Abstract
Data from a wide variety of mammalian species indicate that feeding behavior can be influenced by changes in endogenous estrogens and exogenous estrogenic treatments. Ghrelin is an important physiological signal for the regulation of energy balance, and ghrelin treatment increases eating and body weight in male rodents. The following studies evaluated the hypothesis that the inhibitory effects of estradiol on feeding involve interactions with orexigenic peptides by examining the ability of estradiol to modulate the behavioral effects of ghrelin in female rats. In these experiments, adult rats were ovariectomized and assigned to an estradiol benzoate (EB) or oil (control) group. Three weeks after ovariectomy, animals received two daily subcutaneous injections of EB or the oil vehicle. Animals then received intraperitoneal (ip) injections of ghrelin (6.0 or 12.0 nmol) or saline during the nocturnal and diurnal periods three days after the first injection of estradiol or oil. Food intake, meal size, and meal number were determined during the 2-hour period following ghrelin or saline treatments. Ghrelin significantly increased food intake during nocturnal tests in oil-treated but not estradiol-treated rats. The hyperphagic effects of ghrelin on nocturnal food intake were also accompanied by an increase in meal size, and this effect of ghrelin on meal size was attenuated in estradiol-treated females. These findings support the hypothesis that the effects of estradiol on feeding behavior involve an attenuation of orexigenic signals, possibly by modulating the effects of the peripheral ghrelin signal on hypothalamic neuropeptides involved in the control of food intake.
1. Introduction
Apart from their well-known effects on reproductive physiology and behavior, ovarian hormones also play an important role in the control of feeding behavior in female mammals. For example, food intake fluctuates across the ovarian cycle in rats and guinea pigs, with a decline in daily food intake seen during behavioral estrus, which occurs after the rise in plasma estradiol (Blaustein & Wade, 1976; Czaja & Goy, 1975; ter Haar, 1972). In women, caloric intake is reduced during the follicular phase of the menstrual cycle, following the rise in estradiol levels prior to ovulation (Gong et al., 1989; Lyons et al., 1989). Thus, food intake is significantly reduced at a point in estrous and menstrual cycles when estradiol levels have been increasing. Although the relationship between ovarian hormones and ingestive behavior has been studied in a wide range of mammalian species, estradiol’s effects on feeding behavior and body weight have been best characterized in female rats. For example, withdrawal of estradiol via ovariectomy causes a rapid increase in food intake, weight gain, and adiposity (Asarian & Geary, 2002; Blaustein & Wade, 1976; Holt et al., 1936; Wade, 1975). These changes in eating and body weight seen in ovariectomized (OVX) rats can be reversed by peripheral treatment with physiological doses of estradiol (Asarian & Geary, 2002; Wade, 1975).
Although these effects of estradiol on food intake appear to be mediated in part by interactions with cholecystokinin (CCK) systems that participate in the control of satiety and meal size (Asarian & Geary, 1999; Butera et al., 1993; Geary et al., 1994), the observation that CCK antagonists do not completely reverse the anorectic action of estradiol indicates that CCK is not the only factor involved in mediating estrogenic effects on feeding (Eckel & Geary, 1999). Along these lines, research conducted in our lab and by other investigators has focused on the ability of estradiol to attenuate the effects of orexigenic peptides like ghrelin. Discovered in 1999 as an endogenous ligand for the growth hormone secretagogue receptor (Kojima et al., 1999), ghrelin is a peptide produced by the stomach that is best known for its effects on hunger (Cummings et al., 2001). Fasting increases ghrelin levels in rodents and humans whereas eating suppresses ghrelin secretion (Beck et al., 2002). Administration of ghrelin also increases eating in animals and humans (Kojima et al, 1999; van der Leley et al., 2004), and peripheral or central administration of antibodies to ghrelin inhibits food intake in rats (Bagnasco et al., 2003; Nakazato et al., 2001). Although the studies evaluating the effects of ghrelin on feeding cited above used male rodents and humans, research indicates that there are sex differences in the orexigenic action of ghrelin. Intact male rats and OVX female rats are more responsive to the stimulatory effects of ghrelin on feeding than intact females. In addition, this sex difference appeared to be mediated by estradiol, as the effects of ghrelin on food intake were attenuated in OVX rats treated with estradiol relative to oil-treated controls (Butera, 2010; Clegg et al., 2007).
Whereas the orexigenic effects of ghrelin have been well documented, few studies have examined how ghrelin affects spontaneous meal patterns, and those findings have been mixed. For example, Azzara et al. (2005) found that ip injections of ghrelin significantly increased nocturnal food intake and meal size in male mice. Ghrelin had no significant effects on meal frequency in that experiment. In contrast, Clegg et al. (2007) showed that estradiol decreased the orexigenic effect of ghrelin by an attenuation of ghrelin’s ability to decrease the latency to eat during diurnal feeding tests. This finding was unexpected, as it differs from the well-characterized behavioral mechanism by which estradiol decreases food intake, which is through a selective decrease in meal size.
In the following experiments, OVX rats were treated with a cyclic regimen of estradiol replacement (or the oil vehicle) and changes in food intake, meal size, and meal number were measured following intraperitoneal injections of ghrelin. In addition, the effects of ghrelin on both nocturnal and diurnal food intake were assessed in order to determine whether the orexigenic effects of ghrelin are modulated by estradiol and/or the time of day when the peptide is given. Therefore, one of the goals of the present study was to provide a more comprehensive analysis of the effects of ghrelin on meal patterns during both the diurnal and nocturnal periods in order to address some of the discrepancies described above. It is hypothesized that ghrelin will increase food intake in oil-treated animals and that this orexigenic effect of ghrelin will be attenuated by estradiol. These planned comparisons will be incorporated into the data analyses.
2. Materials and methods
2.1 Animals, housing, and ovariectomy
Twenty female Long-Evans rats obtained from Taconic Farms, Inc. (Hudson, NY) served as subjects in these experiments. Animals were approximately 50 days of age upon arrival and were housed in pairs in Plexiglas cages (10 ½″ × 19″ × 8″) in a windowless colony room. The room was maintained at 21 +/− 3 deg. C with a 12:12 h light-dark cycle (lights on at 0400 h). A white noise generator (Lafayette Instruments, Lafayette, IN) was used to mask any outside noise. Pelleted rodent chow (Mazuri, Brentwood, MO) and tap water were available ad libitum. Following the end of the experiment, animals were euthanized by i.p. injections of sodium pentobarbital (65 mg/kg; FatalPlus, Vortech Pharmaceuticals, Dearborn, MI).
Following a one-week acclimation period to their new environment, the rats were ovariectomized via a single midline abdominal incision under ketamine (75 mg/kg; Ketaset, Fort Dodge, IA) and xylazine (5 mg/kg, Xyla-ject, Phoenix Scientific, St. Joseph, MO) anesthesia via ip injections. Each ovary and respective uterine horn was located, the uterus tied off, and the ovary in addition to approximately 1 cm of the uterus was excised. The incision was then closed with 4 sutures. Animals were given a subcutaneous injection of butorphanol (0.5 mg/kg, Torbugesic, Fort Dodge, IA) after surgery as an analgesic. Ten days after surgery, animals were transferred to the test cages described below. All procedures were approved by the Niagara University Institutional Animal Care and Use Committee and were consistent with the ethical standards of the American Psychological Association.
2.2 Spontaneous meal patterns
Animals were individually housed in Plexiglas cages (43.2 × 43.2 × 19 cm) equipped with computer-controlled food dispensers (Med Associates, St. Albans, VT). Rodent food pellets (45 mg, NOYES Precision Pellets, Research Diets, Inc., New Brunswick, NJ) were dispensed in response to the activation of a photosensor placed above each food cup. Animals were allowed free access to food throughout the study. Tap water was provided by a water bottle adjacent to the food cup and refilled daily. The Plexiglas cages were connected via an interface (Med Associates, St. Albans, VT) to an IBM-compatible computer. The software provided with this system (MED-PC IV) records the number of pellets dispensed and converts the data into spontaneous feeding patterns (e.g., time and bouts of feeding, amount consumed during each interval). A meal was defined as any feeding bout of at least 0.2 g that is separated from other feeding bouts by at least 15 minutes. Using these criteria, recorded meals account for 96% of daily food intake in female rats (Eckel et al., 1998). The data collected were saved to disk for subsequent analyses. The raw data were converted into Excel files using MED-PC 2 Excel and translated into meal size and meal number with the Tongue Twister program (T.A. Houpt, Florida State University, Tallahassee, FL) on a Macintosh G4 computer. Animals were given a 10-day acclimation period in the test cages with the new diet before the onset of hormone treatments and data collection.
2.3 Treatment protocol
For the diurnal tests, the MED-PC system was shut down at 0900 h each day for maintenance and data collection. During this time animals were weighed to the nearest gram on an electronic balance and water bottles were refilled. The MED-PC system was then restarted at 10:00 h.
Following the acclimation period described above, animals were matched for body weight and assigned to one of two groups: estradiol benzoate (EB, 5.0 μg, n = 10), oil (control, n = 10). This was done to insure that there were no pre-existing differences in body weight between the estradiol and control groups prior to the start of the experiment. Previous findings in our lab indicate that this dose of EB can suppress food intake and meal size in OVX rats (Butera et al., 2010). Animals received subcutaneous injections of 5.0 μg EB (Sigma, St. Louis, MO, dissolved in 0.1 ml sesame oil) or the oil vehicle (0.1 ml) at 0930 for two days. This cyclic pattern of estradiol replacement produces behavioral and neurochemical changes associated with estrus without neuroendocrine and behavioral carryover effects that accompany continuous hormone replacement paradigms (Schumacher et al., 1991). Three days after the first EB or oil injection, the animals were given an intraperitoneal (ip) injection of either 6.0 or 12.0 nmol of octanoylated rat ghrelin (Bachem, Temecula, CA) dissolved in 0.9% endotoxin-free saline or the saline vehicle (0.1 ml). The doses of ghrelin were chosen based on our preliminary findings and a previous report showing that they increase food intake in male rats (Davidson et al., 2005). The injections were administered at 0950 h. and food intake, meal size, and meal number was determined for the subsequent 2-hour period following the restart of the MED-PC program at 10:00 h.
For the nocturnal tests, the Med-PC system was turned off at 14:00 h. for cleaning, maintenance, and data collection as described above, and the MED-PC system was then restarted at 15:00 h. Animals received subcutaneous injections of 5.0 μg EB or the oil vehicle (0.1 ml) at 14:30 for two days. Three days after the first EB or oil injection, the animals were given an intraperitoneal (ip) injection of either 6.0 or 12.0 nmol of octanoylated rat ghrelin dissolved in 0.9% endotoxin-free saline or the saline vehicle (0.1 ml). The injections were administered at 14:50 h. (just before the onset of the nocturnal period) and food intake, meal size, and meal number was determined for the subsequent 2-hour period following the restart of the MED-PC program at 15:00 h. The procedure was repeated each week until all animals were tested with saline and both doses of ghrelin during the diurnal and nocturnal periods (half of the animals in the EB and oil groups received the diurnal tests first and half received the nocturnal tests first). The order in which the animals in the EB and oil groups received the two doses of ghrelin and saline was counterbalanced. Data obtained during the 2-hour period following ghrelin or saline administration for animals in the EB and oil groups were analyzed with the SPSS statistical software package (SPSS Inc., Chicago, IL) using a 2 (EB or oil) × 3 (saline and both doses of ghrelin) ANOVA for repeated measures. When the ANOVA detected significant effects (p < 0.05), differences between individual means were compared with the Tukey-Kramer honestly significant difference test and considered significant when p < 0.05. Body weight data were analyzed with a t-test for independent samples.
3. Results
3.1. 23-h food intake and body weight
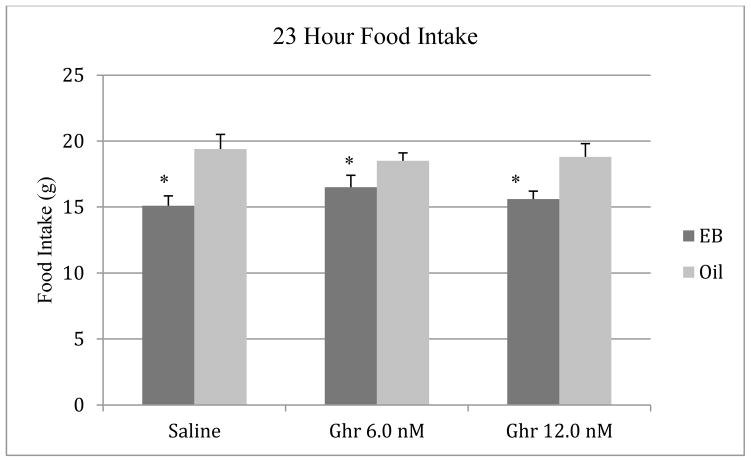
To evaluate the effects of estradiol on daily food intake and body weight gain, the data collected during the 72 hr period following the first EB or oil injection were analyzed, as this time point models behavioral estrus in the cyclic hormone replacement regimen that was used (Geary et al., 1994). Data from the nocturnal feeding tests were used for these analyses. The body weight data during tests with the saline vehicle were expressed as grams change from the animals’ weight the day before the onset of oil or EB injections (baseline) and analyzed with a t-test for independent samples, since acute ghrelin treatment would not be expected to alter 24-hour weight gain. As expected, estradiol treatment decreased food intake (F [1, 18] = 9.18, p < 0.01) and body weight gain (t [18] = 7.92, p < 0.001) relative to the oil condition (Figure 1). Animals in the oil group gained 5.2 ± .98 g relative to baseline (mean ± SE) whereas animals in the EB group lost 4.9 ± 1.1 g compared to baseline (data not shown).
Figure 1.
Effects of EB (5.0 μg) and oil injections on 23-h food intake in ovariectomized rats. The data were obtained three days after the first oil or EB injection. Bars represent means ± SE intake. * P < 0.05 relative to the oil group.
3.2 Food intake and meal patterns during the nocturnal feeding tests
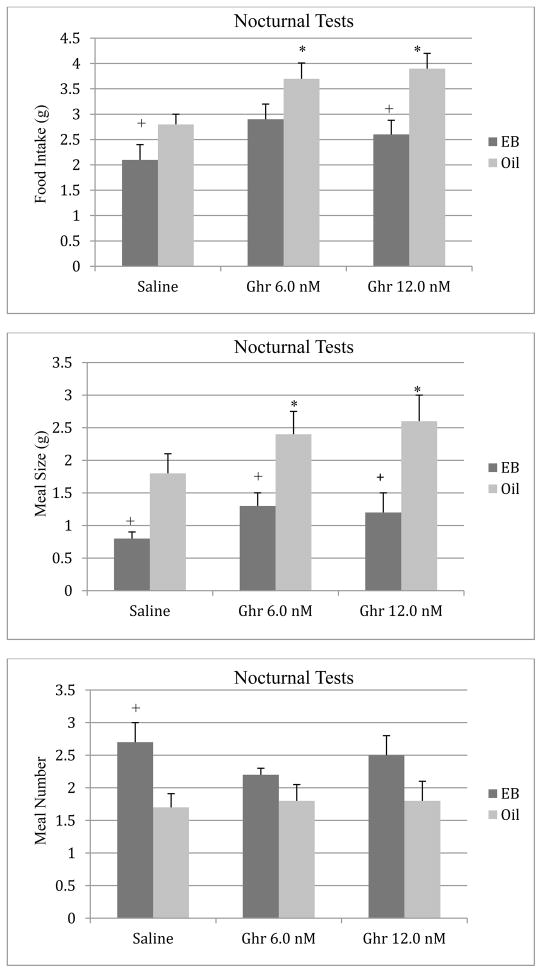
The effects of ghrelin on food intake during the 2-hour period of the nocturnal feeding tests are depicted in Figure 2. There were significant main effects of estradiol treatment (F [1, 18] = 10.73, p < 0.01) and ghrelin on food intake (F [2, 36] = 6.14, p < 0.01). The ANOVA also revealed a significant interaction between estradiol and ghrelin (F [2, 36] = 3.67, p < 0.05). Post hoc tests indicated that although both doses of ghrelin increased food intake relative to the saline condition in oil-treated animals (p < 0.05), this orexigenic effect of ghrelin was attenuated in EB-treated females. Estradiol-treated animals also consumed less food during tests with the saline vehicle relative to the oil control group (p < 0.05). Subsequent analyses indicated that the increase in food intake following ghrelin administration was greater in oil-treated than in EB-treated animals. Compared to the saline condition, the 6 nmol dose of ghrelin increased food intake by 34% ± 16 in the oil group and by 27% ± 18 in the EB group (t[19] = 2.12, p < 0.05). Similarly, the 12 nmol dose of ghrelin increased food intake by 42% ± 10 in the oil group and by 15% ± 14 in the EB group (t[19] = 2.34, p < 0.01).
Figure 2.
Effects of ip injections of ghrelin or saline on nocturnal food intake (top), meal size (middle), and meal number (bottom) in oil- or EB-treated ovariectomized rats. Bars represent means ± SE intake during a 2-h test. * P < 0.05 relative to saline condition; and + P < 0.05 relative to the oil group.
Analyses of the meal pattern data revealed significant main effects of estradiol (F [1,18] = 20.22, p < 0.001) and ghrelin (F [2, 36] = 3.73, p < 0.05) on meal size (Figure 2). Post hoc tests indicated that both doses of ghrelin significantly increased meal size in oil-treated animals compared to the saline condition. Although the 6 nmol dose of ghrelin increased meal size for animals in the EB group, the group difference failed to reach statistical significance (p = 0.05). Similar to the food intake data, meal size was significantly smaller in EB-treated females during tests with the saline vehicle relative to the oil control group. Although the 6 nmol dose of ghrelin produced comparable increases in meal size in oil- and EB-treated females compared to the saline condition (33% ± 12 and 32% ± 14, respectively), the increase in meal size following the 12 nmol dose of ghrelin was greater in the oil group. Compared to the saline condition, the 12 nmol dose of ghrelin increased meal size by 45% ± 10 in the oil group and by 30% ± 12 in the EB group (t[19] = 2.18, p < 0.05).
Analyses of the data on meal number shown in Figure 2 revealed a significant main effect of estradiol (F [1,18] = 7.28, p < 0.01). There was no main effect of ghrelin or a significant estradiol by ghrelin interaction (p > 0.05) on meal number. Post hoc tests indicated that estradiol increased meal number relative to the oil condition during tests with the saline vehicle.
3.3 Food intake and meal patterns during the diurnal feeding tests
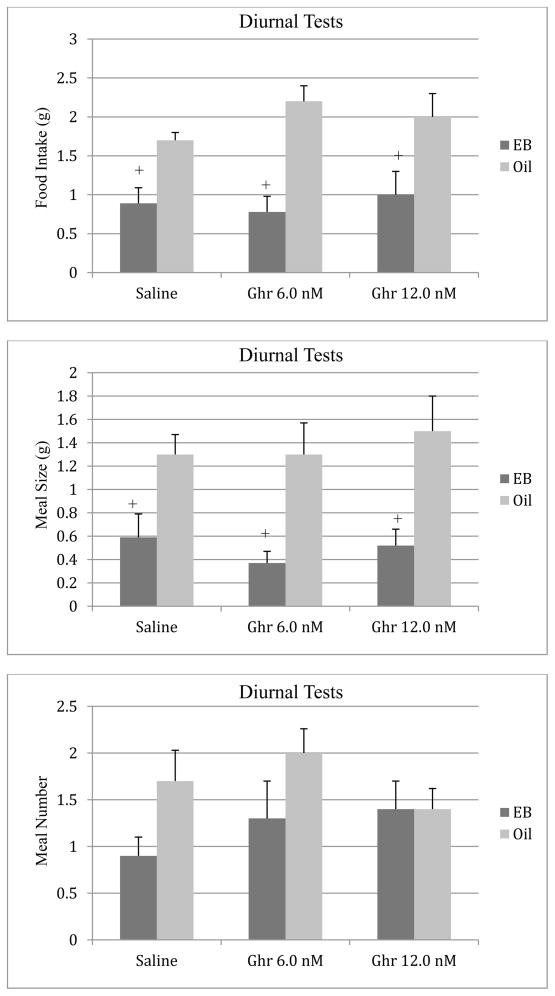
The effects of ghrelin on food intake during the 2-hour period of the diurnal feeding tests are depicted in Figure 3. The ANOVA revealed a significant main effect of estradiol (F [1, 18] = 22.82, p < 0.001) on diurnal food intake. Similar to the data obtained during the nocturnal tests, estradiol-treated animals consumed less food during tests with the saline vehicle relative to the oil control group (p < 0.05). There was no significant main effect of ghrelin on food intake during the diurnal tests or any interaction effects (p > 0.05).
Figure 3.
Effects of ip injections of ghrelin or saline on diurnal food intake (top), meal size (middle), and meal number (bottom) in oil- or EB-treated ovariectomized rats. Bars represent means ± SE intake during a 2-h test. + P < 0.05 relative to the oil group.
Analyses of the meal pattern data revealed a significant main effect of estradiol (F [1, 18] = 18.64, p < 0.001) on meal size, and post hoc tests indicated that estradiol decreased meal size relative to oil-treated animals across all treatment conditions (Figure 3). Ghrelin had no significant effects on meal size (p > 0.05) during the diurnal tests. There were no significant main or interaction effects on meal number during the diurnal feeding tests (p > 0.05, Figure 3).
4. Discussion
The results of these experiments indicate that the hyperphagic effects of ghrelin are attenuated in OVX rats treated with a cyclic pattern of estradiol replacement, as ghrelin increased food intake in untreated, OVX rats but not in EB-treated animals. Although ghrelin increased food intake during the 2-hour period of spontaneous, nighttime feeding, the animals appeared to compensate for this brief orexigenic effect by decreasing the amount of food consumed during the latter part of the nocturnal period leading to no change in 24 hour food intake. Analysis of the meal pattern data indicated that ghrelin increased meal size during the nocturnal feeding tests, and that this effect of ghrelin on meal size was attenuated by estradiol. Ghrelin did not appear to change meal frequency during the nocturnal feeding tests. Although ghrelin produced a slight increase in food intake relative to the saline condition in untreated, OVX females during the diurnal tests, this main effect was not statistically significant in the ANOVA. In addition, ghrelin did not increase meal size as was found when ghrelin was administered during the nocturnal period. The fact that the amount of food consumed following ghrelin treatment during the diurnal tests was approximately 50% less than the amount consumed during the nocturnal tests may have contributed to the inability to detect an effect of ghrelin on diurnal meal size.
The results of the present experiment confirm and extend the findings of a previous report on sex differences in the orexigenic effects of ghrelin (Clegg et al., 2007). In that study, male rats and OVX females were more sensitive to ip and icv injections of ghrelin than were intact females. The results of our experiment are in agreement with those findings, as we found that a cyclic pattern of estradiol replacement attenuated the orexigenic effect of ghrelin in OVX rats. However, in contrast to the study by Clegg et al. (2007), the results of the present experiment indicate that estradiol reduced the orexigenic effects of ghrelin via a decrease in nocturnal meal size. In that experiment, estradiol blocked the ability of ghrelin to decrease the latency to eat during the diurnal phase, thereby reducing the effects of ghrelin on feeding (Clegg et al., 2007). The present findings are more in line with the literature on the behavioral mechanism underlying the effects of estradiol on feeding, which indicates that estradiol inhibits food intake via a selective decrease in meal size (Eckel, 2004). In addition, we conducted feeding tests and meal pattern analyses during the nocturnal period, when the inhibitory effects of estradiol on meal size are usually observed and the orexigenic effect of ghrelin is more robust. Thus, the results of the present experiment are consistent with the theoretical framework indicating that the anorectic action of estradiol stems from a decrease in meal size, and with a previous report showing that peripheral treatment with ghrelin increased food and meal size during the nocturnal period (Azzara et al., 2005).
Along these lines, the results of the present experiment, together with those from other studies, suggest that the inhibitory effects of estradiol on food intake involve interactions with ghrelin and other orexigenic peptides. In adult rats, OVX increased plasma ghrelin levels during the 4-week period of hyperphagia following surgery (Kemp et al., 2005). These investigators, along with others, also reported that OVX increased whole hypothalamic gene expression for neuropeptide Y (NPY) and agouti-related protein (AGRP) that persisted during the period of hyperphagia (Clegg et al., 2007). Ghrelin’s effects on feeding are believed to result from increased production of NPY and AGRP following the activation of ghrelin-GH secretagogue receptors on neurons in the arcuate nucleus that project to the paraventricular nucleus of the hypothalamus (PVN) and regions (Hillebrand et al., 2002; Olszewski et al., 2003). Consistent with these findings, peripheral estradiol treatment has been shown to attenuate the orexigenic effects of lateral ventricle infusions of NPY, but not AGRP, in OVX rats (Santollo & Eckel, 2008). Taken together, these findings suggest that estradiol may modulate the effects of ghrelin on feeding behavior by decreasing hypothalamic orexigenic peptide signaling normally induced by ghrelin. Consistent with this hypothesis, preliminary findings from our lab indicate that estradiol decreases ghrelin-induced c-fos expression in the PVN and arcuate nucleus of OVX rats (Peters et al., 2013). However, the fact that estradiol and ghrelin can also affect food intake through actions on hindbrain neurons (e.g., nucleus tractus solitarius, NTS) [Faulconbridge et al., 2003; Thammacharoen et al., 2008]) suggests that the results of the present experiment may also involve estrogenic actions on ghrelin signaling in the NTS. It will be important for future research to elucidate the mechanism and site of action for this estradiol-ghrelin interaction.
In summary, the results of these experiments indicate that estradiol attenuates the hyperphagic effects of ghrelin on nocturnal food intake in OVX rats. The effect of the ghrelin on nocturnal feeding was associated with increase in meal size, an effect that was reduced by estradiol. Comparable effects of ghrelin food intake and meal size during the diurnal period were not found. These findings add to the growing literature on the mechanisms underlying estrogenic effects on feeding by demonstrating that the ability of estradiol to decrease food intake involves interactions with orexigenic peptides like ghrelin in addition to its actions on CCK satiety systems. Gaining a better understanding of the roles that orexigenic and anorexigenic peptides play in the control of food intake by estradiol may also provide useful information on the factors that lead to increased obesity after menopause (American College of Obstetricians and Gynecologists, 2005) and contribute to sex differences in eating disorders, which occur more frequently in young women (Sodersten & Bergh, 2003).
Highlights.
Evidence suggests that there may be sex differences in orexigenic effects of ghrelin.
These experiments examined the ability of estradiol to modulate ghrelin’s on feeding.
Estradiol attenuated ghrelin’s effects on nocturnal food intake and meal size.
Acknowledgments
We thank Dr. Irene Rykaszewski for her helpful comments on earlier versions of this article. This research was supported by NIH Grant R15-HD053382.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American College of Obstetricians and Gynecologists. Body mass index and insulin resistance. Obstetrics Gynecol. 2005;104:5–10s. doi: 10.1097/01.AOG.0000138805.07080.5e. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin’s satiating action in ovariectomized rats. Peptides. 1999;20:445–50. doi: 10.1016/s0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- Azzara A, Schuss B, Hong S, Schwartz GJ. Peripheral ghrelin administration increases food intake, meal size, and progressive-ratio responding for food. Appetite. 2005;44:332–38. [Google Scholar]
- Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul Pept. 2003;111:161–67. doi: 10.1016/s0167-0115(02)00283-5. [DOI] [PubMed] [Google Scholar]
- Beck B, Musse N, Stricker-Kongrad A. Ghrelin, macronutrient intake and dietary prefrences in long-evans rats. Biochem Biophys Res Comm. 2002;292:1031–35. doi: 10.1006/bbrc.2002.6737. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on meal patterns of female rats. Physiol Behav. 1976;17:201–8. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–80. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–38. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- Butera PC, Wojcik DM, Clough SJ. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physio Behav. 2010;99:142–45. doi: 10.1016/j.physbeh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Cummings D, Purnell J, Frayo R, Schmidova K, Wisse B, Weigle D. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–19. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–36. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–10. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Geary N. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–56. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Eckel LA, Langhans WL, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;44:R186–98. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2265–71. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. Am J Clin Nutr. 1989;49:252–59. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- Hillebrand JJ, de Wied D, Adan RA. Neuropeptides, food intake, and body weight regulation: a hypothalamic focus. Peptides. 2002;23:2283. doi: 10.1016/s0196-9781(02)00269-3. [DOI] [PubMed] [Google Scholar]
- Holt H, Keeton RW, Vennesland B. The effect of gonadectomy on body structure and body weight in albino rats. Am J Physiol. 1936;114:515–22. [Google Scholar]
- Kemp C, Benoit SC, Clegg DJ. Estrogen signaling through estrogen receptor alpha regulates food intake, body weight, and leptin sensitivity. Appetite. 2005;44:357. [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matauo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Lyons PM, Truswell AS, Mira M, Vizzard J, Abraham SF. Reduction of food intake in the ovulatory phase of the menstrual cycle. Am J Clin Nutr. 1989;49:1164–8. doi: 10.1093/ajcn/49.6.1164. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojiima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–98. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Olszewski P, Grace M, Billington C, Levine A. Hypothalamic paraventricular injections of ghrelin: effect on feeding and c-fos immunoreactivity. Peptides. 2003;24:919–23. doi: 10.1016/s0196-9781(03)00159-1. [DOI] [PubMed] [Google Scholar]
- Peters AR, Plyler KS, Daniels D, Butera PC. Modulation of ghrelin-induced c-fos expression by estradiol. Proceedings and Abstracts of the Annual Meeting of the Eastern Psychological Association. 2013;84:26. [Google Scholar]
- Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Behav Brain Res. 2008;191:173–77. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light-dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–92. doi: 10.1037//0735-7044.105.3.487. [DOI] [PubMed] [Google Scholar]
- Sodersten P, Bergh C. Anorexia nervosa: towards a neurobiologically based therapy. Eur J Pharmacol. 2003;480:67–74. doi: 10.1016/j.ejphar.2003.08.093. [DOI] [PubMed] [Google Scholar]
- ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Horm Behav. 1972;3:213–20. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–17. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Leley AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Rev. 2004;25:426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–91. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]