Summary
The melanocortin 1 receptor (MC1R) is a G protein-coupled receptor crucial for the regulation of melanocyte proliferation and function. Upon binding melanocortins, MC1R activates several signaling cascades, notably the cAMP pathway leading to synthesis of photoprotective eumelanin. Polymorphisms in the MC1R gene are a major source of normal variation of human hair color and skin pigmentation, response to ultraviolet radiation (UVR) and skin cancer susceptibility. The identification of a surprisingly high number of MC1R natural variants strongly associated with pigmentary phenotypes and increased skin cancer risk has prompted research on the functional properties of the wild-type receptor and frequent mutant alleles. We summarize current knowledge on MC1R structural and functional properties, as well as on its intracellular trafficking and signaling. We also review the current knowledge about the function of MC1R as a skin cancer, particularly melanoma, susceptibility gene and how it modulates the response of melanocytes to UVR.
Keywords: Melanocortin 1 receptor, MC1R variants, cAMP, melanocytes, melanoma, UVR
Introduction
The melanocortins (MCs) α-, β- and γ-melanocortin (melanocyte-stimulating hormone; MSH) and adrenocorticotropic hormone (ACTH) are bioactive peptides derived from the large precursor protein pro-opiomelanocortin (POMC). α-MSH is known to play an important and evolutionarily conserved role in the regulation of integumental pigmentation, inducing rapid color changes in poikilotherms, fish, amphibians and reptiles, and stimulating melanogenesis in mammalian species (Geschwind, 1966; Sawyer et al., 1982). Genetic studies conducted on mice provided unequivocal evidence that the receptor for α-MSH is the product of the extension locus, which stimulates the synthesis of eumelanin, and that loss-of-function (LOF) mutation in this locus, recessive yellow (e/e), results in yellow coat color (Robbins et al., 1993; Tamate and Takeuchi, 1984). In the 1960’s, Lerner and McGuire reported that injection of human subjects with α- or β-MSH increased skin darkening (Lerner and McGuire, 1961; Lerner and McGuire, 1964). However this increase in pigmentation could not unequivocally be attributed to a direct effect of MCs on melanocytes. Prior to the cloning of the human melanocortin 1 receptor (MC1R) gene from cultured human melanocytes (Chhajlani and Wikberg, 1996; Mountjoy et al., 1992) and the demonstration that these cells express functional MC1R on their cell surface, there was skepticism about a direct effect of MCs on human melanocytes. That melanocytes are a direct target for MCs and that MCs are synthesized in the skin, mainly by epidermal keratinocytes and melanocytes, strongly suggest that MCs act as physiological paracrine/autocrine regulators of human melanocytes (Abdel-Malek et al., 1995; Fuller and Meyskens, Jr., 1981; Halaban et al., 1993; Hunt et al., 1994; Suzuki et al., 1996).
Epidemiological studies revealed that the human MC1R is highly polymorphic, with around 200 coding region allelic variants with protein sequence alterations expressed in different human populations (Box et al., 1997; Garcia-Borron et al., 2005; Smith et al., 1998). A seminal paper by Valverde et al. (Valverde et al., 1995) first reported the association of specific MC1R polymorphisms with the red hair color (RHC) phenotype which also includes fair and freckled skin, impaired or absent tanning response to UVR and propensity to sunburn. MC1R variants, mainly the RHC alleles, are also associated with increased melanoma and nonmelanoma skin cancer risk (Davies et al., 2012; Dessinioti et al., 2011; Scherer and Kumar, 2010). These variants are natural models of genotype-phenotype associations and their study provides information on MC1R structure-function relationships, intracellular trafficking and functional regulation. The MC1R RHC alleles result in LOF of the receptor (Frandberg et al., 1998; Herraiz et al., 2009; Nakayama et al., 2006; Newton et al., 2005; Ringholm et al., 2004; Roberts et al., 2008; Schioth et al., 1999; Scott et al., 2002b).
It is now recognized that the MC1R has effects that extend beyond pigmentation, and involve activation of the DNA damage response, including DNA repair pathways in human melanocytes (Bohm et al., 2005; Kadekaro et al., 2005; Kadekaro et al., 2010; Kadekaro et al., 2012; Maresca et al., 2010; Song et al., 2009). Linking the MC1R not only to the regulation of skin pigmentation, but also to DNA repair pathways, which are pivotal for prevention of photocarcinogenesis, represented a shift in paradigm, and provided an explanation for how MC1R functions as a melanoma predisposition gene, and for why expression of RHC variants increases melanoma risk. This current knowledge about the various effects of MC1R makes it an attractive target for chemoprevention of photocarcinogenesis, including melanoma.
Genetic studies describing the penetrance and interactions of common allelic MC1R variants have been recently reviewed (Beaumont et al., 2011), and key aspects of MC1R structure were discussed in a previous review (Garcia-Borron et al., 2005). Interest in understanding the functions of the MC1R, regulation of its expression, its signaling pathways, and the mechanisms by which it affects the UVR response has led to important findings that substantiate its central role in regulating human melanocytes and skin cancer (particularly melanoma) predisposition. Therefore, we will focus this review on the regulation of MC1R gene expression and the biosynthesis and intracellular trafficking of the receptor as well as on the various signaling pathways downstream of MC1R and their regulation by specific endogenous ligands or promiscuous GPCR partners such as GPCR kinases (GRKs) and cytosolic β-arrestins (ARRBs). We also present a comprehensive list of mutant alleles corresponding to the natural protein sequence variants described to date. Finally, we will summarize recent insights on the intracellular pathways responsible for the protective role of MC1R against UVR-induced genotoxic effects and melanomagenesis.
Endogenous ligands of MC1R and their role in pigmentation
The melanin pigments responsible for the color of the skin and hair of mammals are synthesized within melanocytes. In human skin, melanin synthesized in epidermal melanocytes is transferred to the surrounding keratinocytes to allow for homogenous pigmentation (Lin and Fisher, 2007). Two types of melanins are synthetized in all human melanocytes, regardless of the pigmentary phenotype of the skin (Hunt et al., 1995). The brown/black eumelanin, and the yellow/red pheomelanin, are synthesized within specific organelles called melanosomes, and their ratio is dependent on the catalytic activity of the rate-limiting melanogenic enzyme tyrosinase, and the availability of low molecular weight thiol compounds such as cysteine or glutathione (Wakamatsu et al., 2006). High tyrosinase activity and/or low concentrations of thiolic compounds lead to synthesis of the photoprotective eumelanins (Sakai et al., 1997). Conversely, low tyrosinase activity and high availability of thiol compounds lead to the less photoprotective and possibly phototoxic pheomelanins (Brenner and Hearing, 2008; d'Ischia et al., 2013). The main determinant of the amount and type of pigments formed within epidermal melanocytes is the MC1R (Garcia-Borron et al., 2005). MC1R is a member of the largest family of cell surface receptors in the mammalian genome, the G protein-coupled receptor (GPCR) superfamily, with more than 800 members (Venkatakrishnan et al., 2013). MC1R belongs to a small subfamily of GPCRs, the MC receptors (MCR) 1 to 5. The MCRs are activated by a group of biosynthetically related peptide hormones, the MCs, which contribute to the regulation of diverse and important physiological processes such as production of glucocorticoid hormones in the adrenal glands (MC2R), feeding and energy homeostasis (mainly MC4R), sebaceous gland activity (MC5R) and others (Slominski et al., 2000; Yang, 2011). The MCs are bioactive peptides generated upon cleavage of the prohormone precursor POMC at multiple sites by two endoproteases, PC1 and PC2, which also yields β-endorphin and β-lipotropic hormone (Reviewed in Slominski et al., 2000). POMC-derived peptides may be further processed by N-terminal acetylation and C-terminal amidation, and these post-translational modifications modulate their stability and/or pharmacological properties. The exact sequence of events leading from transcription of the POMC gene to biologically active peptides is both species- and tissue-specific, and may be influenced by environmental cues (Bicknell, 2008; Slominski et al., 2007).
Upon stimulation by its endogenous agonists, MC1R initiates a complex series of events, mainly mediated by the cAMP signaling cascade, ultimately leading to an increase in tyrosinase activity, increased protein levels of tyrosinase, tyrosinase-related protein (TRP)-1 and -2, and, subsequently, activation of biosynthesis of eumelanin pigments (Abdel-Malek et al., 1995; Slominski et al., 2004). Human MC1R binds α-MSH and ACTH with equal affinity, while the mouse MC1R recognizes only α-MSH as its agonist (Mountjoy et al., 1992; Suzuki et al., 1996). Human MC1R has a lower affinity to β-MSH than to α-MSH or ACTH, and least affinity to γ-MSH (Suzuki et al., 1996). Accordingly, ACTH and α-MSH are considered the physiological agonists of MC1R.
The MC1R is a unique GPCR due to the diversity of endogenous ligands that regulate its functional status, with identified physiological agonists and antagonists. The best described MC1R antagonist is agouti signaling protein (ASIP) (Walker and Gunn, 2010). Mutations in the mouse Agouti gene that cause increased and ectopic expression of ASIP (viable yellow, Avy) result in yellow coat color, similar to the phenotype of e/e mice, and obesity due to ASIP binding to MC4R (Lu et al., 1994; Voisey and van Daal, 2002; Yen et al., 1994). ASIP decreases proliferation of mouse melanocytes and inhibits expression of the TRP-1 and TRP-2 genes, as well as total melanin production and content (Aberdam et al., 1998; Hida et al., 2009; Hunt and Thody, 1995; Le et al., 2008; Sakai et al., 1997; Siegrist et al., 1997). Binding of ASIP to the MC1R prevents the stimulation of eumelanin synthesis in response to α-MSH. This shifts dramatically the ratio of melanins synthesized towards pheomelanins, which accounts for the banding pattern in hair, characteristic of the agouti phenotype, and yellow coat color in agouti mutant mice (Lu et al., 1994; Siegrist et al., 1997; Suzuki et al., 1997; Yen et al., 1994).
The cloning of the human Agouti gene and the purification of its product allowed for investigating its role in human pigmentation (Miller et al., 1993). Unlike the highly polymorphic MC1R, only two variants of the human Agouti gene have been identified. These polymorphisms correspond to nucleotide changes in untranslated regions of the gene and do not cause protein sequence alterations, but at least the 3’UTR g.8818A→G variant most likely results in decreased levels of ASIP expression (Voisey et al., 2006). The Agouti variants are associated with skin, hair and eye pigmentation traits (Bonilla et al., 2005; Kanetsky et al., 2002; Meziani et al., 2005; Voisey et al., 2006) and with skin cancer risk (Gudbjartsson et al., 2008; Maccioni et al., 2013; Stefanaki et al., 2013), underscoring the physiological significance of the gene in human pigmentation and predisposition to skin cancer. Moreover, ASIP also modulates expression of many genes involved in redox metabolism, cell adhesion, and other cellular processes other than pigmentation (Le Pape et al., 2009). Human melanocytes respond to ASIP in the same manner as mouse melanocytes with complete abrogation of the stimulatory effects of α-MSH on cAMP formation, tyrosinase activity, and proliferation (Suzuki et al., 1997; Swope et al., 2012). ASIP proved to be an inverse agonist of human and mouse MC1R, evidenced by its ability to reduce basal tyrosinase activity (Chai et al., 2003; Hida et al., 2009; Patel et al., 2010; Siegrist et al., 1997).
A newly identified MC1R antagonist is β-defensin-3 (BD3). This peptide belongs to a family of small secreted proteins with antimicrobial properties. Studies on genetic determinants of black coat color in dogs identified canine β-defensin 103 (CBD103) as a specific MC1R ligand with nanomolar affinity constants (Candille et al., 2007). Mature CBD103 and its human orthologue HBD3 are highly conserved, with only 7 conservative substitutions out of 45 residues. The proteins share a common and compact fold with two patches of positively charged residues important for high affinity binding to MC1R and MC4R (Nix et al., 2013). Initial biochemical studies showed that CBD103 neither activated MC1R in melan-a mouse melanocytes or dog MC1R transiently overexpressed in HEK 293, nor did it decrease its agonist-independent signaling, thereby acting as a classical competitive antagonist. This led to the suggestion that CBD103 would also displace ASIP from MC1R, which may result in a paradoxical induction of eumelanogenesis by an “anti-inhibitor” mechanism abrogating the negative effect of ASIP on basal signaling (Candille et al., 2007). If this is the case, then the final signaling output of BD3 would depend on the relative levels of the 3 types of ligands: MC full agonists, BD3 partial agonist and ASIP inverse agonist.
When tested on normal human melanocytes (NHMs), HBD3 alone, up to 100 nM, had no effect on basal cAMP formation. Conversely, in the presence of α-MSH, HBD3 significantly inhibited α-MSH-induced cAMP production, stimulation of proliferation and tyrosinase activity (Swope et al., 2012). These data led to the conclusion that, HBD3 is a neutral antagonist. Different results were obtained upon testing HBD3 in HEK293 cells overexpressing human MC1R, which responded by a weak increase in basal cAMP and partial inhibition of ASIP action (Beaumont et al., 2012). The discrepancy between the results of these two studies can be attributed to the source of HBD3 and also to the experimental model used, i.e. NHMs that express endogenously MC1R vs HEK293 cells overexpressing the receptor.
In addition to its role in the regulation of constitutive human melanin pigmentation, MC1R is a major determinant of the facultative pigmentation induced by UVR. Indeed, UVR-induced tanning is largely dependent on the production and release of α-MSH and ACTH by irradiated keratinocytes (Chakraborty et al., 1996; Cui et al., 2007) resulting in paracrine activation of MC1R in melanocytes. Moreover, activation of the cAMP pathway, the main signaling pathway for the MC1R, is critical for UV-induced melanogenesis in human melanocytes in vitro, and humanized mouse skin in vivo (D'Orazio et al., 2006; Im et al., 1998).
MC1R gene and protein structure
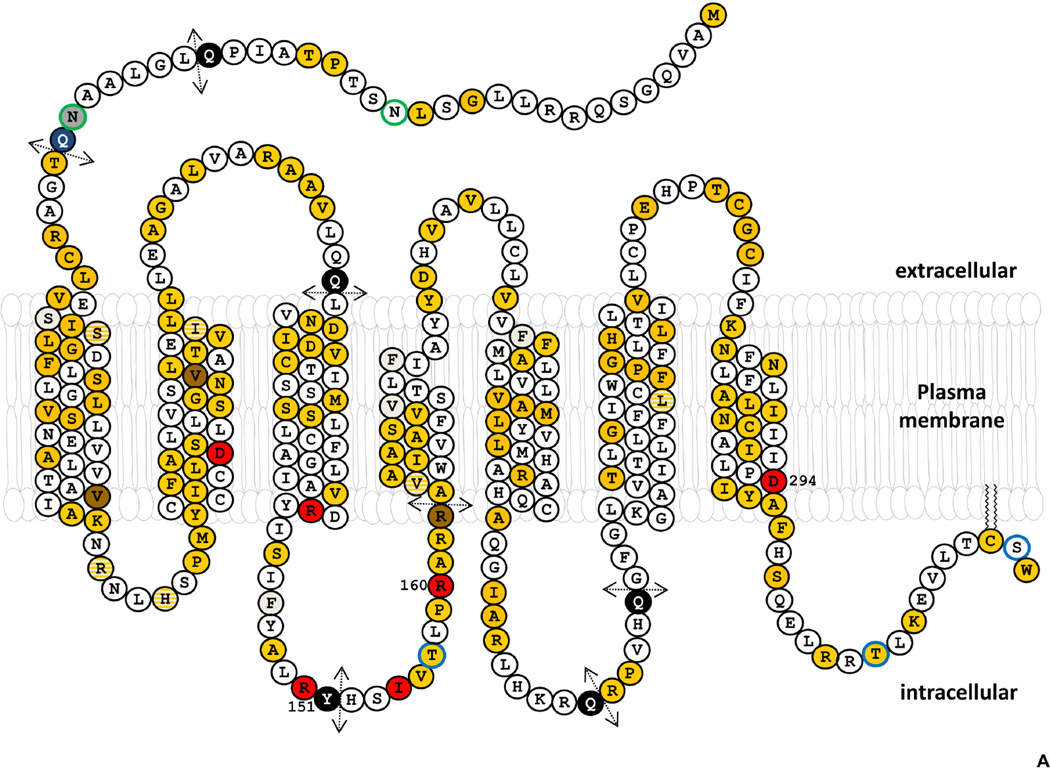
Compared with most GPCR genes, the structure of MC1R is relatively complex, with occurrence of several splice variants, and a bewildering degree of polymorphism. Although the genes encoding for most GPCRs were for a long time considered intronless, it now appears that approximately 50% of GPCR genes possess at least one intron and can undergo alternative splicing (Markovic, 2013; Markovic and Challiss, 2009). The MC1R (MIM# 155555, ID ENSG00000258839) located at 16q24.3 was initially reported as intronless, with a major 2.3 kb transcript and a 951 nucleotides (nt) coding region (Smith et al., 2001). This canonical MC1R transcript (ID ENST00000555147, named MC1R-001) encodes for a 317 amino acid integral transmembrane protein with all the hallmarks of Class A GPCRs (Katritch et al., 2013; Venkatakrishnan et al., 2013), namely seven transmembrane fragments, a potentially glycosylated extracellular N-terminus, three extracellular and three intracellular (il) loops and a cytosolic C-terminal extension (Figure 1). The MC1R-001 open reading frame (ORF) predicts that the N-terminal fragment and the extracellular loops are short, compared with most other GPCRs, which may have functional consequences by contributing to a high constitutive activity (Garcia-Borron et al., 2005; Holst and Schwartz, 2003). The cytosolic tail of MC1R-001, predicted to extend from His301 to Trp317, is also short.
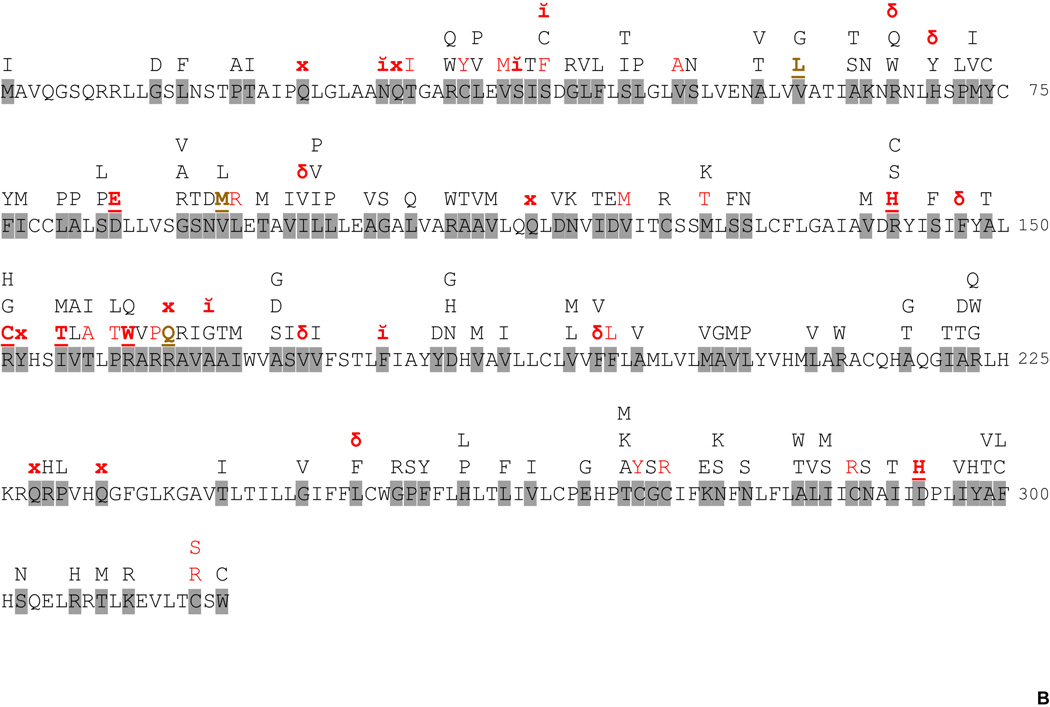
Figure 1. Structure of MC1R and protein sequence changing polymorphisms.
A. Primary structure and transmembrane topology. The sequence corresponds to transcript MC1R-001 (ID number ENST00000555147). Polymorphic positions for which no reliable association studies are available are indicated in yellow. Positions of R and r variants are shown in red and brown, respectively. Residues shown in gray correspond to indels, and black circles with white lettering followed by broken arrows to premature stop codons. Positions where both an indel and a point mutation have been found are shown as yellow circles hatched in gray. Ser/Thr residues presumably phosphorylated are highlighted with a blue border. The two Asn residues glycosylated in WT MC1R are indicated with a green border.
B. MC1R protein sequence polymorphisms. Variant positions with described sequence alterations are shown on a gray background. x stands for premature stop, ĭ for insertion and δ for deletion. Known R (red letters) and r (brown letters) variants are indicated with bold underlined letters specifying the mutant amino acid. Substitutions presumably behaving as either R or r mutations based on functional studies, but for which no genetic association data are available are shown in red capital letters. Further information is provided in Supplementary Table 1.
Further studies established that the MC1R gene comprises 4 exons and yields at least one more protein coding transcript. Tan and coworkers first reported an alternative spliced form, designated MC1R-002 (ID ENST00000555427), with a 1149 nt-long ORF (Tan et al., 1999). The resulting 382 amino acids protein is identical to the canonical MC1R up to Ser316, followed by a 66 amino acids C-terminal extension. At least another splice variant has been reported (Rouzaud et al., 2006), which shares with MC1R-001 and MC1R-002 the sequence up to residue Ser316, but the additional C-terminal extension is different from MC1R-002. This structure suggests splicing of the same 5’ exon to a different acceptor site. The occurrence of the new splice variant, named MC1R350, was confirmed in cultured NHMs and skin sections from donors of different ethnic origin (Rouzaud et al., 2006). No specific polymorphisms have been described to date in association with the MC1R splice variants.
The 16q24 region is a high density area containing the NULP1, MC1R and Tubulin beta III (TUBB3) genes (Smith et al., 2001), with less than 8 kb separating the coding 3’ end for the upstream NULP1 gene and the MC1R initiation codon, and just 2.5 kb of intergenic DNA between MC1R and its downstream TUBB3 neighbor. In addition, human MC1R has an unusual and inefficient polyadenylation signal (Dalziel et al., 2007). These characteristics are compatible with intergenic splicing, and two intergenic splice products have been reported (Dalziel et al., 2011). One of them contains 2 MC1R exons fused to 3 TUBB3 exons. The resulting 797 amino acids protein is an in-frame fusion containing the first 366 residues of MC1R-002 and most of the TUBB3 sequence (MC1R-TUBB3 gene, ID ENSG00000198211). The other intergenic chimera arises by out-of-frame fusion of MC1R and exon 2 of TUBB3, yielding a protein where the first 316 residues correspond to the MC1R sequence and the remaining 116 C-terminal residues share no significant homology with known proteins.
The relative levels of the various MC1R-derived transcripts may be modified by external signals. The canonical MC1R-001 transcript is induced in α-MSH-treated NHM more strongly than MC1R350 (Rouzaud et al., 2006). Stimulation of melanoma cells with α-MSH or activation of p38 increases the expression of MC1R-TUBB3 fusion chimeras (Dalziel et al., 2011). MC1R-001 and the alternatively spliced MC1R-002 isoform share most of the functionally relevant structural elements, suggesting that the proteins might be pharmacologically similar (Tan et al., 1999). However, expression of the MC1R350 isoform was negatively correlated with total melanin contents in NHM cultures, and forced expression of MC1R350 led to decreased levels of MITF and tyrosinase, suggesting that this splice variant is inactive or even behave as a dominant-negative form (Rouzaud et al., 2006). On the other hand, the properties of MC1R-TUBB3 chimeric proteins are still poorly characterized, but it would appear that whereas the longer in-frame fusion product retains at least part of the signaling potential, the out-of-frame chimera is not functional. Therefore, environmental cues might regulate MC1R signaling by modifications of the relative proportions of different transcripts. However, a detailed comparison of the signaling properties of the resulting proteins is still lacking, and the relative levels of the various transcripts have not yet been determined. Accordingly, the function and physiological relevance of non-canonical MC1R forms remains uncertain.
With approximately 200 protein sequence altering allelic variants (Figure 1 and Supplemental Table 1), MC1R is probably the most polymorphic GPCR gene in the human genome. The association of variant alleles with increased risk for melanoma and nonmelanoma skin cancers (Box et al., 1997; Healy et al., 2000; Ichii-Jones et al., 1998; Valverde et al., 1995) and with the UVR-sensitive RHC phenotype is firmly established (Rees, 2004; Sturm et al., 2003a; Sturm et al., 2003b). The properties of the most frequent variants that have been analyzed for function are discussed below.
MC1R gene expression, biosynthesis, processing and trafficking
The MC1R gene is preferentially expressed in melanocytes but, particularly in the skin, low levels of expression are also detected in many non-melanocytic cells including human keratinocytes, fibroblasts, and immune cells (Bohm et al., 2006). MC1R-dependent generation of cAMP in these cells is most often weak or undetectable, and MC1R expression is very low and possibly irrelevant in some cases (Roberts et al., 2006). However, the MC1R protein has been detected by immunohistochemical techniques in a number of non-melanocytic cell types and tissues (Salazar-Onfray et al., 2002), and MC1R expression is upregulated in human burn wounds and hypertrophic scars in the epidermis and in dermal stromal cells (Muffley et al., 2011). The inducibility of non-melanocytic MC1R gene expression, together with the finding that several cutaneous non-pigmentary actions of α-MSH involving skin fibroblasts are lost or impaired in C57BL/6J-MC1Re/e mice homozygous for an inactivating MC1R mutation (Bohm and Stegemann, in press) suggests that, at least in the skin, MC1R is active in cells other than melanocytes. However its precise function in these locations is yet to be determined, which should be best approached by functional assays, since detection of receptor expression by PCR or immunostaining without evidence of agonist binding and signaling is not sufficient for making conclusions about the physiological relevance of the receptor (Roberts et al., 2007).
The MC1R gene has a minimal promoter consisting of 150 bp upstream of the initiation codon which is sufficient to support transcription and assembly of tissue-specific regulatory complexes (Miccadei et al., 2008). This minimal promoter bears an E-box (CATGTG) matching the core sequence of the M-box (AGTCATGTGCT) targeted by MITF (Moro et al., 1999), which stimulates MC1R promoter activity (Aoki and Moro, 2002). Treatment of human melanocytes with NDP-MSH (Funasaka et al., 1998) or α-MSH (Kadekaro et al., 2010; Scott et al., 2002a; Swope et al., 2012), or mouse melanocytes with α-MSH (Rouzaud et al., 2003; Rouzaud et al., 2006) increases MC1R mRNA levels, at least in part due to activation of MITF. Conversely, short treatment of B16 mouse melanoma cells with low concentrations of hydrogen peroxide inhibits tyrosinase activity and protein levels, represses Mitf and also downregulates MC1R expression (Jimenez-Cervantes et al., 2001a).
Pioneering work from John Pawelek’s laboratory established more than two decades ago that cell surface expression of MC1R in melanocytic cells is upregulated by UVR via redistribution of receptors from an internal pool to the plasma membrane and increased mRNA levels indicative of transcriptional activation (Bolognia et al., 1989; Chakraborty et al., 1991; Funasaka et al., 1998). UVR-mediated induction of MC1R gene expression was confirmed in mouse melanocytes and human melanoma cells. The mechanism of this transcriptional activation appears to depend on p38-mediated phosphorylation of the USF1 transcription factor, acting through the conserved E-box in the MC1R promoter (Corre et al., 2004; Corre et al., 2009; Galibert and Corre, 2010). In addition to a direct action on MC1R gene expression in cultured melanocytic cells, UVR also activates MC1R transcription in human epidermis in vivo (Corre et al., 2006). In this case, p53-dependent upregulation of POMC gene expression in keratinocytes and melanocytes (Corre et al., 2004; Cui et al., 2007; D'Orazio et al., 2006) may mediate a paracrine and autocrine activation of MC1R, thus leading to increased cAMP signaling, activation of MITF and induction of MC1R transcription. Accordingly, a p53-dependent, POMC-based regulatory mechanism connecting keratinocytes and melanocytes may cooperate with the activation of p38 in melanocytes to achieve a strong upregulation of MC1R gene expression central to the tanning response in UV-irradiated skin.
In addition to POMC-derived MCs, several paracrine factors expressed by epidermal cells also affect MC1R gene expression. Endothelin 1 (EDN1) (Scott et al., 2002a) and interleukin-1 (Funasaka et al., 1998) upregulate MC1R mRNA in NHMs. Conversely, TNFα and TGFβ, two cytokines that inhibit melanogenesis in NHMs (Swope et al., 1991) and melanoma cells (Martinez-Esparza et al., 1997; Martinez-Esparza et al., 1998), moderately repress MC1R expression (Funasaka et al., 1998; Martinez-Esparza et al., 1999). In conclusion, UVR modulates the ratio of stimulatory (mostly EDN1 and POMC-derived MCs) or inhibitory (TNFα, TGFβ) signals, and may also have a p38-dependent direct effect on MC1R expression (Tada et al., 2002).
It has been reported that ASIP downregulates MC1R protein expression in mouse melanocytes without a parallel decrease in MC1R mRNA levels (Rouzaud et al., 2003). This effect was attributed to a significantly different structure of the 5’ untranslated region (UTR) of the MC1R transcript in treated cells compared to control or MSH-stimulated melanocytes, which may lead to lower translation efficiency for the ASIP-induced transcript. In NHMs, neither ASIP nor HBD3 had any effect on MC1R expression (Swope et al., 2012). In summary, post-transcriptional and translational regulatory mechanisms may cooperate with transcriptional regulation to fine-tune the functional output of mouse MC1R gene expression, but human MC1R appears mostly regulated at the transcriptional level.
Post-translational processing
After being assembled in the rough endoplasmic reticulum (ER), newly synthesized MC1R undergoes a series of post-translational modifications including oligomerization, glycosylation and, most likely, palmitoylation and phosphorylation. Since the density of MC1R molecules on the cell surface of NHMs is rather low, with around 1000 receptors per cell (Roberts et al., 2006), and most likely a limiting factor for generation of cAMP (Mas et al., 2003), alterations in MC1R processing have a significant impact on the cellular responses to agonists.
Many GPCRs exist as dimeric or oligomeric species (Audet and Bouvier, 2012). Oligomerization of newly synthesized monomers is important for anterograde trafficking and may modulate the functional properties of the protein. Concerning the MCR subfamily, dimerization has been demonstrated for MC1R (Mandrika et al., 2005; Sanchez-Laorden et al., 2006a; Zanna et al., 2008), MC2R (Roy et al., 2010; Sebag and Hinkle, 2009), MC3R (Mandrika et al., 2005) and MC4R (Biebermann et al., 2003; Piechowski et al., 2013). SDS-resistant MC1R dimers and oligomers are found in detergent-solubilized extracts from melanoma cells or heterologous cells overexpressing the MC1R (Sanchez-Laorden et al., 2006a). The monomeric units may establish both non-covalent domain swap-type interactions and covalent intermolecular disulphide bonds (Zanna et al., 2008). The minor effect of agonist treatment on the ratio of monomeric and oligomeric species detected by SDS-PAGE suggests that dimerization is constitutive. Dimeric species are found in the ER, showing that oligomerization is an early step in MC1R maturation. Co-immunoprecipitation and functional experiments performed in heterologous cell models show that MC1R variants can heterodimerize with the WT protein, thereby modulating its functional properties (Sanchez-Laorden et al., 2006a). This may account for dominant-negative effects detected in genetic studies (Beaumont et al., 2007).
Human MC1R contains two potential N-glycosylation sequons, 15NST17 and 29NQT31, whose occupancy has been analyzed (Herraiz et al., 2011b). Complete removal of oligosaccharide chains with peptide-N4-(acetyl-β-glucosaminyl)-Asparagine amidase (PNGaseF), or simultaneous mutation to Gln of the two potential acceptor residues Asn15 and Asn29, lowers the apparent molecular weight of the protein, determined by SDS-PAGE, from ~35 to ~ 29 kDa. Mutation of each potential acceptor individually causes a smaller change, thus showing that both sites are occupied in the WT protein. The glycan chains bound to each glycosylation site are not functionally equivalent in that cell surface expression is severely compromised for the N29Q mutant, whereas the N15Q mutation has, at best, a marginal effect. Occupancy of the 15NST17 glycosylation sequon is striking in that is does not adhere to the C-terminal Pro rule stating that a Pro residue immediately C-terminal to a potential glycosylation site abolishes its occupancy (Bause, 1983). Also unexpectedly, mature and active WT MC1R is sensitive to endoglycosidase H (Endo H) digestion (Herraiz et al., 2011b; Perez Oliva et al., 2009). This enzyme cleaves core high-mannose N-glycan chains and hybrid-type chains present in incompletely processed forms of glycoproteins found in the ER, but not the complex oligosaccharides found in the Golgi network. Thus, resistance to EndoH digestion is often taken as indicative of normal processing and trafficking beyond the ER, but this rule does not hold for MC1R. Natural MC1R variants are differentially glycosylated, at least in terms of the ratios of glycosylated to native protein (Herraiz et al., 2011b). Unfortunately, sensitivity to EndoH of WT MC1R excludes this enzyme as a reliable tool to compare trafficking and subcellular compartmentalization of mutant forms.
Acylation of specific residues in the C-terminal cytosolic extension is frequent in GPCRs (Qanbar and Bouvier, 2003), but there is no direct evidence that it occurs in the MC1R. Nevertheless, in silico analysis suggests that Cys315 is a high probability palmitoylation site. Mutation of Cys315 to Ala impairs significantly the number of MC binding sites and agonist-mediated stimulation of cAMP synthesis (Sanchez-Mas et al., 2005b), and a Cys315Gly mutation causes complete LOF by disrupting the signaling of the receptor but not its binding to agonists (Frandberg et al., 2001).
Phosphorylation of specific residues seems to be critical for MC1R trafficking and cell surface expression, and known MC1R mutations might inhibit phosphorylation and thus reduce the number of MC1R per cell. Eight Ser/Thr residues located in intracellular segments of MC1R provide potential phosphorylation targets (Figure 1). Except for Ser71, which lays in il1, these Ser/Thr residues form two clusters. One is located in the short C-terminal tail (residues Ser302, Thr308, Thr314, Ser316) and the other in il2 (Ser145, Ser154 and Thr157). Phosphorylation of C-terminal Thr308 and Ser316 appears to be involved in receptor internalization and desensitization (Sanchez-Laorden et al., 2007) as discussed below. There is evidence that the phosphorylation status of Thr157 located in il2 is a major determinant of MC1R export. A T157A artificial mutant is misrouted and completely retained in an intracellular compartment (Sanchez-Laorden et al., 2009) and the T157I natural mutant is inactive (Nakayama et al., 2006). In contrast, a T157D mutant mimicking the phosphorylated state of Thr157 undergoes normal trafficking to the cell surface. Moreover, immunoprecipitation and Western blot analysis suggest that WT MC1R is constitutively phosphorylated at Thr157 (Sanchez-Laorden et al., 2009). Several natural mutations in il2 also impair MC1R export trafficking. These include the 2 RHC alleles R151C and R160W (Beaumont et al., 2007; Ringholm et al., 2004; Sanchez-Laorden et al., 2006a; Schioth et al., 1999), the I155T mutant (Herraiz et al., 2012), and the R162P inactive variant (Jimenez-Cervantes et al., 2001b), with dramatically reduced cell surface levels (Sanchez-Laorden et al., 2006b; Sanchez-Laorden et al., 2009). Thr157 is part of a 157TLPR160 target sequence for PKC (Siegrist et al., 1994) and in silico analysis suggests that the likelihood of T157 phosphorylation is highly decreased by the R160W mutation. Importantly, the T157D mutation partially rescues forward trafficking of R160W and other il2 mutants.
A model for MC1R export trafficking
The information summarized above suggests a model for MC1R processing and forward trafficking (Figure 2). MC1R is synthesized in the rough ER where several quality control systems check for completion of post-translational processing and determine the intracellular retention of missfolded or missprocessed mutants. As opposed to other MCRs, particularly MC2R, MC1R does not require the interaction with specific accessory proteins for efficient anterograde transport (Rodrigues et al., 2013). Within the ER, newly synthesized MC1R molecules undergo dimerization, and maybe even formation of higher order oligomers. Oligomerization is one of the earliest steps in the biosynthesis of the protein, and the electrophoretic mobility of oligomeric species suggests that it can occur even in the absence of glycosylation. Another key maturation step initiated within the ER is N-glycosylation of two Asn residues in the N-terminal peptide. This peptide is not removed by proteolysis, as shown by persistence of epitopes fused to the N-terminus in the mature protein (Sanchez-Laorden et al., 2006a). Glysosylated oligomers are then transported to the Golgi apparatus where the glycan chains in residues Asn15 and Asn29 may not be processed to complex-type carbohydrates, since mature and fully active MC1R remains sensitive to EndoH. Efficient anterograde trafficking would depend on phosphorylation of Thr157 by an unidentified kinase. This phosphorylation would be a critical regulatory step in MC1R export. Its efficiency would depend on other elements in il2, including R160 and maybe also residues R151, and R162. Interestingly, the il2 of MC1R is particularly well conserved among vertebrate species, and also shows a striking degree of homology between the five MCR subtypes. Thus, the MCRs might share common mechanisms of regulation of plasma membrane expression (Rodrigues et al., 2013). Of note, this discussion is based on data obtained for the MC1R-001 splice variant. The trafficking properties of other isoforms have not been analyzed in detail. However, preliminary data suggest that trafficking of chimeric MC1R-TUBB3 proteins is impaired, resulting in severe intracellular retention (Dalziel et al., 2011).
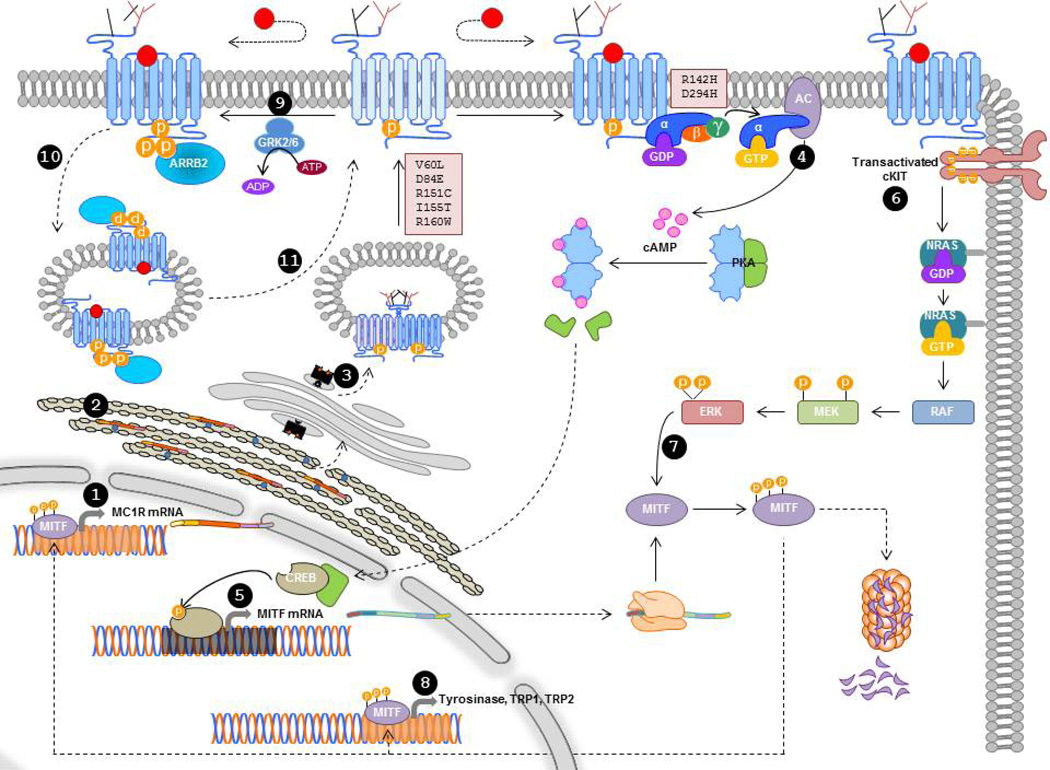
Figure 2. Scheme of the main steps in MC1R biosynthesis and functional coupling.
Transcription of the MC1R gene leads preferentially to the MC1R-001 transcript (1). Translation occurs in the rough ER, where post-translational modifications including oligomerization and glycosylation are also performed (2). Glycosylated oligomers proceed to the Golgi, then to the plasma membrane, and forward trafficking is dependent on Thr157 phosphorylation (3). For simplicity, the receptor is depicted as a monomer on the cell surface, but its structure is likely dimeric/oligomeric. Upon agonist binding, MC1R activates AC via the Gs protein, thus triggering cAMP synthesis and PKA activation (4). Active PKA catalytic subunits move to the nucleus to activate CREB transcription factors, which in turn increase the rate of transcription of the MITF gene (5). Agonist-activated MC1R also transactivates cKIT or a related RTK to trigger the NRAS-BRAF-MEK-ERK cascade (6). Active ERKs can phosphorylate MITF to increase its transcriptional activity and its proteasome-dependent degradation (7). Active MITF promotes transcription of the genes encoding for melanogenic enzymes (8), and for MC1R. Agonist-bound MC1R can also be desensitized by GRK2 or GRK6-dependent phosphorylation and ARRB2 recruitment (9), followed by sequestration in endocytic vesicles (10) whose likely destination is recycling to the cell surface (11).
Signaling pathways
Like most GPCRs, MC1R is coupled to several intracellular signaling cascades. It is established that binding of α-MSH (or ACTH in the case of the human receptor) to MC1R results in its functional coupling to the cAMP pathway, evidenced by sequential activation of the Gs protein and adenylate cyclase (AC), leading to increased cAMP formation (Figure 2) (Pawelek et al., 1973; Suzuki et al., 1996; Wong and Pawelek, 1973). Increased levels of cAMP lead to the activation of PKA, followed by phosphorylation of members of the cAMP responsive-element binding protein (CREB) family of transcription factors, and CREB-mediated activation of Microphthalmia transcription factor (MITF) gene expression (Bertolotto et al., 1998; Price et al., 1998). MITF is a key positive regulator of melanocyte differentiation markers including the melanogenic enzymes (Levy et al., 2006). Thus, the second messenger cAMP is critically involved in the regulation of mammalian pigmentation and it also plays a central role in UVR-induced tanning (Busca and Ballotti, 2000; D'Orazio and Fisher, 2011; Im et al., 1998).
Several lines of evidence indicate that cAMP is the main intracellular messenger responsible for the melanogenic actions of α-MSH (Busca and Ballotti, 2000). cAMP-elevating agents such as the natural diterpene forskolin (FSK) stimulate tyrosinase activity, which triggers the switch from pheomelanogenesis to synthesis of eumelanins and mimics many of the effects of α-MSH (Im et al., 1998; Ito and Wakamatsu, 2003; Ito and Wakamatsu, 2011; Kadekaro et al., 2010; Scott et al., 2002a). Application of FSK onto recessive yellow mice harbouring the LOF mutation MC1Re/e stimulated eumelanogenesis, and rescued the yellow color phenotype by directly activating AC (D'Orazio et al., 2006). Similarly, treatment of NHMs expressing LOF RHC MC1R alleles with FSK markedly increased tyrosinase activity, indicative of stimulation of eumelanin synthesis (Kadekaro et al., 2010; Scott et al., 2002a).
Mouse and human MC1R display significant agonist-independent constitutive coupling to the cAMP pathway in heterologous cells overexpressing the WT proteins (Sanchez-Mas et al., 2004). For human MC1R overexpressed in HEK cells, agonist-independent cAMP production can reach 40% maximal values obtained after agonist stimulation, within the range of the constitutively active E92K natural MC1REso-3J (somber) allele associated with hypermelanized coats, or other artificial constitutively active mutants (Lu et al., 1998; Sanchez et al., 2002). Constitutive coupling of MC1R to cAMP has been recently confirmed (Benned-Jensen et al., 2011), and may account for certain direct effects of the inhibitory ligand ASIP, which behaves as an inverse agonist rather than a competitive antagonist (Chluba-de et al., 1996; Graham et al., 1997; Hida et al., 2009; Le Pape et al., 2009; Ollmann et al., 1998; Sakai et al., 1997; Siegrist et al., 1997; Suzuki et al., 1997). Interestingly, POMC-null mice show significant eumelanin pigmentation, suggesting that constitutive signaling from MC1R is high enough to support eumelanogenesis (Slominski et al., 2005). Alternatively, other paracrine factor might compensate for loss of POMC. The situation for human melanocytes is less clear-cut. Constitutive levels of cAMP in melanocytes expressing functional or non-functional MC1R were found to be similar (Kadekaro et al., 2010). These conflicting results might be explained by the level of expression of MC1R, which is low in human melanocytes, with around 1000 receptors per cell (Roberts et al., 2006), and is expected to be much higher in MC1R transfected cells. Whereas expression of MC1R rescues the pigmentation phenotype of MC1Re/e mice bearing a completely inactive truncated mutant (Healy et al., 2001), patients with mutations in the POMC gene abolishing translation, or interfering with the production or activity of MCs have red hair (Krude et al., 1998; Samuels et al., 2013). However, these studies did not report the MC1R genotype of the patients, thus complicating the interpretation of the phenotype.
The cAMP pathway is surprisingly complex, given that there are 10 different AC isoenzymes (Cooper, 2003), 4 functionally non-redundant PKA regulatory subunits, and 3 catalytic PKA (Taylor et al., 2012). The cAMP-degrading phosphodiesterases (PDEs) also form a relatively large family of proteins with different catalytic and regulatory properties, encoded by 11 different genes (Conti and Beavo, 2007; Houslay, 2010). A further layer of complexity and specificity is provided by the prevalence of scaffold proteins that mediate formation of signaling complexes to regulate not only the substrates of PKA but also the spatiotemporal organization of specific hubs of cAMP signalling (Houslay, 2010; Taylor et al., 2012). Despite the significance of the cAMP pathway in melanocyte biology, the specific components and organization of this signaling system and many aspects of its functional regulation in melanocytic cells are largely unknown. It has been reported that B16 mouse melanoma cells express the widespread AC isoforms 6, 7 and 9, and that their relative levels may be regulated by extracellular signals, including α-MSH (Cho et al., 2000). Human melanoma cells express PDE1, PDE3, PDE4 and PDE5 and these proteins contribute to the regulation of cellular differentiation and proliferation (Marquette et al., 2011; Murata et al., 2010; Narita et al., 2007). Interestingly, the PDE4D3 gene is a direct target of MITF and, accordingly, PDE4D3 expression is upregulated by cAMP (Khaled et al., 2010). Thus, a negative feedback loop that downregulates cAMP-dependent MC1R signaling has been proposed, and its relevance in vivo is underscored by the synergism of specific PDE4 inhibitors and FSK in stimulating UVR-independent pigmentation in mice.
Increased levels of cAMP lead to PKA activation, and this in turn activates the mitogen-activated protein kinase (MAPK) module leading to p38. The stress activated p38 MAPK was first reported to be stimulated upon activation of MC1R by Smalley and Eisen (Smalley and Eisen, 2000). The observation that FSK also triggered p38 phosphorylation in B16 mouse melanoma cells suggested that p38 activation was cAMP-dependent. These findings were extended to human melanoma cells (Smalley and Eisen, 2002) and NHMs of defined MC1R genotype (Kadekaro et al., 2012; Newton et al., 2007; Wong et al., 2012). Consistent with a role of cAMP, p38 activation by MC1R agonists was much less efficient in NHMs harboring mutant MC1R with impaired coupling to the cAMP pathway, compared with WT melanocytes. HBD3 has also been shown to activate p38, at least in heterologous HEK cells stably expressing the human receptor (Beaumont et al., 2012).
The consequences of p38 activation downstream of MC1R have been investigated. In B16 mouse melanoma cells and COLO 853 human melanoma cells, α-MSH-mediated induction of melanin synthesis and inhibition of cellular proliferation were impaired by the p38 inhibitor SB 203580 (Smalley and Eisen, 2000; Smalley and Eisen, 2002). Although the specificity of this pharmacological agent is not absolute and off-target effects of pyridinyl imidazole inhibitors of p38 have been described in B16 cells (Bellei et al., 2010; Bellei et al., 2012), a melanogenic effect of p38 signaling has been confirmed by finding that a placental total lipid extract which activated p38 in B16 cells enhanced Tyrosinase gene expression and stimulated melanogenesis (Singh et al., 2005), probably via cAMP-independent phosphorylation and activation of CREB (Saha et al., 2006). In addition, signaling molecules not related to MCs, such as endocannabinoids (Pucci et al., 2012) or members of the bone morphogenetic protein family (Singh et al., 2012) may also engage p38 to activate melanogenesis in NHMs.
Importantly, p38 activity seems involved in the tanning response, a protective mechanism against photocarcinogenesis. It has been shown that UVR-induced p38 activation leads to phosphorylation and activation of the USF-1 transcription factor with subsequent stimulation of Tyrosinase gene expression in mouse melanocytes and melanoma cells (Galibert et al., 2001). A similar USF-1-dependent mechanism apparently contributes to activation of POMC and MC1R gene expression in irradiated mouse melanocytes and human melanoma cells (Corre et al., 2004), and most likely in human skin in vivo as well (Corre et al., 2006). UVR also promotes p38-dependent CREB phosphorylation in irradiated NHM cultures (Tada et al., 2002). Of note, α-MSH acts synergistically with UVR to promote p38 activation and p53 expression in melanocytes expressing WT MC1R (Kadekaro et al., 2012; Newton et al., 2007; Wong et al., 2012). The transcription factor p53, known to be activated by p38, increases the expression of Tyrosinase (Khlgatian et al., 2002), and upregulates the expression of POMC in mouse skin keratinocytes (Cui et al., 2007). The POMC derivatives α-MSH and ACTH upregulate the expression of MC1R in human melanocytes (Kadekaro et al., 2010; Scott et al., 2002a; Swope et al., 2012). These observations suggest a positive feedback loop involving p38, p53, POMC-derived MCs and MC1R that is important for tanning and for the induction of potent DNA repair responses in UV-irradiated skin (see below).
The signaling module leading to the extracellular signal-regulated kinases (ERK) ERK1 and ERK2 is also activated downstream of MC1R (Busca et al., 2000; Herraiz et al., 2011a). The ERK pathway is classically associated with mitogenic signaling by growth factors, which activate cell surface tyrosine kinase receptors (RTKs) followed by Grb2 and Sos-dependent activation of RAS GTPase. The GTP-bound active form of RAS activates members of the RAF family of protein kinases to trigger a phosphorylation cascade involving the MAPK kinase MEK and ultimately leading to ERK1 and ERK2 (Wellbrock et al., 2004). ERK substrates include cytoplasmic and cytoskeletal proteins, and transcription factors. Mutations in BRAF or NRAS are found in roughly 50% or 20–30% of human melanomas, respectively (Hodis et al., 2012; Krauthammer et al., 2012), consistent with the relevance of the ERK pathway to the regulation of melanocyte proliferation.
In mouse melanoma cells, ERK activation by MC agonists of MC1R was reported to involve cAMP-dependent but PKA-independent activation of NRAS and BRAF (Busca et al., 2000). However, the mouse MC1R E92K mutation, associated with darker eumelanic pigmentation, confers agonist-independent coupling to the cAMP pathway but has no effect on ERK activity (Benned-Jensen et al., 2011). Moreover, treatment of NHMs or human melanoma cells with the strong cAMP inducer FSK does not trigger ERK activation (Tada et al., 2002). In addition, the AC inhibitor 2’,5’-dideoxyadenosine does not impair MC-dependent ERK phosphorylation, even at concentrations that abolish cAMP production (Herraiz et al., 2011a). Thus, in human melanocytic cells cAMP is neither sufficient nor necessary to achieve functional coupling of MC1R to the ERKs. In keeping with this conclusion, ERKs are efficiently activated by certain MC1R mutant alleles with impaired or absent cAMP signaling in NHMs or melanoma cells, as well as in heterologous systems (Herraiz et al., 2009; Herraiz et al., 2012). Conversely, the R163Q mutation with little or no effect on cAMP production strongly impairs ERK activation (Doyle et al., 2012). Overall, these observations indicate that functional coupling to the cAMP and ERK pathways are independent events that can be dissociated by specific mutations in MC1R and that most likely involve different intracellular effectors (Herraiz et al., 2012).
The mechanism of MC1R signaling to the ERK module in human melanocytic cells has been investigated (Herraiz et al., 2011a). In the HBL human melanoma cell line WT for MC1R, NRAS and BRAF, activation of MC1R by NDP-MSH was rapidly followed by an increase in tyrosine phosphorylation of cKIT, a RTK that plays major roles in melanocyte proliferation, migration and survival, as well as in pigmentation. Several cKIT inhibitors and specific siRNA-mediated silencing of cKIT expression also abolished MC-dependent ERK activation, without an effect on cAMP production. These data strongly suggest that MC peptides trigger ERK activation by transactivation of cKIT or another related RTK (Figure 2).
ERK signaling is important to sustain melanocyte proliferation and is activated by several melanocyte-specific mitogens, such as basic FGF, stem cell factor, and EDN1 (Bohm et al., 1995; Tada et al., 1998). MC1R-dependent ERK activation by HBD3 has also been reported (Beaumont et al., 2012). Active ERKs catalyze MITF phosphorylation to increase its transactivating activity (Hemesath et al., 1998). In addition to its role as a positive regulator of melanogenesis (Levy et al., 2006), MITF also modulates expression of the cell cycle regulatory proteins p21 and p27 (Carreira et al., 2005; Carreira et al., 2006). Thus, the cAMP and ERK signals originating independently from MC1R might converge on activating MITF in a synergistic manner (Figure 2).
In addition to the cAMP pathway, a few reports have raised the possible involvement of other second messengers in MC1R signaling, including calcium and PKC (reviewed by Garcia-Borron et al., 2005). However, in the case of the human receptor naturally expressed on NHMs, binding of α-MSH did not cause increased calcium mobilization after loading with Fura-2 whereas treatment with Endothelin-1 showed the expected positive response (Abdel-Malek, unpublished data). Although several members of the MCR subfamily of GPCRs may activate PKC, it is controversial whether this is the case for MC1R. It was reported that α-MSH activates PKCβ in melanocytes (Park et al., 1996). However, Herraiz et al. (Herraiz et al., 2011a) could not detect activation of the downstream target of PKC, MARKS, in NHMs.
Termination of MC1R signaling
MC1R activity is regulated by intracellular proteins acting on most GPCRs, in particular the β-arrestins (ARRBs), as well as by more specific partners that may be restricted to the MCR subfamily. Two such MCR-specific regulatory proteins, attractin and mahogunin ring finger 1, are particularly important determinants of the relative proportions of eumelanins and pheomelanins. Their role has been recently reviewed (Walker and Gunn, 2010). Accordingly, this section discusses the desensitization of cell surface MC1R.
Signaling by most if not all GPCRs is regulated by the concerted action of dedicated GPCR kinases (GRKs) and cytosolic ARRBs. GRKs are Ser/Thr kinases with specificity for occupied receptors and little affinity for their unbound receptors (Gurevich et al., 2012). The GRK family comprises seven members (GRK1 to GRK7), of which GRK1 and GRK7 expression is restricted to the retina and GRK4 is mostly found in testes. The ARRBs are scaffolding proteins belonging to a small family of 4 members (Gurevich and Gurevich, 2006), which act as endocytic adaptors and signal terminators to promote agonist-dependent homologous desensitization. The current model involves rapid phosphorylation of activated receptors by GRKs and recruitment of ARRBs to phosphorylated Ser/Thr residues located on intracellular segments of the receptor. Two non-specific ARRB isoforms, most often called β-arrestin1 and β-arrestin2 (ARRB1 and ARRB2) are ubiquitously expressed in virtually all cell types (DeFea, 2011; Luttrell and Gesty-Palmer, 2010). Receptor-bound ARRBs uncouple the active receptor from signal transducing G proteins and interact with clathrin and AP2 (DeWire et al., 2007; Moore et al., 2007; Wolfe and Trejo, 2007). The result of this series of events is termination of G protein-mediated signaling and receptor endocytosis via clathrin-coated vesicles (DeFea, 2011; Moore et al., 2007).
Human MC1R undergoes homologous desensitization in human melanoma and heterologous cells (Sanchez-Mas et al., 2005a), and in NHMs (Swope et al., 2012). NHM cultures established from donors of different pigmentation phenotype and human melanoma cells express the ubiquitous GRK2, GRK3, GRK5 and GRK6 (Sanchez-Mas et al., 2005a; Swope et al., 2012). However, no correlation between GRK expression in NHMs and pigmentation phenotype has been found (Swope et al., 2012). Co-expression of MC1R with either GRK2 or GRK6 in heterologous cells showed that both kinases inhibit functional coupling to the cAMP pathway, whereas dominant-negative forms increase the cAMP response (Sanchez-Mas et al., 2005a). GRK2 seems to be also involved in the desensitization of other MCRs such as MC2R (Baig et al., 2002) and MC4R (Shinyama et al., 2003). As opposed to GRK2 which appeared specific for agonist-dependent MC1R signaling, GRK6 also impaired constitutive signaling and promoted MC1R internalization, most likely by phosphorylation of the C-terminal Thr308 and Ser316 (Sanchez-Laorden et al., 2007). Accordingly, among the GRKs, GRK2 and GRK6 may play a major role in MC1R desensitization.
The role of ARRB isoforms in MC1R desensitization has also been investigated (Abrisqueta et al., 2013). NHMs and human melanoma cells express ARRB1 and ARRB2, and both isoforms were able to bind to MC1R even in the absence of agonists, in a competitive and mutually exclusive manner. Lack of selectivity for a specific ARRB isoform has also been reported for MC2R (Kilianova et al., 2006), MC3R (Breit et al., 2006; Nyan et al., 2008), MC4R (Breit et al., 2006) and MC5R (Rodrigues et al., 2012). The functional effects of ARRB binding to MC1R seemed isoform-specific, in that ARRB1 did not show any inhibitory or uncoupling activity, whereas ARRB2 inhibited agonist-dependent cAMP production. Interestingly ARRB2 had no effect on functional coupling to the ERK pathway. A certain selectivity of ARRB2 on functional coupling has also been reported for MC2R (Roy et al., 2011b). Interestingly, the effect of ARRB2 on MC2R functional coupling was also biased, as cAMP production was inhibited without impairment of ERK activation (Roy et al., 2011a).
ARRB2, but not ARRB1 triggered MC1R internalization, with prolonged co-localization in endocytic vesicles. The fate of internalized ARRB2-MC1R complexes has not yet been directly investigated. Mouse MC1R is most likely degraded in lysosomes following internalization (Wong and Minchin, 1996). However, the density of MC binding sites on the cell surface of human melanoma cell lines can even increase after prolonged exposure to the agonists (Siegrist et al., 1994), and in NHMs continuous agonist treatment for several hours yielded a steady rise in cAMP (Swope et al., 2012), which suggests that ARRB2-mediated MC1R internalization was followed by slow recycling to the cell surface. Given a possible differential expression of the desensitization machinery in different cell types, using the appropriate cell model (i.e. cells belonging to the melanocytic lineage naturally expressing MC1R) to study MC1R desensitization is important for accurate data interpretation.
Variant alleles and mutations of human MC1R
The observation that some NHMs in culture failed to respond to α-MSH suggested that this might be due to a LOF mutation in the MC1R, and led to the first investigation of SNPs in the MC1R gene in British and Irish individuals (Valverde et al., 1995). This was followed by a large twin study in Australia that solidified the association of three MC1R variants, R151C, R160W, and D294H, with red hair color (Box et al., 1997). Other studies ensued from different countries, which established the association of the above variants with RHC phenotype and also with increased melanoma risk (Ichii-Jones et al., 1998; Kennedy et al., 2001; Palmer et al., 2000). Subsequent studies have shown that the MC1R is extraordinarily polymorphic (Figure 1) (Gerstenblith et al., 2007; Guan et al., 2013; Kanetsky et al., 2006; Savage et al., 2008), with around 200 coding region variants altering the protein sequence. In addition, 50 synonymous changes and another 50 polymorphisms in the 5´- and 3´’ UTRs have been described to date. Nonsynonymous coding region polymorphisms and the corresponding nucleotide changes are listed in Supplemental Table 1. This unusual nucleotide diversity has been attributed to a high CpG content which increases mutation rates, in combination with some degree of positive selection for mutant alleles associated with lighter skin pigmentation in regions of lower UVR incidence (Jablonski and Chaplin, 2010; Martinez-Cadenas et al., 2013). Indeed, the frequencies of specific alleles show significant variations in different populations. The consensus sequence for the MC1R coding region is more common in African populations, whereas nonsynonymous variants are at higher frequency in Eurasian individuals (Harding et al., 2000; Martinez-Cadenas et al., 2013; Rana et al., 1999).
Following the discovery of the association of variant alleles with the RHC phenotype (Valverde et al., 1995) and melanoma risk (Valverde et al., 1996), MC1R variants have been reported to be associated with other cutaneous phenotypes such as oculocutaneous albinism (Saleha et al., 2013), congenital melanocytic nevi (Kinsler et al., 2012), vitiligo (Szell et al., 2008), piebaldism (Oiso et al., 2009) or severe photoaging of facial skin (Elfakir et al., 2010). Moreover, the increase in the density of melanocytes in human skin exposed to UVR is significantly reduced in carriers of MC1R variants (Hacker et al., 2013). Associations with unrelated traits or diseases have also been described, including glucocorticoid deficiency (Turan et al., 2012), follicular lymphoma (Kane et al., 2010), depression and antidepressant response (Wu et al., 2011), radiotherapy side effects (Fogarty et al., 2010), anesthetic requirements and recovery time after surgery (Myles et al., 2012), acute pain and pain from inflammatory origin (Delaney et al., 2010; Oertel and Lotsch, 2008), multiple sclerosis (Strange et al., 2010) and Parkinson’s disease risk (Gao et al., 2009).
MC1R variants whose frequency is high enough to allow for statistically significant association studies have been classified into groups according to their penetrance for the RHC phenotype: strong and penetrant “R” alleles, and the weaker “r” forms, and the pseudoalleles that do not have any noticeable phenotype (Duffy et al., 2004). Extensive evidence shows that all the R alleles yield hypomorphic proteins with decreased or even absent ability to activate the cAMP pathway, when assayed in heterologous expression systems, human melanoma cells or NHMs of defined genotype (Frandberg et al., 1998; Herraiz et al., 2009; Kadekaro et al., 2010; Nakayama et al., 2006; Newton et al., 2005; Ringholm et al., 2004; Roberts et al., 2008; Schioth et al., 1999; Scott et al., 2002b). For the R alleles overexpressed in heterologous systems, both basal and agonist-dependent coupling to cAMP synthesis is impaired, with varying degrees of residual activity ranging from an almost complete loss of functional coupling for D84E and D294H, to 25–50% residual activity for other forms, particularly R160W.
Decreased cell surface expression seems the primary cause of LOF for the major RHC alleles D84E, R151C, I155T and R160W (Beaumont et al., 2005; Herraiz et al., 2012; Schioth et al., 1999). Evidence for extensive intracellular retention with preferential association of R151C with the ER and R160W with the Golgi apparatus has also been provided (Sanchez-Laorden et al., 2009). However, plasma membrane density of R142H is normal and D294H is expressed at even higher cell surface levels than WT. Moreover, both variants bind MC agonists with high affinity, thus suggesting that for these R alleles the molecular defect lies in the inability to undergo the agonist-induced transition to an active conformation.
The best characterized r alleles are V60L, V92M and R163Q. The V60L variant is found at frequencies as high as 20% in some European populations, but is much rarer in Asian or African individuals. Conversely, V92M and particularly R163Q are common in individuals of Asian descent, with frequencies around 14% and higher than 60%, respectively (Gerstenblith et al., 2007; Savage et al., 2008). The effects of these two variants on functional coupling to the cAMP cascade are not significant (Kadekaro et al., 2010; Scott et al., 2002b). Only the V60L mutation has been consistently found to result in significant LOF, with a residual ability to activate cAMP synthesis approximately 50% of WT when assessed in heterologous cells (Beaumont et al., 2007; Herraiz et al., 2012; Schioth et al., 1999), as well as in homozygous human melanocytes (Kadekaro et al., 2010). This moderate functional impairment can be accounted for by misstrafficking with significant intracellular retention and reduced cell surface levels. However, the residual activity of the V60L variant is apparently sufficient to sustain normal dendricity and dopachrome tautomerase responses in homozygous NHMs, upon activation of MC1R (Ainger et al., 2011). For V92M, slightly impaired (Herraiz et al., 2012; Ringholm et al., 2004) or WT coupling (Beaumont et al., 2007) has been reported, and both the dose-response curves for agonist-induced cAMP and the maximal stimulation appear normal for R163Q (Nakayama et al., 2006; Ringholm et al., 2004).
Impaired functional coupling of the R alleles to the cAMP pathway accounts for their phenotypic association: red hair, fair skin and poor tanning ability. Low or absent activation of cAMP synthesis by these variants would decrease MITF induction, resulting in inefficient stimulation of eumelanogenesis by MC peptides, pheomelanic red hair and poorly pigmented, fair skin. Within this framework, the lower penetrance of the r forms for the RHC phenotype would be explained by their higher residual signaling. Surprisingly, RHC alleles are also expressed in individuals with olive skin color, such as in Southern Italy and Greece, which suggests alternative compensatory mechanisms for regulating eumelanin synthesis (Landi et al., 2005; Palmer et al., 2000; Stratigos et al., 2006).
A recent report described the structural impact and possible functional consequences of rare MC1R mutations (Ibarrola-Villava et al., 2014). In another study, an algorithm has been proposed to classify nonsynonymous rare variants whose low frequency excludes significant genetic association studies as “r” or “R” (Davies et al., 2012). This algorithm is primarily based on the PMut and SIFT software. Given that all the R forms analyzed thus far have levels of α-MSH-induced cAMP production below 50% of WT, whereas the r alleles retain higher residual activities, we propose the evaluation of agonist-promoted cAMP synthesis as an alternative and maybe more reliable in vitro method for the classification of rare alleles, setting as a first approximation a 50% residual signaling potential as the threshold between r and R alleles. This simple rule would rank as R the R307G variant found in two Neanderthal specimens (Lalueza-Fox et al., 2007), and is therefore consistent with the suggestion that these individuals might be red-haired. Other potential R alleles are S41F (Perez Oliva et al., 2009), L93R (Sanchez et al., 2002) and R162P (Jimenez-Cervantes et al., 2001b) all of which exhibit severe intracellular retention, or M128T and C289R with impaired agonist binding properties in spite of significant cell surface density (Perez Oliva et al., 2009).
Although most of the functional studies of variant MC1R reported to date focused on the cAMP pathway, the effects of common R and r mutations on ERK activation downstream MC1R have also been investigated. Herraiz et al (2009) first reported that in spite of major impairment in triggering the cAMP pathway, the R151C, R160W and D294H alleles are comparable to the wild type MC1R in activating ERK. The effect of these mutations on ERK signaling was confirmed in NHMs (Herraiz et al., 2011a), and extended to other natural and artificial variants (Herraiz et al., 2012). A minor impairment of signaling to the ERKs, as compared to a major LOF in the cAMP pathway was later on confirmed for R and r variants (Beaumont et al., 2012). Intriguingly, the R163Q mutation with minimal effect on cAMP production strongly decreases ERK activation (Doyle et al., 2012). Overall, these observations show that R and r variants are best described as imbalanced signaling mutants, rather than LOF forms. Moreover, they are consistent with a major role of the cAMP pathway, as opposed to the ERK module, as a determinant of the pigmentary phenotype.
Modulation of the UV-induced DNA damage response by MC1R
Exposure to solar UVR is the main etiological factor for melanoma. A role of the MC1R and α-MSH in the UVR response of melanocytes was first suggested by Pawelek and his team (Chakraborty et al., 1996; Funasaka et al., 1998). Later, using NHMs, it was demonstrated that activation of the cAMP pathway is required for the melanogenic (tanning) response to UVR (Im et al., 1998). Given that pigmentation is the main photoprotective mechanism in the skin, it was presumed for a long time that stimulation of melanogenesis is the sole mechanism by which the activated MC1R reduces the extent of UVR-induced DNA damage, and consequently photocarcinogenesis. An important discovery was that treatment of cultured human melanocytes with α-MSH reduces the burden of UV-induced DNA damage by enhancing repair of cyclobutane pyrimidine dimers, the major form of DNA photoproducts (Bohm et al., 2005; Kadekaro et al., 2005). These results were further substantiated by the findings that α-MSH up regulates the NR4A nuclear receptor, which is involved in nucleotide excision repair pathway, the main pathway for removal of DNA photoproducts (Smith et al., 2008). In addition to induction of DNA photoproducts, UVR also results in the generation of reactive oxygen species (ROS) that cause oxidative DNA damage in human melanocytes, as well as damage to cellular lipids and proteins. Treatment with α-MSH reduces oxidative DNA damage by immediately inhibiting the generation of hydrogen peroxide and by activating antioxidant enzymes, exemplified by catalase (Maresca et al., 2010), and up regulating the expression of antioxidant and base excision repair enzymes (Kadekaro et al., 2010; Kadekaro et al., 2012; Song et al., 2009). Further evidence for the antioxidant effects of α-MSH was provided by the finding that it increases the expression and activity of Nrf-2, an important transcription factor that regulates the expression of phase-2 antioxidant genes, such as heme-oxygenase II, and NQO-1 (Kokot et al., 2009). Since melanoma tumors often have an aberrant redox state, the antioxidant effects of α-MSH might have a critical role in prevention of melanoma formation or progression (Meyskens, Jr. et al., 2001). The study by Kadekaro et al (2005) showed that the effect of α-MSH on DNA repair on UVR-irradiated melanocytes was not due to increased melanogenesis, and was accompanied with inhibition of the cytotoxic effect of UVR, evidenced by reduced apoptosis. Taken together, it can be concluded that the effect of α-MSH on DNA repair enables melanocytes to survive with genomic stability, and this is not strictly dependent on increased melanin content. FSK, a direct activator of AC, mimicked the above effects of α-MSH on UVR-irradiated melanocytes, indicating that activation of the cAMP pathway mediates these effects (Kadekaro et al., 2005; Kadekaro et al., 2010). Based on the time course of the effects of MC1R activation on the repair of DNA damage and stimulation of melanogenesis in response to UVR, the authors proposed the paradigm that the activated MC1R protects melanocytes from the DNA damaging effects of UVR, and hence malignant transformation to melanoma, by rapid enhancement of DNA repair to ensure melanocyte survival, followed by stimulating melanogenesis in order to protect from subsequent UVR exposure that induces DNA damage.
The above-described effects of α-MSH on UV-irradiated melanocytes absolutely require expression of functional MC1R, as expression of 2 RHC alleles, which results in LOF of the receptor (lack of signaling via the cAMP pathway), compromises the DNA repair capacity and sustains oxidative stress in melanocytes. Using a panel of NHM cultures with different MC1R genotypes, it was shown that cultures homozygous or compound heterozygous for RHC alleles fail to respond to α-MSH with enhanced repair of cyclobutane pyrimidine dimers (CPDs) or reduction of ROS. To unequivocally prove the significance of functional MC1R in the UVR response of NHMs, melanocytes expressing LOF receptor were transfected with WT MC1R (Kadekaro et al., 2010). The response to α-MSH was normalized by restoring the ability of the receptor to signal by stimulating cAMP formation, and reducing UVR-induced DNA damage and apoptosis. The aberrant UVR response of melanocytes with mutant MC1R explains how inheritance of RHC alleles increases the predisposition to melanoma, and substantiates the role of the MC1R as a melanoma susceptibility gene. Interestingly, this aberrant response is not always associated with the RHC phenotype. For instance, Kadekaro and coworkers reported a primary NHM culture compound heterozygous for 2 MC1R RHC alleles but with a high constitutive eumelanin content, which exhibited diminished capacity to repair UV-induced DNA damage or overcome oxidative stress in response to α-MSH treatment (Kadekaro et al., 2010). This observation explains why individuals with olive skin color who harbor 2 RHC have a high risk for melanoma, despite their dark pigmentation (Palmer et al., 2000; Stratigos et al., 2006), and emphasize that melanoma risk cannot be accurately assessed based only on pigmentary phenotype
It has been suggested that expression of MC1R variant alleles increases the risk for the somatic activating BRAF mutation BRAFv600E (Landi et al., 2006), that is very commonly found in nevi and melanoma tumors (Pollock et al., 2003). Moreover, co-inheritance of an MC1R RHC allele with a mutation in p16 exacerbates the risk for melanoma above that resulting from mutation in either gene (Box et al., 2001; Demenais et al., 2010; Fargnoli et al., 2010). Elucidating the mechanism(s) by which MC1R interacts with other melanoma-associated genes will lead to improved understanding of how NHMs become transformed to melanoma cells.
Microarray experiments that were carried out on melanocytes with functional vs. non-functional MC1R clearly showed that the latter are refractory to α-MSH, with hardly any genes altered in expression in response to α-MSH treatment (Kadekaro et al, 2010). In contrast, using melanocytes with functional MC1R identified many genes that were altered in expression by α-MSH, including genes involved in regulating apoptosis, DNA repair, and antioxidant genes, as well as melanocyte-specific genes, such as MC1R, MITF, and TYROSINASE. Additionally, the microarray data revealed that treatment of melanocytes expressing functional MC1R with α-MSH reversed the effects of UVR on many genes, including those belonging to the above functional categories, thus underscoring the significance of MC1R in modulating the response of melanocytes to UV.
The above photoprotective effects of activated MC1R on NHMs led to further studies to elucidate the role of MC1R in their DNA damage response. Swope et al. recently showed that activation of MC1R by its agonist α-MSH leads to the phosphorylation, hence activation of the DNA damage sensors ataxia telangiectasia-related (ATR) and ataxia telangiectasia-mutated (ATM), and the subsequent phosphorylation of their main downstream targets check point kinase (Chk) 1 and 2, respectively, in UVR-irradiated NHMs (Swope et al., 2014). Similar treatment also led to phosphorylation of the DNA damage sensor DNA-PK (Kadekaro et al., 2012). Treatment of melanocytes expressing functional MC1R with α-MSH increases the expression of UVR-induced γ-H2AX, the phosphorylated form of histone 2AX (Swope et al., 2014), which marks DNA lesions and helps recruit DNA repair proteins to the site of damage. Furthermore, activation of MC1R resulted in increased protein levels of XPC, the DNA damage recognition enzyme that catalyzes the first step in the nucleotide excision repair pathway. These effects provide compelling evidence for the role of MC1R in enhancing repair of UVR-induced DNA damage. That these effects are absent in melanocytes expressing 2 RHC alleles further supports the link between MC1R activity and DNA repair capacity of melanocytes.
Another means by which activation of MC1R modulates the UVR-induced DNA damage response is by enhancing the phosphorylation of the stress-activated MAPK p38, as well as p53 in human melanocytes (Kadekaro et al., 2012). These events are important for repair of UVR-induced DNA damage, and were found to be particularly critical for repair of oxidative DNA damage (Kadekaro et al., 2010). Increased phosphorylation of p38 and p53 in response to α-MSH treatment was observed in melanocytes with functional MC1R, but not in melanocytes of R/R genotype.
Perspectives
Since the early 1990’s, there has been great advancement in our knowledge about MC1R, the MCs, and the significance of their physiological role, particularly in prevention of photocarcinogenesis. It is now established that human MC1R is a main determinant of the diversity of human pigmentation. That human melanocytes express functional MC1R, and that the activated MC1R enhances the repair of UVR-induced DNA damage in addition to its classic role in regulating eumelanin synthesis, strongly suggests that MC1R can be directly targeted in a skin cancer, including melanoma chemoprevention strategy. For many years, there has been interest in developing potent α-MSH analogs for safer sunless tanning. The best known α-MSH analog is the tridecapeptide [Nle4, D-Phe7]-α-MSH (NDP-MSH; melanotan or afamelanotide). A clinical trial with systemic application of melanotan showed that in addition to the desired stimulation of pigmentation in the absence of any sun exposure, the analog had several unexpected effects, including nausea and loss of appetite (Levine et al., 1991). These side effects were due to the ability of the analog to bind and activate other MCRs. Nonetheless, NDP-MSH is currently being used on patients with polymorphic light eruption, and tested for treatment of vitiligo (Fabrikant et al., 2013).
To alleviate the systemic effects of non-selective α-MSH analogs, novel analogs that are highly selective and specific to MC1R, small in size and lipophilic need to be developed to allow for topical delivery to the skin, and for primarily targeting the melanocytes. Towards this goal, potent tetrapeptide analogs of α-MSH, n-capped-His-D-Phe-Arg-Trp-NH2 have been developed and tested on NHMs in vitro. Two of these tetrapeptides, 4-phenylbutyryl- and n-pentadecanoyl-His-D-Phe-Arg-Trp-NH2, proved to be 100 fold more potent than the full-length physiological hormone α-MSH in activating the MC1R, based on activating cAMP formation, and stimulation of tyrosinase activity, and surpassed α-MSH in enhancing the repair of DNA photoproducts and reducing ROS generation and apoptosis in UVR-irradiated melanocytes. These effects were mediated by activation of the MC1R, and were not evident in melanocytes expressing LOF MC1R (Abdel-Malek et al., 2006). Further testing of n-pentadecanoyl-His-D-Phe-Arg-Trp-NH2 showed that it is efficacious in stimulating pigmentation, and repairing UVR-induced CPDs in a 3D-skin model. This analog also proved to be highly selective to MC1R, and to have the ability to diffuse through human skin, providing confidence that it can be employed in a strategy to protect from the mutagenic and carcinogenic effects of UVR. Tripeptide α-MSH analogs have also been developed, and found to retain full melanotropic activity, being only 10 fold less potent that α-MSH (Abdel-Malek et al., 2009). This can be compensated for by the low molecular weight of these peptides that should allow for using then at a high concentration in the micromolar range. The significance of targeting the MC1R on melanocytes by selective MC analogs lies in their efficacy in protecting individuals with high risk to skin cancer, including melanoma, from the carcinogenic effects of UVR. Such individuals include those heterozygous for a RHC allele, or harboring mutations in known melanoma-associated genes, such as p16.
The identification of MC1R alleles strongly associated with the RHC phenotype and with increased skin cancer risk has fuelled research on the functional properties of WT and variant MC1R, as well as on downstream targets involved in the regulation of pigmentary and non-pigmentary mechanisms to deal with UVR-induced damage. These studies are urgent given the importance of MC1R signaling in prevention of UV-induced skin cancers, mainly melanoma. The signaling properties of the various intra- and intergenic splice forms of the MC1R gene need further analysis to fully understand the physiological output of changes in their relative levels of expression. Our current knowledge of the MC1R life cycle still has important gaps, notably concerning the site and the molecular machinery of its proteolytic degradation. Given that recent research underlined important differences in the behavior of mouse MC1R compared with human MC1R, several current paradigms call for reevaluation. In this respect the functional coupling to the ERK pathway needs further research, since the molecular event(s) leading to transactivation of the cKIT receptor, the differential regulation of signaling to ERKs or cAMP, and the physiological consequences of ERK activation downstream of MC1R remain poorly understood. Efforts will also be made to fully understand the molecular basis of the different penetrance of r versus R alleles, and to find functional criteria to classify rare alleles in one of these two categories. An immediate task will be to define the possible role of MC1R as the hub of a signaling complex undergoing dynamic changes. For instance, receptor-bound ARRBs are expected to act as scaffolds orchestrating the formation of signaling complexes, whose precise composition will depend on the ARRB isoform linked to the MC1R. Moreover, there is some evidence that the specific partners of MC1R in multiprotein complexes might be regulated by UVR as well as by the MC1R genotype (Cao et al., 2013). Importantly, new downstream targets of MC1R signaling with a significant effect on pigmentation and cellular metabolism have been recently identified, including PPARγ coactivator-1α (Ronai, 2013).The involvement of this and other possible newcomers on key physiological processes including the responses to UVR will be dissected. Overall, the study of the MC1R and its many natural variants will continue to provide a model of genotype-phenotype associations, an understanding of the basis of normal human pigment variation, and of the interplay of genetic and environmental factors in carcinogenesis.
Supplementary Material
Acknowledgements
This work is partially supported by grant SAF2012-32134 from the Mineco (Spain) and FEDER (European Community) for JCGB and CJC, and by NIH grants R01 ES 009110, R01 CA114095 and R01 ES017561 for ZAM. The authors are grateful to C Olivares Sánchez, M Abrisqueta and J Sirés for support and critically reading the manuscript. We apologize to colleagues whose relevant work could not be cited due to space limitations.
Abbreviations
- AC
adenylyl cyclase
- ACTH
adrenocorticotropic hormone
- ARRB
β-arrestin
- ASIP
agouti signaling protein
- CBD103
dog β-defensin-3
- CPD
cyclobutane pyrimidine dimer
- CREB
cAMP responsive-element binding protein
- EDN1
endothelin 1
- Endo H
endoglycosidase H
- ER
endoplasmic reticulum
- ERK
extracellular signal-regulated protein kinase
- FSK
forskolin
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- HBD3
human β-defensin-3
- il
intracellular loop
- LOF
loss-of-function
- MAPK
mitogen-activated protein kinase
- MC
melanocortin
- MCR
melanocortin receptor
- MITF
Microphthalmia transcription factor
- MSH
melanocyte-stimulating hormone
- NHM
normal human melanocyte
- ORF
open reading frame
- PDE
phosphodiesterase
- POMC
proopiomelanocortin
- RHC
red hair color
- ROS
reactive oxygen species
- RTK
receptor tyrosine kinase
- TRP
tyrosinase related protein
- UTR
untranslated region
- UV
ultraviolet
- UVR
ultraviolet radiation
- WT
wildtype.
References
- 1.Abdel-Malek Z, Swope VB, Suzuki I, Akcali C, Harriger MD, Boyce ST, Urabe K, Hearing VJ. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc. Natl. Acad. Sci. U. S. A. 1995;92:1789–1793. doi: 10.1073/pnas.92.5.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdel-Malek ZA, Kadekaro AL, Kavanagh RJ, et al. Melanoma prevention strategy based on using tetrapeptide alpha-MSH analogs that protect human melanocytes from UV-induced DNA damage and cytotoxicity. FASEB J. 2006;20:1561–1563. doi: 10.1096/fj.05-5655fje. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Malek ZA, Ruwe A, Kavanagh-Starner R, Kadekaro AL, Swope V, Haskell-Luevano C, Koikov L, Knittel JJ. alpha-MSH tripeptide analogs activate the melanocortin 1 receptor and reduce UV-induced DNA damage in human melanocytes. Pigment Cell Melanoma Res. 2009;22:635–644. doi: 10.1111/j.1755-148X.2009.00598.x. [DOI] [PubMed] [Google Scholar]
- 4.Aberdam E, Bertolotto C, Sviderskaya EV, de TV, Hemesath TJ, Fisher DE, Bennett DC, Ortonne JP, Ballotti R. Involvement of microphthalmia in the inhibition of melanocyte lineage differentiation and of melanogenesis by agouti signal protein. J. Biol. Chem. 1998;273:19560–19565. doi: 10.1074/jbc.273.31.19560. [DOI] [PubMed] [Google Scholar]
- 5.Abrisqueta M, Herraiz C, Perez Oliva AB, Sanchez-Laorden BL, Olivares C, Jimenez-Cervantes C, Garcia-Borron JC. Differential and competitive regulation of human melanocortin 1 receptor signaling by beta-arrestin isoforms. J. Cell Sci. 2013;126:3724–3737. doi: 10.1242/jcs.128322. [DOI] [PubMed] [Google Scholar]
- 6.Ainger SA, Wong SS, Roberts DW, Leonard JH, Sturm RA. Effect of MC1R variant allele status on MSH-ligand induction of dopachrome tautomerase in melanocytes co-cultured with keratinocytes. Exp Dermatol. 2011;20:681–684. doi: 10.1111/j.1600-0625.2011.01293.x. [DOI] [PubMed] [Google Scholar]
- 7.Aoki H, Moro O. Involvement of microphthalmia-associated transcription factor (MITF) in expression of human melanocortin-1 receptor (MC1R) Life Sci. 2002;71:2171–2179. doi: 10.1016/s0024-3205(02)01996-3. [DOI] [PubMed] [Google Scholar]
- 8.Audet M, Bouvier M. Restructuring G-protein-coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Baig AH, Swords FM, Szaszak M, King PJ, Hunyady L, Clark AJ. Agonist activated adrenocorticotropin receptor internalizes via a clathrin-mediated G protein receptor kinase dependent mechanism. Endocr. Res. 2002;28:281–289. doi: 10.1081/erc-120016798. [DOI] [PubMed] [Google Scholar]
- 10.Bause E. Structural requirements of N-glycosylation of proteins. Studies with proline peptides as conformational probes. Biochem. J. 1983;209:331–336. doi: 10.1042/bj2090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaumont KA, Newton RA, Smit DJ, Leonard JH, Stow JL, Sturm RA. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum. Mol. Genet. 2005;14:2145–2154. doi: 10.1093/hmg/ddi219. [DOI] [PubMed] [Google Scholar]
- 12.Beaumont KA, Shekar SN, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum. Mol. Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- 13.Beaumont KA, Smit DJ, Liu YY, Chai E, Patel MP, Millhauser GL, Smith JJ, Alewood PF, Sturm RA. Melanocortin-1 receptor-mediated signalling pathways activated by NDP-MSH and HBD3 ligands. Pigment Cell Melanoma Res. 2012;25:370–374. doi: 10.1111/j.1755-148X.2012.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaumont KA, Wong SS, Ainger SA, et al. Melanocortin MC(1) receptor in human genetics and model systems. Eur. J. Pharmacol. 2011;660:103–110. doi: 10.1016/j.ejphar.2010.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellei B, Maresca V, Flori E, Pitisci A, Larue L, Picardo M. p38 regulates pigmentation via proteasomal degradation of tyrosinase. J. Biol. Chem. 2010;285:7288–7299. doi: 10.1074/jbc.M109.070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellei B, Pitisci A, Izzo E, Picardo M. Inhibition of melanogenesis by the pyridinyl imidazole class of compounds: possible involvement of the Wnt/beta-catenin signaling pathway. PLoS. One. 2012;7:e33021-. doi: 10.1371/journal.pone.0033021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benned-Jensen T, Mokrosinski J, Rosenkilde MM. The E92K melanocortin 1 receptor mutant induces cAMP production and arrestin recruitment but not ERK activity indicating biased constitutive signaling. PLoS. One. 2011;6:e24644-. doi: 10.1371/journal.pone.0024644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertolotto C, Abbe P, Hemesath TJ, Bille K, Fisher DE, Ortonne JP, Ballotti R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998;142:827–835. doi: 10.1083/jcb.142.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bicknell AB. The tissue-specific processing of pro-opiomelanocortin. J. Neuroendocrinol. 2008;20:692–699. doi: 10.1111/j.1365-2826.2008.01709.x. [DOI] [PubMed] [Google Scholar]
- 20.Biebermann H, Krude H, Elsner A, Chubanov V, Gudermann T, Gruters A. Autosomal-dominant mode of inheritance of a melanocortin-4 receptor mutation in a patient with severe early-onset obesity is due to a dominant-negative effect caused by receptor dimerization. Diabetes. 2003;52:2984–2988. doi: 10.2337/diabetes.52.12.2984. [DOI] [PubMed] [Google Scholar]
- 21.Bohm M, Luger TA, Tobin DJ, Garcia-Borron JC. Melanocortin receptor ligands: new horizons for skin biology and clinical dermatology. J. Invest Dermatol. 2006;126:1966–1975. doi: 10.1038/sj.jid.5700421. [DOI] [PubMed] [Google Scholar]
- 22.Bohm M, Moellmann G, Cheng E, Alvarez-Franco M, Wagner S, Sassone-Corsi P, Halaban R. Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differ. 1995;6:291–302. [PubMed] [Google Scholar]
- 23.Bohm M, Wolff I, Scholzen TE, Robinson SJ, Healy E, Luger TA, Schwarz T, Schwarz A. alpha-Melanocyte-stimulating hormone protects from ultraviolet radiation-induced apoptosis and DNA damage. J. Biol. Chem. 2005;280:5795–5802. doi: 10.1074/jbc.M406334200. [DOI] [PubMed] [Google Scholar]
- 24.Bolognia J, Murray M, Pawelek J. UVB-induced melanogenesis may be mediated through the MSH-receptor system. J. Invest Dermatol. 1989;92:651–656. doi: 10.1111/1523-1747.ep12696836. [DOI] [PubMed] [Google Scholar]
- 25.Bonilla C, Boxill LA, Donald SA, et al. The 8818G allele of the agouti signaling protein (ASIP) gene is ancestral and is associated with darker skin color in African Americans. Hum. Genet. 2005;116:402–406. doi: 10.1007/s00439-004-1251-2. [DOI] [PubMed] [Google Scholar]
- 26.Box NF, Duffy DL, Chen W, Stark M, Martin NG, Sturm RA, Hayward NK. MC1R genotype modifies risk of melanoma in families segregating CDKN2A mutations. Am. J. Hum. Genet. 2001;69:765–773. doi: 10.1086/323412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum. Mol. Genet. 1997;6:1891–1897. doi: 10.1093/hmg/6.11.1891. [DOI] [PubMed] [Google Scholar]
- 28.Breit A, Wolff K, Kalwa H, Jarry H, Buch T, Gudermann T. The natural inverse agonist agouti-related protein induces arrestin-mediated endocytosis of melanocortin-3 and -4 receptors. J. Biol. Chem. 2006;281:37447–37456. doi: 10.1074/jbc.M605982200. [DOI] [PubMed] [Google Scholar]
- 29.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busca R, Abbe P, Mantoux F, Aberdam E, Peyssonnaux C, Eychene A, Ortonne JP, Ballotti R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000;19:2900–2910. doi: 10.1093/emboj/19.12.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 32.Candille SI, Kaelin CB, Cattanach BM, et al. A -defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao J, Wan L, Hacker E, et al. MC1R is a potent regulator of PTEN after UV exposure in melanocytes. Mol. Cell. 2013;51:409–422. doi: 10.1016/j.molcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carreira S, Goodall J, Aksan I, La Rocca SA, Galibert MD, Denat L, Larue L, Goding CR. Mitf cooperates with Rb1 and activates p21Cip1 expression to regulate cell cycle progression. Nature. 2005;433:764–769. doi: 10.1038/nature03269. [DOI] [PubMed] [Google Scholar]
- 35.Carreira S, Goodall J, Denat L, Rodriguez M, Nuciforo P, Hoek KS, Testori A, Larue L, Goding CR. Mitf regulation of Dia1 controls melanoma proliferation and invasiveness. Genes Dev. 2006;20:3426–3439. doi: 10.1101/gad.406406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai BX, Neubig RR, Millhauser GL, et al. Inverse agonist activity of agouti and agouti-related protein. Peptides. 2003;24:603–609. doi: 10.1016/s0196-9781(03)00104-9. [DOI] [PubMed] [Google Scholar]
- 37.Chakraborty AK, Funasaka Y, Slominski A, Ermak G, Hwang J, Pawelek JM, Ichihashi M. Production and release of proopiomelanocortin (POMC) derived peptides by human melanocytes and keratinocytes in culture: regulation by ultraviolet B. Biochim. Biophys. Acta. 1996;1313:130–138. doi: 10.1016/0167-4889(96)00063-8. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty AK, Orlow SJ, Bolognia JL, Pawelek JM. Structural/functional relationships between internal and external MSH receptors: modulation of expression in Cloudman melanoma cells by UVB radiation. J. Cell Physiol. 1991;147:1–6. doi: 10.1002/jcp.1041470102. [DOI] [PubMed] [Google Scholar]
- 39.Chhajlani V, Wikberg JE. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA (FEBS 11553) FEBS Lett. 1996;390:238-. doi: 10.1016/0014-5793(96)81375-5. [DOI] [PubMed] [Google Scholar]
- 40.Chluba-de TJ, Bagutti C, Cotti R, Eberle AN. Induction of constitutive melanogenesis in amelanotic mouse melanoma cells by transfection of the human melanocortin-1 receptor gene. J. Cell Sci. 1996;(Pt 8):2023–2030. doi: 10.1242/jcs.109.8.2023. [DOI] [PubMed] [Google Scholar]
- 41.Cho DH, Bae CD, Juhnn YS. Multi-facet expressions of adenylate cyclase isoforms in B16-F10 melanoma cells differentiated by forskolin treatment. Exp. Mol. Med. 2000;32:235–242. doi: 10.1038/emm.2000.39. [DOI] [PubMed] [Google Scholar]
- 42.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 43.Cooper DM. Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 2003;375:517–529. doi: 10.1042/BJ20031061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corre S, Mekideche K, Adamski H, Mosser J, Watier E, Galibert MD. In vivo and ex vivo UV-induced analysis of pigmentation gene expressions. J. Invest Dermatol. 2006;126:916–918. doi: 10.1038/sj.jid.5700190. [DOI] [PubMed] [Google Scholar]
- 45.Corre S, Primot A, Baron Y, Le SJ, Goding C, Galibert MD. Target gene specificity of USF-1 is directed via p38-mediated phosphorylation-dependent acetylation. J. Biol. Chem. 2009;284:18851–18862. doi: 10.1074/jbc.M808605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corre S, Primot A, Sviderskaya E, Bennett DC, Vaulont S, Goding CR, Galibert MD. UV-induced expression of key component of the tanning process, the POMC and MC1R genes, is dependent on the p-38-activated upstream stimulating factor-1 (USF-1) J. Biol. Chem. 2004;279:51226–51233. doi: 10.1074/jbc.M409768200. [DOI] [PubMed] [Google Scholar]
- 47.Cui R, Widlund HR, Feige E, et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 48.d'Ischia M, Wakamatsu K, Napolitano A, et al. Melanins and melanogenesis: methods, standards, protocols. Pigment Cell Melanoma Res. 2013;26:616–633. doi: 10.1111/pcmr.12121. [DOI] [PubMed] [Google Scholar]
- 49.D'Orazio J, Fisher DE. Central role for cAMP signaling in pigmentation and UV resistance. Cell Cycle. 2011;10:8–9. doi: 10.4161/cc.10.1.14292. [DOI] [PubMed] [Google Scholar]
- 50.D'Orazio JA, Nobuhisa T, Cui R, et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature. 2006;443:340–344. doi: 10.1038/nature05098. [DOI] [PubMed] [Google Scholar]
- 51.Dalziel M, Kolesnichenko M, das Neves RP, Iborra F, Goding C, Furger A. Alpha-MSH regulates intergenic splicing of MC1R and TUBB3 in human melanocytes. Nucleic Acids Res. 2011;39:2378–2392. doi: 10.1093/nar/gkq1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dalziel M, Nunes NM, Furger A. Two G-rich regulatory elements located adjacent to and 440 nucleotides downstream of the core poly(A) site of the intronless melanocortin receptor 1 gene are critical for efficient 3' end processing. Mol. Cell. Biol. 2007;27:1568–1580. doi: 10.1128/MCB.01821-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies JR, Randerson-Moor J, Kukalizch K, et al. Inherited variants in the MC1R gene and survival from cutaneous melanoma: a BioGenoMEL study. Pigment Cell Melanoma Res. 2012;25:384–394. doi: 10.1111/j.1755-148X.2012.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeFea KA. Beta-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23:621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Delaney A, Keighren M, Fleetwood-Walker SM, Jackson IJ. Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin. PLoS. One. 2010;5:e12498-. doi: 10.1371/journal.pone.0012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demenais F, Mohamdi H, Chaudru V, et al. Association of MC1R variants and host phenotypes with melanoma risk in CDKN2A mutation carriers: a GenoMEL study. J. Natl. Cancer Inst. 2010;102:1568–1583. doi: 10.1093/jnci/djq363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dessinioti C, Antoniou C, Katsambas A, Stratigos AJ. Melanocortin 1 receptor variants: functional role and pigmentary associations. Photochem. Photobiol. 2011;87:978–987. doi: 10.1111/j.1751-1097.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 58.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 59.Doyle JR, Fortin JP, Beinborn M, Kopin AS. Selected melanocortin 1 receptor single-nucleotide polymorphisms differentially alter multiple signaling pathways. J. Pharmacol. Exp. Ther. 2012;342:318–326. doi: 10.1124/jpet.112.194548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duffy DL, Box NF, Chen W, Palmer JS, Montgomery GW, James MR, Hayward NK, Martin NG, Sturm RA. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum. Mol. Genet. 2004;13:447–461. doi: 10.1093/hmg/ddh043. [DOI] [PubMed] [Google Scholar]
- 61.Elfakir A, Ezzedine K, Latreille J, et al. Functional MC1R-gene variants are associated with increased risk for severe photoaging of facial skin. J. Invest Dermatol. 2010;130:1107–1115. doi: 10.1038/jid.2009.366. [DOI] [PubMed] [Google Scholar]
- 62.Fabrikant J, Touloei K, Brown SM. A review and update on melanocyte stimulating hormone therapy: afamelanotide. J. Drugs Dermatol. 2013;12:775–779. [PubMed] [Google Scholar]
- 63.Fargnoli MC, Gandini S, Peris K, Maisonneuve P, Raimondi S. MC1R variants increase melanoma risk in families with CDKN2A mutations: a meta-analysis. Eur. J. Cancer. 2010;46:1413–1420. doi: 10.1016/j.ejca.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 64.Fogarty GB, Muddle R, Sprung CN, Chen W, Duffy D, Sturm RA, McKay MJ. Unexpectedly severe acute radiotherapy side effects are associated with single 33 nucleotide polymorphisms of the melanocortin-1 receptor. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:1486–1492. doi: 10.1016/j.ijrobp.2009.07.1690. [DOI] [PubMed] [Google Scholar]
- 65.Frandberg PA, Doufexis M, Kapas S, Chhajlani V. Human pigmentation phenotype: a point mutation generates nonfunctional MSH receptor. Biochem. Biophys. Res. Commun. 1998;245:490–492. doi: 10.1006/bbrc.1998.8459. [DOI] [PubMed] [Google Scholar]
- 66.Frandberg PA, Doufexis M, Kapas S, Chhajlani V. Cysteine residues are involved in structure and function of melanocortin 1 receptor: Substitution of a cysteine residue in transmembrane segment two converts an agonist to antagonist. Biochem. Biophys. Res. Commun. 2001;281:851–857. doi: 10.1006/bbrc.2001.4429. [DOI] [PubMed] [Google Scholar]
- 67.Fuller BB, Meyskens FL., Jr Endocrine responsiveness in human melanocytes and melanoma cells in culture. J. Natl. Cancer Inst. 1981;66:799–802. [PubMed] [Google Scholar]
- 68.Funasaka Y, Chakraborty AK, Hayashi Y, Komoto M, Ohashi A, Nagahama M, Inoue Y, Pawelek J, Ichihashi M. Modulation of melanocyte-stimulating hormone receptor expression on normal human melanocytes: evidence for a regulatory role of ultraviolet B, interleukin-1alpha, interleukin-1beta, endothelin-1 and tumour necrosis factor-alpha. Br. J. Dermatol. 1998;139:216–224. doi: 10.1046/j.1365-2133.1998.02357.x. [DOI] [PubMed] [Google Scholar]
- 69.Galibert MD, Carreira S, Goding CR. The Usf-1 transcription factor is a novel target for the stress-responsive p38 kinase and mediates UV-induced Tyrosinase expression. EMBO J. 2001;20:5022–5031. doi: 10.1093/emboj/20.17.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galibert MD, Corre S. In vivo and in vitro tools to identify and study transcriptional regulation of USF-1 target genes. Methods Mol. Biol. 2010;647:339–355. doi: 10.1007/978-1-60761-738-9_21. [DOI] [PubMed] [Google Scholar]
- 71.Gao X, Simon KC, Han J, Schwarzschild MA, Ascherio A. Genetic determinants of hair color and Parkinson's disease risk. Ann. Neurol. 2009;65:76–82. doi: 10.1002/ana.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res. 2005;18:393–410. doi: 10.1111/j.1600-0749.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 73.Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum. Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- 74.Geschwind II. Change in hair color in mice induced by injection of alpha-MSH. Endocrinology. 1966;79:1165–1167. doi: 10.1210/endo-79-6-1165. [DOI] [PubMed] [Google Scholar]
- 75.Graham A, Wakamatsu K, Hunt G, Ito S, Thody AJ. Agouti protein inhibits the production of eumelanin and phaeomelanin in the presence and absence of alpha-melanocyte stimulating hormone. Pigment Cell Res. 1997;10:298–303. doi: 10.1111/j.1600-0749.1997.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 76.Guan X, Niu J, Liu Z, Wang LE, Amos CI, Lee JE, Gershenwald JE, Grimm EA, Wei Q. Variants in melanocortin 1 receptor gene contribute to risk of melanoma--a direct sequencing analysis in a Texas population. Pigment Cell Melanoma Res. 2013;26:422–425. doi: 10.1111/pcmr.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gudbjartsson DF, Sulem P, Stacey SN, et al. ASIP and TYR pigmentation variants associate with cutaneous melanoma and basal cell carcinoma. Nat. Genet. 2008;40:886–891. doi: 10.1038/ng.161. [DOI] [PubMed] [Google Scholar]
- 78.Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236-. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gurevich EV, Tesmer JJ, Mushegian A, Gurevich VV. G protein-coupled receptor kinases: more than just kinases and not only for GPCRs. Pharmacol. Ther. 2012;133:40–69. doi: 10.1016/j.pharmthera.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hacker E, Boyce Z, Kimlin MG, Wockner L, Pollak T, Vaartjes SA, Hayward NK, Whiteman DC. The effect of MC1R variants and sunscreen on the response of human melanocytes in vivo to ultraviolet radiation and implications for melanoma. Pigment Cell Melanoma Res. 2013;26:835–844. doi: 10.1111/pcmr.12157. [DOI] [PubMed] [Google Scholar]
- 81.Halaban R, Tyrrell L, Longley J, Yarden Y, Rubin J. Pigmentation and proliferation of human melanocytes and the effects of melanocyte-stimulating hormone and ultraviolet B light. Ann. N. Y. Acad. Sci. 1993;680:290–301. doi: 10.1111/j.1749-6632.1993.tb19691.x. [DOI] [PubMed] [Google Scholar]
- 82.Harding RM, Healy E, Ray AJ, et al. Evidence for variable selective pressures at MC1R. Am. J. Hum. Genet. 2000;66:1351–1361. doi: 10.1086/302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Healy E, Flannagan N, Ray A, Todd C, Jackson IJ, Matthews JN, Birch-Machin MA, Rees JL. Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet. 2000;355:1072–1073. doi: 10.1016/S0140-6736(00)02042-0. [DOI] [PubMed] [Google Scholar]
- 84.Healy E, Jordan SA, Budd PS, Suffolk R, Rees JL, Jackson IJ. Functional variation of MC1R alleles from red-haired individuals. Hum. Mol. Genet. 2001;10:2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- 85.Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- 86.Herraiz C, Jimenez-Cervantes C, Zanna P, Garcia-Borron JC. Melanocortin 1 receptor mutations impact differentially on signalling to the cAMP and the ERK mitogen-activated protein kinase pathways. FEBS Lett. 2009;583:3269–3274. doi: 10.1016/j.febslet.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 87.Herraiz C, Journe F, Abdel-Malek Z, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol. Endocrinol. 2011a;25:138–156. doi: 10.1210/me.2010-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herraiz C, Journe F, Ghanem G, Jimenez-Cervantes C, Garcia-Borron JC. Functional status and relationships of melanocortin 1 receptor signaling to the cAMP and extracellular signal-regulated protein kinases 1 and 2 pathways in human melanoma cells. Int. J. Biochem. Cell Biol. 2012;44:2244–2252. doi: 10.1016/j.biocel.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Herraiz C, Sanchez-Laorden BL, Jimenez-Cervantes C, Garcia-Borron JC. N-glycosylation of the human melanocortin 1 receptor: occupancy of glycosylation sequons and functional role. Pigment Cell Melanoma Res. 2011b;24:479–489. doi: 10.1111/j.1755-148X.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 90.Hida T, Wakamatsu K, Sviderskaya EV, et al. Agouti protein, mahogunin, and attractin in pheomelanogenesis and melanoblast-like alteration of melanocytes: a cAMP-independent pathway. Pigment Cell Melanoma Res. 2009;22:623–634. doi: 10.1111/j.1755-148X.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150:251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holst B, Schwartz TW. Molecular mechanism of agonism and inverse agonism in the melanocortin receptors: Zn(2+) as a structural and functional probe. Ann. N. Y. Acad. Sci. 2003;994:1–11. doi: 10.1111/j.1749-6632.2003.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 93.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Hunt G, Donatien PD, Lunec J, Todd C, Kyne S, Thody AJ. Cultured human melanocytes respond to MSH peptides and ACTH. Pigment Cell Res. 1994;7:217–221. doi: 10.1111/j.1600-0749.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 95.Hunt G, Kyne S, Ito S, Wakamatsu K, Todd C, Thody A. Eumelanin and phaeomelanin contents of human epidermis and cultured melanocytes. Pigment Cell Res. 1995;8:202–208. doi: 10.1111/j.1600-0749.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 96.Hunt G, Thody AJ. Agouti protein can act independently of melanocyte-stimulating hormone to inhibit melanogenesis. J. Endocrinol. 1995;147:R1–R4. doi: 10.1677/joe.0.147r001. [DOI] [PubMed] [Google Scholar]
- 97.Ichii-Jones F, Lear JT, Heagerty AH, et al. Susceptibility to melanoma: influence of skin type and polymorphism in the melanocyte stimulating hormone receptor gene. J. Invest Dermatol. 1998;111:218–221. doi: 10.1046/j.1523-1747.1998.00287.x. [DOI] [PubMed] [Google Scholar]
- 98.Ibarrola-Villava M, Peña-Chilet M, Llorca-Cardeñosa MJ, Oltra S, Cadenas CM, Bravo J, Ribas G. Modeling MC1R rare variants: a structural evaluation of variants detected in a Mediterranean case-control study. J. Invest Dermatol. 2014;134:1146–1149. doi: 10.1038/jid.2013.469. [DOI] [PubMed] [Google Scholar]
- 99.Im S, Moro O, Peng F, Medrano EE, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Activation of the cyclic AMP pathway by alpha-melanotropin mediates the response of human melanocytes to ultraviolet B radiation. Cancer Res. 1998;58:47–54. [PubMed] [Google Scholar]
- 100.Ito S, Wakamatsu K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: a comparative review. Pigment Cell Res. 2003;16:523–531. doi: 10.1034/j.1600-0749.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 101.Ito S, Wakamatsu K. Human hair melanins: what we have learned and have not learned from mouse coat color pigmentation. Pigment Cell Melanoma Res. 2011;24:63–74. doi: 10.1111/j.1755-148X.2010.00755.x. [DOI] [PubMed] [Google Scholar]
- 102.Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc. Natl. Acad. Sci. U. S. A. 2010;107(Suppl 2):8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jimenez-Cervantes C, Martinez-Esparza M, Perez C, Daum N, Solano F, Garcia-Borron JC. Inhibition of melanogenesis in response to oxidative stress: transient downregulation of melanocyte differentiation markers and possible involvement of microphthalmia transcription factor. J. Cell Sci. 2001a;114:2335–2344. doi: 10.1242/jcs.114.12.2335. [DOI] [PubMed] [Google Scholar]
- 104.Jimenez-Cervantes C, Olivares C, Gonzalez P, Morandini R, Ghanem G, Garcia-Borron JC. The Pro162 variant is a loss-of-function mutation of the human melanocortin 1 receptor gene. J. Invest Dermatol. 2001b;117:156–158. doi: 10.1046/j.0022-202x.2001.01393.x. [DOI] [PubMed] [Google Scholar]
- 105.Kadekaro AL, Chen J, Yang J, Chen S, Jameson J, Swope VB, Cheng T, Kadakia M, Abdel-Malek Z. Alpha-melanocyte-stimulating hormone suppresses oxidative stress through a p53-mediated signaling pathway in human melanocytes. Mol. Cancer Res. 2012;10:778–786. doi: 10.1158/1541-7786.MCR-11-0436. [DOI] [PubMed] [Google Scholar]
- 106.Kadekaro AL, Kavanagh R, Kanto H, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–4299. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 107.Kadekaro AL, Leachman S, Kavanagh RJ, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB. J. 2010;24:3850–3860. doi: 10.1096/fj.10-158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kane EV, Painter D, Roman E, Allan J, Law G, Lightfoot T. Melanocortin 1 receptor (MC1R), pigmentary characteristics and sun exposure: findings from a case-control study of diffuse large B-cell and follicular lymphoma. Cancer Epidemiol. 2010;34:136–141. doi: 10.1016/j.canep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Kanetsky PA, Rebbeck TR, Hummer AJ, et al. Population-based study of natural variation in the melanocortin-1 receptor gene and melanoma. Cancer Res. 2006;66:9330–9337. doi: 10.1158/0008-5472.CAN-06-1634. [DOI] [PubMed] [Google Scholar]
- 110.Kanetsky PA, Swoyer J, Panossian S, Holmes R, Guerry D, Rebbeck TR. A polymorphism in the agouti signaling protein gene is associated with human pigmentation. Am. J. Hum. Genet. 2002;70:770–775. doi: 10.1086/339076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013;53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kennedy C, ter HJ, Berkhout M, Gruis N, Bastiaens M, Bergman W, Willemze R, Bavinck JN. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J. Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 113.Khaled M, Levy C, Fisher DE. Control of melanocyte differentiation by a MITF-PDE4D3 homeostatic circuit. Genes Dev. 2010;24:2276–2281. doi: 10.1101/gad.1937710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khlgatian MK, Hadshiew IM, Asawanonda P, Yaar M, Eller MS, Fujita M, Norris DA, Gilchrest BA. Tyrosinase gene expression is regulated by p53. J. Invest Dermatol. 2002;118:126–132. doi: 10.1046/j.0022-202x.2001.01667.x. [DOI] [PubMed] [Google Scholar]
- 115.Kilianova Z, Basora N, Kilian P, Payet MD, Gallo-Payet N. Human melanocortin receptor 2 expression and functionality: effects of protein kinase A and protein kinase C on desensitization and internalization. Endocrinology. 2006;147:2325–2337. doi: 10.1210/en.2005-0991. [DOI] [PubMed] [Google Scholar]
- 116.Kinsler VA, Abu-Amero S, Budd P, et al. Germline melanocortin-1-receptor genotype is associated with severity of cutaneous phenotype in congenital melanocytic nevi: a role for MC1R in human fetal development. J. Invest Dermatol. 2012;132:2026–2032. doi: 10.1038/jid.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kokot A, Metze D, Mouchet N, Galibert MD, Schiller M, Luger TA, Bohm M. Alpha-melanocyte-stimulating hormone counteracts the suppressive effect of UVB on Nrf2 and Nrf-dependent gene expression in human skin. Endocrinology. 2009;150:3197–3206. doi: 10.1210/en.2008-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat. Genet. 2012;44:1006–1014. doi: 10.1038/ng.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 120.Lalueza-Fox C, Rompler H, Caramelli D, et al. A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science. 2007;318:1453–1455. doi: 10.1126/science.1147417. [DOI] [PubMed] [Google Scholar]
- 121.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–522. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 122.Landi MT, Kanetsky PA, Tsang S, et al. MC1R, ASIP, and DNA repair in sporadic and familial melanoma in a Mediterranean population. J. Natl. Cancer Inst. 2005;97:998–1007. doi: 10.1093/jnci/dji176. [DOI] [PubMed] [Google Scholar]
- 123.Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1802–1807. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Le Pape E, Wakamatsu K, Ito S, Wolber R, Hearing VJ. Regulation of eumelanin/pheomelanin synthesis and visible pigmentation in melanocytes by ligands of the melanocortin 1 receptor. Pigment Cell Melanoma Res. 2008;21:477–486. doi: 10.1111/j.1755-148X.2008.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lerner AB, McGuire JS. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- 126.Lerner AB, McGuire JS. Melanocyte-stimulating hormone and adrenocorticotrophic hormone. their relation to pigmentation. N. Engl. J. Med. 1964;270:539–546. doi: 10.1056/NEJM196403122701101. [DOI] [PubMed] [Google Scholar]
- 127.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 129.Lu D, Vage DI, Cone RD. A ligand-mimetic model for constitutive activation of the melanocortin-1 receptor. Mol. Endocrinol. 1998;12:592–604. doi: 10.1210/mend.12.4.0091. [DOI] [PubMed] [Google Scholar]
- 130.Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 131.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maccioni L, Rachakonda PS, Scherer D, Bermejo JL, Planelles D, Requena C, Hemminki K, Nagore E, Kumar R. Variants at chromosome 20 (ASIP locus) and melanoma risk. Int. J. Cancer. 2013;132:42–54. doi: 10.1002/ijc.27648. [DOI] [PubMed] [Google Scholar]
- 133.Mandrika I, Petrovska R, Wikberg J. Melanocortin receptors form constitutive homo- and heterodimers. Biochem. Biophys. Res. Commun. 2005;326:349–354. doi: 10.1016/j.bbrc.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 134.Maresca V, Flori E, Bellei B, Aspite N, Kovacs D, Picardo M. MC1R stimulation by alpha-MSH induces catalase and promotes its re-distribution to the cell periphery and dendrites. Pigment Cell Melanoma Res. 2010;23:263–275. doi: 10.1111/j.1755-148X.2010.00673.x. [DOI] [PubMed] [Google Scholar]
- 135.Markovic D. Alternative mRNA splicing of G protein-coupled receptors. Methods Enzymol. 2013;520:323–335. doi: 10.1016/B978-0-12-391861-1.00015-0. [DOI] [PubMed] [Google Scholar]
- 136.Markovic D, Challiss RA. Alternative splicing of G protein-coupled receptors: physiology and pathophysiology. Cell Mol. Life Sci. 2009;66:3337–3352. doi: 10.1007/s00018-009-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marquette A, Andre J, Bagot M, Bensussan A, Dumaz N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat. Struct. Mol. Biol. 2011;18:584–591. doi: 10.1038/nsmb.2022. [DOI] [PubMed] [Google Scholar]
- 138.Martinez-Cadenas C, Lopez S, Ribas G, et al. Simultaneous purifying selection on the ancestral MC1R allele and positive selection on the melanoma-risk allele V60L in south Europeans. Mol. Biol. Evol. 2013;30:2654–2665. doi: 10.1093/molbev/mst158. [DOI] [PubMed] [Google Scholar]
- 139.Martinez-Esparza M, Jimenez-Cervantes C, Beermann F, Aparicio P, Lozano JA, Garcia-Borron JC. Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1. J. Biol. Chem. 1997;272:3967–3972. doi: 10.1074/jbc.272.7.3967. [DOI] [PubMed] [Google Scholar]
- 140.Martinez-Esparza M, Jimenez-Cervantes C, Solano F, Lozano JA, Garcia-Borron JC. Mechanisms of melanogenesis inhibition by tumor necrosis factor-alpha in B16/F10 mouse melanoma cells. Eur. J. Biochem. 1998;255:139–146. doi: 10.1046/j.1432-1327.1998.2550139.x. [DOI] [PubMed] [Google Scholar]
- 141.Martinez-Esparza M, Solano F, Garcia-Borron JC. Independent regulation of tyrosinase by the hypopigmenting cytokines TGF beta1 and TNF alpha and the melanogenic hormone alpha-MSH in B16 mouse melanocytes. Cell Mol. Biol. (Noisy. -le-grand) 1999;45:991–1000. [PubMed] [Google Scholar]
- 142.Mas JS, Gerritsen I, Hahmann C, Jimenez-Cervantes C, Garcia-Borron JC. Rate limiting factors in melanocortin 1 receptor signalling through the cAMP pathway. Pigment Cell Res. 2003;16:540–547. doi: 10.1034/j.1600-0749.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 143.Meyskens FL, Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–154. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 144.Meziani R, Descamps V, Gerard B, et al. Association study of the g.8818A>G polymorphism of the human agouti gene with melanoma risk and pigmentary characteristics in a French population. J. Dermatol. Sci. 2005;40:133–136. doi: 10.1016/j.jdermsci.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 145.Miccadei S, Pascucci B, Picardo M, Natali PG, Civitareale D. Identification of the minimal melanocyte-specific promoter in the melanocortin receptor 1 gene. J. Exp. Clin. Cancer Res. 2008;27:71-. doi: 10.1186/1756-9966-27-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miller MW, Duhl DM, Vrieling H, Cordes SP, Ollmann MM, Winkes BM, Barsh GS. Cloning of the mouse agouti gene predicts a secreted protein ubiquitously expressed in mice carrying the lethal yellow mutation. Genes Dev. 1993;7:454–467. doi: 10.1101/gad.7.3.454. [DOI] [PubMed] [Google Scholar]
- 147.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 148.Moro O, Ideta R, Ifuku O. Characterization of the promoter region of the human melanocortin-1 receptor (MC1R) gene. Biochem. Biophys. Res. Commun. 1999;262:452–460. doi: 10.1006/bbrc.1999.1228. [DOI] [PubMed] [Google Scholar]
- 149.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 150.Muffley LA, Zhu KQ, Engrav LH, Gibran NS, Hocking AM. Spatial and temporal localization of the melanocortin 1 receptor and its ligand alpha-melanocyte-stimulating hormone during cutaneous wound repair. J. Histochem. Cytochem. 2011;59:278–288. doi: 10.1369/0022155410397999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murata T, Shimizu K, Watanabe Y, Morita H, Sekida M, Tagawa T. Expression and role of phosphodiesterase 5 in human malignant melanoma cell line. Anticancer Res. 2010;30:355–358. [PubMed] [Google Scholar]
- 152.Myles PS, Buchanan FF, Bain CR. The effect of hair colour on anaesthetic requirements and recovery time after surgery. Anaesth. Intensive Care. 2012;40:683–689. doi: 10.1177/0310057X1204000415. [DOI] [PubMed] [Google Scholar]
- 153.Nakayama K, Soemantri A, Jin F, et al. Identification of novel functional variants of the melanocortin 1 receptor gene originated from Asians. Hum. Genet. 2006;119:322–330. doi: 10.1007/s00439-006-0141-1. [DOI] [PubMed] [Google Scholar]
- 154.Narita M, Murata T, Shimizu K, Nakagawa T, Sugiyama T, Inui M, Hiramoto K, Tagawa T. A role for cyclic nucleotide phosphodiesterase 4 in regulation of the growth of human malignant melanoma cells. Oncol. Rep. 2007;17:1133–1139. [PubMed] [Google Scholar]
- 155.Newton RA, Roberts DW, Leonard JH, Sturm RA. Human melanocytes expressing MC1R variant alleles show impaired activation of multiple signaling pathways. Peptides. 2007;28:2387–2396. doi: 10.1016/j.peptides.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 156.Newton RA, Smit SE, Barnes CC, Pedley J, Parsons PG, Sturm RA. Activation of the cAMP pathway by variant human MC1R alleles expressed in HEK and in melanoma cells. Peptides. 2005;26:1818–1824. doi: 10.1016/j.peptides.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 157.Nix MA, Kaelin CB, Ta T, Weis A, Morton GJ, Barsh GS, Millhauser GL. Molecular and functional analysis of human beta-defensin 3 action at melanocortin receptors. Chem. Biol. 2013;20:784–795. doi: 10.1016/j.chembiol.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nyan DC, Anbazhagan R, Hughes-Darden CA, Wachira SJ. Endosomal colocalization of melanocortin-3 receptor and beta-arrestins in CAD cells with altered modification of AKT/PKB. Neuropeptides. 2008;42:355–366. doi: 10.1016/j.npep.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 159.Oertel B, Lotsch J. Genetic mutations that prevent pain: implications for future pain medication. Pharmacogenomics. 2008;9:179–194. doi: 10.2217/14622416.9.2.179. [DOI] [PubMed] [Google Scholar]
- 160.Oiso N, Kishida K, Fukai K, Motokawa T, Hosomi N, Suzuki T, Mitsuhashi Y, Tsuboi R, Kawada A. A Japanese piebald patient with auburn hair colour associated with a novel mutation p.P832L in the KIT gene and a homozygous variant p.I120T in the MC1R gene. Br. J. Dermatol. 2009;161:468–469. doi: 10.1111/j.1365-2133.2009.09138.x. [DOI] [PubMed] [Google Scholar]
- 161.Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998;12:316–330. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palmer JS, Duffy DL, Box NF, Aitken JF, O'Gorman LE, Green AC, Hayward NK, Martin NG, Sturm RA. Melanocortin-1 receptor polymorphisms and risk of melanoma: is the association explained solely by pigmentation phenotype? Am. J. Hum. Genet. 2000;66:176–186. doi: 10.1086/302711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Park HY, Russakovsky V, Ao Y, Fernandez E, Gilchrest BA. Alpha-melanocyte stimulating hormone-induced pigmentation is blocked by depletion of protein kinase C. Exp. Cell Res. 1996;227:70–79. doi: 10.1006/excr.1996.0251. [DOI] [PubMed] [Google Scholar]
- 164.Patel MP, Cribb Fabersunne CS, Yang YK, Kaelin CB, Barsh GS, Millhauser GL. Loop-swapped chimeras of the agouti-related protein and the agouti signaling protein identify contacts required for melanocortin 1 receptor selectivity and antagonism. J. Mol. Biol. 2010;404:45–55. doi: 10.1016/j.jmb.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pawelek J, Wong G, Sansone M, Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J. Biol. Med. 1973;46:430–443. [PMC free article] [PubMed] [Google Scholar]
- 166.Perez Oliva AB, Fernendez LP, Detorre C, et al. Identification and functional analysis of novel variants of the human melanocortin 1 receptor found in melanoma patients. Hum. Mutat. 2009 doi: 10.1002/humu.20971. [DOI] [PubMed] [Google Scholar]
- 167.Piechowski CL, Rediger A, Lagemann C, et al. Inhibition of melanocortin-4 receptor dimerization by substitutions in intracellular loop 2. J. Mol. Endocrinol. 2013;51:109–118. doi: 10.1530/JME-13-0061. [DOI] [PubMed] [Google Scholar]
- 168.Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat. Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 169.Price ER, Horstmann MA, Wells AG, Weilbaecher KN, Takemoto CM, Landis MW, Fisher DE. alpha-Melanocyte-stimulating hormone signaling regulates expression of microphthalmia, a gene deficient in Waardenburg syndrome. J. Biol. Chem. 1998;273:33042–33047. doi: 10.1074/jbc.273.49.33042. [DOI] [PubMed] [Google Scholar]
- 170.Pucci M, Pasquariello N, Battista N, et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012;287:15466–15478. doi: 10.1074/jbc.M111.314880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qanbar R, Bouvier M. Role of palmitoylation/depalmitoylation reactions in G-protein-coupled receptor function. Pharmacol. Ther. 2003;97:1–33. doi: 10.1016/s0163-7258(02)00300-5. [DOI] [PubMed] [Google Scholar]
- 172.Rana BK, Hewett-Emmett D, Jin L, et al. High polymorphism at the human melanocortin 1 receptor locus. Genetics. 1999;151:1547–1557. doi: 10.1093/genetics/151.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rees JL. The genetics of sun sensitivity in humans. Am. J. Hum. Genet. 2004;75:739–751. doi: 10.1086/425285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ringholm A, Klovins J, Rudzish R, Phillips S, Rees JL, Schioth HB. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J. Invest Dermatol. 2004;123:917–923. doi: 10.1111/j.0022-202X.2004.23444.x. [DOI] [PubMed] [Google Scholar]
- 175.Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 176.Roberts DW, Newton RA, Beaumont KA, Helen LJ, Sturm RA. Quantitative analysis of MC1R gene expression in human skin cell cultures. Pigment Cell Res. 2006;19:76–89. doi: 10.1111/j.1600-0749.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- 177.Roberts DW, Newton RA, Leonard JH, Sturm RA. Melanocytes expressing MC1R polymorphisms associated with red hair color have altered MSH-ligand activated pigmentary responses in coculture with keratinocytes. J. Cell Physiol. 2008;215:344–355. doi: 10.1002/jcp.21318. [DOI] [PubMed] [Google Scholar]
- 178.Roberts DW, Newton RA, Sturm RA. MC1R expression in skin: is it confined to melanocytes? J. Invest Dermatol. 2007;127:2472–2473. doi: 10.1038/sj.jid.5700881. [DOI] [PubMed] [Google Scholar]
- 179.Rodrigues AR, Almeida H, Gouveia AM. Melanocortin 5 receptor signaling and internalization: role of MAPK/ERK pathway and beta-arrestins 1/2. Mol. Cell Endocrinol. 2012;361:69–79. doi: 10.1016/j.mce.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 180.Rodrigues AR, Sousa D, Almeida H, Gouveia AM. Structural determinants regulating cell surface targeting of melanocortin receptors. J. Mol. Endocrinol. 2013;51:R23–R32. doi: 10.1530/JME-13-0055. [DOI] [PubMed] [Google Scholar]
- 181.Ronai Z. The masters talk: the PGC-1alpha-MITF axis as a melanoma energizer. Pigment Cell Melanoma Res. 2013 doi: 10.1111/pcmr.12090. [DOI] [PubMed] [Google Scholar]
- 182.Rouzaud F, Annereau JP, Valencia JC, Costin GE, Hearing VJ. Regulation of melanocortin 1 receptor expression at the mRNA and protein levels by its natural agonist and antagonist. FASEB J. 2003;17:2154–2156. doi: 10.1096/fj.03-0206fje. [DOI] [PubMed] [Google Scholar]
- 183.Rouzaud F, Costin GE, Yamaguchi Y, et al. Regulation of constitutive and UVR-induced skin pigmentation by melanocortin 1 receptor isoforms. FASEB J. 2006;20:1927–1929. doi: 10.1096/fj.06-5922fje. [DOI] [PubMed] [Google Scholar]
- 184.Roy S, Perron B, Gallo-Payet N. Role of asparagine-linked glycosylation in cell surface expression and function of the human adrenocorticotropin receptor (melanocortin 2 receptor) in 293/FRT cells. Endocrinology. 2010;151:660–670. doi: 10.1210/en.2009-0826. [DOI] [PubMed] [Google Scholar]
- 185.Roy S, Pinard S, Chouinard L, Gallo-Payet N. Adrenocorticotropin hormone (ACTH) effects on MAPK phosphorylation in human fasciculata cells and in embryonic kidney 293 cells expressing human melanocortin 2 receptor (MC2R) and MC2R accessory protein (MRAP)beta. Mol. Cell Endocrinol. 2011a;336:31–40. doi: 10.1016/j.mce.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 186.Roy S, Roy SJ, Pinard S, Taillefer LD, Rached M, Parent JL, Gallo-Payet N. Mechanisms of melanocortin-2 receptor (MC2R) internalization and recycling in human embryonic kidney (hek) cells: identification of Key Ser/Thr (S/T) amino acids. Mol. Endocrinol. 2011b;25:1961–1977. doi: 10.1210/me.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Saha B, Singh SK, Sarkar C, Bera R, Ratha J, Tobin DJ, Bhadra R. Activation of the Mitf promoter by lipid-stimulated activation of p38-stress signalling to CREB. Pigment Cell Res. 2006;19:595–605. doi: 10.1111/j.1600-0749.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 188.Sakai C, Ollmann M, Kobayashi T, Abdel-Malek Z, Muller J, Vieira WD, Imokawa G, Barsh GS, Hearing VJ. Modulation of murine melanocyte function in vitro by agouti signal protein. EMBO J. 1997;16:3544–3552. doi: 10.1093/emboj/16.12.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Salazar-Onfray F, Lopez M, Lundqvist A, et al. Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Br. J. Cancer. 2002;87:414–422. doi: 10.1038/sj.bjc.6600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Saleha SB, Ajaml M, Jamil M, Nasir M, Hameed A. MC1R gene mutation and its association with oculocutaneous albinism type (OCA) phenotype in a consanguineous Pakistani family. J. Dermatol. Sci. 2013;70:68–70. doi: 10.1016/j.jdermsci.2012.11.591. [DOI] [PubMed] [Google Scholar]
- 191.Samuels ME, Gallo-Payet N, Pinard S, et al. Bioinactive ACTH causing glucocorticoid deficiency. J. Clin. Endocrinol. Metab. 2013;98:736–742. doi: 10.1210/jc.2012-3199. [DOI] [PubMed] [Google Scholar]
- 192.Sanchez MJ, Olivares SC, Ghanem G, Haycock J, Lozano Teruel JA, Garcia-Borron JC, Jimenez-Cervantes C. Loss-of-function variants of the human melanocortin-1 receptor gene in melanoma cells define structural determinants of receptor function. Eur. J. Biochem. 2002;269:6133–6141. doi: 10.1046/j.1432-1033.2002.03329.x. [DOI] [PubMed] [Google Scholar]
- 193.Sanchez-Laorden BL, Herraiz C, Valencia JC, Hearing VJ, Jimenez-Cervantes C, Garcia-Borron JC. Aberrant trafficking of human melanocortin 1 receptor variants associated with red hair and skin cancer: Steady-state retention of mutant forms in the proximal golgi. J. Cell Physiol. 2009;220:640–654. doi: 10.1002/jcp.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sanchez-Laorden BL, Jimenez-Cervantes C, Garcia-Borron JC. Regulation of human melanocortin 1 receptor signaling and trafficking by Thr-308 and Ser-316 and its alteration in variant alleles associated with red hair and skin cancer. J. Biol. Chem. 2007;282:3241–3251. doi: 10.1074/jbc.M606865200. [DOI] [PubMed] [Google Scholar]
- 195.Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, Martinez-Menarguez JA, Garcia-Borron JC, Jimenez-Cervantes C. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J. Invest Dermatol. 2006a;126:172–181. doi: 10.1038/sj.jid.5700036. [DOI] [PubMed] [Google Scholar]
- 196.Sanchez-Laorden BL, Sanchez-Mas J, Turpin MC, Garcia-Borron JC, Jimenez-Cervantes C. Variant amino acids in different domains of the human melanocortin 1 receptor impair cell surface expression. Cell Mol. Biol. (Noisy. -le-grand) 2006b;52:39–46. [PubMed] [Google Scholar]
- 197.Sanchez-Mas J, Guillo LA, Zanna P, Jimenez-Cervantes C, Garcia-Borron JC. Role of G protein-coupled receptor kinases in the homologous desensitization of the human and mouse melanocortin 1 receptors. Mol. Endocrinol. 2005a;19:1035–1048. doi: 10.1210/me.2004-0227. [DOI] [PubMed] [Google Scholar]
- 198.Sanchez-Mas J, Hahmann C, Gerritsen I, Garcia-Borron JC, Jimenez-Cervantes C. Agonist-independent, high constitutive activity of the human melanocortin 1 receptor. Pigment Cell Res. 2004;17:386–395. doi: 10.1111/j.1600-0749.2004.00160.x. [DOI] [PubMed] [Google Scholar]
- 199.Sanchez-Mas J, Sanchez-Laorden BL, Guillo LA, Jimenez-Cervantes C, Garcia-Borron JC. The melanocortin-1 receptor carboxyl terminal pentapeptide is essential for MC1R function and expression on the cell surface. Peptides. 2005b;26:1848–1857. doi: 10.1016/j.peptides.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 200.Savage SA, Gerstenblith MR, Goldstein AM, Mirabello L, Fargnoli MC, Peris K, Landi MT. Nucleotide diversity and population differentiation of the melanocortin 1 receptor gene, MC1R. BMC. Genet. 2008;9:31-. doi: 10.1186/1471-2156-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sawyer TK, Hruby VJ, Wilkes BC, Draelos MT, Hadley ME, Bergsneider M. Comparative biological activities of highly potent active-site analogues of alpha-melanotropin. J. Med. Chem. 1982;25:1022–1027. doi: 10.1021/jm00351a004. [DOI] [PubMed] [Google Scholar]
- 202.Scherer D, Kumar R. Genetics of pigmentation in skin cancer--a review. Mutat. Res. 2010;705:141–153. doi: 10.1016/j.mrrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 203.Schioth HB, Phillips SR, Rudzish R, Birch-Machin MA, Wikberg JE, Rees JL. Loss of function mutations of the human melanocortin 1 receptor are common and are associated with red hair. Biochem. Biophys. Res. Commun. 1999;260:488–491. doi: 10.1006/bbrc.1999.0935. [DOI] [PubMed] [Google Scholar]
- 204.Scott MC, Suzuki I, Abdel-Malek ZA. Regulation of the human melanocortin 1 receptor expression in epidermal melanocytes by paracrine and endocrine factors and by ultraviolet radiation. Pigment Cell Res. 2002a;15:433–439. doi: 10.1034/j.1600-0749.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- 205.Scott MC, Wakamatsu K, Ito S, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J. Cell Sci. 2002b;115:2349–2355. doi: 10.1242/jcs.115.11.2349. [DOI] [PubMed] [Google Scholar]
- 206.Sebag JA, Hinkle PM. Opposite effects of the melanocortin-2 (MC2) receptor accessory protein MRAP on MC2 and MC5 receptor dimerization and trafficking. J. Biol. Chem. 2009;284:22641–22648. doi: 10.1074/jbc.M109.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shinyama H, Masuzaki H, Fang H, Flier JS. Regulation of melanocortin-4 receptor signaling: agonist-mediated desensitization and internalization. Endocrinology. 2003;144:1301–1314. doi: 10.1210/en.2002-220931. [DOI] [PubMed] [Google Scholar]
- 208.Siegrist W, Drozdz R, Cotti R, Willard DH, Wilkison WO, Eberle AN. Interactions of alpha-melanotropin and agouti on B16 melanoma cells: evidence for inverse agonism of agouti. J. Recept. Signal. Transduct. Res. 1997;17:75–98. doi: 10.3109/10799899709036595. [DOI] [PubMed] [Google Scholar]
- 209.Siegrist W, Stutz S, Eberle AN. Homologous and heterologous regulation of alpha-melanocyte-stimulating hormone receptors in human and mouse melanoma cell lines. Cancer Res. 1994;54:2604–2610. [PubMed] [Google Scholar]
- 210.Singh SK, Abbas WA, Tobin DJ. Bone morphogenetic proteins differentially regulate pigmentation in human skin cells. J. Cell Sci. 2012;125:4306–4319. doi: 10.1242/jcs.102038. [DOI] [PubMed] [Google Scholar]
- 211.Singh SK, Sarkar C, Mallick S, Saha B, Bera R, Bhadra R. Human placental lipid induces melanogenesis through p38 MAPK in B16F10 mouse melanoma. Pigment Cell Res. 2005;18:113–121. doi: 10.1111/j.1600-0749.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 212.Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–1253. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 214.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 215.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Mol. Cell Endocrinol. 2007:265–266. 143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smalley K, Eisen T. The involvement of p38 mitogen-activated protein kinase in the alpha-melanocyte stimulating hormone (alpha-MSH)-induced melanogenic and antiproliferative effects in B16 murine melanoma cells. FEBS Lett. 2000;476:198–202. doi: 10.1016/s0014-5793(00)01726-9. [DOI] [PubMed] [Google Scholar]
- 217.Smalley KS, Eisen TG. Differentiation of human melanoma cells through p38 MAP kinase is associated with decreased retinoblastoma protein phosphorylation and cell cycle arrest. Melanoma Res. 2002;12:187–192. doi: 10.1097/00008390-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 218.Smith AG, Box NF, Marks LH, Chen W, Smit DJ, Wyeth JR, Huttley GA, Easteal S, Sturm RA. The human melanocortin-1 receptor locus: analysis of transcription unit, locus polymorphism and haplotype evolution. Gene. 2001;281:81–94. doi: 10.1016/s0378-1119(01)00791-0. [DOI] [PubMed] [Google Scholar]
- 219.Smith AG, Luk N, Newton RA, Roberts DW, Sturm RA, Muscat GE. Melanocortin-1 receptor signaling markedly induces the expression of the NR4A nuclear receptor subgroup in melanocytic cells. J. Biol. Chem. 2008;283:12564–12570. doi: 10.1074/jbc.M800480200. [DOI] [PubMed] [Google Scholar]
- 220.Smith R, Healy E, Siddiqui S, et al. Melanocortin 1 receptor variants in an Irish population. J. Invest Dermatol. 1998;111:119–122. doi: 10.1046/j.1523-1747.1998.00252.x. [DOI] [PubMed] [Google Scholar]
- 221.Song X, Mosby N, Yang J, Xu A, Abdel-Malek Z, Kadekaro AL. alpha-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment Cell Melanoma Res. 2009;22:809–818. doi: 10.1111/j.1755-148X.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 222.Stefanaki I, Panagiotou OA, Kodela E, et al. Replication and predictive value of SNPs associated with melanoma and pigmentation traits in a Southern European case-control study. PLoS. One. 2013;8:e55712-. doi: 10.1371/journal.pone.0055712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Strange RC, Ramachandran S, Zeegers MP, Emes RD, Abraham R, Raveendran V, Boggild M, Gilford J, Hawkins CP. The Multiple Sclerosis Severity Score: associations with MC1R single nucleotide polymorphisms and host response to ultraviolet radiation. Mult. Scler. 2010;16:1109–1116. doi: 10.1177/1352458510373784. [DOI] [PubMed] [Google Scholar]
- 224.Stratigos AJ, Dimisianos G, Nikolaou V, et al. Melanocortin receptor-1 gene polymorphisms and the risk of cutaneous melanoma in a low-risk southern European population. J. Invest Dermatol. 2006;126:1842–1849. doi: 10.1038/sj.jid.5700292. [DOI] [PubMed] [Google Scholar]
- 225.Sturm RA, Duffy DL, Box NF, Chen W, Smit DJ, Brown DL, Stow JL, Leonard JH, Martin NG. The role of melanocortin-1 receptor polymorphism in skin cancer risk phenotypes. Pigment Cell Res. 2003a;16:266–272. doi: 10.1034/j.1600-0749.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 226.Sturm RA, Duffy DL, Box NF, Newton RA, Shepherd AG, Chen W, Marks LH, Leonard JH, Martin NG. Genetic association and cellular function of MC1R variant alleles in human pigmentation. Ann. N. Y. Acad. Sci. 2003b;994:348–358. doi: 10.1111/j.1749-6632.2003.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 227.Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology. 1996;137:1627–1633. doi: 10.1210/endo.137.5.8612494. [DOI] [PubMed] [Google Scholar]
- 228.Suzuki I, Tada A, Ollmann MM, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, Abdel-Malek ZA. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. J. Invest Dermatol. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 229.Swope VB, Abdel-Malek Z, Kassem LM, Nordlund JJ. Interleukins 1 alpha and 6 and tumor necrosis factor-alpha are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J. Invest Dermatol. 1991;96:180–185. doi: 10.1111/1523-1747.ep12460991. [DOI] [PubMed] [Google Scholar]
- 230.Swope VB, Jameson JA, McFarland KL, et al. Defining MC1R regulation in human melanocytes by its agonist alpha-melanocortin and antagonists agouti signaling protein and beta-defensin 3. J. Invest Dermatol. 2012;132:2255–2262. doi: 10.1038/jid.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Swope VB, Alexander C, Starner R, et al. Significance of the Melanocortin 1 Receptor in the DNA Damage Response of Human Melanocytes to Ultraviolet Radiation. Pigment Cell Mel Res. 2014 doi: 10.1111/pcmr.12252. In press. [DOI] [PubMed] [Google Scholar]
- 232.Szell M, Baltas E, Bodai L, et al. The Arg160Trp allele of melanocortin-1 receptor gene might protect against vitiligo. Photochem. Photobiol. 2008;84:565–571. doi: 10.1111/j.1751-1097.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- 233.Tada A, Pereira E, Beitner-Johnson D, Kavanagh R, Abdel-Malek ZA. Mitogen- and ultraviolet-B-induced signaling pathways in normal human melanocytes. J. Invest Dermatol. 2002;118:316–322. doi: 10.1046/j.0022-202x.2001.01694.x. [DOI] [PubMed] [Google Scholar]
- 234.Tada A, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575–584. [PubMed] [Google Scholar]
- 235.Tamate HB, Takeuchi T. Action of the e locus of mice in the response of phaeomelanic hair follicles to alpha-melanocyte-stimulating hormone in vitro. Science. 1984;224:1241–1242. doi: 10.1126/science.6328651. [DOI] [PubMed] [Google Scholar]
- 236.Tan CP, McKee KK, Weinberg DH, et al. Molecular analysis of a new splice variant of the human melanocortin-1 receptor. FEBS Lett. 1999;451:137–141. doi: 10.1016/s0014-5793(99)00525-6. [DOI] [PubMed] [Google Scholar]
- 237.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: lessons from PKA. Nat. Rev. Mol. Cell Biol. 2012;13:646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Turan S, Hughes C, Atay Z, Guran T, Haliloglu B, Clark AJ, Bereket A, Metherell LA. An atypical case of familial glucocorticoid deficiency without pigmentation caused by coexistent homozygous mutations in MC2R (T152K) and MC1R (R160W) J. Clin. Endocrinol. Metab. 2012;97:E771–E774. doi: 10.1210/jc.2011-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat. Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 240.Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, Jackson IJ, Rees JL. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum. Mol. Genet. 1996;5:1663–1666. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- 241.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 242.Voisey J, Gomez-Cabrera MC, Smit DJ, Leonard JH, Sturm RA, van DA. A polymorphism in the agouti signalling protein (ASIP) is associated with decreased levels of mRNA. Pigment Cell Res. 2006;19:226–231. doi: 10.1111/j.1600-0749.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 243.Voisey J, van Daal A. Agouti: from mouse to man from skin to fat. Pigment Cell Res. 2002;15:10–18. doi: 10.1034/j.1600-0749.2002.00039.x. [DOI] [PubMed] [Google Scholar]
- 244.Wakamatsu K, Kavanagh R, Kadekaro AL, Terzieva S, Sturm RA, Leachman S, Abdel-Malek Z, Ito S. Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 2006;19:154–162. doi: 10.1111/j.1600-0749.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 245.Walker WP, Gunn TM. Shades of meaning: the pigment-type switching system as a tool for discovery. Pigment Cell Melanoma Res. 2010;23:485–495. doi: 10.1111/j.1755-148X.2010.00721.x. [DOI] [PubMed] [Google Scholar]
- 246.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 247.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 248.Wong G, Pawelek J. Control of phenotypic expression of cultured melanoma cells by melanocyte stimulating hormones. Nat. New Biol. 1973;241:213–215. doi: 10.1038/newbio241213a0. [DOI] [PubMed] [Google Scholar]
- 249.Wong SS, Ainger SA, Leonard JH, Sturm RA. MC1R variant allele effects on UVR-induced phosphorylation of p38, p53, and DDB2 repair protein responses in melanocytic cells in culture. J. Invest Dermatol. 2012;132:1452–1461. doi: 10.1038/jid.2011.473. [DOI] [PubMed] [Google Scholar]
- 250.Wong W, Minchin RF. Binding and internalization of the melanocyte stimulating hormone receptor ligand [Nle4, D-Phe7] alpha-MSH in B16 melanoma cells. Int. J. Biochem. Cell Biol. 1996;28:1223–1232. doi: 10.1016/s1357-2725(96)00074-x. [DOI] [PubMed] [Google Scholar]
- 251.Wu GS, Luo HR, Dong C, Mastronardi C, Licinio J, Wong ML. Sequence polymorphisms of MC1R gene and their association with depression and antidepressant response. Psychiatr. Genet. 2011;21:14–18. doi: 10.1097/YPG.0b013e32834133d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yang Y. Structure, function and regulation of the melanocortin receptors. Eur. J. Pharmacol. 2011;660:125–130. doi: 10.1016/j.ejphar.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/− mice: ectopic expression of the agouti gene. FASEB J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 254.Zanna PT, Sanchez-Laorden BL, Perez-Oliva AB, Turpin MC, Herraiz C, Jimenez-Cervantes C, Garcia-Borron JC. Mechanism of dimerization of the human melanocortin 1 receptor. Biochem. Biophys. Res. Commun. 2008;368:211–216. doi: 10.1016/j.bbrc.2008.01.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.