Abstract
Sensory adaptation in the E. coli chemosensory pathway has been the subject of interest for decades, with investigation focusing on the receptors that process extracellular inputs. Recent studies demonstrate that the flagellar motors responsible for cell locomotion also play a role, adding or subtracting FliM subunits to maximise sensitivity to pathway signals. It is difficult to reconcile this FliM remodelling with the observation that partner FliN subunits are relatively static fixtures in the motor. By fusing a fluorescent protein internally to FliN, we show that there is in fact significant FliN remodelling. The kinetics and stoichiometry of FliN in steady-state and in adapting motors are investigated and found to match the behaviour of FliM in all respects except for timescale, where FliN rates are about four times slower. We notice that motor adaptation is slower in the presence of the fluorescent protein, indicating a possible source for the difference. The behaviour of FliM and FliN is consistent with a kinetic and stoichiometric model that contradicts the traditional view of a packed, rigid motor architecture.
Keywords: Escherichia coli, bacterial motility, signal transduction, sensory adaptation, protein subunit exchange
Introduction
Bacterial flagellar motors in E. coli switch between clockwise (CW) and counterclockwise (CCW) rotation1–3. The probability of CW rotation (the CW bias) is controlled by the level of the phosphorylated chemotaxis response regulator, CheY-P4. The switch complex in the motor is a protein ring composed of subunits of FliG, FliM and FliN5. FliG interfaces with the motor’s torque-generating units at the periphery of the complex6. Binding of CheY-P to FliM7 and FliN8 promotes conformational changes in FliG that result in CW rotation9.
Recent studies have identified the ability of the motor to adapt to the steady-state concentration of CheY-P, set by the chemotaxis network10. FliM subunits continuously exchange between the motor and the cytoplasm11. The details of exchange differ between the rotational states of the motor, such that CCW motors are able to host more subunits than CW motors12. When steady-state [CheY-P] is low, the motor spends more time in the CCW state and remodels by adding FliM subunits10. With more ligand binding sites present, the motor is able to sense lower levels of CheY-P and bias is partially restored.
We would like to understand how the architecture of the switch complex changes during motor adaptation. Studies have revealed mismatches in the exchange kinetics of FliG, FliM and FliN, raising questions about how remodelling proceeds. FliG appears to be anchored to the motor: fluorescence studies have not observed any exchange of the protein between motor and cytoplasm13,14. In fluorescent studies of FliN, ~10% of the protein exchanges in ~1 hr13. In comparison, upwards of ~25% of FliM exchanges in ~2 min12. This discrepancy between FliM and FliN kinetics is surprising, given the position of FliN at the base of the complex below FliM5, and its role in binding CheY-P in conjunction with FliM8. Here, we re-visit the topic of FliN exchange and investigate its role in motor adaptation.
Results
FliN fluorescent fusions
We investigated the functionality of various FliN fluorescent fusions. The fluorescent protein eYFPA206K (eYFP with alanine at residue 206 substituted for lysine, to prevent aggregation, referred to hereafter as YFP) was fused with a [Gly Gly Gly] linker to the N-terminus or C-terminus of FliN. Three internal fusions also were constructed. Locations were chosen by considering the known interactions between FliN, FliM and FliG, and by inspection of the primary, secondary and tertiary structure of FliN. The [linker][YFP][linker] insertion [Gly Gly Gly][YFPSer…YFPLys][Ser Gly Gly Gly Gly] was placed between FliN codons 45 and 46, 93 and 94, and 115 and 116 (the notation used here labels the N-terminal methionine as codon 1). Cells carrying a genomic fliN deletion and expressing the N-terminal fusion (YFP-FliN) or the internal fusion between codons 45 and 46 (FliN-YFPINT) were motile and fluorescent. The other fusions did not rescue fliN function in a ΔfliN background. These results can be understood in the context of a FliN docking model where a FliN tetramer forms a torus, with C-termini pointing into the hole and N-termini facing out15.
We tested the functional fusions for tethered-cell rotation and fluorescence localization using Total Internal Reflection Fluorescence (TIRF) microscopy. Results are shown in Table 1. YFP-FliN motors rotated more slowly and were much dimmer than FliN-YFPINT motors, and were also defective for switching (CW bias = 0). The functionality of FliN-YFPINT motors was similar to parent strain (RP437) motors containing WT FliN. (We note that our RP437 rotation statistics are similar to previous measurements16 but dissimilar to those of AW40517). Furthermore, FliM-YFP motors had an intensity relative to that of FliN-YFPINT motors of 0.20±0.11, consistent with the expected FliM:FliN stoichiometry of ~1:45. We chose to proceed in our investigation using FliN-YFPINT.
Table 1.
Properties of motors containing either YFP-FliN, FliN-YFPINT or WT FliN. Numbers are mean ± standard error.
| YFP-FliN (n=9) | FliN-YFPINT (n=10) | WT FliN (n=13) | |
|---|---|---|---|
| CCW rotation rate (Hz) | 2.36±1.02 | 5.40±2.24 | 5.60±1.80 |
| CW bias | 0.00±0.00 | 0.02±0.04 | 0.04±0.08 |
| Relative brightness | 0.23±0.03 (n=11) | 1±0.10 (n=11) | n/a |
Kinetic Model
The model developed here for the interpretation of our results in later sections is based on previous work10,12. The model describes the subunit kinetics of steady-state and adapting motors, accounting for exchanging and non-exchanging fractions, and including the effects of bleaching of subunits in both the motor and cytoplasm.
The total number of subunits in the motor is N(t) = NT + NW(t), where NT(t) is the number of tightly bound (non-exchanging) subunits, NW(t) is the number of weakly bound (exchanging) subunits, and t is time. Rates of change are:
| (eq1) |
| (eq2) |
where B is the total number of weak binding sites, U is the number of subunits in the cytoplasm, kon is the subunit on-rate, and koff is the subunit off-rate. We define the pseudo on-rate Ukon. For motors at steady-state, eq1 gives:
| (eq3) |
where N is the steady-state number of subunits in the motor and E is the fraction of motor subunits undergoing exchange.
The number of fluorescent subunits in the motor is F(t) = FT(t) + FW(t), where FT(t) is the number of fluorescent tightly bound subunits and FW(t) is the number of fluorescent weakly bound subunits. Rates of change are:
| (eq4) |
| (eq5) |
where λ1 is the fluorescence bleaching rate of subunits in the motor, and L(t) is the number of fluorescent subunits in the cytoplasm, with rate of change:
| (eq6) |
where λ2 is the fluorescence bleaching rate of subunits in the cytoplasm. We do not consider rates of change in space - we assume the intervals between exposures in our experiments are large enough that cytoplasmic fluorescence becomes uniform. For motors at steady-state, eq4 becomes
| (eq7) |
FliN-YFPINT exchange kinetics
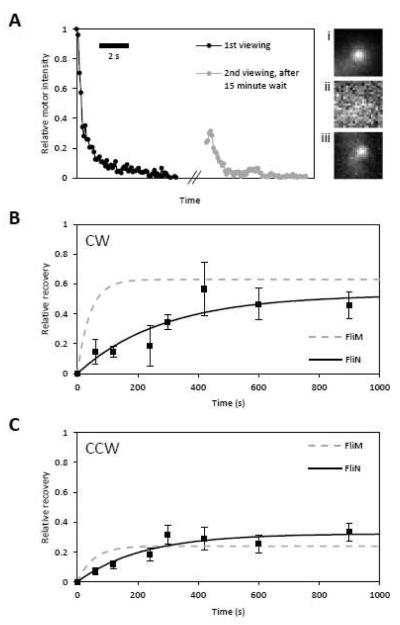
Previous studies have used Fluorescence Recovery After Photobleaching (FRAP) to investigate the exchange kinetics of FliM fluorescent fusions11–13. The motor at the centre of rotation of a tethered cell can be bleached with a high-intensity pulse11 or a TIRF field12. Motor fluorescence recovers as bleached subunits in the motor exchange with fluorescent subunits in the cytoplasm. Fig. 1A illustrates a FRAP experiment on a motor containing FliN-YFPINT. The apparent recovery (~30% in ~15 min) is much greater than observed in previous work (~10% in ~1 hr)13, and indicates appreciable exchange of FliN-YFPINT between motor and cytoplasm.
Figure 1.
FliN-YFPINT exchange kinetics. A) FRAP experiment on a motor containing FliN-YFPINT. During TIRF imaging, motor fluorescence intensity decays due to photobleaching and is fit with A1exp(−B1t)+C1. After an interval of 15 min, the motor is imaged again and fitted with A2exp(−B2t)+C2. For direct comparison to the data presented in reference 12, the apparent recovery of motor fluorescence is calculated as (A2+C2−C1)/A1 ~ 0.3. Each viewing comprises 70×100ms exposures. Images i, ii, and iii are frames from the beginning of the first viewing, end of the first viewing, and beginning of the second viewing, respectively. Pixel values are scaled between 0 and 64 within each frame. B) Timecourse of recovery in CW motors. From left to right: sample sizes are 7, 6, 8, 5, 4, 16 and 9 motors. Black squares are means and bars are standard errors. Black curve is the fit to the data with eq8/α. The grey dashed curve is the fit to CW FliM-YFP motor data, reproduced from Fig 2E of reference 12. See main text for fit parameter values ± standard errors. C) The same as (B) but with CCW motors (sample sizes are 15, 15, 8, 11, 8, 15 and 15 motors).
Lele et al.12 identified the rotational state as an important factor when considering FliM-YFP exchange. We overexpressed CheY to measure recoveries in CW motors and used a cheY deletion strain to measure recoveries in CCW motors. To quantify the kinetics of exchange we measured recovery as a function of time. FRAP experiments were conducted as described in Fig. 1A, but with a wait interval of either 1, 2, 4, 5, 7, 10 or 15 min. The theoretical time-course of recovery is obtained by solving eq5 and eq7 for post-bleach conditions FT(t) = 0, FW(0) = 0, λ1 = 0, and L(t) = αU (where α is the fraction of subunits in the cytoplasm that are fluorescent). The relative recovery is:
| (eq8) |
For our setup, the relative cytoplasmic fluorescence after bleaching was previously measured to be 0.712. We divide experimental recoveries by α = 0.7 and fit the time-courses with eq8/α to obtain exchanging fractions and off-rates. Time-courses for CW and CCW motors are shown in Figs. 1B and C. For FliM-YFP, the exchanging fraction in CW motors (0.63±0.02) is higher than the exchanging fraction in CCW motors (0.24±0.01) and the off-rate is independent of rotation direction (0.024±0.003 s−1 for CW rotation and 0.019±0.005 s−1 for CCW rotation)12. Except for the magnitude of the off-rate, the details of exchange for FliN-YFPINT are similar: the exchanging fraction in CW motors (0.53±0.12) is greater than the exchanging fraction in CCW motors (0.32±0.05) and the off-rate is independent of rotation direction (0.004±0.002 s−1 for CW rotation and 0.005±0.002 s−1 for CCW rotation). The FliN-YFPINT off-rate is ~4-5 times lower than the FliM-YFP off-rate.
These data demonstrate that, like FliM-YFP, the population of FliN-YFPINT in the motor is divided between subunits that are tightly bound (non-exchanging) and subunits that are weakly bound (exchanging), and that the tightly bound fraction is larger in the CCW state. The independence of off-rate on rotation direction demonstrates that off-rate does not play a role in remodelling, contradicting the suggestion of an earlier study10. For FliM-YFP, the difference in tightly bound fractions results in CCW motors hosting more FliM-YFP subunits than CW motors12. This difference in stoichiometry forms the basis of adaptation. We proceed to investigate the stoichiometry of FliN-YFPINT.
FliN-YFPINT stoichiometry
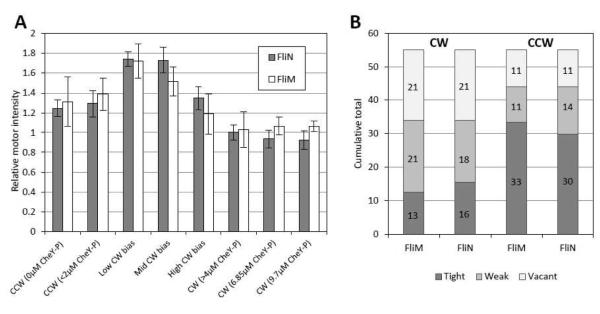
We measured FliN-YFPINT motor intensity with TIRF as a function of [CheY-P] to explore FliN-YFPINT stoichiometry (Fig. 2A). The variation in the FliN-YFPINT motor intensity matches the variation in FliMYFP motor intensity, demonstrating that the ratio of the proteins in the motor remains constant. As reported for FliM-YFP motors12, FliN-YFPINT CCW motors are brighter than CW motors, indicating the presence of more subunits in the CCW state. There is no dependence of intensity on [CheY-P] per se – intensity is constant below 2 μM CheY-P and above 4 μM CheY-P – suggesting that CheY-P is not involved in the remodelling process11.
Figure 2.
FliN-YFPINT stoichiometry. (A) Relative motor intensity as a function of [CheY-P]. See Methods for estimation of [CheY-P]. Low bias motors have 0 < CW bias <= 0.33, mid bias motors have 0.33 < CW bias <= 0.66 and high bias motors have 0 < CW bias < 0.33. FliN motor intensity curves were fitted with Aexp(−Bt)+C and motor intensity was calculated as A+C. FliM-YFP values were calculated from the datasets used to produce figures 1C and 1D in reference 12. From left to right: FliN-YFPINT sample sizes are 29, 20, 13, 19, 17, 25, 25 and 23; FliM-YFP sample sizes are 63, 4, 6, 10, 10, 6, 19 and 28. Bar heights are means and error bars are standard errors. FliN-YFPINT motor intensities are relative to the ‘CW (>4 μM CheY-P)’ dataset. FliM-YFP motor intensities are relative to the CW mutant dataset in Figure 1D of reference 12. (B) Model for motor stoichiometry. The motor has ~55 FliM binding sites. In the CW state, ~13 of these sites are occupied by tightly bound units, and ~half of the remaining sites are occupied by weakly bound units. In the CCW state, ~33 of the sites are occupied by tightly bound units and ~half of the remaining sites are occupied by weakly-bound subunits. The same model applies to FliN tetramers. Numbers of weakly and tightly bound units, along with numbers of vacant sites, are indicated on the bars.
We develop the model for CW and CCW motor stoichiometry outlined by Lele et al.12. The CW motor is known to host ~34 FliM subunits18 and ~34 FliN tetramers19 (our model and data cannot distinguish between monomeric or tetrameric FliN – we consider tetramers for convenience). From the measurements of relative motor intensity (Fig. 2A), the CCW motor hosts ~1.3 times as many subunits as the CW motor. Assuming proportionality between stoichiometry and fluorescence intensity, this equates to ~44 FliM subunits and ~44 FliN tetramers. The exchanging fractions determined earlier can be used to calculate the number of weakly bound and tightly bound subunits in each rotational state. When the off-rate and pseudo on-rate for weakly bound subunits are equal (which was described to be the case for CCW FliM-YFP motors12), the weakly bound subunits occupy half of the available binding sites (see eq3). This indicates ~55 FliM binding sites in total for the CCW motor, and by extension ~55 FliN tetramer binding sites. We assume the CW motor also has a total of ~55 binding sites. The model is visualized in Fig. 2B.
FliN-YFPINT adaptation kinetics
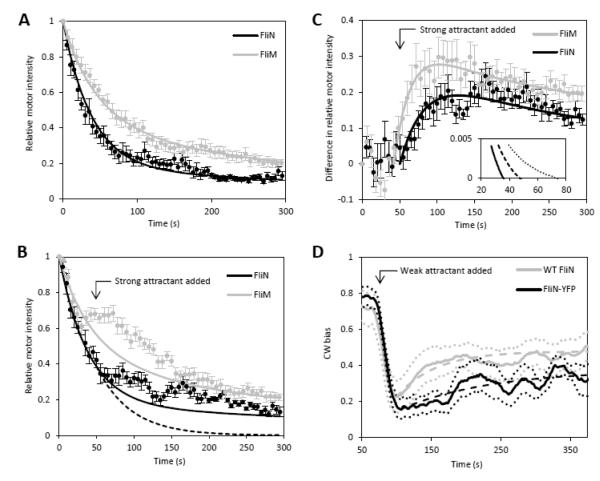
Yuan et al.10 observed the real-time increase in FliM-YFP motor intensity associated with the change in stoichiometry when motors switch from CW to CCW rotation. We performed the same experiment using FliN-YFPINT. A cheR cheB deletion strain was used, where motor adaptation can be observed in the absence of receptor adaptation. The cheR cheB strain yields motors with a wide range of biases. We selected CW motors and monitored motor intensity with TIRF (Fig. 3A). An expression for the decay is obtained by solving eqs 5,6 and 7 for initial conditions FW(0) = EN, FT(0) = (1 – E)N and L(0) = U:
| (eq9) |
The data are fitted with eq9. Parameters are described in the figure caption. The difference between the FliM-YFP and FliN-YFPINT decay curves is partly due to a difference in the YFP bleaching rates (laser and exposure settings were different between the two experiments), but also due to the FliN-YFPINT off-rate being lower than FliM-YFP off-rate. FliN-YFPINT motors are not replenished with fluorescent subunits from the cytoplasm as quickly as FliM-YFP motors. Hence, the decay in the intensity of FliN-YFPINT motors is greater than in FliM-YFP motors.
Figure 3.
FliN-YFPINT adaptation kinetics. (A) Relative intensity of CW FliN-YFPINT motors and high CW bias FliM-YFP motors (reproduced from Fig. 4C of reference 10) as a function of time. The relative intensities of 8 FliN-YFPINT motors were averaged. Circles are means and bars are standard errors. Curves are fits to the data with eq9. The data do not constrain fitting of all parameters. We allowed the exchanging fractions and off-rates to vary within the 95% confidence bounds determined in the FRAP experiments and fitted for bleaching rates. Final values for FliM-YFP and FliN-YFPINT exchanging fractions were 0.59 (95% confidence lower bound) and 0.77 (95% confidence upper bound), respectively; final values for off-rates were 0.018 s−1 (95% confidence lower bound) and 0.007 s−1 (95% confidence upper bound), respectively; final values for motor bleaching rates were 0.015±0.0002 s−1 and 0.024±0.0004 s−1, respectively; final values for cytoplasmic bleaching rates were 0.0021±0.0002 s−1 and 0.0018±0.0006 s−1, respectively. We note that these values are consistent with the cytoplasmic bleaching rate calculated from α=0.7=exp(−λ2t), once scaled for the difference in exposure rate between our experiment and the experiment in (12). (B) Relative intensities during motor adaptation. The relative intensities of 8 FliN-YFPINT motors were averaged. Circles are means and bars are standard errors. FliM-YFP motor data is from reference 10. A switch from CW to CCW rotation was induced at ta≈50 s in FliN-YFPINT experiments. A switch from high CW bias rotation to CCW rotation was induced at ta≈42 s in FliM-YFP experiments. Solid curves are fits from A, plotted for comparison. Dashed curve is eq11. (C) Data in B re-plotted to show fluorescent signal of additional subunits. Curves are fits to the data with eq12. The value of N in eq12 was 41 for the fit to FliM data, and 34×4 for the fit to FliN data (values based on data in Figure 2A).See main text for fit parameter values. Inset: parameter space search. Abscissa is steady-state weakly bound number of tetramers, ordinate is fitted pseudo on-rate. Off-rate was either 0.003 s−1 (dotted line), 0.005 s−1 (dashed line) or 0.007 s−1 (solid line), consistent with the FRAP experiment off-rate 0.005±0.002 s−1. Only parameter sets resulting in fits with R-squared>0.7 are plotted. (D) Partial recovery of bias in cheR cheB cells after the addition of weak attractant. Solid lines are means, dotted lines are means ± standard errors. Dashed lines are fits to the data with A(1-exp(−Bt)). WT motor data is reproduced from reference 10. FliN-YFPINT sample size is 5 motors.
We repeated the above experiment, but introduced strong attractant (2mM MeAsp + 0.5 mM L-serine) after time ta to induce CCW rotation. The results are shown in Figure 3B, together with the fits from Figure 3A for comparison. Following the switch, the rate of decay is reduced, indicating the addition of both FliM-YFP and FliN-YFPINT subunits. We interpret the result in terms of the model illustrated in Fig. 2B. When the switch occurs, the weakly bound subunits in the motor become tightly bound, and vacant sites begin to fill up with new, weakly bound subunits. A new steady-state is reached when ~half of these sites are occupied. A formal description is provided by solving eq1-5 with conditions NW(ta) = 0, NT(ta) = N, FW(ta) = 0, FT(ta) = F(ta) and the approximation L(ta) = U:
| (eq10) |
| (eq11) |
| (eq12) |
where NW is the new steady-state number of weakly bound subunits. The sum of eq11 and eq12 describe the fluorescent signal in Fig. 3B as the motor adapts and reaches a new steady-state.
To isolate the fluorescent contribution of the new subunits from the fluorescent contribution of the old subunits, previous work10 subtracted the CW decay from the CCW decay. However, the present model indicates that this will underestimate the fluorescent contribution of the new subunits. According to the model, all old subunits are tightly bound following the switch. Consequently, the fluorescence of the old subunits will decay at a greater rate than the CW decay, where about two-thirds of the subunits are undergoing exchange and replenishing motor fluorescence. The theoretical decay for the case that all old subunits are tightly bound (eq11) is plotted for the FliN case as a dashed line in Figure 3B. This is the decay that should be subtracted from CCW decay in order to isolate the fluorescence of the new subunits.
The fluorescent signal attributed to the new subunits is presented in Fig. 3C. The increase in motor intensity following the addition of strong attractant is evident. We fit the data with eq12. For the FliM-YFP data the fit provides values for the off-rate (0.019±0.006 s−1), pseudo on-rate (0.013±0.002 s−1) and the steady-state number of weakly bound subunits (20±2). We note that the off-rate agrees with the value obtained in FRAP experiments12, and that the pseudo on-rate is similar to the off-rate, as concluded before. For the FliN-YFPINT data, a free parameter fit could not be achieved with confidence. Given the longer timescale associated with FliN-YFPINT kinetics, a longer recording time might be required to obtain a dataset that can be fitted. We conducted a parameter space search (Fig. 3C, inset). For values of the off-rate close to the value determined in the FRAP experiment, acceptable fits to the data are achieved with a pseudo on-rate in the range 0-0.004 s−1 and steady-state values of the number of weakly bound tetramers greater than 26. The particular fit shown in Fig. 3C is with pseudo on-rate, off-rate and steady-state values of 0.004 s−1, 0.007 s−1 and 27 tetramers, respectively. As with FliM-YFP, the pseudo on-rate and off-rate are the same order of magnitude.
Motor adaptation has been investigated in WT motors10: using a bead assay, motors with high CW bias were selected and bias was monitored following the addition of weak attractant (1 mM MeAsp). The CW bias dropped to a lower value and then partially recovered (Fig. 3D, grey curve). The rate of adaptation is obtained by fitting the recovery with A(1-exp(−Bt)). For WT motors, the rate of adaptation is 0.018±0.002 s−1. The number of weakly-bound subunits in the motor as a function of time during adaptation is given by eq10. The rate of remodelling is koff + Ukon. For FliM-YFP, the rate of remodelling is ~0.04 s−1. For FliN-YFPINT the rate of remodelling is less than 0.009 s−1 - less than half the rate of adaptation. If FliN is involved in adaptation, we would expect the rate of remodelling to be at least as great as the rate of adaptation. It is possible that the presence of YFPINT slows down FliN kinetics. We measured adaptation in FliN-YFPINT motors (Fig. 3D, black curve). The rate of adaptation was 0.008±0.002 s−1, indicating that adaptation was rate-limited by FliN-YFPINT remodelling. This suggests an active role for FliN in adaptation, and that FliN kinetics may be faster in the absence of YFPINT.
Discussion
This study extends our understanding of motor adaptation, demonstrating that the whole base of the motor undergoes remodelling. Motors contain an exchanging and non-exchanging population of FliM and FliN subunits. The fraction of non-exchanging subunits changes with rotational state, resulting in changes in stoichiometry and, consequently, changes in sensitivity to CheY-P. The behaviour of FliM and FliN are separated only by timescale. The faster timescale of FliM would indicate that this protein is the key player in motor adaptation, but our data show that the presence of YFPINT perturbs adaptation and might be slowing down FliN kinetics. Indeed, results appear to be dependent on the particulars of the fluorescent fusion: Fukuoka et al. reported much less exchange using GFP-FliN13. In light of this, it might be worth re-visiting the statement that FliG does not undergo exchange13,14.
The defining parameters of our model for motor remodelling are listed in Table 2. Values for subunit pseudo on-rate and total number of binding sites in the CW state are missing; these can be determined by conducting the experiments of Figure 3A and B, but on motors switching from CCW to CW rotation. In contrast to the traditional view of a packed protein ring, the model describes a switch complex with an excess of binding sites, with gaps permitted between proteins in the FliM and FliN architecture. In this sense, the architecture does not need to grow or shrink to accommodate more or fewer units during adaptation. Gaps in the FliM and FliN architecture would not necessarily interfere with the act of switching. A consideration of the MWC model indicates the need to energetically decouple the binding element of the complex from the switching element of the complex12. In this context, FliM and FliN may serve simply as CheY-P receptors that relay occupancy information to the intact, bistable FliG ring. Recent studies describe inner and outer FliM (and by extension, FliN) binding sites on FliG20,21, where the inner and outer locations correspond to tightly and weakly binding sites, respectively. With 26-34 FliG subunits per motor22,23, this scheme could account for the excess of binding sites.
Table 2.
Defining parameters of the kinetic and stoichiometric models. Numbers are mean ± standard error.
| FliM-YFP | FliN-YFP|NT | ||||
|---|---|---|---|---|---|
| Parameter | CW | CCW | CW | CCW | Reference |
| N | 34 | ~44 | ~4×34 | ~4×44 | 12, 17, this work |
| E | 0.63±0.02 | 0.24±0.01 | 0.53±0.12 | 0.32±0.05 | 12, this work |
| k off (s ) | 0.024±0.003 | 0.019±0.005 | 0.004±0.002 | 0.005±0.002 | 12, this work |
| Ukon (s’1) | - | 0.013±0.002 | - | 0-0.004 | 10, this work |
| binding sites | - | ~55 | - | ~4×55 | 12, this work |
These arguments apply to CW and CCW motors. In Fig. 2A, we note that the brightest motors are found in the range 0 < CW bias < 1, i.e. for motors that switch. Based on the relative intensity, the number of units in low CW bias motors is ~58. Interestingly, this is similar to the steady state number of units estimated from our adaptation experiments (Fig. 3C), after motors transitioned from the CW or high CW bias state to the CCW state. Remodelling might be enhanced in motors that switch. Further investigation is required to determine whether the observed relationship between stoichiometry and bias (Fig. 2A) is physiologically accurate or a consequence of the fusion. The exact relationship will provide information about the dynamic range of motor operation and also the precision of motor adaptation. In our data, brightness reduces with increasing CW bias, but the resolution is not sufficient to draw conclusions about whether the relationship is non-linear, as predicted by a recent model describing precise motor adaptation24.
Methods
Strains and plasmids
For motility tests, FliN fluorescent fusions were cloned separately into pTrc99A25 under an isopropyl-β-D-thiogalactoside (IPTG)-inducible promoter. The fliN deletion strain DFB22326 was transformed separately with each construct. For tethered assay tests, DFB223 was deleted for fliC and transformed with pKAF131 carrying the sticky fliC allele under control of the native fliC promoter27. This strain was transformed separately with the constructs carrying yfp-fliN and fliN-yfpINT. Induction was with 50 mM IPTG to yield WT levels of FliN. FliM-YFP motor data came from our previous study10.
For CCW motor FRAP experiments, we replaced WT fliN in VS124 (cheB cheZ cheY)28 with fliN-yfpINT. All gene replacements in this investigation were with the lambda Red protocol29. For CW motor FRAP experiments, we transformed the CCW motor strain with pWB5, a gift from B. Wang carrying cheY on a pTrc99A vector and overexpressed CheY. Induction was with 100 μM IPTG. Due to the deletion of cheB and cheZ, the CheY pool in these strains is almost completely phosphorylated4.
In FliN stoichiometry experiments, the ‘CCW (0 μM CheY-P)’ dataset was collected with the CCW motor strain above. The ‘CCW (<2 μM CheY-P)’ dataset was collected from the CW motor strain above with 0 μM IPTG -- [CheY-P] is greater than zero due to background expression from the plasmid, but less than 2 μM based on the CW bias vs [CheY-P] relationship4. The ‘Low/Mid/High CW bias’ datasets were collected with the CCW motor strain transformed with pVS7, a gift from V. Sourjik carrying cheY on a pBAD18-Kan vector30. Induction with 0-0.001% arabinose provided motors with 0 < CW bias ≤ 1. Motors with CW bias = 1 contributed to the CW (>4 μM CheY-P) dataset -- [CheY-P] > 4 μM based on the CW bias vs [CheY-P] relationship4. The ‘CW (6.85/9.7 μM CheY-P)’ datasets were collected with the CW motor strain above induced with 30/50 μM IPTG. [CheY-P] values are from the calibration curve in reference 31.
For adaptation experiments we replaced fliN in JY35 (cheR cheB fliC)10 with fliN-yfpINT. For bead assay adaptation experiments, we transformed this strain with pKAF131.
Assays
Cells were grown at 33 °C in 10ml T-broth (1% tryptone, 0.5% NaCl) supplemented with the appropriate antibiotics (ampicillin: 100 μg ml−1, kanamycin: 50 μg ml−1) and inducers to an OD600 of 0.5. Cells with filaments were sheared to truncate flagella by passing ~1 mL of culture 50 times between two syringes with 23-gauge needles connected by polyethylene tubing. Cells were collected by centrifugation (2 min at 4000g), washed twice in 1 ml of motility medium (10 mM potassium phosphate, 0.1 mM EDTA, 10 mM lactate, pH 7.0) and resuspended in 0.2 mL motility medium. Cells were incubated with the appropriate antibody (anti-FlgE or anti-FliC, at ~0.5 μg ml−1) for 20 min, washed twice in 0.3 mL of motility medium and resuspended in 0.2 mL motility medium. Antibodies were purified from antiserum using Protein A sepharose CL-4B beads (Amersham Biosciences) and a Bio Rad #731-1550 10 mL chromatographic column. Dialysis was carried out with #66810 10000 MWCO Slide-A-Lyzer dialysis cassettes (Pierce Biotechnology). Purified antibody was preadsorbed using hookless strain HCB137.
Cell suspension was flowed into a custom tunnel slide for FRAP and stoichiometry experiments, and a custom flow slide for adaptation experiments. The suspension was left for 15 min to allow cell tethering and then the chamber was rinsed with motility medium. For adaptation experiments with the bead assay, 0.01% poly-L-lysine solution (Sigma) was flowed into a custom flow slide and left for 1 min to allow coating of the coverslip, followed by rinsing. Cell suspension was flowed in and left for 15 min, followed by rinsing. A 1.0-μm polystyrene bead suspension (Polysciences) was flowed in and left for 10 min to allow attachment to sheared flagella, followed by rinsing. In adaptation experiments, the chamber was kept under constant flow (60 μL min−1 for tethered assays or 400 μL min−1 for bead assays) by syringe pump (Harvard apparatus), with either motility medium or attractant medium.
Microscopy
For TIRF work, a 25 mW Cobolt Fandango diode-pumped solid-state laser provided 515 nm light. The laser beam was gated on and off with a shutter (Vincent Associates LS6-ZM-1 with VMM-D1 driver). A fiber port (Thorlabs PAF-X-5-A) and fiber (Oz Optical QSMJ-3AF3U-488-3.5/125-3AS-3”) coupled the excitation light into a Nikon TI-TIRF TIRF/Epi-fl Illuminator unit fitted on a Nikon Eclipse TI-U inverted microscope. Excitation light passed through a Z514/10 bandpass filter and was reflected by a ZT514RDC dichroic into a Nikon CFI Apo 60× 1.49 oil TIRF objective. All dichroics and filters were from Chroma Technology. When focused in the middle of the back focal plane (BFP) of the objective, the laser beam exited directly upward out of the objective at ~9.5 mW. The field was an elliptical Gaussian in shape with widths at half maximum of ~165 μm and ~220 μm. Thus the field intensity was ~8 W/cm2.
The 1/e decay depth of the TIR evanescent wave is a function of the laser angle of incidence at the coverslip/sample interface, θ. The exit-angle of the laser from the objective as a function of the position (p) of the laser focus within the objective BFP was measured with a marked prism atop the objective, in contact by Cargille DF immersion oil. A calibration curve between p and θ was constructed, accounting for differences in refractive index between oil, prism and coverslip. The 1/e decay depth, d, was set to ~100 nm using the relation d=(λ/4π)(ncover2sin2θ–nsample2)−1/2, where λ is the laser wavelength, and ncover and nsample are the refractive indices of the coverslip and sample, respectively.
Emission light passed through the ZT514RDC dichroic and an ET520LP longpass filter, and was reflected by a T680LPXXR dichroic through a ET650sp-3p shortpass filter into a Nikon VM Lens C-4x telescope fitted to the back port of the TI-U. Imaging was with a 512×512 pixel EMCCD (Andor iXon Model DV887ECS-BV). Resolution was ~65 nm/pixel. The iXon peltier was set to −55°C and fan-cooled. Electron multiplier gain was set to 145. iXon control and acquisition was with Andor Solis software. For FRAP and stoichiometry experiments, acquisition was under ‘frame-transfer’ mode, with 70×100 ms exposures. For adaptation experiments, acquisition was under ‘kinetic’ mode, with 200 ms exposures every 5 s for 60 exposures. For synchronization of laser excitation and exposures, the iXon ‘shutter’ output provided input to the VMM-D1 driver. Recordings were saved as .sif files and exported as .txt files for analysis with custom Matlab scripts. Motor intensities were calculated using a Gaussian mask method, as described10.
A Nikon TI T BPH Eyepiece tube base unit allowed for ‘external’ phase contrast microscopy. An image on the primary image plane is refocused on a secondary image plane via relay optics. This allows the TI C TPH 60x/PH4 phase ring (matching the 60x Ph4 annular ring of the Nikon CLWD 0.72 condenser) to be placed at a conjugate objective BFP instead of at the objective BFP. The result is unobstructed fluorescence microscopy. Light for external phase contrast microscopy was provided by the 100-W Ti-U halogen lamp system. After diffuser filtering, an HQ740/80 bandpass filter allowed passage of infra-red light, which passed all mentioned filters and dichroics and was imaged on a Thorlabs DCC1240M CMOS camera mounted on the TI T BPH port at the secondary image plane. Resolution was ~105 nm/pixel and at least 120 Hz. Acquisition was with custom LabView software.
Highlights.
Adaptive remodelling has been observed in rotary motors for FliM but not FliN
Using an internal fluorescent fusion, we find that remodelling does occur with FliN
Remodelling is enhanced by motor switching
This work reconciles the apparent mismatch between the activities of FliM and FliN
Acknowledgements
We thank Pushkar Lele for input on genetic work and Junhua Yuan for useful discussions. This work was supported by National Institutes of Health Grant AI016478. M. N. S. acknowledges financial support from the Harvard Physics Department and Harvard College Program for Research in Science and Engineering.
Footnotes
Contributions R. W. B. and H. C. B. designed research. R. W. B., M. N. S. and C. S. conducted experiments. R. W. B., M. N. S. and C. S. analyzed data. V. S. J. N., R. W. B. and M. N. S. conducted genetic work. R. W. B and H. C. B. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silverman M, Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974;249:73–4. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- 2.Larsen SH, Reader RW, Kort EN, Tso WW, Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974;249:74–7. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- 3.Berg HC. Dynamic properties of bacterial flagellar motors. Nature. 1974;249:77–9. doi: 10.1038/249077a0. [DOI] [PubMed] [Google Scholar]
- 4.Cluzel P, Surette M, Leibler S. An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Science. 2000;287:1652–5. doi: 10.1126/science.287.5458.1652. [DOI] [PubMed] [Google Scholar]
- 5.Brown PN, Terrazas M, Paul K, Blair DF. Mutational analysis of the flagellar protein FliG: sites of interaction with FliM and implications for organization of the switch complex. J. Bacteriol. 2007;189:305–12. doi: 10.1128/JB.01281-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou J, Lloyd SA, Blair DF. Electrostatic interactions between rotor and stator in the bacterial flagellar motor. Proc. Natl. Acad. Sci. 1998;95:6436–6441. doi: 10.1073/pnas.95.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bren A, Eisenbach M. The N terminus of the flagellar switch protein, FliM, is the binding domain for the chemotactic response regulator, CheY. J. Mol. Biol. 1998;278:507–14. doi: 10.1006/jmbi.1998.1730. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar MK, Paul K, Blair D. Chemotaxis signaling protein CheY binds to the rotor protein FliN to control the direction of flagellar rotation in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9370–5. doi: 10.1073/pnas.1000935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer CM, Vartanian AS, Zhou H, Dahlquist FW. A molecular mechanism of bacterial flagellar motor switching. J. Mol. Biol. 2009;388:71–84. doi: 10.1016/j.jmb.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan J, Branch RW, Hosu BG, Berg HC. Adaptation at the output of the chemotaxis signalling pathway. Nature. 2012;484:233–6. doi: 10.1038/nature10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delalez NJ, Wadhams GH, Rosser G, Xue Q, Brown MT, Dobbie IM, Berry RM, Leake MC, Armitage JP. Signal-dependent turnover of the bacterial flagellar switch protein FliM. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11347–51. doi: 10.1073/pnas.1000284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lele PP, Branch RW, Nathan VSJ, Berg HC. Mechanism for adaptive remodeling of the bacterial flagellar switch. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20018–22. doi: 10.1073/pnas.1212327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuoka H, Inoue Y, Terasawa S, Takahashi H, Ishijima A. Exchange of rotor components in functioning bacterial flagellar motor. Biochem. Biophys. Res. Commun. 2010;394:130–5. doi: 10.1016/j.bbrc.2010.02.129. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Sourjik V. Assembly and stability of flagellar motor in Escherichia coli. Mol. Microbiol. 2011;80:886–99. doi: 10.1111/j.1365-2958.2011.07557.x. [DOI] [PubMed] [Google Scholar]
- 15.Sowa Y, Berry RM. Bacterial flagellar motor. Q. Rev. Biophys. 2008;41:103–32. doi: 10.1017/S0033583508004691. [DOI] [PubMed] [Google Scholar]
- 16.Lapidus IR, Welch M, Eisenbach M. Pausing of flagellar rotation is a component of bacterial motility and chemotaxis. J. Bacteriol. 1988;170:3627–32. doi: 10.1128/jb.170.8.3627-3632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg HC, Tedesco PM. Transient response to chemotactic stimuli in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 1975;72:3235–9. doi: 10.1073/pnas.72.8.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DR, Francis NR, Xu C, DeRosier DJ. The three-dimensional structure of the flagellar rotor from a clockwise-locked mutant of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2006;188:7039–48. doi: 10.1128/JB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao R, Pathak N, Jaffe H, Reese TS, Khan S. FliN is a major structural protein of the C-ring in the Salmonella typhimurium flagellar basal body. J. Mol. Biol. 1996;261:195–208. doi: 10.1006/jmbi.1996.0452. [DOI] [PubMed] [Google Scholar]
- 20.Passmore SE, Meas R, Marykwas DL. Analysis of the FliM/FliG motor protein interaction by two-hybrid mutation suppression analysis. Microbiology. 2008;154:714–24. doi: 10.1099/mic.0.2007/014597-0. [DOI] [PubMed] [Google Scholar]
- 21.Paul K, Brunstetter D, Titen S, Blair DF. A molecular mechanism of direction switching in the flagellar motor of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 2011;108:17171–6. doi: 10.1073/pnas.1110111108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee LK, Ginsburg MA, Crovace C, Donohoe M, Stock D. Structure of the torque ring of the flagellar motor and the molecular basis for rotational switching. Nature. 2010;466:996–1000. doi: 10.1038/nature09300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paul K, Gonzalez-Bonet G, Bilwes AM, Crane BR, Blair D. Architecture of the flagellar rotor. EMBO J. 2011;30:2962–71. doi: 10.1038/emboj.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu Y, Berg HC. Tandem adaptation with a common design in Escherichia coli chemotaxis. J. Mol. Biol. 2012;423:782–8. doi: 10.1016/j.jmb.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 26.Tang H, Billings S, Wang X, Sharp L, Blair DF. Regulated underexpression and overexpression of the FliN protein of Escherichia coli and evidence for an interaction between FliN and FliM in the flagellar motor. J. Bacteriol. 1995;177:3496–3503. doi: 10.1128/jb.177.12.3496-3503.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Fahrner KA, Turner L, Berg HC. Asymmetry in the clockwise and counterclockwise rotation of the bacterial flagellar motor. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12846–12849. doi: 10.1073/pnas.1007333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sourjik V, Berg HC. Receptor sensitivity in bacterial chemotaxis. Proc. Natl. Acad. Sci. U. S. A. 2002;99:123–7. doi: 10.1073/pnas.011589998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sourjik V, Berg HC. Binding of the Escherichia coli response regulator CheY to its target measured in vivo by fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12669–74. doi: 10.1073/pnas.192463199. [DOI] [PMC free article] [PubMed] [Google Scholar]