). This could mean that the absence of gD-HVEM interaction in HVEM−/− animals further augments the scenario of diminished FoxP3 expression on Tregs already existing due to lack of HVEM. Perhaps in HVEM−/− animals, HSV-1 is unable to bind HVEM and modulate the immune system (to activate Tregs and inhibit antiviral responses) in its favor. These findings were consistent with our in-vitro experiments that utilized soluble gD with the effects abolished with anti HVEM antibody. It is still not clear whether Tregs that suppress pathogen specific T-cell responses have to be antigen -specific themselves. In several infectious diseases, such as Leishmania [27] or hepatitis C virus [28] pathogen specific Tregs have been found and in some auto-immune diseases, only antigen specific Tregs were able to suppress autoreactive T cells, whereas non-specific Tregs were ineffective [29]. However several studies have reported that non-specific Tregs are efficiently recruited and proliferate in the organs after virus-induced inflammation and can suppress virus-specific T cell responses [30]. Our report provides one mechanism by which non-specific Treg activation and expansion might occur. HSV-1 remains a significant cause of ocular infections and blindness and Tregs have been convincingly reported to influence the course of HSV-1 induced corneal immunopathology [18, 31–34]. In our attempt to delineate the impact of defective Treg responses in HVEM−/− animals we observed a higher susceptibility of these animals to HSV-1 induced ocular immunopathology. This observation was unexpected as HVEM is an entry mediator for HSV and is in contrast to some other reports [35, 36]. It could be argued that the differences might be due to the strain and dose of virus used. Furthermore in previous report nectin-1 knockout animals were more susceptible to HSV compared to HVEM−/− animals. However our results are consistent with a study that reported that absence of nectin-1 (but not HVEM) reduced efficiency of infection [37].
In conclusion we have shown that HSV can mediate Treg activation and proliferation by up-regulating HVEM on Tregs, a molecule that has been attributed to increase the suppressive ability of Tregs [10]. Additionally HVEM can also serve as a potential target for the development of immune therapeutic interventions.
Supplementary Material
Figure 1S. CD4 T cells were enriched from the splenocytes of naïve mice using miltenyi biotech kit and FoxP3+ cells were sorted from enriched CD4 T cell population based on GFP expression. CD4+FoxP3+ Treg populations were incubated with 100ng gD and IL-2 for 36–48hrs. The cells were than stained with activation markers CD69 (1S-A) and CD25 (IS-B) and acquired on FACS caliber.
Figure 2S. CD8+ T cell responses were compared among age and gender matched HSV-1 infected WT and HVEM−/− animals at indicated time points post infection. (A) Representative FACS plots show Kb gB tet+CD8 T cells from WT and HVEM−/− animals in the draining PLN at given time points post HSV kos infection in the foot pad. Kb gB tet+ CD44hi CD8 T cell responses at day 5.5 (B) and day 8 (C) post HSV infection is shown. Kb gB tet+ CD62LO CD8 T cell responses at day 5.5 (D) and day 8 (E) post HSV infection is shown. Frequencies (F) and absolute numbers (G) of IFNγ+CD8T cell responses in the draining PLN are shown in WT and HVEM−/− animals at day 5.5 post HSV kos infection. (H) Viral titers in the foot pad of WT and HVEM deficient animals is shown following HSV- kos infection
Acknowledgments
We thank Dr. Gary Cohen and Dr. Roselyn Eisenberg University of Pennsylvania, Philadelphia, for providing us recombinant HSV-gD and antiHVEM antibody. We are also thankful to Dr. Carl Ware at Sanford Burnham medical research institute, La Jolla, California for HVEM knockout animals and Dr. Paul G Thomas at St Jude Children’s research hospital, Memphis, for help with manuscript editing. This work was supported by National Institute of Allergy and Infectious Diseases Grant AI 063365 and National Eye Institute Grant EY 005093
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 4.Veiga-Parga T, Sehrawat S, Rouse BT. Role of regulatory T cells during virus infection. Immunol Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedý JR, Spear PG, Ware CF. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol. 2008;8:861–873. doi: 10.1038/nri2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, Flies AS, Flies DB, Zhu G, Anand S, Flies SJ, Xu H, Anders RA, Hancock WW, Chen L, Tamada K. Selective targeting of the LIGHT-HVEM costimulatory system for the treatment of graft-versus-host disease. Blood. 2007;109:4097–4104. doi: 10.1182/blood-2006-09-047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon M, Kopp SJ, Taylor JM, Storti CS, Spear PG, Muller WJ. Functional interaction between herpes simplex virus type 2 gD and HVEM transiently dampens local chemokine production after murine mucosal infection. PLoS One. 2011;6:e16122. doi: 10.1371/journal.pone.0016122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang J, Liu X, Mink K, Degrandi D, Pfeffer K, Fu YX. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffrè-Cuculletto M, Venuti A, Grelli S, Bramanti P, Mastino A. Involvement of gD/HVEM interaction in NF-kB-dependent inhibition of apoptosis by HSV-1 gD. Biochem Pharmacol. 2008;76:1522–1532. doi: 10.1016/j.bcp.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Tao R, Wang L, Murphy KM, Fraser CC, Hancock WW. Regulatory T cell expression of herpesvirus entry mediator suppresses the function of B and T lymphocyte attenuator-positive effector T cells. J Immunol. 2008;180:6649–6655. doi: 10.4049/jimmunol.180.10.6649. [DOI] [PubMed] [Google Scholar]
- 11.De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, Pfeffer K, Benedict CA, Ware CF. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma S, Mulik S, Kumar N, Suryawanshi A, Rouse BT. An anti-inflammatory role of VEGFR2/Src kinase inhibitor in herpes simplex virus 1-induced immunopathology. J Virol. 2011;85:5995–6007. doi: 10.1128/JVI.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sisk WP, Bradley JD, Leipold RJ, Stoltzfus AM, Ponce de Leon M, Hilf M, Peng C, Cohen GH, Eisenberg RJ. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J Virol. 1994;68:766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Sundararajan A, Suryawanshi A, Kumar N, Veiga-Parga T, Kuchroo VK, Thomas PG, Sangster MY, Rouse BT. T cell immunoglobulin and mucin protein-3 (Tim-3)/Galectin-9 interaction regulates influenza A virus-specific humoral and CD8 T-cell responses. Proc Natl Acad Sci U S A. 2011;108:19001–19006. doi: 10.1073/pnas.1107087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumaraguru U, Rouse BT. Application of the intracellular gamma interferon assay to recalculate the potency of CD8(+) T-cell responses to herpes simplex virus. J Virol. 2000;74:5709–5711. doi: 10.1128/jvi.74.12.5709-5711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNally JM, Dempsey D, Wolcott RM, Chervenak R, Jennings SR. Phenotypic identification of antigen-dependent and antigen-independent CD8 CTL precursors in the draining lymph node during acute cutaneous herpes simplex virus type 1 infection. J Immunol. 1999;163:675–681. [PubMed] [Google Scholar]
- 17.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 18.Sehrawat S, Suvas S, Sarangi PP, Suryawanshi A, Rouse BT. In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions. J Virol. 2008;82:6838–6851. doi: 10.1128/JVI.00697-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Jiang S, Manczak M, Sugden B, Adamus G. Phenotypes of T cells infiltrating the eyes in autoimmune anterior uveitis associated with EAE. Invest Ophthalmol Vis Sci. 2002;43:1499–1508. [PubMed] [Google Scholar]
- 20.Kopp SJ, Storti CS, Muller WJ. Herpes simplex virus-2 glycoprotein interaction with HVEM influences virus-specific recall cellular responses at the mucosa. Clin Dev Immunol. 2012;2012:284104. doi: 10.1155/2012/284104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrop JA, McDonnell PC, Brigham-Burke M, Lyn SD, Minton J, Tan KB, Dede K, Spampanato J, Silverman C, Hensley P, DiPrinzio R, Emery JG, Deen K, Eichman C, Chabot-Fletcher M, Truneh A, Young PR. Herpesvirus entry mediator ligand (HVEM-L), a novel ligand for HVEM/TR2, stimulates proliferation of T cells and inhibits HT29 cell growth. J Biol Chem. 1998;273:27548–27556. doi: 10.1074/jbc.273.42.27548. [DOI] [PubMed] [Google Scholar]
- 22.Harrop JA, Reddy M, Dede K, Brigham-Burke M, Lyn S, Tan KB, Silverman C, Eichman C, DiPrinzio R, Spampanato J, Porter T, Holmes S, Young PR, Truneh A. Antibodies to TR2 (herpesvirus entry mediator), a new member of the TNF receptor superfamily, block T cell proliferation, expression of activation markers, and production of cytokines. J Immunol. 1998;161:1786–1794. [PubMed] [Google Scholar]
- 23.Cheung TC, Humphreys IR, Potter KG, Norris PS, Shumway HM, Tran BR, Patterson G, Jean-Jacques R, Yoon M, Spear PG, Murphy KM, Lurain NS, Benedict CA, Ware CF. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc Natl Acad Sci U S A. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sciortino MT, Medici MA, Marino-Merlo F, Zaccaria D, Giuffrè-Cuculletto M, Venuti A, Grelli S, Mastino A. Involvement of HVEM receptor in activation of nuclear factor kappaB by herpes simplex virus 1 glycoprotein D. Cell Microbiol. 2008;10:2297–2311. doi: 10.1111/j.1462-5822.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 25.La S, Kim J, Kwon BS, Kwon B. Herpes simplex virus type 1 glycoprotein D inhibits T-cell proliferation. Mol Cells. 2002;14:398–403. [PubMed] [Google Scholar]
- 26.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 27.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–788. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westendorf AM, Fleissner D, Groebe L, Jung S, Gruber AD, Hansen W, Buer J. CD4+Foxp3+ regulatory T cell expansion induced by antigen-driven interaction with intestinal epithelial cells independent of local dendritic cells. Gut. 2009;58:211–219. doi: 10.1136/gut.2008.151720. [DOI] [PubMed] [Google Scholar]
- 30.Zelinskyy G, Dietze KK, Hüsecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009;114:3199–3207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- 31.Veiga-Parga T, Suryawanshi A, Rouse BT. Controlling viral immuno-inflammatory lesions by modulating aryl hydrocarbon receptor signaling. PLoS Pathog. 2011;7:e1002427. doi: 10.1371/journal.ppat.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sehrawat S, Rouse BT. Anti-inflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J Immunol. 2008;180:7636–7647. doi: 10.4049/jimmunol.180.11.7636. [DOI] [PubMed] [Google Scholar]
- 34.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004;172:4123–4132. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 35.Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator is a serotype specific determinant of pathogenesis in ocular herpes. Proc Natl Acad Sci U S A. 2012;109:20649–20654. doi: 10.1073/pnas.1216967109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karaba AH, Kopp SJ, Longnecker R. Herpesvirus entry mediator and nectin-1 mediate herpes simplex virus 1 infection of the murine cornea. J Virol. 2011;85:10041–10047. doi: 10.1128/JVI.05445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor JM, Lin E, Susmarski N, Yoon M, Zago A, Ware CF, Pfeffer K, Miyoshi J, Takai Y, Spear PG. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host Microbe. 2007;2:19–28. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1S. CD4 T cells were enriched from the splenocytes of naïve mice using miltenyi biotech kit and FoxP3+ cells were sorted from enriched CD4 T cell population based on GFP expression. CD4+FoxP3+ Treg populations were incubated with 100ng gD and IL-2 for 36–48hrs. The cells were than stained with activation markers CD69 (1S-A) and CD25 (IS-B) and acquired on FACS caliber.
Figure 2S. CD8+ T cell responses were compared among age and gender matched HSV-1 infected WT and HVEM−/− animals at indicated time points post infection. (A) Representative FACS plots show Kb gB tet+CD8 T cells from WT and HVEM−/− animals in the draining PLN at given time points post HSV kos infection in the foot pad. Kb gB tet+ CD44hi CD8 T cell responses at day 5.5 (B) and day 8 (C) post HSV infection is shown. Kb gB tet+ CD62LO CD8 T cell responses at day 5.5 (D) and day 8 (E) post HSV infection is shown. Frequencies (F) and absolute numbers (G) of IFNγ+CD8T cell responses in the draining PLN are shown in WT and HVEM−/− animals at day 5.5 post HSV kos infection. (H) Viral titers in the foot pad of WT and HVEM deficient animals is shown following HSV- kos infection