Abstract
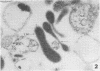
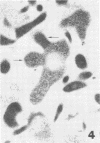
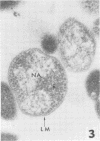
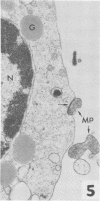
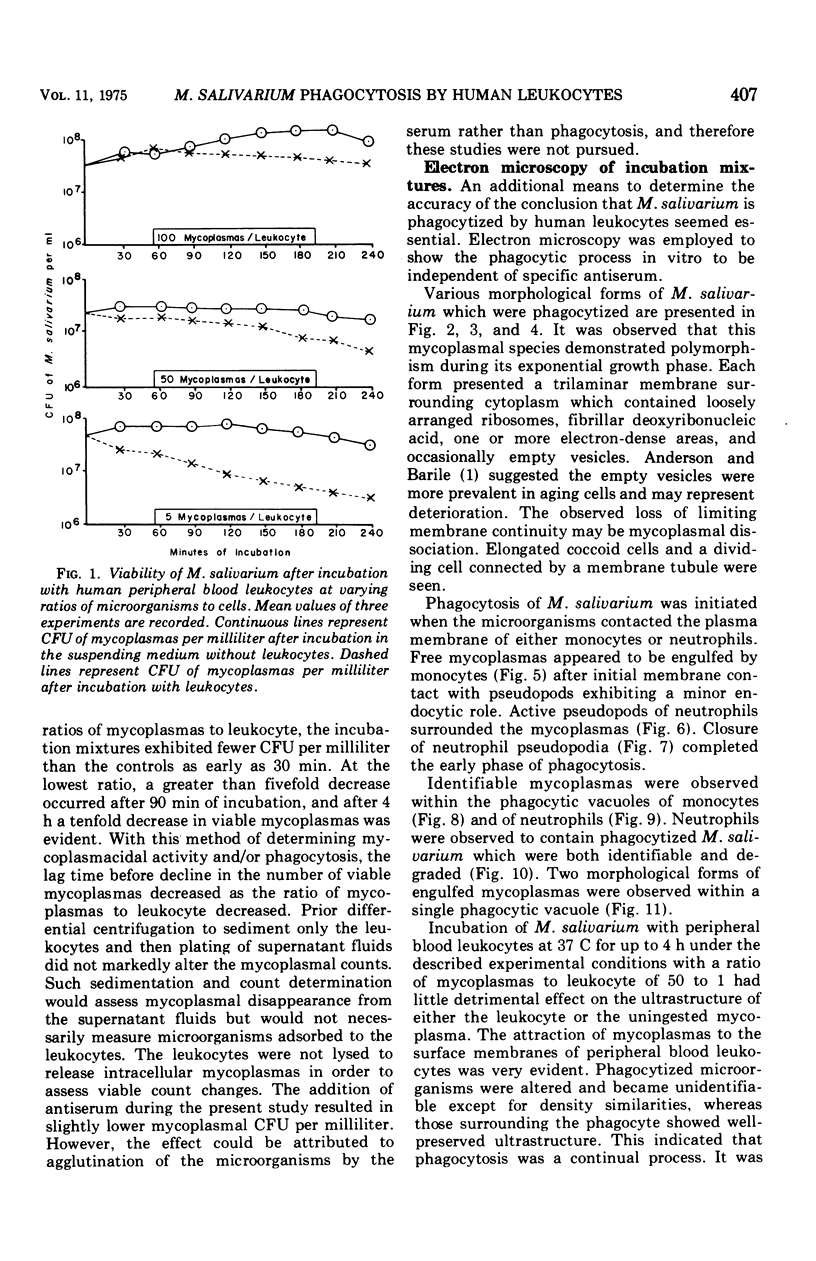
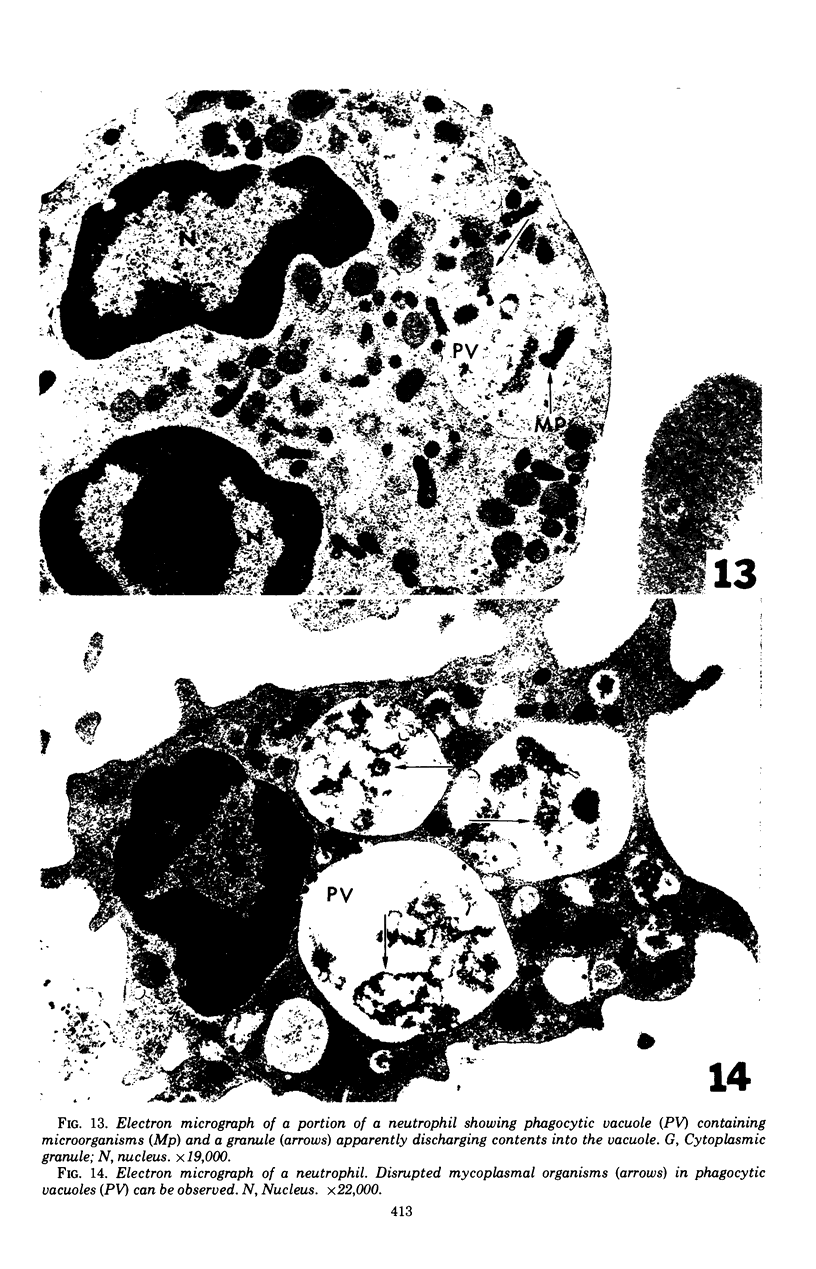
Mycoplasmacial activity was exhibited by human peripheal blood leukocytes in the absence of detectable specific antiserum. After incubation of varying concentrations of Mycoplasma salivarium with leukocytes, changes in colony-forming units (CFU) of this species per millilter occurred. The most noticeable decrease in CFU per milliliter was then the incubation mixtures contained five mycoplasmas per leukocyte. At this ratio, the mycoplasmacial min of incubation. Continued incubation demonstrated a tenfold decrease in CFU per milliliter by 4 h. Electron micrographs of incubated mixtures of human leukocytes and M. salivarium showed this mycoplasma to be phagocytized by monocytes and neutrophils whenever mutual contact or pseudopodial formation occurred. The process was continuous. Numerous phagocytic vacuoles developed which contained multiple ingested microorganisms. After the cytoplasmic granules of the leukocytes fused with the phagocytic vacuole, the phagocytized mycoplasmas became disrupted and unrecognizable.
Full text
PDF

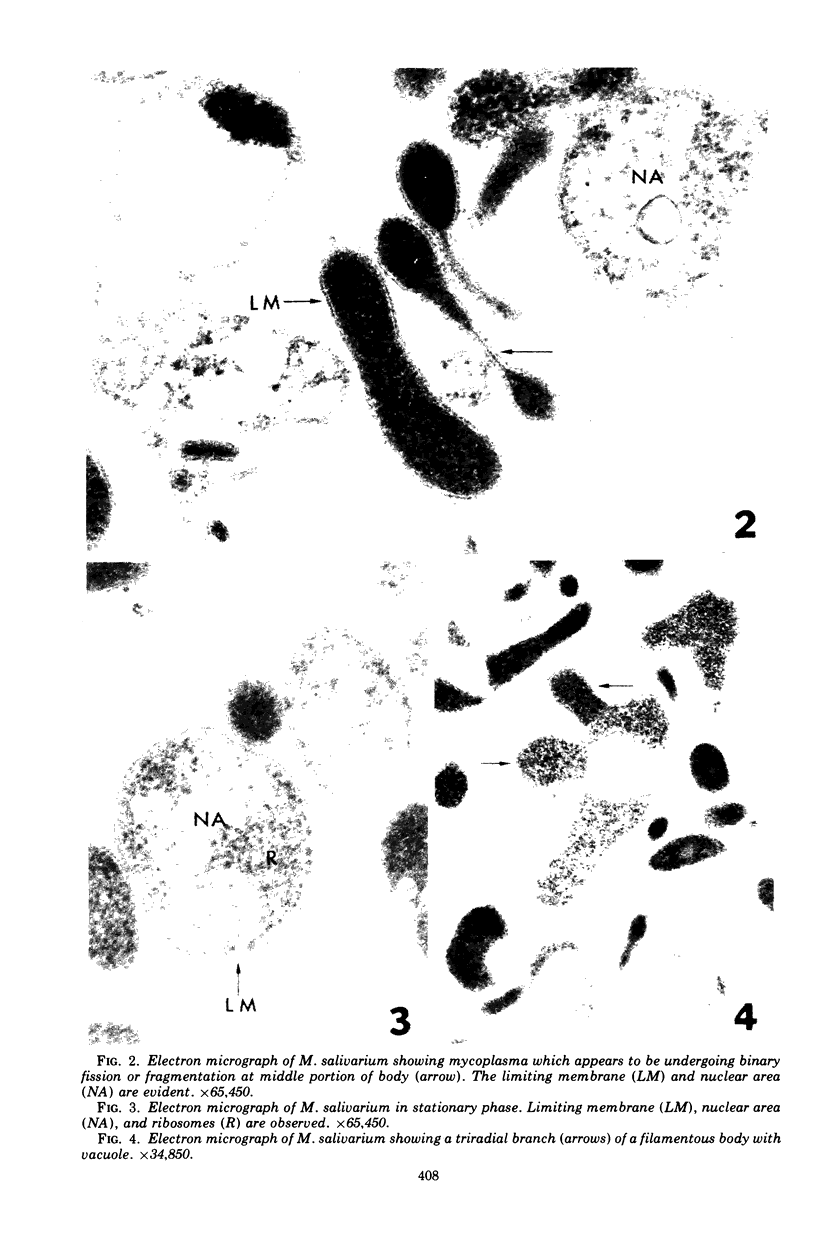
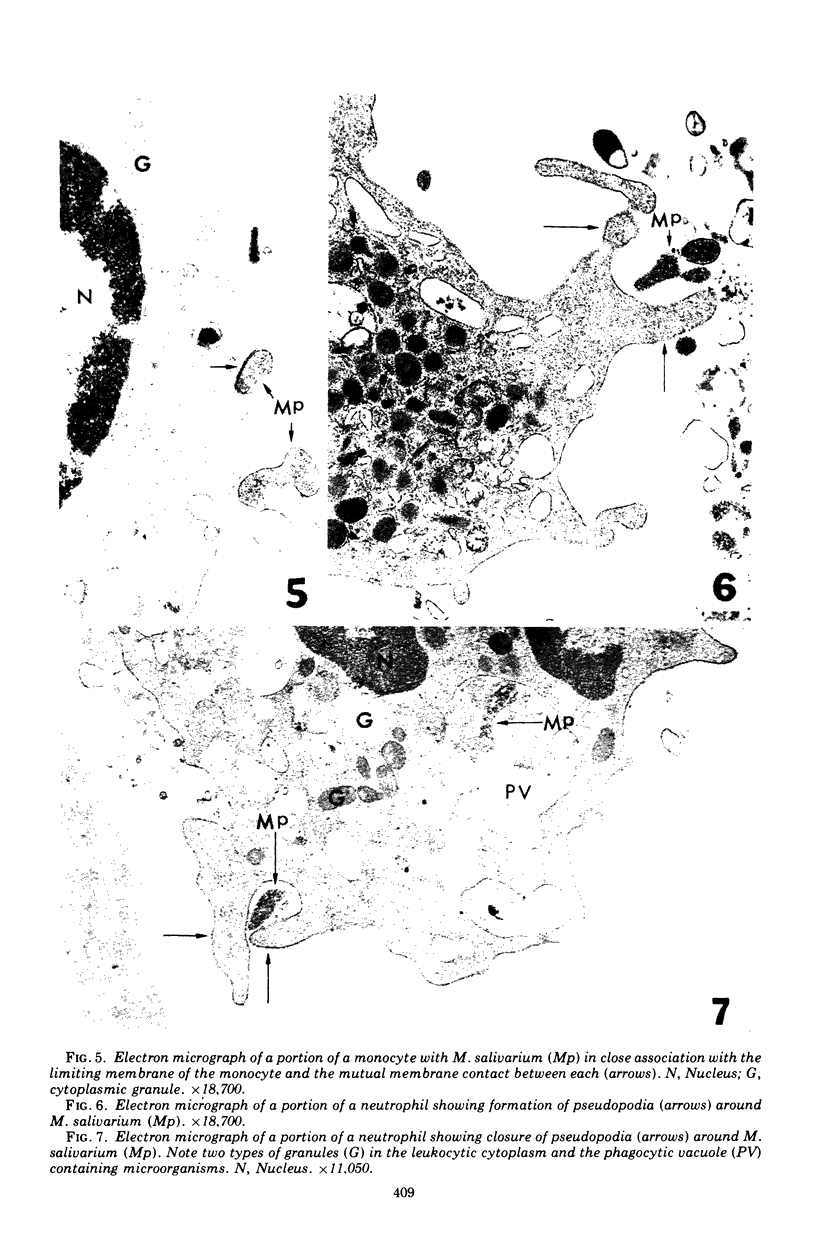
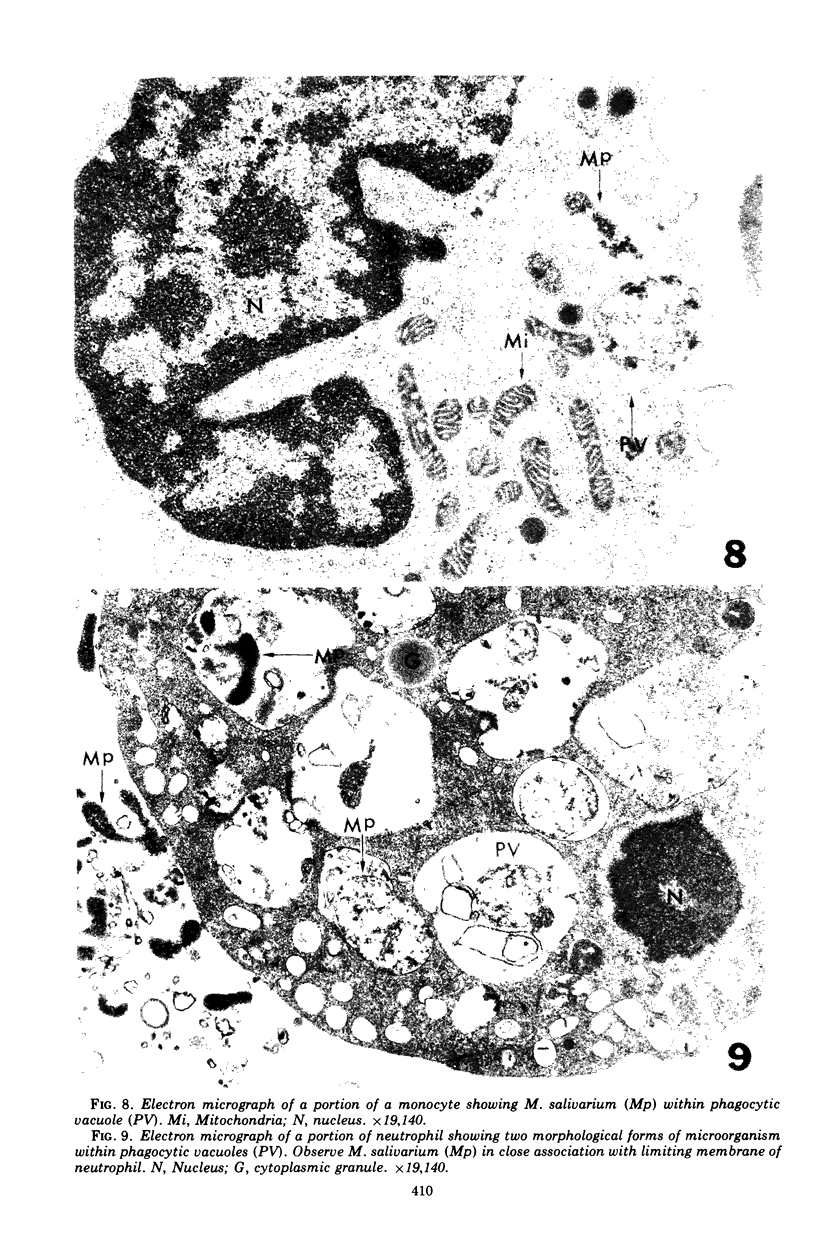

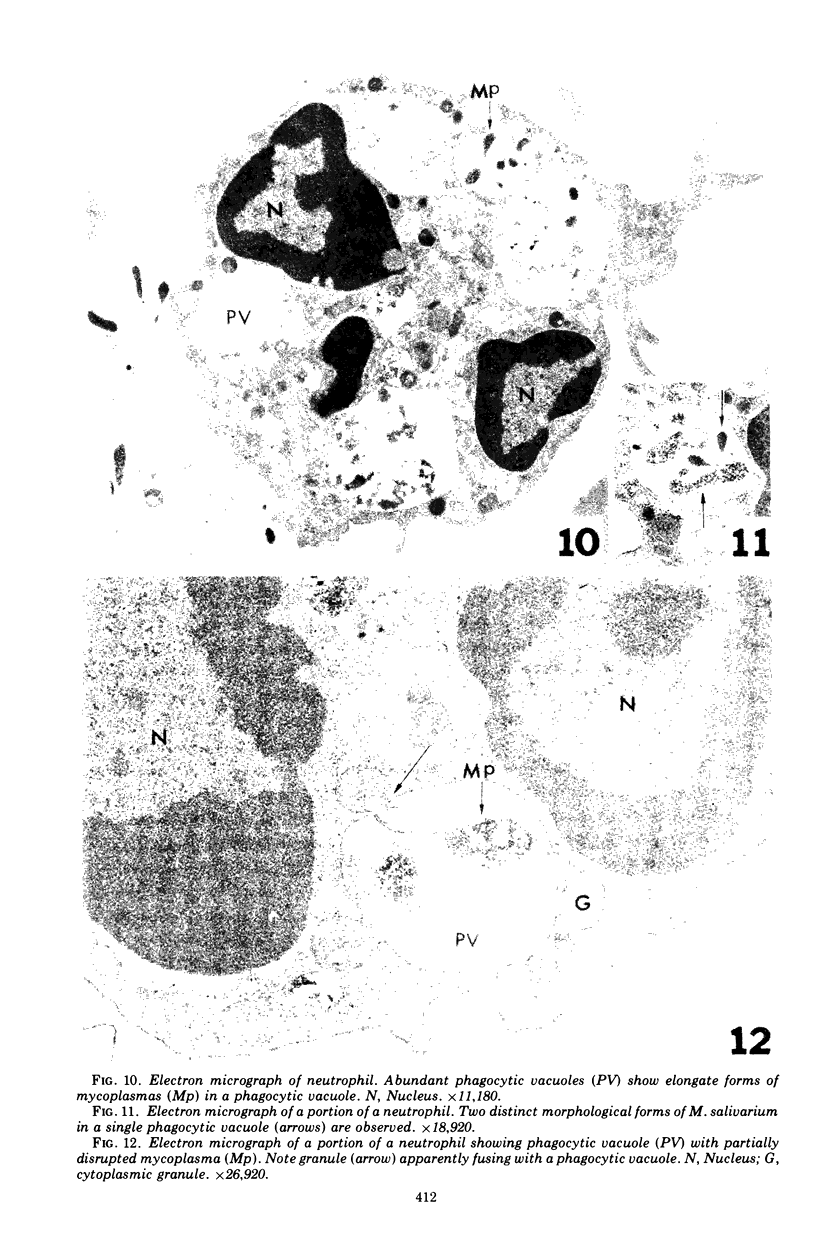

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY J. S., CLARK H. W., FELTS W. R., FOWLER R. C., BROWN T. M. Antigenic properties of pleuropneumonia-like organisms from tissue cell cultures and the human genital area. J Bacteriol. 1961 Oct;82:542–547. doi: 10.1002/path.1700820236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Cochrane C. G. Immunologic tissue injury mediated by neutrophilic leukocytes. Adv Immunol. 1968;9:97–162. doi: 10.1016/s0065-2776(08)60442-3. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajani A. S., Ayoub E. M. Mycoplasmacidal effect of polymorphonuclear leukocyte extract. J Immunol. 1969 Mar;102(3):698–702. [PubMed] [Google Scholar]
- Engel L. D., Kenny G. E. Mycoplasma salivarium in human gingival sulci. J Periodontal Res. 1970;5(3):163–171. doi: 10.1111/j.1600-0765.1970.tb00711.x. [DOI] [PubMed] [Google Scholar]
- Folds J. D., Welsh I. R., Spitznagel J. K. Neutral proteases confined to one class of lysosomes of human polymorphonuclear leukocytes. Proc Soc Exp Biol Med. 1972 Feb;139(2):461–463. doi: 10.3181/00379727-139-36164. [DOI] [PubMed] [Google Scholar]
- GOODMAN J. R., MOORE R. E. Electron microscopic study of phagocytosis of Staphylococcus by human leukocytes. J Bacteriol. 1956 May;71(5):547–556. doi: 10.1128/jb.71.5.547-556.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Jensen A. Mycoplasma: growth precipitation as a serodiagnostic method. Appl Microbiol. 1972 Mar;23(3):553–558. doi: 10.1128/am.23.3.553-558.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Iwabuchi T., Hinuma Y., Yuri K., Ishida N. Incidence, species, and significance of Mycoplasma species in the mouth. J Infect Dis. 1971 Jan;123(1):16–21. doi: 10.1093/infdis/123.1.16. [DOI] [PubMed] [Google Scholar]
- Low I. E., Jacobs A. A., Sbarra A. J. Mycoplasmacidal activity of a leukocytic myeloperoxidase-hydrogen peroxide-halide system. J Infect Dis. 1973 Mar;127(Suppl):S72–S76. doi: 10.1093/infdis/127.supplement_1.s72. [DOI] [PubMed] [Google Scholar]
- RAZIN S., MICHMANN J., SHIMSHONI Z. THE OCCURRENCE OF MYCOPLASMA (PLEUROPNEUMONIA-LIKE ORGANISMS, PPLO) IN THE ORAL CAVITY OF DENTULOUS AND EDENTULOUS SUBJECTS. J Dent Res. 1964 May-Jun;43:402–405. doi: 10.1177/00220345640430031101. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R. Periodontal disease: a model for the study of inflammation. J Infect Dis. 1971 Jun;123(6):676–677. doi: 10.1093/infdis/123.6.676. [DOI] [PubMed] [Google Scholar]
- Woolweaver D. A., Koch G. G., Crawford J. J., Lundblad R. L. Relation of the orogranulocytic migratory rate to periodontal disease and blood leukocyte count: a clinical study. J Dent Res. 1972 Jul-Aug;51(4):929–939. doi: 10.1177/00220345720510043401. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. I. HeLa cells, neutrophils, and eosinophils. J Exp Med. 1966 Sep 1;124(3):521–532. doi: 10.1084/jem.124.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker-Franklin D., Davidson M., Thomas L. The interaction of mycoplasmas with mammalian cells. II. Monocytes and lymphocytes. J Exp Med. 1966 Sep 1;124(3):533–542. doi: 10.1084/jem.124.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]