Abstract
GP88 (progranulin) is a growth and survival factor implicated in tumorigenesis and drug resistance. Previous studies showed that GP88 was expressed in breast cancer tissue in inverse correlation with survival. This study evaluates GP88 tissue expression in localized/locally advanced lung cancer and GP88 serum levels in advanced disease. GP88 expression was determined by immunohistochemistry in tumor tissue from non-small cell carcinoma (NSCLC) patients, 85 with localized (Stage I-II) and 40 with locally advanced disease (Stage IIIa) and correlated with clinical outcome. Serum GP88 levels from Stage IIIb/IV patients, quantified by Enzyme Immunoassay (EIA) were compared to GP88 levels from patients with Chronic Obstructive Pulmonary Disease (COPD) and healthy individuals. GP88 was expressed in >80% adenocarcinoma and squamous cell carcinoma in contrast to normal lung or small cell lung cancer. There was a statistically significant inverse association of GP88 expression (GP88 Immunohistochemistry score 3+ vs. <3+) with survival for patients with localized resected NSCLC with Hazard Ratio (HR)=2.28 (p=0.0076) for DFS and HR=2.17 (p=0.014) for OS. A statistically significant decrease in progression-free survival (HR=2.9, p=0.022) for GP88 scores of 3+ vs. <3+ was observed for Stage IIIa after chemoradiotherapy. In addition, serum GP88 was significantly elevated in Stage IIIb/IV NSCLC compared to control subjects (49.9ng/ml vs. 28.4ng/ml, p<0.0001). This is the first study demonstrating GP88 tissue and serum expression as a prognostic biomarker in localized and advanced disease. Future research will determine utility of monitoring GP88 and the potential of GP88 expression as a predictive marker for anti-GP88 therapeutics.
Keywords: GP88/Progranulin, non-small cell carcinoma, prognostic factor, serum progranulin, serum GP88, immunohistochemistry
Introduction
Lung cancer is the leading cause of cancer related deaths in the US. 159,480 deaths and 228,190 new cases of lung cancer were recorded in 2013 of which 85% were non-small cell lung carcinoma (NSCLC). (1) In terms of therapeutic options NSCLC is generally divided into three groups of patients: localized (Stage I/II), locally advanced (Stage III) and metastatic (Stage IV). Outside of radiographic testing there are no validated diagnostic tools to distinguish patients with localized or locally advanced disease who are at high risk of relapse from those who are cured using current treatment modalities, nor are there non-imaging methods to detect recurrence or monitor response to therapy. The identification of biomarkers that have prognostic potential in lung cancer could provide a strategy to address these unmet needs.
The 88,000 kDa, cysteine-rich glycoprotein GP88 (Progranulin, PCDGF, granulin/epithelin precursor or acrogranin) is the largest member of a unique family of growth factors called granulin/epithelin characterized by a unique double cysteine rich motif (2–4). Initially identified as being overexpressed in breast cancer, GP88 has since been reported by other investigators to be overexpressed in several human cancers while normal tissues have been shown to express little or no GP88 (1, 5, 6).
In breast cancer, GP88 is associated with increased tumorigenesis and mediates, at least in part, cancer cell growth, survival, resistance to therapy (anti-estrogen, Herceptin and doxorubicin) and many hallmarks of metastasis such as invasion, angiogenesis and migration (6–13). Immunohistochemistry (IHC) studies of formalin fixed paraffin- embedded (FFPE) tumor specimens demonstrated that GP88 expression is low or negative in normal tissues whereas it is elevated in corresponding malignant tissues (5).
Analysis of GP88 tissue expression in 600 cases of estrogen receptor positive, invasive ductal carcinoma in relation with clinical outcomes demonstrated that high GP88 expression was statistically associated with a 5.9-fold higher hazard of disease recurrence (p< 0.0001) and a 2.5-fold higher mortality hazard (p=0.0002) compared to patients with no or low expression of tumor GP88 (14). GP88 remained an independent risk predictor after considering age, ethnicity, nodal status, tumor size, tumor grade, disease stage, progesterone receptor expression and treatments (14). Since GP88 is a secreted protein, a prospective longitudinal clinical study to measure circulating level of GP88 with an Enzyme Immunoassay (EIA) developed in our laboratory demonstrated the performance of the serum GP88 EIA by establishing a basal range for GP88 in serum from healthy volunteers of 28 ng/ml and showing that serum GP88 levels in breast cancer patients was elevated to 40 ng/ml in early stage and over 100 ng/ml in later stages of breast cancer (15).
Based on the above data and since GP88 can be measured both in tumor tissue and in biological fluids, we hypothesized that GP88 tissue expression might be a prognostic marker in localized and locally advanced NSCLC and that serum GP88 would be elevated in advanced disease. Specifically we measured GP88 tissue expression in correlation with survival outcome in tumor biopsies from local and locally advanced patients. Additionally we examined GP88 serum level in patients with advanced disease when compared to control subjects.
Materials and Methods
GP88 Expression in normal lung and lung cancer lesions
GP88 expression in normal and lung cancer tissues was examined by IHC using a tissue microarray (TMA) (US Biomax Inc. Rockville, MD). The TMA consisted of 72 cases (normal tissue, adenocarcinoma, squamous cell carcinoma, small cell carcinoma) from subjects with a median age of 59 (range 30 – 77), of which 71% were male and 29% female. Tissue sections were 5 micron with a 1.5 mm core diameter.
GP88 expression in local resected Stage I & Stage II NSCLC
A clinically annotated TMA of FFPE NSCLC was obtained from the University of Michigan. This TMA was constructed using triplicate cores from 96 Stage I (81%) and Stage IIa (13%) NSCLC patients, who had undergone potentially curative tumor resection. This TMA consisted of 13 squamous carcinoma and 77 adenocarcinoma and 6 cases being either small cell carcinoma or indeterminate tumors. Of the 77 adenocarcinomas 32(42%) and 36(47%) were acinar or solid sub-types respectively. The remaining 9 (11%) were lepidic, papillary or adenosquamous carcinomas. Upon GP88 staining, 11 cases had no detectable tumor, so statistical analysis was carried out with the remaining 85 cases. All patients underwent surgery and none received adjuvant therapy. Patient demographics are described in Table 2. Clinical and pathological parameters provided with the cases included age at diagnosis, disease stage, tumor size, smoking status, race, sex and clinical outcomes (recurrence, death).
Table 2.
Patient demographics for Local Resectable and Locally Advance Cases
| Demographic | Local Resected (n=85) | Locally Advanced (n=40) |
|---|---|---|
| Sex | ||
| Male | 39 | 23 |
| Female | 38 | 17 |
| Unknown | 8 | 0 |
| Age (years) | ||
| Median | 65 | 62 |
| Range | 17 – 85 | 44 – 91 |
| Stage | ||
| I | 69 | 0 |
| II | 11 | 0 |
| III | 1 | 40 (all IIIa) |
| Unknown | 4 | 0 |
| Tumor size | ||
| <3 | 48 | NA* |
| 3 – 5 | 21 | NA* |
| >5 | 7 | NA* |
| Unknown | 9 | NA* |
| Race | ||
| Caucasian | 37 | 21 |
| Afr. American | 3 | 18 |
| Unknown | 45 | 1 |
| Smoking history | ||
| Present/Former | 49 | 37 |
| Never | 6 | 1 |
| Unknown | 30 | 2 |
| Treatment | ||
| Surgery | 85 | 0 |
| Chemo/radiation | 0 | 39 |
| Other | 0 | 1 |
NA – Not applicable for locally advanced cases
GP88 expression in Stage III NSCLC patients
FFPE specimens were obtained from 40 patients with locally advanced disease who had undergone potentially curative treatment at the University of Maryland Greenebaum Cancer Center (UMGCC) with combined modality therapy (surgery followed by adjuvant chemotherapy, chemoradiotherapy or chemoradiotherapy and surgery) for pathologically staged Stage IIIa NSCLC(16). The demographics of these patients are described (Table 2). Tissue specimens were limited and there was one core per patient.
GP88 expression by Immunohistochemistry (IHC)
GP88 tissue expression was measured by IHC on 5 micron sections of FFPE lung TMA using previously validated and described IHC methodology (5, 14). Briefly, TMA sections were deparaffinized with xylene and rehydrated through a graded ethanol series. Antigen retrieval was conducted for 25 minutes in 0.2 M citrate buffer pH 6.0 in a 94°C water bath. GP88 was detected in tissue sections using the Oncostain 88™ immunohistochemistry kit (A&G Pharmaceutical, Columbia, MD) consisting of incubation with an anti-human GP88 mouse monoclonal antibody (clone 6B3), followed by washing, and incubation with HRP-conjugated secondary goat anti-mouse antibody (Dako, Carpinteria, CA). Bound antibody was detected using DAB as chromogen (Dako). Slides were then washed and counter-stained with Mayer’s Hematoxylin.
Evaluation of GP88 immunohistochemistry results
GP88 cytoplasmic immunoreactivity was semi-quantitatively scored as: <10% of cells staining: negative (0); >10 % of cells staining: positive with positive staining graded from weak/focal (1+) to moderate/focal or diffuse (2+) to strong/diffuse (3+) as described previously (5, 14). The immunostained slides were evaluated and scored by two board certified pathologists who (independently examined the tissue sections while) were blinded to the clinical data.
GP88 Serum levels
Serum specimens were obtained from patients undergoing treatment for advanced disease (Stage IIIb/IV, M1a or M1b) and patients with Chronic Obstructive Pulmonary Disease (COPD) without evidence of cancer attending the UMGCC thoracic oncology and pulmonary clinics, respectively. Specimens were obtained under an IRB approved protocol (UMGCC 0429). Serum specimens were also collected from healthy subjects enrolled at the UMGCC. Written informed consent was obtained from enrolled subjects. GP88 level in serum samples run in triplicates were determined using GP88 sandwich EIA assay (A&G Pharmaceutical, Columbia, MD) as described previously (15). Concentrations of GP88 in patient sera were determined using a standard curve established using known amounts of human GP88. Additional controls consisted of human serum alone or spiked with a known amount of GP88 as internal calibrators.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics for the local and the locally advanced cohorts. For localized (Stage I, II) and locally advanced (Stage III) patients, the statistical analysis of the results was based on evaluating the GP88 test performance for its ability to predict disease-free survival (DFS) and overall survival (OS) using Kaplan-Meier curves and the Cox proportional hazard (CPH) models for quantification of risk. DFS was defined as the time interval from date of diagnosis to first event being either recurrence (local or distant) or death if no recurrence. OS was defined as the time interval from date of diagnosis to time of last follow-up or death. Time to recurrence (local, regional and distant) was censored at the time of last disease-free follow-up. Survival curves for DFS and OS were derived from Kaplan-Meier estimates and the curves were compared using the log rank tests and Gehan-Breslau-Wilcoxon analyses. A CPH model was applied to quantify the hazard associated with GP88 scores. Additional potential correlations between GP88 expression and prognosis were carried out using analysis of deviance of a sequence of CPH models as described in the results section. The statistical analyses were performed using SAS V9.2.
GP88 serum levels between controls and patients with advanced lung cancer were compared utilizing an unpaired t-test. All statistical tests were two-sided and P values less than 0.05 were considered as statistically significant. Analysis and graphing were performed utilizing Graph Pad Prism software, version 6.02 (San Diego, CA).
Results
GP88 is expressed in NSCLC whereas it is negative in normal lung tissues
A TMA that contained representative cases of adenocarcinoma, squamous cell carcinoma, small cell carcinoma and normal lung tissue was stained for GP88 by IHC and scored by board certified pathologist as described above. As previously observed for breast carcinoma (14) GP88 staining pattern of lung cancer cases appeared cytoplasmic. As shown in Table 1, squamous cell carcinoma (70%) and adenocarcinoma (74%) stained positive for GP88 with 21% of squamous and 30% of adenocarcinoma showing a GP88 score of 3+. In contrast small cell carcinoma were mostly negative for GP88 staining (80% scored 0) whereas normal lung tissues were totally GP88 negative. Based on these results, subsequent studies focused on examining the effect of GP88 tissue expression on clinical outcomes for NSCLC patients with either localized (Stage I-IIa) or locally advanced (Stage III) disease.
Table 1.
GP88 Staining analysis in normal and lung cancer tissues
| Histologic Source of tissue for analysis | # of cases | GP88 Scoring | |||
|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | ||
| Squamous Cell Carcinoma | 28 | 8 (29%) | 9 (32%) | 5 (18%) | 6 (21%) |
| Adenocarcinoma | 27 | 7 (26%) | 5 (19%) | 7 (26%) | 8 (30%) |
| Small Cell Carcinoma | 5 | 4 (80%) | 1 (20%) | 0 | 0 |
| Normal Lung | 5 | 5 (100%) | 0 | 0 | 0 |
TMA with representative cases of normal lung, Non-Small Cell carcinoma and Small Cell carcinoma was obtained and used to examine the distribution of GP88 IHC score by staining for GP88 using kit as described in the method section.
High GP88 expression in local resected NSCLC (Stage Ia-IIb) is associated with worse outcome
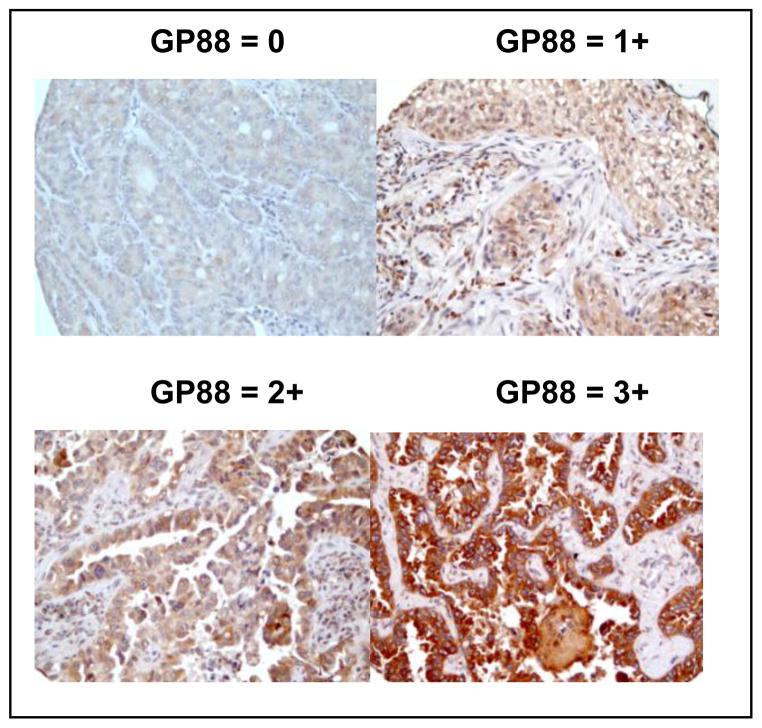
GP88 expression in tumor tissues from 85 patients with Stage Ia-IIb NSCLC was determined by IHC staining and scored as 0, 1+, 2+ and 3+ by the certified pathologists as described above and in the method section. Figure 1 provides photomicrograph examples of the different GP88 scores. No difference of GP88 expression pattern was observed in the different sub-types of adenocarcinoma represented in this TMA and described in the method section.
Figure 1. Immunohistochemical staining for GP88 expression in paraffin-embedded local NSCLC tissue sections.
Representative photomicrographs (magnification x200) of lung cancer tissue sections from local resected NSCLC showing various levels of GP88 expression with 0, 1+, 2+, and 3+ scores, respectively are provided.
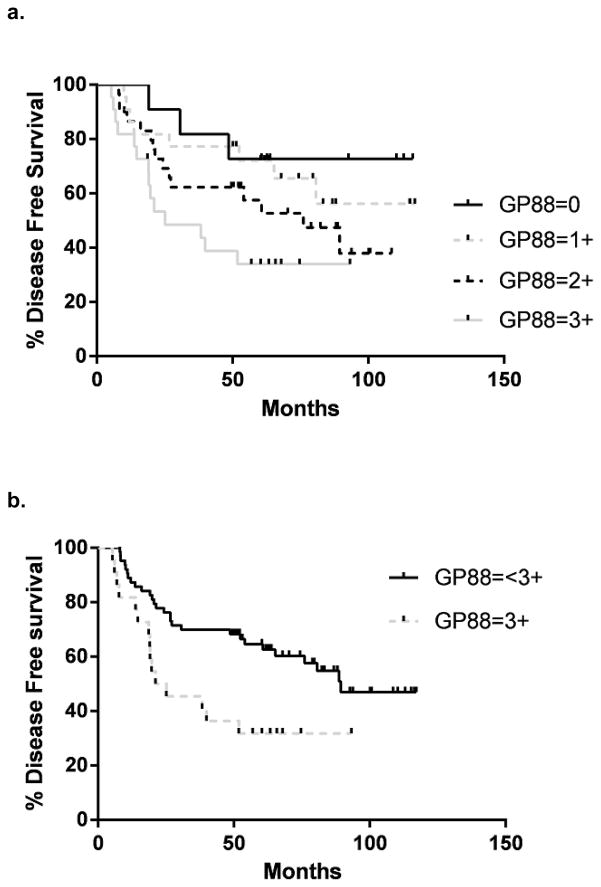
Patient demographics are described in Table 2. Survival curves for DFS and OS for patients grouped by their GP88 staining scores are provided in figure 2a and c. There was a significant inverse relationship between both DFS and OS and the degree of GP88 staining (log rank test for trend p=0.0049 and p=0.0027, respectively). There were significant differences noted for 0 vs. 1+ to 3+; 0–1 vs. 2–3+ and 0–2 vs 3+ for DFS and OS. Given the results for the Stage III patients (see below), we focused on DFS for patients with a tumor GP88 of 3+ and compared these to patients with tumor GP88 < 3+ (23.1 months vs. 89.4 months, log rank, p= 0.0076, Wilcoxon, p= 0.0045), figure 2b. The CPH analysis of both groups indicated that the DFS HR for GP88 3+ was 2.28 (95% CI = 1.33, 6.11). Similarly, there was a significant association between GP88 scores (scores from 0 to 3+) and decreased OS (logrank test for trend p=0.0027), figure 2c. As shown in figure 2d, a highly significant association was observed between GP8 score 3+ vs. <3+ and decreased OS (36.7 vs. 97.1 months, log rank p =0.014, Wilcoxon p =0 .012) The HR for OS was 2.17 (95% CI = 1.24, 5.83), Sex and smoking status were also investigated for their possible value as additional survival indicators using CPH models. First an analysis to test for possible interaction was done using age, sex, smoking, elevated GP88, along with two terms for interaction between sex and GP88 and between smoking status and GP88. These runs showed no evidence of interaction on either survival measure: the sex*GP88 indicator that tests whether the effect of gender differs between GP88 <3+ and 3+ groups had a p value of 0.8183 for OS and 0.5744 for event-free survival. The smoking*GP88 indicator that tests whether the effect of smoking status differs between GP88 <3+ and 3+ groups has a p value of 0.8709 for OS and 0.6838 for event-free survival. In the light of this additivity, a main effect model was fitted. In this, gender had a p value of 0.7015 for OS and 0.8310 for event-free survival. Smoking had a p value of 0.5751 for OS and 0.8083 for event-free survival. Thus neither sex nor smoking status has any value as an indicator of OS or event-free survival suggesting that GP88 is equally prognostic of both OS and event-free survival, regardless of patient sex and smoking status.
Figure 2. Kaplan-Meier estimates for Disease-free survival and Overall survival by GP88 scores for patients with localized NSCLC.
GP88 expression for the 85 NSCLC cases was examined by IHC and reported as GP88 scores of 0, 1+, 2+ or 3+. Kaplan-Meier estimates for DFS (a) and OS (c) were determined for each GP88 score group. The difference between the curves was estimated by the log-rank test and were p= 0.0049 for DFS and p= 0.0027 for OS, respectively. Kaplan-Meier estimates for DFS (b) and OS (d) were determined for GP88 staining scores grouped as 3+ and < 3+ (0, 1+ and 2+).
A separate analysis explored the predictive power of tumor size used in addition to the GP88 indicator by fitting a CPH model using GP88, the tumor size, and an interaction. For both DFS and OS, there was a highly significant interaction between tumor size and GP88 (p=0.0012 and 0.0037 respectively). Separate CPH models were then fitted in the GP88<3+ and GP88=3+ groups, relating survival to tumor size. In the GP88<3+ group, tumor size was not statistically significant for either endpoint (p=0.1198 and 0.0916 for DFS and OS). Interestingly, in the GP88=3+ group, tumor size remains highly significant (p=0.0180 and 0.0104) for DFS and OS. Thus tumor size is highly significantly predictive of poor outcome in the GP88=3+ group, but not in the lower risk GP88<3+ group. Since 83% of the cases were lymph node negative, it was not possible to examine the association between GP88 expression and lymph node status.
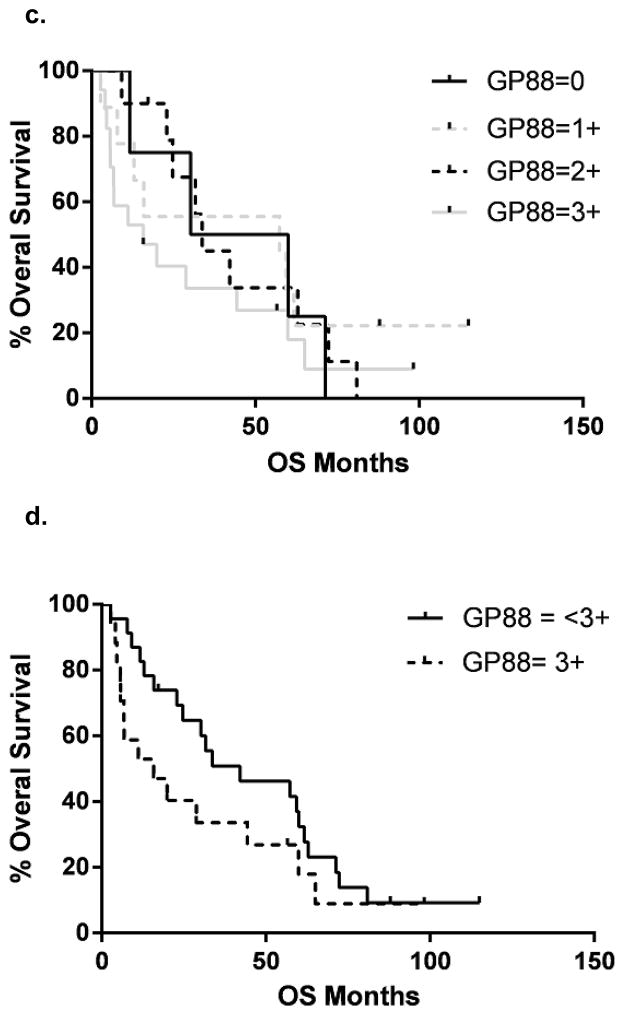
GP88 expression is associated with worse clinical outcome in Stage IIIa disease
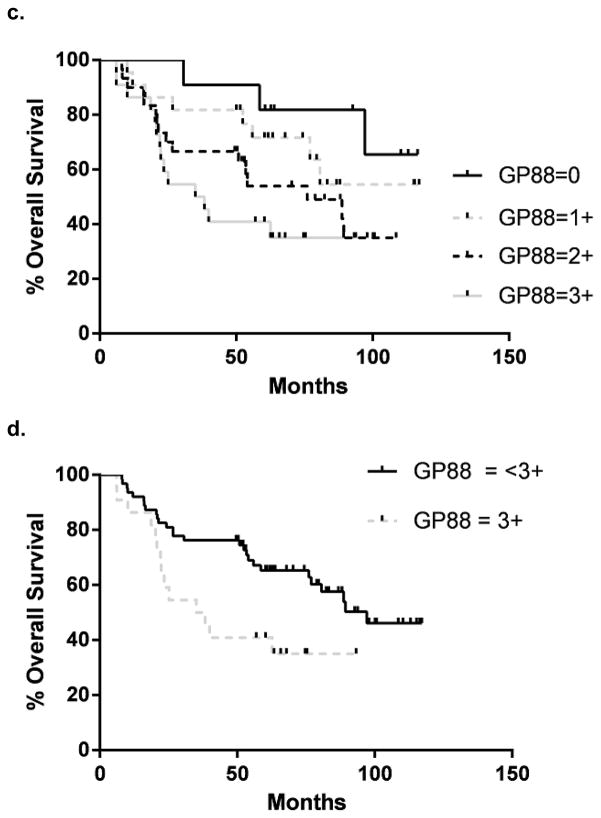
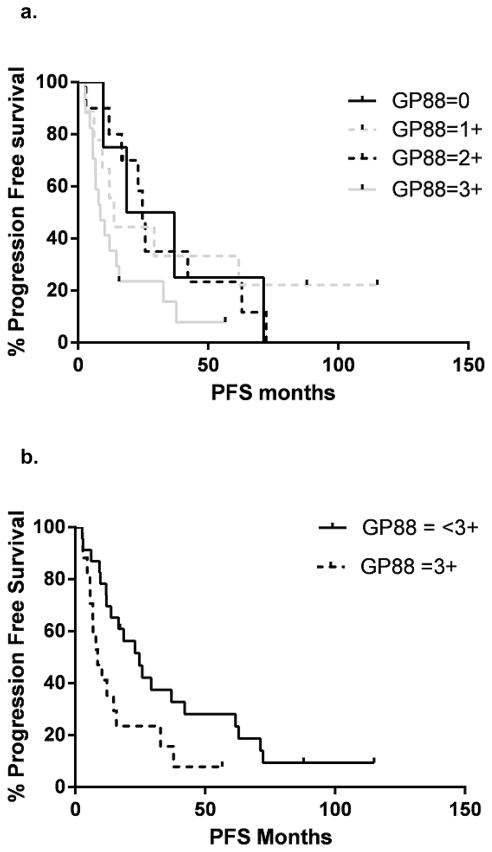
40 NSCLC samples from patients with locally advanced disease Stage IIIa were analyzed for the GP88 IHC expression. Patient demographics are presented in Table 2. Progression free and OS curves for all GP88 scores are presented in figure 3a & c Samples with a GP88 score of 0–1 vs 2–3 and 0 vs 1–3 showed no significant difference in progression free or OS (data not shown). However, there was a statistically significant difference in progression free survival (24.7 vs. 8.5 months) between GP88 < 3+ (n=23) and GP88 = 3+ (n=17) by both log rank (p=0.022) and Gehan-Breslau-Wilcoxon analysis (p=0.016) with an HR = 2.09 (95% CI = 1.14, 5.47) (figure 3b). For OS, the survival again favored GP88 <3+ (42.1 months) compared to GP88=3+ (15.8 months), respectively. This difference was significant by Gehan-Breslau-Wilcoxon analysis (p=0.044), but not by the log rank (p=0.164), figure 3d. Determination of OS hazard (figure 3d) indicated an HR of 1.61 (95% CI =0.81, 3.51).
Figure 3. Kaplan-Meier estimates for progression-free survival and overall survival by GP88 scores for patients with localized advanced (Stage III) NSCLC.
a: Kaplan-Meier estimates for progression-free survival for each GP88 score (0, 1+, 2+ 3+); b: Kaplan-Meier estimates for progression-free survival for GP88 scores grouped as < 3+ and 3+; c: Kaplan-Meier estimates for OS for each GP88 score; d: Kaplan-Meier estimates for overall survival for GP88 scores grouped as < 3+ and 3+
Increased GP88 serum level in NSCLC patients with advanced disease
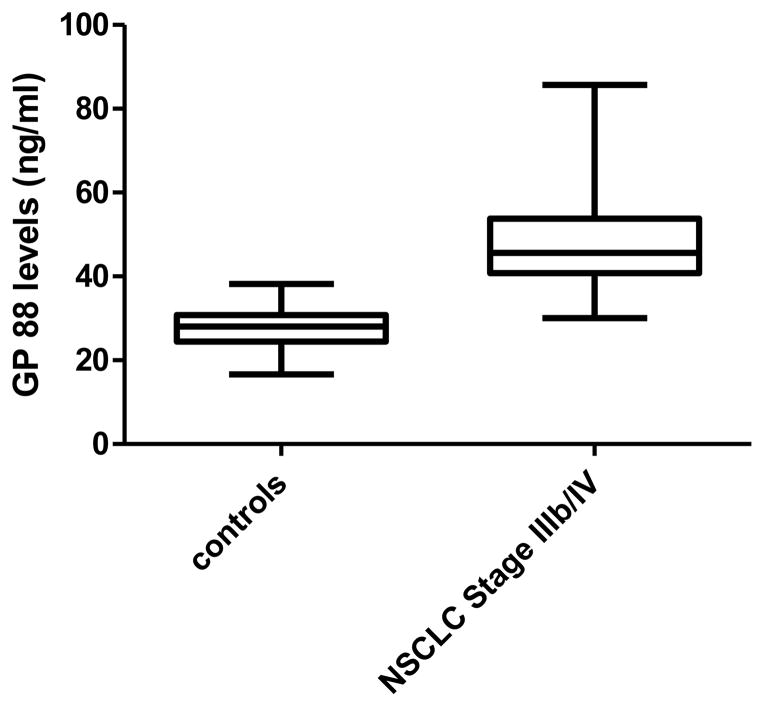
Serum GP88 levels of 19 patients with advanced disease (Stage IIIb, pleural effusion and Stage IV) were measured by GP88 sandwich EIA as described in the method section and compared with 20 controls (healthy individuals or patients with COPD). As shown in figure 4, there was a significant difference in GP88 serum levels between the two groups with the median serum GP88 value for controls of 28.4 ± 5.6 ng/ml (range 16.6–38.2 ng/ml) and 49.9 ± 14.7 ng/ml (range 30.0–85.7) for patients with advanced disease (p<0.0001).
Figure 4. Comparison of Serum GP88 levels in Stage IIIb/IV NSCLC and non-lung cancer control subjects.
Serum was collected from Stage IIIb/IV NSCLC (n=19) and control subjects that did not have lung cancer (n=20). Serum GP88 levels was determined by sandwich EIA as described in the method section.
Discussion
GP88 (progranulin) has been shown to play a role in the pathogenesis of several cancers and has been demonstrated to be an autocrine growth and survival factor in cancer cells and also to stimulate angiogenesis, migration and invasion (7, 10). Increased GP88 expression is associated with increased tumorigenicity and drug resistance. We show here that GP88 is expressed in 70% of lung adenocarcinoma and squamous cell carcinoma whereas it is negative in normal lung tissue and in small cell carcinoma. Correlation studies of GP88 IHC tissue expression with clinical outcomes described in this paper demonstrate that GP88 levels in NSCLC tissue as measured by IHC has potential use as a prognostic marker in localized and locally advanced disease. Furthermore, serum GP88 levels are elevated in advanced disease (in comparison to normal controls) and future investigations will determine if the level of GP88 is prognostic or associated with tumor response. The current report is limited by its retrospective nature, however, it provides support for further investigation of GP88 prognostic value in prospective studies.
The results presented here are interesting as they would suggest that measuring tissue and circulating GP88 levels may provide strategies to not only assess recurrence risk, but also to provide real time monitoring of recurrence in NSCLC patients.
It is well documented that over 50% of early stage NSCLC (Stage I, II) patients treated with curative intent will recur within 5 years. This emphasizes the need to stratify this population for risk of recurrence. The GP88 IHC test has the potential to stratify patients at high risk of recurrence and/or death.
The fact that GP88 serum level is elevated in Stage IIIb/IV patients compared to controls is potentially promising due to the lack of a serum based biomarker for diagnosing or monitoring NSCLC during treatment and follow-up. Future studies are necessary to examine whether elevated serum GP88 levels are also observed in early stage NSCLC patients when compared to controls. Furthermore, since GP88 expression has been associated with drug resistance in models of NSCLC, one key question would be to examine whether GP88 expression can help to prospectively identify patients who may benefit from other treatment strategies in advanced disease or who may not benefit from conventional chemotherapy in the adjuvant setting. Inhibition of GP88 expression by antisense or SiRNA leads to reduction in tumor growth and tumor incidence in mouse xenograft studies (8). A neutralizing anti-human GP88 antibody was shown to have efficacy in pre-clinical studies including mouse xenografts (11). Therefore, there is significant potential that anti-GP88 may have therapeutic potential and that tissue and serum GP88 assays may be used as companion diagnostics to identify subsets of patients that are potentially suitable for such treatment. In summary, we have demonstrated that GP88 is expressed in NSCLC and that it is not expressed in normal tissues or in Small Cell Lung Cancer and that the level of expression is prognostic. Furthermore, we have demonstrated that elevated blood levels of GP88 are observed in NSCLC Stages IIIb (pleural effusion) and IV patients. Future studies will determine whether GP88 levels are elevated in earlier disease stages and whether GP88 levels can be monitored for response to therapy in advanced disease. These findings, coupled with data from other disease entities and preclinical work, indicate that GP88 may be useful as both a biomarker for disease prognosis and a potential therapeutic target in NSCLC.
Acknowledgments
Source of Funding
This work was supported by grant 1R43CA162629-01A1 from National Cancer Institute
Footnotes
Conflicts of Interest
G Serrero, B Yue, D Hicks are full-time employees of A&G Pharmaceutical, Inc. M Edelman, J Feliciano, P Bejarano, O Ioffe, D Reisman, D Hawkins and Q Gai : no conflict of interest concerning the data contained in, or preparation of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 3.Serrero G. Autocrine growth factor revisited: PC-cell-derived growth factor (progranulin), a critical player in breast cancer tumorigenesis. Biochem Biophys Res Commun. 2003;308:409–413. doi: 10.1016/s0006-291x(03)01452-9. [DOI] [PubMed] [Google Scholar]
- 4.Tolkatchev D, Malik S, Vinogradova A, et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 2008;17:711–724. doi: 10.1110/ps.073295308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serrero G, Ioffe O. Expression of the novel autocrine growth factor PC-Cell Derived Growth Factor in human breast cancer tissue. Hum Pathol. 2003;34:1148–1154. doi: 10.1016/s0046-8177(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 6.Pizarro GO, Zhou XC, Koch A, et al. Prosurvival function of the granulin-epithelin precursor is important in tumor progression and chemoresponse. Int J Cancer. 2007;120:2339–2343. doi: 10.1002/ijc.22559. [DOI] [PubMed] [Google Scholar]
- 7.Tangkeangsirisin W, Serrero G. PC-Cell Derived Growth Factor [PCDGF/GP88] Stimulates Migration, Invasiveness and VEGF expression in Breast Cancer Cells. Carcinogenesis. 2004;25:1587–1589. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 8.Lu R, Serrero G. Inhibition of PCDGF expression by antisense cDNA transfection inhibits tumorigenicity of the human breast carcinoma cell line MDA-MB-486 P. Natl Acad Sci USA. 2000;97:3993–98. doi: 10.1073/pnas.97.8.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 10.Halper J. Growth Factors as active participants in carcinogenesis: a perspective. Vet Pathol. 2010;47:77–97. doi: 10.1177/0300985809352981. 2010. [DOI] [PubMed] [Google Scholar]
- 11.Lu R, Serrero G. Mediation of estrogen mitogenic effect in human BC MCF-7 cells by PCDGF P. Natl Acad Sci USA. 2001;98:142–47. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangkeangsirisin W, Serrero G. PC-Cell Derived Growth Factor [PCDGF/GP88] Stimulates Migration, Invasiveness and VEGF expression in Breast Cancer Cells. Carcinogenesis. 2004;25:1587–1589. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 13.Kim WE, Serrero G. PC-Cell Derived Growth Factor [PCDGF/GP88, progranulin] stimulates proliferation and confers Herceptin resistance to Her-2 overexpressing breast cancer cells. Clin Cancer Res. 2006;12:4192. doi: 10.1158/1078-0432.CCR-05-2663. [DOI] [PubMed] [Google Scholar]
- 14.Serrero G, Hawkins DM, Yue B, et al. Progranulin (GP88) tumor tissue expression is associated with increased risk of recurrence in breast cancer patients diagnosed with estrogen receptor positive invasive ductal carcinoma. Breast Cancer Res. 2012;14:R26–35. doi: 10.1186/bcr3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkaczuk KR, Yue B, Zhan M, et al. Increased circulating level of the survival factor GP88 (Progranulin) in the serum of breast cancer patients when compared to healthy subjects. BCBCR. 2011;5:155–162. doi: 10.4137/BCBCR.S7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6. New York: Springer-Verlag; 2002. Staging of Lung Cancers; pp. 166–174. [Google Scholar]