Abstract
Pin1 is an evolutionarily conserved peptidyl-prolyl isomerase that binds and changes the three dimensional conformation of specific phospho-proteins. By regulating protein structure and folding, Pin1 affects the stability, interaction and activity of a broad spectrum of target proteins thus impacting upon diverse cellular processes. This review discusses the pivotal role Pin1 plays in regulating cardiac pathophysiology by functioning as a “molecular orchestrator” of a myriad of signal transduction pathways in the heart.
Keywords: Pin1, cardiac hypertrophy, cardiac progenitor cells, heart, aging
Introduction: A Place for Pin1 in the Molecular Circuitry of a Cell
Post-translational modifications such as reversible protein phosphorylation form the basis of several complex signaling cascades governing cellular fate during cardiac pathophysiological conditions. The dual actions of kinases and phosphatases on serine/threonine sites determine when and which signal transduction pathways can be initiated or silenced in a cell, with protein phosphorylation being traditionally considered a mechanism to turn on signaling and dephosphorylation as means to terminate the signal. However, beyond this “binary on/off switching mechanism”, a layer of complexity is added by cis-trans isomerases that act as rheostats in the molecular circuitry and regulate the intensity and duration of signals by simply modulating the three-dimensional conformation of phospho-proteins (1, 2). When multiple synergistic or oppositional signaling cascades are triggered, determining which signal should be amplified or sustained dictates cellular outcome and governs the difference between cell survival, growth, proliferation or death. An enzyme with the unique potential to collectively synchronize signals and determine cellular fate is the peptidyl-prolyl isomerase Pin1 (Peptidyl-prolyl cis/trans Isomerase, NIMA-interacting 1). The regulatory role of Pin1 in signaling has received increasing attention in the cancer field but remains relatively unexplored in the context of cardiac biology. This review discusses the significance of Pin1 that functions as a “molecular timer” to fine tune the signal amplitude and duration of a myriad of cardiovascular signaling networks (1, 3, 4).
Pin1 Structure and Regulation: Small Molecule, Big Impact
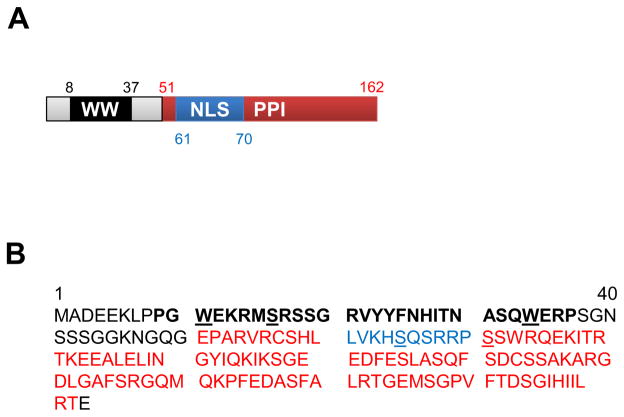
Pin1 is a small protein (163 amino acids) with a simple structure comprising of a N terminal WW domain, bearing two Tryptophans 22 amino acids apart, and a C terminal catalytic peptidyl prolyl isomerase (PPI) domain (Figure 1). The WW domain allows Pin1 to selectively recognize and bind to phospho-proteins with a Serine/Threonine adjacent to a Proline residue (5, 6). The presence of Proline generates kinks in the polypeptide chain and following addition of the phosphate group on the Serine/Threonine residue of the target substrate, Pin1 catalyzes the isomerization of the protein (5). A novel nuclear import sequence in the PPI domain permits targeting action of nuclear substrates (Figure 1) (7).
Figure 1.
Pin1 is a small enzyme with a simple protein structure. A. Important structural domains of human Pin1 with their corresponding amino acid start and end sites. B. Amino acid sequence of human Pin1 indicating the structural domains shown in A. WW domain (amino acids 8–37, black bold font), peptidyl prolyl isomerase domain (PPI, amino acids 51–162, red font) and nuclear localization sequence (NLS, amino acids 61–70, blue font) are shown. The two invariant tryptophans in the WW domain and S16, S65 and S71 residues, important for Pin1 stability and inactivation are underlined.
Regulation of Pin1 is facilitated by transcriptional, translational and post-translational modifications (8). Transcription factor E2F binds to Pin1 promoter and increases Pin1 transcription (9). Growth stimuli like insulin growth factor (IGF) activate Pin1 protein expression via the phosphatidylinositol 3-kinase (PI3K) and mitogen activated protein kinase (MAPK) pathways (10). Pin1 is phosphorylated by different protein kinases which affects its catalytic activity and stability. Polo-like kinase 1 known to be a critical regulator of mitosis, phosphorylates Pin1 on Ser65 residue which increases Pin1 stability without affecting isomerase activity (11). On the other hand, phosphorylation of Pin1 at the Ser16 residue by protein kinase A (PKA) inhibits its interaction with target substrates and consequentially inhibits Pin1 function (12). Similarly, phosphorylation at the Ser71 residue by death associated protein kinase-1 inhibits Pin1 catalytic activity (13) (Figure 1). Pin1 affects the stability, interaction, activity, and subcellular localization of a broad spectrum of target proteins thus impacting upon diverse cellular processes (14). Not surprisingly therefore, deregulation of Pin1 is associated the pathogenesis of cancer, aging, neuro-degenerative diseases such as Alzheimer’s and Parkinson’s, rheumatoid arthritis as well as many other inflammatory and autoimmune diseases (8, 15–19).
Pin1 and Cardiac Hypertrophy: Choosing Between Signals
Over the past couple of years, our lab has been actively studying the impact of Pin1 signaling in the heart and characterizing a cell specific role in cardiac myocytes and c-kit+ cardiac progenitor cells (CPCs) (1, 4). Pin1is highly expressed and mainly localized to the nucleus in the neonatal heart; transition into adulthood is associated with a reduction in expression and switch to cytosolic localization in the myocardium (1). Consistent with a role in growth and survival signaling, Pin1 expression increases upon cardiac injury such as pressure overload induced cardiac hypertrophy (1). Cardiac hypertrophy is caused and effected by the simultaneous action of numerous signaling cascades (20) and to fully appreciate the impact of Pin1 mediated signaling, we studied effects of Pin1 deregulation upon cardiac hypertrophy. Global Pin1 knockout mice (Pin1KO) are protected against pathological hypertrophy, evident as attenuated hypertrophic response, preserved cardiac function, and higher survival rates at 4 weeks after thoracic aortic constriction (1). Consistent inhibition of hypertrophy is also seen in cultured cardiac myocytes with Pin1 silencing following stimulation with serum or phenylephrine, the α1-adrenergic receptor agonist. Our work has identified inhibition of Akt and MEK as mechanistic targets for the attenuated hypertrophic response observed upon Pin1 silencing (Table 1) (1). Serine threonine kinase Akt (or Protein Kinase B) promotes cardiac growth and hypertrophy downstream of IGF/PI3K signaling pathway (21), while MEK (mitogen-activated protein kinase/extracellular signal-regulated kinase kinase) is a part of the Raf/MEK/ERK signaling cascade, also established to be pro-hypertrophic (22). Pin1 stabilizes Akt (23) and positively regulates MEK phosphorylation and activity (24) through direct interactions, resulting in cell transformation and carcinogenesis in tumor cells. Indeed, increased interaction of Akt and MEK with Pin1 is seen under conditions resembling physiological (serum treatment) or pathological growth (phenylephrine treatment) in cultured cardiac myocytes (1). Reduced interaction as well as hypo-phosphorylation of Akt and ERK occurs upon Pin1 deletion, attributing to the diminished hypertrophic response in cardiac myocytes in vitro and in vivo.
Table 1.
Pin1 target regulation in cardiac myocytes and cardiac progenitor cells (CPCs)
| Pin1 Manipulation | Cell type | Target | Target Regulation | Cellular Outcome | Ref. |
|---|---|---|---|---|---|
| Knockdown (siRNA) | Cardiac myocytes | Akt | Decreased phosphorylation; Inhibition of downstream signaling | Decreased hypertrophy | (1) |
| Knockdown (siRNA); Overexpression (Adenovirus) | Cardiac myocytes | MEK | Decreased phosphorylation; Inhibition of downstream signaling | Decreased hypertrophy | (1) |
| Overexpression (Adenovirus) | Cardiac myocytes | Raf-1 | Increased phosphorylation (S259); Inhibition of downstream signaling | Decreased hypertrophy | (1) |
| Overexpression (Adenovirus) | Cardiac myocytes | Pin1 | Increased phosphorylation (S16) Inhibition of binding and function |
Increased hypertrophy | (25) |
| Knockdown (siRNA) | CPCs | Cyclin D | Decreased protein expression | Cell cycle arrest (G1/S) | (4) |
| Knockdown (siRNA); Overexpression (lentivirus) | CPCs | Cyclin B | Decreased protein expression | Cell cycle arrest (G1/S) Cell cycle arrest (G2/M) |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | p53 | Increased protein expression Decreased protein expression |
Senescence Inhibits senescence |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | Rb | Increased protein expression Decreased protein expression |
Senescence Inhibits senescence |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | α-Smooth muscle actin | Decreased protein expression Increased protein expression |
Decreased differentiation Increased differentiation |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | Cardiac Troponin T | Decreased mRNA expression Increased mRNA expression |
Decreased differentiation Increased differentiation |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | c-kit | Decreased protein expression | Decreased stemness | (4) |
| Knockdown (siRNA) | CPCs | c-Myc | Decreased protein expression | Decreased proliferation, multipotency | (4) |
| Knockdown (siRNA) | CPCs | Oct4 | Decreased protein expression | Decreased multipotency | (4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | KLF4 | Decreased protein expression Increased protein expression |
Decreased multipotency Differential regulation of multipotency |
(4) |
| Knockdown (siRNA) Overexpression (lentivirus) |
CPCs | Nanog | Decreased protein expression Increased protein expression |
Decreased multipotency Differential regulation of multipotency |
(4) |
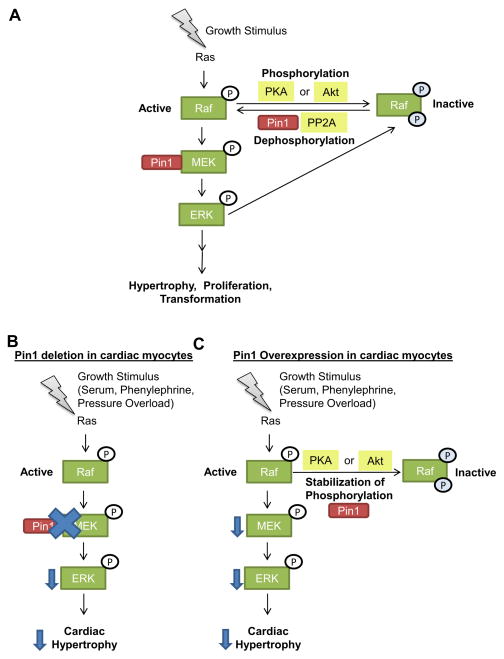
The regulation of hypertrophy upon Pin1 gain of function is seemingly debatable (1, 25). Our work demonstrates that overexpression of Pin1 prevents loss of cardiac function and significantly blunts the hypertrophic response during heart failure occurring at 6 weeks after thoracic aortic constriction in mice (1). Reduced cardiac myocyte size concomitantly associated with decreased phosphorylation and inactivation of MEK, but not Akt, occurs in cardiac myocytes overexpressing Pin1 when challenged with growth stimuli (1). The mechanism behind Pin1 overexpression-mediated inhibition of MEK in cardiac myocytes may be explained by closer examination of the complex Raf/MEK/ERK signaling cascade (Figure 2). Mitogen mediated-phosphorylation of Raf in its kinase domain is required for activation of downstream signaling targets including MEK and ERK, while phosphorylation of Raf on Ser259 residue by PKA or Akt inhibits activation of MEK in cancer cells (26, 27) and cardiac myocytes (1). Upon Pin1 overexpression, phosphorylation of Raf on Ser259 residue is stabilized, consequentially leading to inhibition of downstream MEK and ERK signaling (1). It is tempting to speculate that Akt may be directly involved in the phosphorylation of Raf (Ser259) upon Pin1 overexpression given the evidence that link Pin1 and Akt, however this hypothesis remains to be tested. Interestingly, ERK kinase triggers a negative feedback mechanism by hyper-phosphorylating and inactivating Raf (28). Re-activation of Raf by dephosphorylation through protein phosphastases such as protein phosphatase 2A requires Pin1 interaction and activity (28). Thus Pin1 tightly regulates the phosphorylation/dephosphorylation status of Raf and controls downstream signaling. By selectively choosing the signaling cascades to stabilize and modulate, Pin1 participates in cardiac hypertrophy signaling (Figure 2).
Figure 2.
Pin1 regulation of Raf/MEK/ERK signaling cascade. A. Growth stimuli induce activation of Ras, which leads to phosphorylation and activation of Raf. Active Raf phosphorylates MEK which in turns phosphorylates and activates ERK leading to hypertrophy, proliferation or cellular transformation depending on the cell type and stimulus. Phosphorylation of Raf by Protein kinase A (PKA) or Akt on Ser259 residue leads to inactivation of Raf and inhibition of downstream signaling. ERK kinase also inactivates Raf and triggers a negative feedback mechanism. Re-activation of Raf by dephosphorylation through protein phosphastase 2A (PP2A) requires Pin1. Thus Pin1 tightly regulates the phosphorylation/dephosphorylation status of Raf and controls signaling and cellular outcome downstream of Raf. B and C. Growth stimuli such as serum, phenylephrine or pressure overload induce cardiac myocyte hypertrophy. B. Pin1 deletion in cardiac myocytes leads to decreased phospho MEK, possibly through reduced interaction between Pin1 and MEK, which leads to decreased phospho ERK and attenuated hypertrophy. C. Pin1 overexpression in cardiac myocytes is accompanied by stabilization of phosphorylated Raf (S259) which leads to inactivation of Raf. Consequentially phosphorylation of downstream targets MEK and ERK are decreased leading to blunted hypertrophy.
Contradictory findings have recently been reported by Sakai et al wherein adenoviral mediated overexpression of Pin1 increases cardiac myocyte size and protein synthesis, indicative of increased hypertrophy (25). A correlation between cardiac hypertrophy and hyperactivation of Pin1, determined by reduced phosphorylation at Ser16 (25) and Ser71 (1) residues, is seen upon treatment of cardiac myocytes with Endothelin-1 and thoracic aortic constriction in mice respectively. Endothelin-1 is a secreted protein that functions as a vasoconstrictor and is established to be pro-hypertrophic (25). Endothelin-1-mediated cardiac hypertrophy is accompanied by increases in phosphorylation of c-Jun-N terminal Kinase (JNK) and downstream transcription factor c-Jun, both of which are well established Pin1 targets in non-cardiac cells (29, 30). Interestingly, Endothelin-1-induced hypertrophy and Pin1 activation are inhibited by Fluvastatin, a pharmacologic HMG-coA reductase inhibitor which functions to lower cholesterol levels. The mechanistic involvement of Pin1 in mediating Endothelin-1-induced cardiac hypertrophy remains unexplored in the study. Surprisingly however, overexpression of Pin1 is accompanied by increased phosphorylation at the Ser16 residue, indicative of Pin1 inactivity (25). It is possible that Pin1 overexpression activates PKA as a consequence of increased hypertrophy, which in turn inhibits Pin1 through a phosphorylation-dependent feedback mechanism. A clear understanding of the molecular regulation of endogenous Pin1 in response to physiological and pathological growth stimuli in cardiac myocytes is needed to decipher the context dependent role of Pin1 overexpression in regulating cardiac hypertrophy.
Pin1 in Cardiac Progenitor Cells: Determining Cell Fate
Proliferation, survival, pluripotency, lineage commitment and differentiation are cellular outcomes critical to stem and progenitor cells that are modulated directly by Pin1. Regulation of cell cycle and mitosis remains one of the most well studied roles of Pin1 in non-cardiac cells. Human Pin1 was in fact discovered in a yeast two hybrid screen for proteins regulating mitosis, as an interacting partner of NIMA protein kinase (Never In Mitosis, Gene A) (6), after a study revealed that loss of the yeast homolog of Pin1 causes defects in cytokinesis (31). Over the years, Pin1 has been touted to regulate cell cycle check point kinases and function in centrosome duplication and chromosome condensation (32–34). Pin1 is overexpressed in nearly 38 out of 60 human tumors and is required for survival and cell cycle progression of tumor cells (35), thus serving as an independent prognostic marker in several cancers (16, 36). With the development of pharmacological inhibitors, Pin1 is being tested as viable treatment option for cancer therapy (37). Studies demonstrating the requirement of Pin1 activity for colony formation and differentiation of induced pluripotent stem cells implicate Pin1 in maintenance of stem cell self-renewability and pluripotency (38). By stabilizing Nanog, β-catenin and Notch, Pin1 extends its influences on stem cell differentiation and commitment (8, 39). Corroborating findings in other stem cells, our recent study demonstrates Pin1 as a pivotal regulator of stem cell maintenance in adult c-kit+ CPCs (4).
Endogenous Pin1 expression correlates with the proliferative potential of CPCs, with decline in Pin1 levels observed under growth arrest conditions such as serum starvation and Dexamethasone-induced differentiation (4). A tight control of Pin1 expression is apparently critical for cellular growth and proliferation, as dysregulation of Pin1 via either loss or gain of function approaches causes cell cycle arrest at the G1/S and G2/M phases respectively in CPCs (4). Consistently, Pin1KO mice have fewer cycling CPCs positive for cell cycle marker Ki-67 by 1 week after myocardial infarction. Surprisingly, however, despite the drastic reduction in number of CPCs, Pin1KO mice have preserved cardiac function and geometry after myocardial infarction, which may be explained by a pro-survival role exerted by Pin1. Both Pin1 knockdown and overexpression attenuate cell death in response to serum starvation in CPCs (4). This further emphasizes that Pin1 simultaneously impacts upon several signaling networks bearing unexpected consequences in the cellular and organ level. Loss of Pin1-induced G1/S phase arrest is accompanied by increased cellular senescence, decreased differentiation and loss of multipotency/stemness in CPCs. On the contrary, Pin1 overexpression antagonizes senescence, increases lineage commitment and differentially regulates proteins associated with maintenance of stemness in CPCs (4). By promiscuously impacting upon a broad range of molecular targets such as Cyclins D and B, cell cycle inhibitors associated with senescence such as p53 and retinoblastoma, differentiation markers like smooth muscle actin and cardiac troponin T, as well as stemness markers such as c-kit, c-Myc, Oct4, KLF4 and Nanog, Pin1 determines the fate of stem cells (Table 1) (4).
Pin1 expression is affected by modulation of the stromal cell derived factor -1α - CXCR4 receptor pathway, known to be crucial for stem cell homing and survival (40). Pharmacologic antagonism of the CXCR4 receptor after myocardial infarction increases proliferation and cardiac specific differentiation of CPCs concomitantly associated with upregulation of Pin1, attenuated Akt signaling, increased Cyclin D1 expression and decreased Cyclin D1 phosphorylation (40). With Akt and Cyclin D1 being well established targets of Pin1 (23, 41), it is likely that some of the phenotypic consequences are directly mediated by Pin1. The direct contribution of Pin1 in regulating CPC homing and asymmetric cell division is unknown and remains a topic of future investigations.
Pin1 and Cardiac Aging: Pining to be young
Cardiovascular disease is the leading cause of death in the elderly population and requires significant understanding of molecular mechanisms of aging to antagonize it. Loss of Pin1 has been correlated with and is directly causal to age associated pathologies including neurodegenerative diseases and arthritis (16–18, 41, 42). Inferring from evidences in non-cardiac cells, it is reasonable to postulate that Pin1 is required to antagonize cardiac aging. Expression and activity of Pin1 drastically decline in the aged myocardium (1) and Pin1KO mice significantly upregulate molecular markers of senescence and display accelerated cardiac aging (Hariharan N and Sussman MA, unpublished observations). A clear delineation of the mechanisms of regulation of a central molecule like Pin1 will potentiate design of better therapeutic strategies and use of Pin1 to antagonize cardiac aging.
Conclusion: End of the Beginning in Understanding Pin1
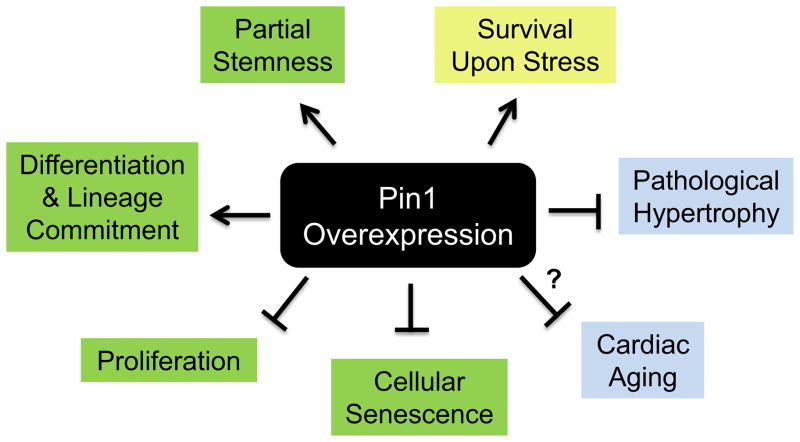
Pin1 functions as a “molecular orchestrator”(1), analogous to a conductor in a musical orchestra, whose principal roles by definition are to set the beat and tempo, lead and synchronously shape the outcome of the ensemble. By simultaneously modulating the timing and intensity of several signaling cascades, Pin1 directs signaling networks with precision and dictates the outcome of the cell, organ and organism as a whole. Our prior publications demonstrate a compelling role for Pin1 as a desirable interventional strategy to enhance myocardial survival, blunt pathological cardiac hypertrophy, antagonize cellular senescence, increase lineage commitment of CPCs and perhaps enhance myocardial regeneration (1, 4) (Figure 3). Future research should be directed at investigating mechanisms by which Pin1 may be used to antagonize cardiac aging and attenuate age associated cardiovascular diseases, focusing on critical aging-related issues such as telomeric attrition, stem cell exhaustion, de-sensitization of beta adrenergic receptors, accumulation of reactive oxygen species, impaired proteostasis and dysfunctional nutrient sensing mechanisms. Manipulating Pin1 levels in signaling cascades that are deregulated during aging and cardiac diseases will perhaps hold the key to a healthy heart. However, the elegance of Pin1 mediated regulation is also a limitation as achieving target specificity is a significant challenge with Pin1. The pivotal influence of Pin1 on myocardial pathophysiology needs further recognition and active research to better manipulate Pin1 levels to yield cardioprotective effects.
Figure 3.
Pleiotropic effects of Pin1 overexpression in the heart. Overexpression of Pin1 in CPCs (green boxes) antagonizes cellular senescence, inhibits cellular proliferation and increases differentiation/lineage commitment, while partially regulating stemness. Pin1 overexpression in cardiac myocytes (blue boxes) attenuates pathological hypertrophy and may contribute to antagonizing cardiac aging, the mechanism behind which remains to be determined (‘?’). Pin1 exerts pro-survival effects in response to stress in both CPCs and cardiac myocytes (yellow box).
Acknowledgments
This study was supported in part by National Institutes of Health grants (1R01HL105759, 1R37HL091102, 5R01HL067245, 1R01HL117163, 1R01HL113647 and 2P01HL085577 to M.A.S) and American Heart Association (12POST12060191 to N.H).
The authors apologize for not being able to cite all the references on Pin1 due to space constraints.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toko H, Konstandin MH, Doroudgar S, Ormachea L, Joyo E, Joyo AY, et al. Regulation of cardiac hypertrophic signaling by prolyl isomerase Pin1. Circ Res. 2013;112(9):1244–52. doi: 10.1161/CIRCRESAHA.113.301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaffe MB, Schutkowski M, Shen M, Zhou XZ, Stukenberg PT, Rahfeld JU, et al. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science. 1997;278(5345):1957–60. doi: 10.1126/science.278.5345.1957. [DOI] [PubMed] [Google Scholar]
- 3.Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nature chemical biology. 2007;3(10):619–29. doi: 10.1038/nchembio.2007.35. [DOI] [PubMed] [Google Scholar]
- 4.Toko H, Hariharan N, Konstandin MH, Ormachea L, McGregor M, Gude NA, et al. Differential regulation of cellular senescence and differentiation by prolyl isomerase pin1 in cardiac progenitor cells. J Biol Chem. 2014;289(9):5348–56. doi: 10.1074/jbc.M113.526442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranganathan R, Lu KP, Hunter T, Noel JP. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell. 1997;89(6):875–86. doi: 10.1016/s0092-8674(00)80273-1. [DOI] [PubMed] [Google Scholar]
- 6.Lu KP, Hanes SD, Hunter T. A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature. 1996;380(6574):544–7. doi: 10.1038/380544a0. [DOI] [PubMed] [Google Scholar]
- 7.Lufei C, Cao X. Nuclear import of Pin1 is mediated by a novel sequence in the PPIase domain. FEBS letters. 2009;583(2):271–6. doi: 10.1016/j.febslet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Liou YC, Zhou XZ, Lu KP. Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends in biochemical sciences. 2011;36(10):501–14. doi: 10.1016/j.tibs.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryo A, Liou YC, Wulf G, Nakamura M, Lee SW, Lu KP. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol Cell Biol. 2002;22(15):5281–95. doi: 10.1128/MCB.22.15.5281-5295.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You H, Zheng H, Murray SA, Yu Q, Uchida T, Fan D, et al. IGF-1 induces Pin1 expression in promoting cell cycle S-phase entry. J Cell Biochem. 2002;84(2):211–6. doi: 10.1002/jcb.10037. [DOI] [PubMed] [Google Scholar]
- 11.Eckerdt F, Yuan J, Saxena K, Martin B, Kappel S, Lindenau C, et al. Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem. 2005;280(44):36575–83. doi: 10.1074/jbc.M504548200. [DOI] [PubMed] [Google Scholar]
- 12.Lu PJ, Zhou XZ, Liou YC, Noel JP, Lu KP. Critical role of WW domain phosphorylation in regulating phosphoserine binding activity and Pin1 function. J Biol Chem. 2002;277(4):2381–4. doi: 10.1074/jbc.C100228200. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Chen CH, Suizu F, Huang P, Schiene-Fischer C, Daum S, et al. Death-associated protein kinase 1 phosphorylates Pin1 and inhibits its prolyl isomerase activity and cellular function. Mol Cell. 2011;42(2):147–59. doi: 10.1016/j.molcel.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou XZ, Lu PJ, Wulf G, Lu KP. Phosphorylation-dependent prolyl isomerization: a novel signaling regulatory mechanism. Cellular and molecular life sciences : CMLS. 1999;56(9–10):788–806. doi: 10.1007/s000180050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeong HG, Pokharel YR, Lim SC, Hwang YP, Han EH, Yoon JH, et al. Novel role of Pin1 induction in type II collagen-mediated rheumatoid arthritis. Journal of immunology. 2009;183(10):6689–97. doi: 10.4049/jimmunol.0901431. [DOI] [PubMed] [Google Scholar]
- 16.Lee TH, Pastorino L, Lu KP. Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert reviews in molecular medicine. 2011;13:e21. doi: 10.1017/S1462399411001906. [DOI] [PubMed] [Google Scholar]
- 17.Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003;424(6948):556–61. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- 18.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399(6738):784–8. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 19.Wulf G, Ryo A, Liou YC, Lu KP. The prolyl isomerase Pin1 in breast development and cancer. Breast cancer research : BCR. 2003;5(2):76–82. doi: 10.1186/bcr572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 21.Sussman MA, Volkers M, Fischer K, Bailey B, Cottage CT, Din S, et al. Myocardial AKT: the omnipresent nexus. Physiological reviews. 2011;91(3):1023–70. doi: 10.1152/physrev.00024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenz K, Schmitt JP, Vidal M, Lohse MJ. Cardiac hypertrophy: targeting Raf/MEK/ERK1/2-signaling. The international journal of biochemistry & cell biology. 2009;41(12):2351–5. doi: 10.1016/j.biocel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y, Wei Y, Zhou X, Yang JY, Dai C, Chen YJ, et al. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene. 2009;28(26):2436–45. doi: 10.1038/onc.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanal P, Namgoong GM, Kang BS, Woo ER, Choi HS. The prolyl isomerase Pin1 enhances HER-2 expression and cellular transformation via its interaction with mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1. Molecular cancer therapeutics. 2010;9(3):606–16. doi: 10.1158/1535-7163.MCT-09-0560. [DOI] [PubMed] [Google Scholar]
- 25.Sakai S, Shimojo N, Kimura T, Tajiri K, Maruyama H, Homma S, et al. Involvement of peptidyl-prolyl isomerase Pin1 in the inhibitory effect of fluvastatin on endothelin-1-induced cardiomyocyte hypertrophy. Life sciences. 2014 doi: 10.1016/j.lfs.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Dumaz N, Marais R. Raf phosphorylation: one step forward and two steps back. Mol Cell. 2005;17(2):164–6. doi: 10.1016/j.molcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286(5445):1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty MK, Muller J, Ritt DA, Zhou M, Zhou XZ, Copeland TD, et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell. 2005;17(2):215–24. doi: 10.1016/j.molcel.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 29.Park JE, Lee JA, Park SG, Lee DH, Kim SJ, Kim HJ, et al. A critical step for JNK activation: isomerization by the prolyl isomerase Pin1. Cell death and differentiation. 2012;19(1):153–61. doi: 10.1038/cdd.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wulf GM, Ryo A, Wulf GG, Lee SW, Niu T, Petkova V, et al. Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1. EMBO J. 2001;20(13):3459–72. doi: 10.1093/emboj/20.13.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanes SD, Shank PR, Bostian KA. Sequence and mutational analysis of ESS1, a gene essential for growth in Saccharomyces cerevisiae. Yeast. 1989;5(1):55–72. doi: 10.1002/yea.320050108. [DOI] [PubMed] [Google Scholar]
- 32.Shen M, Stukenberg PT, Kirschner MW, Lu KP. The essential mitotic peptidyl-prolyl isomerase Pin1 binds and regulates mitosis-specific phosphoproteins. Genes Dev. 1998;12(5):706–20. doi: 10.1101/gad.12.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suizu F, Ryo A, Wulf G, Lim J, Lu KP. Pin1 regulates centrosome duplication, and its overexpression induces centrosome amplification, chromosome instability, and oncogenesis. Mol Cell Biol. 2006;26(4):1463–79. doi: 10.1128/MCB.26.4.1463-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu YX, Manley JL. The prolyl isomerase Pin1 functions in mitotic chromosome condensation. Mol Cell. 2007;26(2):287–300. doi: 10.1016/j.molcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Rippmann JF, Hobbie S, Daiber C, Guilliard B, Bauer M, Birk J, et al. Phosphorylation-dependent proline isomerization catalyzed by Pin1 is essential for tumor cell survival and entry into mitosis. Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research. 2000;11(7):409–16. [PubMed] [Google Scholar]
- 36.Bao L, Kimzey A, Sauter G, Sowadski JM, Lu KP, Wang DG. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am J Pathol. 2004;164(5):1727–37. doi: 10.1016/S0002-9440(10)63731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore JD, Potter A. Pin1 inhibitors: Pitfalls, progress and cellular pharmacology. Bioorganic & medicinal chemistry letters. 2013;23(15):4283–91. doi: 10.1016/j.bmcl.2013.05.088. [DOI] [PubMed] [Google Scholar]
- 38.Nishi M, Akutsu H, Masui S, Kondo A, Nagashima Y, Kimura H, et al. A distinct role for Pin1 in the induction and maintenance of pluripotency. J Biol Chem. 2011;286(13):11593–603. doi: 10.1074/jbc.M110.187989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rustighi A, Zannini A, Tiberi L, Sommaggio R, Piazza S, Sorrentino G, et al. Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast. EMBO molecular medicine. 2014;6(1):99–119. doi: 10.1002/emmm.201302909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, et al. Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49(4):587–97. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, Fujimori F, et al. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci U S A. 2002;99(3):1335–40. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee TH, Tun-Kyi A, Shi R, Lim J, Soohoo C, Finn G, et al. Essential role of Pin1 in the regulation of TRF1 stability and telomere maintenance. Nat Cell Biol. 2009;11(1):97–105. doi: 10.1038/ncb1818. [DOI] [PMC free article] [PubMed] [Google Scholar]