Abstract
COX-2, cyclooxygenase; CXCR4, CXC chemokine receptor 4; ECM, extracellular matrix; ELAM-1, endothelial leukocyte adhesion molecule 1; eNOS, endothelial nitric oxide synthase; GC, guanylyl cyclase; HIF-1, hypoxia-inducible factor-1; HUVECs, human umbilical vascular endothelial cells; IL-33, cytokine interleukin-33; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; nNOS, neuronal nitric oxide synthase; NO•, nitric oxide; NO•-NSAIDs, nitric oxide-releasing non-steroidal anti-inflammatory drugs; NOS, nitric oxide synthase; PCNA, proliferating cell nuclear antigen; PKC, protein kinase C; QUER, quercetin; TPA, 12-O-tetradecanoylphorbol 13-acetate; t-PTER, trans-pterostilbene; VEGF-C, vascular endothelial growth factor-C
Keywords: iNOS, NSAIDs, tumor, signaling, hypoxia
1. Introduction
Cancer metastasis is the spread and growth of tumor cells through angiogenesis, invasion, colonization, and ultimately proliferation from the original neoplasm to other organs,[1] which can be extremely difficult to treat and therefore often lead to death (Fig. 1).[2, 3] Nitric oxide (NO•) is a signaling molecule that plays various roles pathologically and physiologically.[4] In the last two decades, the function of NO• in the regulation of cancer formation, progression and metastasis has been extensively investigated.[5, 6] Activation of nitric oxide synthase (NOS) and elevation of NO• have exhibited an antitumor nature,[7–9] however, NO• may also promote cancer formation and progression.[10–12] Therefore the effect of NO• on metastasis cannot be easily classified as “pro-metastasis” or “anti-metastasis” as it may rely on other factors such as the cell type,[13] dosage,[14, 15] organs involved,[13] or even which step of metastasis NO• influences. This review will summarize the current knowledge of the influence of NO• in tumor progression and metastasis. The potential therapeutic applications of NO• in cancer treatment will also be discussed.
Fig. 1.
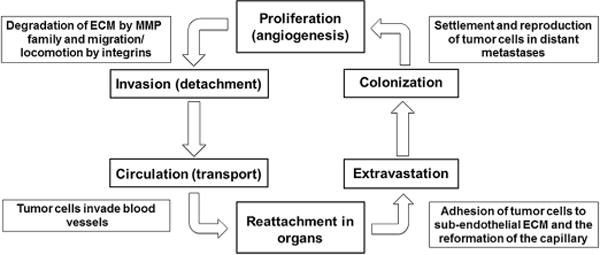
This schematic demonstrates the typical progression of tumor cell metastasis. Abbreviations: extracellular matrix (ECM), matrix metalloproteinase (MMP).
NO• was first discovered as a vasodilator in the cardiovascular system.[16] Recently, NO• has been found to have a pleiotropic effect on platelet aggregation,[17] immune response,[18] and signaling pathways critical to tumor progression,[19] all of which affect tumor cell metastasis.[17, 20, 21] NO• is synthesized from L-Arginine and oxygen by a family of enzymes termed nitric oxide synthases (NOS). The three isoforms of NOS include neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3). Because nNOS and eNOS undergo constitutive expression, they have also been named constitutive NOS.[22] These isoforms are significant because NOS expression in tumors differ from case to case, exhibiting their heterogeneous characteristics on cancer metastasis.[23]
NO• has a paradoxical role in certain malignancies and prognoses. A recent clinical study showed that patients with iNOS-positive tumors had a significantly lower disease-specific survival rate than those with iNOS-negative tumors in various stages of colorectal cancer, suggesting iNOS overexpression is related to increased disease-specific fatality.[24] Analysis of the relationship between angiogenesis and iNOS in primary gallbladder carcinomas has shown that the degree of malignancy is significantly affiliated with the expression level of iNOS.[25] In breast cancer, NO• is shown to decrease aggressiveness of breast tumor cells by inhibiting cell motility and reinforcing cell adhesion, ultimately hindering the cell’s metastatic characteristics.[26] While NO• has been clinically connected to a poor cancer prognosis, not all the effects of NO• are clear and may be impacted by dose or organs involved.
Mice transfected with an iNOS-negative retrovirus led to the formation of multiple lung metastases and aggressive, subcutaneous tumors. However, cells infected with iNOS-positive retrovirus formed few lung metastases and slowly progressing tumors.[27] In addition, when the metastatic cells of murine M5076 were transfected with functional iNOS genes, the established hepatic lesions and tumorigenesis regressed.[8] In syngeneic C57BL/6 mice, lower levels of iNOS produced tumors in the pancreas that metastasized to the liver and formed ascites. However, higher levels of iNOS expression did not result in liver metastases or ascites.[28] These results suggest that NO• may drastically impede or even eliminate metastatic progression. However, the effect of NO• on tumor metastasis appears to be organ-specific. A study exploring the effects of heme oxygenase and NO• on pulmonary or liver metastasis of colon cancer in mice found that the mice receiving NG-nitro-L-arginine methyl ester, an inhibitor of eNOS, had an increased number of tumor cells 24 hours later. Those same mice had an increased number of pulmonary metastases 18 days later, but possessed a similar number of liver metastases as untreated mice.[13] Since eNOS showed anti-metastatic effects on pulmonary metastases but no effect on liver metastases, these results suggest that rather than NO• being correctly labeled “pro-metastasis” or “anti-metastasis,” the setting and organs involved have a great effect on the manifestation of NO•.[13]
During in vitro and in vivo conditions, NO• exercises its anti-tumor nature by inducing cytotoxicity and apoptosis, affectively influencing tumor metastasis.[7, 29–31] Galectin-3 is a carbohydrate-binding protein that is important for cell-cell and cell-matrix interactions and cancer metastasis.[32] NO• is involved in the mechanism by which galectin-3 enhances metastasis. In human breast carcinoma (BT549) cells, galectin-3 improves metastatic potential and protects tumor cells from death through the iNOS cytotoxicity pathway.[33, 34] Paclitaxel, an antineoplastic drug that introduces cytotoxicity against cancer cells, was shown to accomplish its effect through stimulation of NO• production in human liver cancer cells HepG2.[35] In addition, two natural and structurally similar polyphenols, trans-pterostilbene (t-PTER) and quercetin (QUER) were administered to mice and found to impede the metastasis of B16F10 melanoma cells by causing NO• to be released from the vascular endothelium, resulting in the cytotoxicity and death of B16F10 cells (Fig. 2).[36]
Fig. 2.
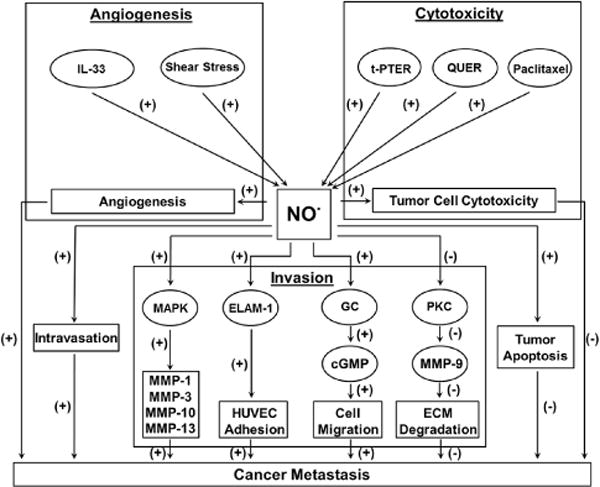
This schematic demonstrates the multitude of mechanisms through which nitric oxide increases or decreases the activation of and prevalence of cancer metastasis. Abbreviations: extracellular matrix (ECM), endothelial leukocyte adhesion molecule 1 (ELAM-1), guanylyl cyclase (GC), human umbilical vascular endothelial cells (HUVECs), Cytokine interleukin-33 (IL-33), mitogen-activated protein kinase (MAPK), matrix metalloproteinase (MMP), Nitric oxide (NO•), protein kinase C (PKC), quercetin (QUER), trans-pterostilbene (t-PTER).
In addition to anti-cancer effects through cytotoxicity, there is sufficient evidence suggesting anti-cancer effects of NO• through apoptosis.[14, 37] Many cancers show resistance to apoptosis by suppressing the genes that promote apoptosis. This resistance largely contributes to poor prognosis by affecting tissue homeostasis and causing failure of treatments.[38, 39] Through the use of a series of adenoviral vectors that expressed different levels of iNOS activity, Xie et al. reported that although NO• has some protumor activity such as mediated gene transfer and up-regulated angiogenetic molecules, the antitumor actions including loss of malignancy due to apoptosis outweigh the protumor factors and result in an overall deregulation of malignancy.[14] These dichotomous effects on cancer progression arise from NO• regulations on specific signaling pathways.[40] Successful cancer metastasis consists of several complex, consecutive, and very particular steps.[1] Numerous evidence suggests that NO• plays important roles in nearly all steps of cancer metastasis.[6]
2. Invasion
Invasion consists of alteration in tumor cell adhesion to the extracellular matrix (ECM), proteolytic degradation of encompassing tissue, and migration of tumor cells (Fig. 1).[1, 41–43] During invasion, the matrix metalloproteinase (MMP) family is responsible for the essential degradation of the ECM[44, 45] while integrins likely assist in locomotion, the forward migration synchronized by operations of actin cytoskeleton filaments.[46]
MMPs exist at a high level in malignant cells, but are expressed at basic levels in normal cells. NO• was found to modulate MMP expression and therefore affects tumor cell invasion.[47–50] The invasion-inhibiting effects of NO• on the 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced MMP-9 expression was examined in human breast cancer cell line MCF-7. It was observed that a supplement of NO• donor leads to a decrease of MMP-9 mRNA level and reduction of MMP-9 translation. A 0.67 kb fragment from a 5′-promoter region of the MMP-9 gene is primarily responsible for the inhibition of MMP-9 by NO•.[50] Furthermore, the TPA-triggered protein kinase C (PKC) activity was significantly inhibited by NO• in MCF-7 cells, indicating that NO• attenuates TPA-induced MMP-9 expression mediated by PKC pathway[50] and therefore avoids invasion. Conversely, an examination of human melanoma cell line C32TG found NO• enhanced MMP-1, -3, -10 and -13 expression transcriptionally via the mitogen-activated protein kinase (MAPK) (ERK/p38) pathway,[49] thereby assisting tumor metastasis (Fig. 2). This difference also suggests a potential cell-type dependent effect of NO• on MMP expression and invasion.
Integrins are a family of cell surface proteins that mediate cell-to-cell and cell-to-matrix attachment, control the adherence of tumor cells to and in the ECM, and likely aid in locomotion, thereby impacting tumor cell invasion.[46, 51–53] NO• specifically inhibits α2β1 integrin-mediated platelet adhesion to immobilized collagen.[52] Activation of integrin α9β1 enhances cell migration through the induction of iNOS expression triggering cGMP generation via guanylyl cyclase (GC).[46] NO• from human umbilical vascular endothelial cells (HUVECs) promote cell adhesion of human fibrosarcoma HT1080 to HUVEC monolayer by enhancing endothelial leukocyte adhesion molecule 1 (ELAM-1) expression on HUVECs. This further increased tumor cell invasion through the HUVEC monolayer (Fig. 2).[54] Therefore, in addition to evidence of pro- and anti-invasion effects, NO• reduces adhesion and promotes migration, and therefore invasion, through inhibition of integrin expression.[46, 51–53]
3. Angiogenesis
Angiogenesis is the growth of new blood vessels from the original vascular bed and is an essential step in tumor progression and metastasis.[55] An analysis using samples from normal liver tissue and hepatocellular carcinoma patients demonstrates that iNOS positively modulate MMP-9 expression, facilitating tumor cell angiogenesis, invasion, and metastasis.[56] Compared to normal tissue, tumor specimens from head and neck cancer showed higher NOS activity, which is associated with a higher level of angiogenesis. When the production of NO• is obstructed, tumor angiogenesis is suppressed.[57, 58] Cytokine interleukin-33 (IL-33), a regulator of vasculature, was also shown to promote proliferation, migration, angiogenesis and vascular permeability by stimulating endothelial NO• generation through the ST2/TRAF6-Akt-eNOS signaling pathway.[59] In addition, the reduction of NO• formation achieved by knockdown eNOS using siRNA markedly decreased endothelial cells migration in shear stress; however, a supplement of external NO• donor led to a 2-fold recovery in angiogenesis (Fig. 2).[60]
4. Intravasation
Although less documented, evidence suggests that NO• is also involved in the process of intravasation, which occurs when tumor cells invade the blood vessels. Leukocytes are circulated throughout the body to defend against tumor cell invasion in blood vessels. Significant to intravasation, the rolling and adhesion, and therefore effectiveness, of leukocytes on the endothelium in tumor vessels is largely reduced in an NO• and ICAM-1 dependent manner. In addition, as shown in Fig. 2, inhibition of NO• partially reversed the lack of adhesion of leukocytes in tumor microvessels, suggesting that NO• has pro-intravasative effects.[61]
5. Extravasation and Colonization
During extravasation and colonization, the tumor cells prompt endothelial retraction that leads to the adhesion of tumor cells to the subendothelial ECM and the reformation of the capillary (Fig. 1). When a tumor cell attaches to coagulation factors such as fibrin, thrombin, or fibrinogen, an embolus is created and, with the assistance of E- and P-selectins, it causes cell arrest in the capillary beds. NO• has been shown to decrease the expression of E-selectins through inactivation of NF-kappaB, thereby resulting in an anti-metastatic outcome.[62] However, a selective iNOS inhibitor (e.g., 1400W dihydrochloride) attenuated the mRNA transcription of E-selectins in pulmonary artery endothelial cells.[63]
6. Lymphatic metastasis
Lymphatic metastasis is a common and critical determinant of cancer pathogenesis.[64] The lymph nodes are the primary metastasis location for most malignancies and they are a critical determinate in the prognosis of the patient. Two important factors in promoting lymphatic metastasis are vascular endothelial growth factor-C (VEGF-C), by which lymphangiogenesis is stimulated,[65] and the CXC chemokine receptor 4 (CXCR4), which is shown to be a contributor to lymph node metastasis.[66] With the treatment of the NO• donor DETA NONOate, the production of VEGF-C increases. Furthermore, when treated with an NO•-synthesis impeder N-nitro-L-arginine, additional VEGF-C production is halted.[67] In addition to VEGF-C, NO• also stimulates cytoplasmic CXCR4 expression and therefore may also be characterized as pro-metastatic due to its possible aiding of lymphatic metastasis in human breast carcinoma cells.[66]
7. Hypoxia evasion
Hypoxia occurs when the entire or part of the body is deprived of oxygen.[68] When this occurs in a tumor, it may lead to further tumor metastasis due to therapy resistance and accelerated invasion, and is not characterized with a promising prognosis for patients with many types of cancer.[69, 70]
NO• evokes hypoxia-inducible factor-1 (HIF-1), a transcription factor that enhances many hypoxia-inducible genes, via PI3k/Akt pathway.[71, 72] As mentioned previously, an open-label 24-month clinical trial shows that NO• annihilates prostate cancer metastasis by weakening hypoxia-induced progression.[73] In B16F10 cells, the presence of low-concentration NO•-mimetics caused an inhibition in hypoxia-induced lung nodule formation. When NOS was repressed, it caused lung nodule formation in a hypoxia-similar fashion. This regulatory effect of NO• involves the cGMP-dependent pathway.[70]
The effect of NO• also relies on the presence of additional substances. Together, expression of iNOS and cyclooxygenase (COX-2) has been found to be responsible for enhanced vascular invasion, lymphatic metastasis and angiogenesis in various cancers. Although we cannot define both iNOS and COX-2 as exclusive pro- or anti-metastatic in gastric adenocarcinoma patients, the combined production of NO• and prostaglandins via iNOS and COX-2 expression can be associated with cancer progression, tumor angiogenesis, and a graver prognosis.[74] Besides COX-2, the Src kinase family, a key regulator of cell invasiveness, is shown to phosphorylate iNOS Tyr (1055) and stabilize iNOS half-life, thereby producing a constantly large amount of NO• to regulate tumor metastasis.[75]
8. Nitric oxide therapy
It is clear that NO• plays a significant role in tumor progression and metastasis. The bimodal effects of NO• have been established through studies focusing on variables affecting NO• impact. These factors include duration of NO• exposure, cellular microenvironment, NO• flux, tumor cell cycle stage, as well as prevalence of oxidizing and reducing agents.[76] Since the influence of NO• is also dependent on the concentration, angiogenesis, proliferation and metastasis can normally be stimulated by lower levels of NO• (<100nM). However, higher concentrations of NO• (>400–500 nM) do not facilitate tumor progression but instead promote cytotoxicity and cell apoptosis.[40, 76] Accordingly, the inhibition of cell survival and anti-apoptosis pathways by NO• donors has been proposed as a novel therapy to various cancers.[4, 77] Most of the research has been in the area of nitric oxide-releasing non-steroidal anti-inflammatory drugs (NO•-NSAIDs), which has been particularly promising because in many cases, NO• appears to exhibit both combinatory and enhancing effects on pre-existing anticancer effects of the parent NSAIDs. Several studies have found a link between NSAIDs use and decreased risk of colorectal cancer[78–80], suggesting a possible chemopreventive role. NO•-NSAIDs have been shown to be more effective in the inhibition of cancer cell growth and metastasis than the parent drug alone.[77, 81]
Evidence suggests NO•-NSAIDs act on cancer cells in several ways. First, they inhibit cell proliferation by decreasing expression of proliferating cell nuclear antigen (PCNA).[82] PCNA is a protein that acts as a processivity factor for DNA polymerase delta in eukaryotic cells. It plays an important role in DNA synthesis, and as such, decreased PCNA expression leads to decreased DNA synthesis and reduced cell proliferation. However, this decrease in PCNA expression has not been shown to correspond linearly to the decrease in cell proliferation, so it appears that other factors are involved.[82] NO•-aspirin exhibits greater pro-apoptotic effects than aspirin itself. Interestingly, NO•-aspirin also induced a large population of “atypical” cells.[82, 83] These “atypical” cells exhibit diminished or no DNA while retaining their basic cellular structure. NO•-NSAIDs also appear to block cell cycle transitions from G0-G1 to S, further inhibiting growth (Fig. 3).[82]
Fig. 3.
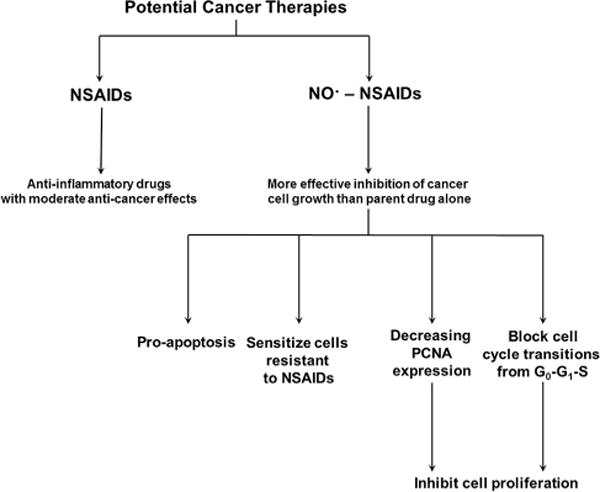
This flowchart highlights the increased effectiveness of NSAIDs contrasted to NO•-NSAIDs in blocking cancer development. Abbreviations: nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NO•-NSAIDs), proliferating cell nuclear antigen (PCNA).
Accordingly, NO•-NSAIDs may likely augment cellular pathways favorable to apoptosis and diminish metastasis in certain cell types. For example, the anti-cancer effects of NO•-NSAIDs may not help reduce metastasis in hepatocytes, endothelial cells, B-cell lymphomas, and other cell lines, where NO• has been shown to obstruct pro-apoptotic mechanisms.[84–86] However, other tumor cells such as breast carcinomas have been shown to undergo apoptosis following prolonged exposure to elevated NO• concentrations (1 mM).[40] In colon adenocarcinomas, NO•-NSAIDs repress tumor proliferation through epigenetic contributions such as DNA methylation and histone modifications.[76, 82]
Another study found that nitric oxide-donating pro-drugs significantly enhanced the efficacy of the chemotherapy drug fludarabine against chronic lymphocytic leukemia cells.[87] Three-dimensional analysis of the combination of fludarabine and DETA-NO• showed that at the optimum combination, cell death of 80–90% should be expected. As shown in Fig. 3, NO• also appears to preferentially sensitize cells that exhibit fludarabine resistance.[87]
9. Conclusion and perspective
In this review, we have identified the underlying biological factors influencing the pro- and anti-metastatic effects of NO•. We discussed the various ways NO• can regulate tumor metastasis, ultimately providing justification for utilization of NO•-NSAIDs against carcinomas due to their ability to hinder cell-to-cell attachment and invasion. Several important aspects of NO• must be considered when assessing the biological outcome of NO• on cancer, such as the duration of NO• exposure, tumor microenvironment that NO• encounters, as well as specific signaling pathways that NO• regulates including MMP, integrin, cGMP-dependent, and COX-2 pathways. The double-edged nature of NO• action facilitates utilization of NO•-NSAIDs against carcinomas complex. Multiple factors including NO• pathways, tumor redox microenvironments, and NO•-mediated signaling cascades, must be considered in vitro and in vivo for potential therapies. Thus, NO•-NSAIDs can be important drugs for anti-cancer treatment if the effect of NO•-NSAIDs is optimized. Although NO• can assist in cancer progression by inducing angiogenesis and cell invasion, we conclude that nitric oxide-donating prodrugs, such as NO•-NSAIDs, are a promising development for further research.
Highlights.
This review summarizes the dichotomous effects of NO• in tumor metastasis.
Biological factors influence the pro- and anti-metastatic impact of NO•.
NO• induces cytotoxicity and apoptosis to hinder cancer cell growth.
NO• promotes intravasation and angiogenesis to enhance cancer cell growth.
The bimodal nature of NO• facilitates the anti-cancer potential of NO•-NSAIDs.
Acknowledgments
This work is partially supported by RO1 CA086928 and HRS-013000. We appreciate Shenali Wickramanayake, Andrew Graef, and Alexander Ziegler for their assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Coulter JA, McCarthy HO, Xiang J, Roedl W, Wagner E, Robson T, Hirst DG. Nitric oxide–a novel therapeutic for cancer. Nitric Oxide. 2008;19(2):192–198. doi: 10.1016/j.niox.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998;19(5):711–721. doi: 10.1093/carcin/19.5.711. [DOI] [PubMed] [Google Scholar]
- 6.Williams EL, Djamgoz MB. Nitric oxide and metastatic cell behaviour. Bioessays. 2005;27(12):1228–1238. doi: 10.1002/bies.20324. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Staroselsky AH, Qi X, Xie K, Fidler IJ. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 1994;54(3):789–793. [PubMed] [Google Scholar]
- 8.Xie K, Huang S, Dong Z, Juang SH, Gutman M, Xie QW, Nathan C, Fidler IJ. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med. 1995;181(4):1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie K, Dong Z, Fidler IJ. Activation of nitric oxide synthase gene for inhibition of cancer metastasis. J Leukoc Biol. 1996;59(6):797–803. doi: 10.1002/jlb.59.6.797. [DOI] [PubMed] [Google Scholar]
- 10.Tozer GM, Prise VE, Chaplin DJ. Inhibition of nitric oxide synthase induces a selective reduction in tumor blood flow that is reversible with L-arginine. Cancer Res. 1997;57(5):948–955. [PubMed] [Google Scholar]
- 11.Felley-Bosco E. Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev. 1998;17(1):25–37. doi: 10.1023/a:1005948420548. [DOI] [PubMed] [Google Scholar]
- 12.Bing RJ, Miyataka M, Rich KA, Hanson N, Wang X, Slosser HD, Shi SR. Nitric oxide, prostanoids, cyclooxygenase, and angiogenesis in colon and breast cancer. Clin Cancer Res. 2001;7(11):3385–3392. [PubMed] [Google Scholar]
- 13.Ishikawa T, Yoshida N, Higashihara H, Inoue M, Uchiyama K, Takagi T, Handa O, Kokura S, Naito Y, Okanoue T, et al. Different effects of constitutive nitric oxide synthase and heme oxygenase on pulmonary or liver metastasis of colon cancer in mice. Clin Exp Metastasis. 2003;20(5):445–450. doi: 10.1023/a:1025448403124. [DOI] [PubMed] [Google Scholar]
- 14.Le X, Wei D, Huang S, Lancaster JR, Jr, Xie K. Nitric oxide synthase II suppresses the growth and metastasis of human cancer regardless of its up-regulation of protumor factors. Proc Natl Acad Sci U S A. 2005;102(24):8758–8763. doi: 10.1073/pnas.0409581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocellin S. Nitric oxide: cancer target or anticancer agent? Curr Cancer Drug Targets. 2009;9(2):214–236. doi: 10.2174/156800909787581015. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddell DR, Owen JS. Nitric oxide and platelet aggregation. Vitam Horm. 1999;57:25–48. doi: 10.1016/s0083-6729(08)60639-1. [DOI] [PubMed] [Google Scholar]
- 18.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukumura D, Kashiwagi S, Jain RK. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6(7):521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 20.DeNardo DG, Johansson M, Coussens LM. Immune cells as mediators of solid tumor metastasis. Cancer Metastasis Rev. 2008;27(1):11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 21.Tsuruo T, Fujita N. Platelet aggregation in the formation of tumor metastasis. Proc Jpn Acad Ser B Phys Biol Sci. 2008;84(6):189–198. doi: 10.2183/pjab/84.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forstermann U, Schmidt HH, Pollock JS, Sheng H, Mitchell JA, Warner TD, Nakane M, Murad F. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem Pharmacol. 1991;42(10):1849–1857. doi: 10.1016/0006-2952(91)90581-o. [DOI] [PubMed] [Google Scholar]
- 23.Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51(22):6073–6078. [PubMed] [Google Scholar]
- 24.Zafirellis K, Zachaki A, Agrogiannis G, Gravani K. Inducible nitric oxide synthase expression and its prognostic significance in colorectal cancer. APMIS. 2010;118(2):115–124. doi: 10.1111/j.1600-0463.2009.02569.x. [DOI] [PubMed] [Google Scholar]
- 25.Niu XJ, Wang ZR, Wu SL, Geng ZM, Zhang YF, Qing XL. Relationship between inducible nitric oxide synthase expression and angiogenesis in primary gallbladder carcinoma tissue. World J Gastroenterol. 2004;10(5):725–728. doi: 10.3748/wjg.v10.i5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahiri M, Martin JH. Nitric oxide decreases motility and increases adhesion in human breast cancer cells. Oncol Rep. 2009;21(2):275–281. [PubMed] [Google Scholar]
- 27.Juang SH, Xie K, Xu L, Wang Y, Yoneda J, Fidler IJ. Use of retroviral vectors encoding murine inducible nitric oxide synthase gene to suppress tumorigenicity and cancer metastasis of murine melanoma. Cancer Biother Radiopharm. 1997;12(3):167–175. doi: 10.1089/cbr.1997.12.167. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Wei D, Crum VE, Richardson EL, Xiong HH, Luo Y, Huang S, Abbruzzese JL, Xie K. A novel model system for studying the double-edged roles of nitric oxide production in pancreatic cancer growth and metastasis. Oncogene. 2003;22(12):1771–1782. doi: 10.1038/sj.onc.1206386. [DOI] [PubMed] [Google Scholar]
- 29.Xie K, Huang S. Contribution of nitric oxide-mediated apoptosis to cancer metastasis inefficiency. Free Radic Biol Med. 2003;34(8):969–986. doi: 10.1016/s0891-5849(02)01364-3. [DOI] [PubMed] [Google Scholar]
- 30.Worthington J, McCarthy HO, Barrett E, Adams C, Robson T, Hirst DG. Use of the radiation-inducible WAF1 promoter to drive iNOS gene therapy as a novel anti-cancer treatment. J Gene Med. 2004;6(6):673–680. doi: 10.1002/jgm.567. [DOI] [PubMed] [Google Scholar]
- 31.Tell RT, Sydiskis RJ, Isaacs RD, Davidson WM. Long-term cytotoxicity of orthodontic direct-bonding adhesives. Am J Orthod Dentofacial Orthop. 1988;93(5):419–422. doi: 10.1016/0889-5406(88)90101-1. [DOI] [PubMed] [Google Scholar]
- 32.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) Int J Oncol. 2004;25(4):983–992. [PubMed] [Google Scholar]
- 33.Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159(3):1055–1060. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song YK, Billiar TR, Lee YJ. Role of galectin-3 in breast cancer metastasis: involvement of nitric oxide. Am J Pathol. 2002;160(3):1069–1075. doi: 10.1016/S0002-9440(10)64927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayed-Ahmad MM, Mohamad MA. Contribution of nitric oxide and epidermal growth factor receptor in anti-metastatic potential of paclitaxel in human liver cancer cell (HebG2) J Egypt Natl Canc Inst. 2005;17(1):35–41. [PubMed] [Google Scholar]
- 36.Ferrer P, Asensi M, Priego S, Benlloch M, Mena S, Ortega A, Obrador E, Esteve JM, Estrela JM. Nitric oxide mediates natural polyphenol-induced Bcl-2 down-regulation and activation of cell death in metastatic B16 melanoma. J Biol Chem. 2007;282(5):2880–2890. doi: 10.1074/jbc.M605934200. [DOI] [PubMed] [Google Scholar]
- 37.Muntane J, De la Rosa AJ, Marin LM, Padillo FJ. Nitric oxide and cell death in liver cancer cells. Mitochondrion. 2013;13(3):257–262. doi: 10.1016/j.mito.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Fulda S. Apoptosis pathways and their therapeutic exploitation in pancreatic cancer. J Cell Mol Med. 2009;13(7):1221–1227. doi: 10.1111/j.1582-4934.2009.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, et al. Apoptosis and cancer: mutations within caspase genes. J Med Genet. 2009;46(8):497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 40.Ridnour LA, Thomas DD, Switzer C, Flores-Santana W, Isenberg JS, Ambs S, Roberts DD, Wink DA. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide. 2008;19(2):73–76. doi: 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, Groom AC. Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 1995;14(4):279–301. doi: 10.1007/BF00690599. [DOI] [PubMed] [Google Scholar]
- 42.Ponta H, Sleeman J, Herrlich P. Tumor metastasis formation: cell-surface proteins confer metastasis-promoting or -suppressing properties. Biochim Biophys Acta. 1994;1198(1):1–10. doi: 10.1016/0304-419x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 43.Pass HI. Biology of metastatic disease. Semin Thorac Cardiovasc Surg. 2002;14(1):10–17. doi: 10.1053/stcs.2002.29534. [DOI] [PubMed] [Google Scholar]
- 44.Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22(2–3):145–152. doi: 10.1023/a:1023039230052. [DOI] [PubMed] [Google Scholar]
- 45.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122(Pt 17):3015–3024. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 46.Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J Cell Sci. 2009;122(Pt 12):2043–2054. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.St-Pierre Y, Couillard J, Van Themsche C. Regulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseases. Expert Opin Ther Targets. 2004;8(5):473–489. doi: 10.1517/14728222.8.5.473. [DOI] [PubMed] [Google Scholar]
- 48.Thompson EW, Yu M, Bueno J, Jin L, Maiti SN, Palao-Marco FL, Pulyaeva H, Tamborlane JW, Tirgari R, Wapnir I, et al. Collagen induced MMP-2 activation in human breast cancer. Breast Cancer Res Treat. 1994;31(2–3):357–370. doi: 10.1007/BF00666168. [DOI] [PubMed] [Google Scholar]
- 49.Ishii Y, Ogura T, Tatemichi M, Fujisawa H, Otsuka F, Esumi H. Induction of matrix metalloproteinase gene transcription by nitric oxide and mechanisms of MMP-1 gene induction in human melanoma cell lines. Int J Cancer. 2003;103(2):161–168. doi: 10.1002/ijc.10808. [DOI] [PubMed] [Google Scholar]
- 50.Jespersen C, Doller A, Akool el S, Bachmann M, Muller R, Gutwein P, Muhl H, Pfeilschifter J, Eberhardt W. Molecular mechanisms of nitric oxide-dependent inhibition of TPA-induced matrix metalloproteinase-9 (MMP-9) in MCF-7 cells. J Cell Physiol. 2009;219(2):276–287. doi: 10.1002/jcp.21658. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Liu J, Qian F, Xu Q. Nitric oxide inhibits T cell adhesion and migration by down-regulation of beta1-integrin expression in immunologically liver-injured mice. Int Immunopharmacol. 2006;6(4):616–626. doi: 10.1016/j.intimp.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Roberts W, Riba R, Homer-Vanniasinkam S, Farndale RW, Naseem KM. Nitric oxide specifically inhibits integrin-mediated platelet adhesion and spreading on collagen. J Thromb Haemost. 2008;6(12):2175–2185. doi: 10.1111/j.1538-7836.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 53.Conran N, Gambero A, Ferreira HH, Antunes E, de Nucci G. Nitric oxide has a role in regulating VLA-4-integrin expression on the human neutrophil cell surface. Biochem Pharmacol. 2003;66(1):43–50. doi: 10.1016/s0006-2952(03)00243-0. [DOI] [PubMed] [Google Scholar]
- 54.Yudoh K, Matsui H, Tsuji H. Nitric oxide induced by tumor cells activates tumor cell adhesion to endothelial cells and permeability of the endothelium in vitro. Clin Exp Metastasis. 1997;15(6):557–567. doi: 10.1023/a:1018487213157. [DOI] [PubMed] [Google Scholar]
- 55.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 56.Sun MH, Han XC, Jia MK, Jiang WD, Wang M, Zhang H, Han G, Jiang Y. Expressions of inducible nitric oxide synthase and matrix metalloproteinase-9 and their effects on angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol. 2005;11(38):5931–5937. doi: 10.3748/wjg.v11.i38.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gallo O, Masini E, Morbidelli L, Franchi A, Fini-Storchi I, Vergari WA, Ziche M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90(8):587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 58.Jiang T, Yu JT, Zhu XC, Zhang QQ, Tan MS, Cao L, Wang HF, Lu J, Gao Q, Zhang YD, et al. Angiotensin-(1–7) Induces Cerebral Ischemic Tolerance by Promoting Brain Angiogenesis in a Mas/eNOS-Dependent Pathway. Br J Pharmacol. 2014 doi: 10.1111/bph.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, Kim J, Kim YM, Kwon YG. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009;114(14):3117–3126. doi: 10.1182/blood-2009-02-203372. [DOI] [PubMed] [Google Scholar]
- 60.Kolluru GK, Sinha S, Majumder S, Muley A, Siamwala JH, Gupta R, Chatterjee S. Shear stress promotes nitric oxide production in endothelial cells by subcellular delocalization of eNOS: A basis for shear stress mediated angiogenesis. Nitric Oxide. 2010;22(4):304–315. doi: 10.1016/j.niox.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Bessa X, Elizalde JI, Mitjans F, Pinol V, Miquel R, Panes J, Piulats J, Pique JM, Castells A. Leukocyte recruitment in colon cancer: role of cell adhesion molecules, nitric oxide, and transforming growth factor beta1. Gastroenterology. 2002;122(4):1122–1132. doi: 10.1053/gast.2002.32369. [DOI] [PubMed] [Google Scholar]
- 62.Spiecker M, Darius H, Kaboth K, Hubner F, Liao JK. Differential regulation of endothelial cell adhesion molecule expression by nitric oxide donors and antioxidants. J Leukoc Biol. 1998;63(6):732–739. [PubMed] [Google Scholar]
- 63.Huang H, Lavoie-Lamoureux A, Moran K, Lavoie JP. IL-4 stimulates the expression of CXCL-8, E-selectin, VEGF, and inducible nitric oxide synthase mRNA by equine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1147–1154. doi: 10.1152/ajplung.00294.2006. [DOI] [PubMed] [Google Scholar]
- 64.Cao Y. Opinion: emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nat Rev Cancer. 2005;5(9):735–743. doi: 10.1038/nrc1693. [DOI] [PubMed] [Google Scholar]
- 65.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61(5):1786–1790. [PubMed] [Google Scholar]
- 66.Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Kodama R, Sanke T, Nakamura Y. Cytoplasmic CXCR4 expression in breast cancer: induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura Y, Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Nakao K, Nakamura M, Kakudo K. Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin Cancer Res. 2006;12(4):1201–1207. doi: 10.1158/1078-0432.CCR-05-1269. [DOI] [PubMed] [Google Scholar]
- 68.Baek JH, Jang JE, Kang CM, Chung HY, Kim ND, Kim KW. Hypoxia-induced VEGF enhances tumor survivability via suppression of serum deprivation-induced apoptosis. Oncogene. 2000;19(40):4621–4631. doi: 10.1038/sj.onc.1203814. [DOI] [PubMed] [Google Scholar]
- 69.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Postovit LM, Adams MA, Lash GE, Heaton JP, Graham CH. Nitric oxide-mediated regulation of hypoxia-induced B16F10 melanoma metastasis. Int J Cancer. 2004;108(1):47–53. doi: 10.1002/ijc.11556. [DOI] [PubMed] [Google Scholar]
- 71.Huang LE, Willmore WG, Gu J, Goldberg MA, Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274(13):9038–9044. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 72.Sandau KB, Zhou J, Kietzmann T, Brune B. Regulation of the hypoxia-inducible factor 1alpha by the inflammatory mediators nitric oxide and tumor necrosis factor-alpha in contrast to desferroxamine and phenylarsine oxide. J Biol Chem. 2001;276(43):39805–39811. doi: 10.1074/jbc.M107689200. [DOI] [PubMed] [Google Scholar]
- 73.Siemens DR, Heaton JP, Adams M, Kawakami J, Graham CH. Phase II Study of Nitric Oxide Donor for Men With Increasing Prostate-specific Antigen Level After Surgery or Radiotherapy for Prostate Cancer. Urology. 2009;74(4):878–883. doi: 10.1016/j.urology.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Chen CN, Hsieh FJ, Cheng YM, Chang KJ, Lee PH. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J Surg Oncol. 2006;94(3):226–233. doi: 10.1002/jso.20372. [DOI] [PubMed] [Google Scholar]
- 75.Tyryshkin A, Gorgun FM, Abdel Fattah E, Mazumdar T, Pandit L, Zeng S, Eissa NT. Src kinase-mediated phosphorylation stabilizes inducible nitric-oxide synthase in normal cells and cancer cells. J Biol Chem. 2010;285(1):784–792. doi: 10.1074/jbc.M109.055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burke AJ, Sullivan FJ, Giles FJ, Glynn SA. The yin and yang of nitric oxide in cancer progression. Carcinogenesis. 2013;34(3):503–512. doi: 10.1093/carcin/bgt034. [DOI] [PubMed] [Google Scholar]
- 77.Burgaud JL, Ongini E, Del Soldato P. Nitric oxide-releasing drugs: a novel class of effective and safe therapeutic agents. Ann N Y Acad Sci. 2002;962:360–371. doi: 10.1111/j.1749-6632.2002.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 78.Stolfi C, De Simone V, Pallone F, Monteleone G. Mechanisms of action of nonsteroidal anti-inflammatory drugs (NSAIDs) and mesalazine in the chemoprevention of colorectal cancer. Int J Mol Sci. 2013;14(9):17972–17985. doi: 10.3390/ijms140917972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaish V, Piplani H, Rana C, Vaiphei K, Sanyal SN. NSAIDs may regulate EGR-1-mediated induction of reactive oxygen species and non-steroidal anti-inflammatory drug-induced gene (NAG)-1 to initiate intrinsic pathway of apoptosis for the chemoprevention of colorectal cancer. Mol Cell Biochem. 2013;378(1–2):47–64. doi: 10.1007/s11010-013-1593-y. [DOI] [PubMed] [Google Scholar]
- 80.Domingo E, Church DN, Sieber O, Ramamoorthy R, Yanagisawa Y, Johnstone E, Davidson B, Kerr DJ, Tomlinson IPM, Midgley R. Evaluation of PIK3CA Mutation As a Predictor of Benefit From Nonsteroidal Anti-Inflammatory Drug Therapy in Colorectal Cancer. J Clin Oncol. 2013;31(34):4297–U4234. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 81.Cheng H, Mollica MY, Lee SH, Wang L, Velazquez-Martinez CA, Wu S. Effects of nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NONO-NSAIDs) on melanoma cell adhesion. Toxicol Appl Pharmacol. 2012;264(2):161–166. doi: 10.1016/j.taap.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams JL, Borgo S, Hasan I, Castillo E, Traganos F, Rigas B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: Implications for colon cancer chemoprevention. Cancer Res. 2001;61(8):3285–3289. [PubMed] [Google Scholar]
- 83.Kashfi K, Ryann Y, Qiao LL, Williams JL, Chen J, Del Soldato P, Traganos F, Rigas B. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: Evidence of a tissue type-independent effect. J Pharm Exp Ther. 2002;303(3):1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 84.Olson SY, Garban HJ. Regulation of apoptosis-related genes by nitric oxide in cancer. Nitric Oxide. 2008;19(2):170–176. doi: 10.1016/j.niox.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pan X, Wang X, Lei W, Min L, Yang Y, Wang X, Song J. Nitric oxide suppresses transforming growth factor-beta1-induced epithelial-to-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology. 2009;50(5):1577–1587. doi: 10.1002/hep.23156. [DOI] [PubMed] [Google Scholar]
- 86.Brune B. Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ. 2003;10(8):864–869. doi: 10.1038/sj.cdd.4401261. [DOI] [PubMed] [Google Scholar]
- 87.Adams DJ, Levesque MC, Weinberg JB, Smith KL, Flowers JL, Moore J, Colvin OM, Silber R. Nitric oxide enhancement of fludarabine cytotoxicity for B-CLL lymphocytes. Leukemia. 2001;15(12):1852–1859. doi: 10.1038/sj.leu.2402291. [DOI] [PubMed] [Google Scholar]


