Abstract
Objectives
To evaluate the feasibility of using unattended, portable polysomnography (PSG) to measure sleep among patients in the medical intensive care unit (MICU).
Background
Accurate measurement of sleep is critical to studies of MICU sleep deprivation. Although PSG is the gold standard, there is limited data regarding the feasibility of utilizing unattended, portable PSG modalities in the MICU.
Methods
MICU based observational pilot study. We conducted unattended, 24-hour PSG studies in 29 patients. Indicators of feasibility included attainment of electroencephalography data sufficient to determine sleep stages, sleep efficiency, and arousal indices.
Results
Electroencephalography data were not affected by electrical interference and were of interpretable quality in 27/29 (93%) of these patients. Overnight sleep efficiency was 48% reflecting a mean overnight sleep duration of 3.7 hours.
Conclusions
Unattended, portable PSG produces high quality sleep data in the MICU and can facilitate investigation of sleep deprivation among critically ill patients. Patient sleep was short and highly fragmented.
Keywords: Sleep deprivation, sleep fragmentation, polysomnography, critical care, feasibility study
INTRODUCTION
Sleep deprivation, characterized by short sleep duration and poor sleep continuity (fragmentation), is virtually universal in critically ill patients.1 A variety of environmental, patient care and physiologic factors influence sleep deprivation. Noise disrupts patient sleep,2 and patients experience high levels of overnight in-room activity.3-5 Furthermore, patients undergoing mechanical ventilation and accompanying continuous sedation suffer particular decrements in sleep quality.6-8 Because sleep deprived patients often demonstrate behaviors that mimic intensive care unit (ICU) delirium,9 there is concern that sleep deprivation potentiates ICU delirium10 and therefore contributes to delirium-related morbidity and mortality.11-13 Additionally, complete sleep deprivation and selective rapid eye movement (REM) sleep and slow wave sleep deprivation are associated with immune system dysfunction.14, 15 Animal models and studies in other patient populations suggest links to other negative outcomes including derangements in glucose metabolism,16, 17 adverse cardiovascular events,18 cognitive dysfunction,19, 20 and all-cause mortality.21
Polysomnography (PSG) is the gold standard of sleep measurement and allows full delineation of sleep duration, continuity, and architecture, characterized by sleep stages and electrophysiological arousals. Reports of PSG use in the ICU have focused primarily on mechanically ventilated patients.8, 22, 23 Studies of non-ventilated patients have most commonly included healthy volunteers in simulated ICU environments.24-27 Barriers such as large amounts of technician time, concerns about lead displacement, electrical interference, and difficulties with scoring sleep in the ICU have been noted by previous investigators but not rigorously studied.28-32
Existing PSG studies demonstrate that the sleep of critically ill patients is severely disrupted. ICU patients have more difficulty falling asleep, more difficulty progressing through sleep stages, more arousals, decreased REM sleep, and decreased N3 sleep.1, 2, 25, 33-38 In addition, sleep is distributed throughout the 24-hour day rather than consolidated during the night, as during normal circadian cycling.2, 35, 38 Several groups have identified a subgroup of ICU patients with atypical sleep patterns of unknown clinical significance.35, 39, 40 Atypical sleep's most prominent characteristic is electroencephalography (EEG) tracings consistent with sleep but lacking sleep spindles, K complexes and other markers of specific non-REM (NREM) sleep stages. REM sleep has also been identified as diminished in this population.
Given the need for PSG measures of ICU sleep and the limited data regarding barriers to data acquisition and scoring, the objective of this study was to establish the feasibility of monitoring the sleep of medical ICU (MICU) patients via unattended portable PSG (type 2 device) for a 24 hour period. Fragmentation of ICU patient sleep across the 24 hour day led us to include both day and night recording periods. Technical feasibility outcomes included the ability to obtain EEG data of sufficient quality to determine sleep stages, sleep efficiency, and arousals from sleep. Acceptability outcomes included patient, family and staff acceptance of PSG, and a review of the reasons given for premature discontinuation.
METHODS
Study Design and Setting
This was an observational, cross-sectional study. We obtained approval for the study from the Institutional Review Board and informed consent was obtained from all participants. The study was conducted at a 1,500 bed academic teaching hospital in New England with a 38 bed closed MICU staffed by university faculty physicians. The MICU is new construction (completed in 2010) with all private rooms separated by solid walls and glass sliding doors. The unit is rectangular in design with a administrative desk at the single main entrance. The central core of the rectangle is comprised of enclosed supply and conference rooms. There is no central nursing station. Most clinical staff work 7:00 AM to 7:00 PM shifts. Lab work, wound care, bathing and routine radiology most typically occur overnight (specifically within the 10:00 PM to 6:00 AM period)
Inclusion and Exclusion Criteria, Patient Characteristics
We included English-speaking patients older than 18 years of age who were admitted to the MICU for less than 72 hours and who were expected to stay for at least an additional 24 hours at the time of screening. Exclusion criteria included terminal illness, receipt of comfort care only, coma or deep sedation (Richmond Agitation Sedation Scale (RASS) score of -4 to -5),41 patient inability to consent and without an identifiable surrogate, anticipated procedure requiring sterile access to the head or neck during the PSG recording time, anticipated procedure requiring movement of patient out of the MICU during the PSG recording time, severe agitation (agitated to touch, hallucinations, uncontrolled pain, violent behavior), and anatomic contraindication to PSG (including infections of the head or neck, recent surgery of the head or neck, recent trauma to the head or neck). We did not exclude patients who were mechanically ventilated and did not use a severity of illness scoring system cut-point for study exclusion.
Medication usage was abstracted for the 48 hours prior to PSG initiation and during the PSG study. All medications, regardless of administration route, from the following classes were recorded: sleep aids, benzodiazepines, opioids, and vasoactive medications. Home medication use was included for study subjects who had their PSG initiated within 48 hours of hospital admission. If an entire day at home fell within the 48 hours prior to PSG initiation, all home medications were presumed to be taken unless this was explicitly contradicted in the chart. If a portion of a day fell within the 48 hours prior to PSG initiation, hospital arrival time was used to estimate what medications were likely taken prior to admission. Daily medications were presumed to be taken in the morning unless prescribed with a per bedtime designation. Twice daily medications were presumed to be taken in the morning and evening and thrice daily medications were presumed to be taken in the morning, midday and evening.
Study Procedures
Patients or their surrogates gave verbal consent for study participation. A waiver of signed consent was granted by the Institutional Review Board. Surrogates were approached for consent in the event of patient delirium (positive Confusion Assessment Method for the ICU (CAM-ICU)) status,42, 43 patient sedation (RASS -1 to -5) and/or inability of the patient to communicate with study staff. No compensation was offered for study participation. Reasons for ineligibility were recorded. Patient demographic and medical data were collected for enrolled and non-enrolled patients to assess for enrollment bias secondary to patient age, sex, diagnosis, severity of illness or ventilator status. The Acute Physiology and Chronic Health Evaluation II (APACHE II)44 scoring algorithm was utilized for estimation of illness severity. Delirium status was also monitored on the day of enrollment and during PSG recording via CAM-ICU42, 43 and chart review.45
Polysomnography
Patients underwent unattended 24-hour PSG obtained with the Safiro Portable Data Acquisition System (Compumedics, Abbotsford, VIC, Australia) in the setting of otherwise usual MICU care. PSG studies were initiated in the evening and terminated at or before 24 hours. Leads were applied by a board certified sleep technician (RPSGT) familiar with the MICU clinical setting. Leads included 6 EEG channels (C3, C4, F3, F4, O1, O2) referenced to the contralateral mastoid. A chin electromyogram (EMG), right and left electrooculograms (EOC), and electrocardiogram (ECG) were also recorded. EEG signals were amplified, recorded at 200 hertz (Hz) sampling frequency and filtered (0.5 to 70 Hz). The signals were recorded on the Safiro device and later transferred to a laptop computer with Profusion 2 software (Compumedics, Abbotsford, VIC, Australia). The studies were not attended by the technician once the leads were applied and recordings were started. The MICU nurses responsible for caring for the patients were instructed on lead removal and safe equipment shut down in the event of transfer or clinical need. They were not instructed to replace leads in the event of disconnection.
Information regarding duration of the PSG study and reasons for early termination of the PSG study were collected. PSG data were scored by RPSGTs according to standard American Academy of Sleep Medicine (AASM) protocols.46 The technologist scoring this data participated in the AASM inter-scorer reliability program and maintained a 93% agreement with AASM epochs over the most recent eight month period reviewed at the time of the study. Individual epochs were scored as wake, sleep stage N1, sleep stage N2, sleep stage N3 or REM sleep. If a determination could not be made for a particular epoch, the epoch was marked “unsure,” and if the epoch could be identified as sleep, but the stage of sleep was unclear, the epoch was marked “sleep” (no stage). EEG scoring and PSG tracings were reviewed by sleep medicine physicians (HKY and MPK). Sleep data obtained during PSG were analyzed for sleep duration, diurnal sleep timing, sleep stage distribution, arousal indices and the presence or absence of atypical sleep features.35, 47 Patients were considered to have atypical sleep following the definitions of Cooper et al.35 and Drouot et al.39 Briefly, these patients had EEG consistent with stage N2 sleep but lacking sleep spindles and K complexes. Definitions for sleep related terminology are provided in Table 1.
Table 1.
| Term | Definition |
|---|---|
| Polysomnography | Sleep study which includes multiple measurements to assess sleep architecture and physiological parameters during sleep. Type 1 studies are attended in-laboratory studies with full sleep staging including: EEG, EOG, heart rate or ECG, Chin EMG, Limb EMG, respiratory and abdominal effort belts, nasal cannula flow thermistor, pulse oximetry. Type 2 studies are unattended sleep studies with a minimum of 7 channels including: EEG, EOG, chin EMG, heart rate or ECG, airflow, respiratory effort, and oxygen saturation. Type 3 and 4 studies have no EEG monitoring and are successively more limited. |
| Sleep architecture | The basic structure of sleep; normally composed of a cyclical pattern of NREM and REM sleep stages (see below). |
| Electrophysiological arousal | Abrupt change from sleep to wakefulness or a lighter stage of sleep (N1 < N2 < N3 sleep depth; NREM < REM sleep depth). Arousals are defined by EEG when there is an abrupt shift of EEG frequency that lasts 3 seconds with at least 10 seconds of stable sleep before the change. Scoring of arousals during REM sleep requires a change in submental EMG tone. |
| Sleep duration | Hours per day of sleep. |
| Sleep continuity | Amount and distribution of sleep versus wake in a given period; it includes elements of sleep initiation and sleep maintenance. |
| Sleep efficiency | Amount of sleep obtained in a time period of sleep opportunity. |
| NREM sleep | Non-REM sleep which includes stage N1, N2 and N3. |
| Stage N1 sleep | NREM stage 1: The stage between sleep and wakefulness; “light sleep;” Muscles are active, and the eyes roll slowly, opening and closing moderately; typically 2 to 5% of total sleep time. |
| Stage N2 sleep | NREM stage 2: The first stage of “real sleep;” sleepers become gradually harder to awaken; characteristic EEG findings of sleep spindles and K-complexes; typically 45 to 55% of total sleep time. |
| Stage N3 sleep | NREM stage 3: Slow wave or restorative sleep; “deep sleep;” N3 was formerly divided into stages 3 and 4; characteristic EEG findings of high amplitude delta waves on EEG; typically 20% of total sleep time. |
| REM sleep | Rapid Eye Movement Sleep: Dream sleep with associated paralysis and stereotypic eye movements on EOG leads; typically 20 to 25% of total sleep time. |
Data Analysis
Data analyses were performed with SAS software V9.3 (SAS Institute, North Carolina). Continuous variables were expressed as a mean with standard deviation (SD) or median with interquartile range. Frequencies and percentages were used to express categorical data. Differences in frequencies or percentages between groups were tested with a chi-square statistic; differences in means were tested with an unpaired Student's t-test; and differences in medians were tested with the Wilcoxon Two Sample statistic. APACHE II score was calculated according to published algorithms.44
PSG studies with a length of less than four hours were examined for feasibility metrics but were excluded from analysis of sleep architecture. For calculation of sleep efficiency, stage proportions, and arousal indices, the whole study was considered to be from the first to last study epoch. Overnight was considered to be from 10:00 PM to 6:00 AM and all other hours were considered as daytime. For each individual patient, sleep efficiency was calculated by dividing total sleep time by total time available for sleep for a given period (whole study, overnight, or daytime). Sleep stage proportions were similarly calculated by dividing the amount of a given stage of sleep by the total sleep time in a given period. Arousal indices were calculated by dividing the total number of arousals in either REM or NREM sleep by the total amount of REM or NREM sleep in hours. A mean (SD) was then calculated across the study population for each sleep variable included in the sleep architecture analysis.
RESULTS
Patient characteristics
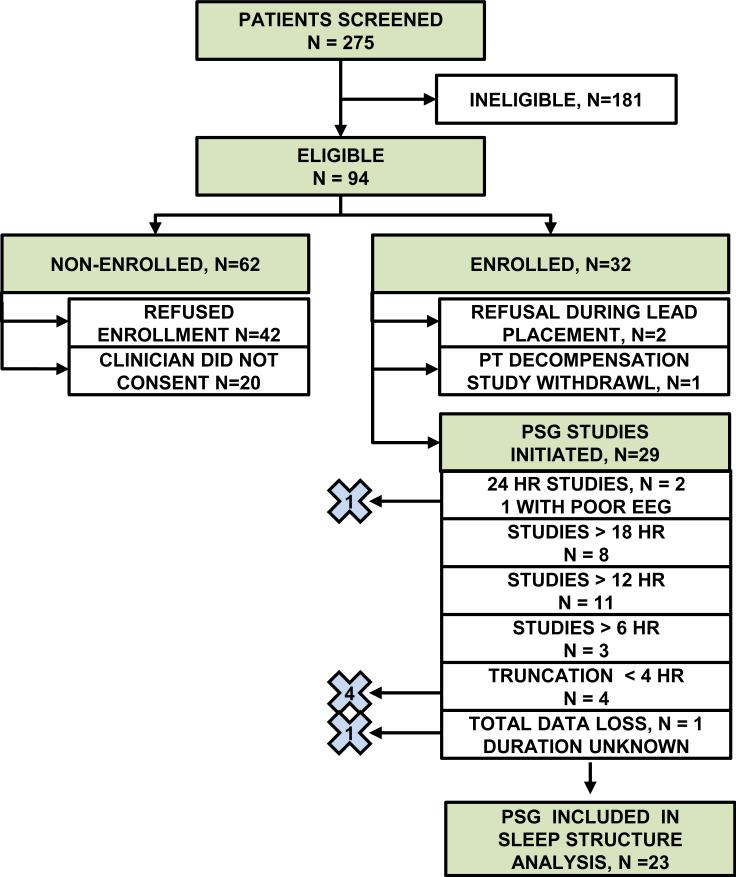
We screened 275 patients, of whom 181 patients were ineligible, yielding 94 eligible patients (see Figure 1). Ineligible status was due to a variety of factors; lack of identifiable surrogate (56), patient was comatose (32) and patient expected to die within the next 24 hours (26) were dominant reasons. Of the 62 eligible, non-enrolled patients, 20 patients were not approached because consent was not granted by a member of the clinical team; the other 42 patients declined to participate. Thirty-two eligible patients were enrolled. Two patients who were initially enrolled subsequently declined further participation once exposed to the lead application process. One patient deteriorated clinically, and the family requested withdrawal from the study. Ultimately 29 patients had PSG studies initiated; feasibility analysis was based on this cohort.
Figure 1.
Patient enrollment, screening, and eligibility flow diagram. “×” with number indicates patients excluded from PSG sleep architecture analysis. HR indicates length of study in hours; PT indicates patient; PSG indicate polysomnogram.
Baseline demographic, medical and severity of illness characteristics of the enrolled patients for whom PSG (N=29) was attempted are described in Table 2. Patients had a mean age of 59.2 years and average length of MICU stay of 10.9 days. Reasons for ICU admission were diverse and dominated by sepsis and respiratory failure. Mean APACHE II score was 13.9 with 24% of patients dependent on mechanical ventilation. Comparison of enrolled and non-enrolled MICU patients reveals no significant differences in age, gender, race, reason for ICU admission, severity of illness or mechanical ventilation status. Furthermore, the patients with PSG studies greater than four hours in length (n=23) and whose PSGs were ultimately included in the sleep architecture analyses did not differ significantly in age, gender, race, reason for ICU admission, severity of illness or mechanical ventilation status compared to the general enrollment group (N=29) or the non-enrolled patients (data not shown).
Table 2.
Clinical and demographic characteristics of enrolled study participants.
| Baseline Demographics | |
|---|---|
| Enrolled N=29 | |
| Age in Years: mean (SD) | 59.2 (17.8) |
| Male Gender: n (%) | 19 (66) |
| Race, Non-white: n (%) | 12 (41) |
| MICU Length of Stay in Days: mean (SD) | 10.9 (7.7) |
| Time in Sleep Study in Days: mean (SD) | 3.6 (2.8) |
| Reason for ICU Admission: n (%) | |
| Enrolled N=29 | |
| Infection, Sepsis: n (%) | 11 (38) |
| Respiratory Failure: n (%) | 5 (17) |
| Heart Failure: n (%) | 1 (3.5) |
| Pulmonary Embolism: n (%) | 1 (3.5) |
| Gastrointestinal Bleed: n (%) | 1 (3.5) |
| Liver Disease: n (%) | 1 (3.5) |
| Acute Kidney Injury: n (%) | 2 (7) |
| Neurologic Injury: n (%) | 2 (7) |
| Other: n (%) | 5 (17) |
| Severity of Illness Parameters | |
| Enrolled N=29 | |
| APACHE II Score: mean (SD) | 13.9 (7.7) |
| Mechanical Ventilation: n (%) | 7 (24) |
| Delirium, n (%) | 10 (34) |
Patient medication use included drugs that influence sleep (see Table 3). Thirty-five percent (10/29) of all enrolled patients received benzodiazepines; sixty-six percent (19/29) received opioids; seven percent (2/29) received sleep aids. Thirty-one percent (9/29) of patients received none of these three classes of medication. No study patients received propofol or dexmedetomidine.
Table 3.
Medication Use: Patients Receiving Medication from Listed Drug Class: n (%).
| Medication Class | |||
|---|---|---|---|
| All Patients N=29 | Patients with Typical Sleep n =14 | Patients with Atypical Sleep n=9 | |
| Sleep Aid | 2 (7) | 1 (7) | 1 (11) |
| Benzodiazepine | 10 (35) | 7 (50) | 1 (11) |
| Opioid | 19 (66) | 10 (71) | 5 (56) |
| NO Sleep, Benzodiazepine or Opioid | 9 (31) | 3 (21) | 4 (44) |
| Vasopressor | 7 (24) | 3 (21) | 3 (33) |
PSG data quality
Unattended PSG sleep data were interpretable using standard AASM criteria46 in the 23 studies included in the sleep architecture analysis; these studies were selected on the basis of length (greater than 4 hours). The sleep data obtained from 4 additional studies were interpretable, but were excluded from sleep architecture analysis on the basis of short duration (less than 4 hours). Two studies had no interpretable data. There was no evidence of electrical (60 Hz) interference from the ICU equipment; devices known to be on or near patients during PSG recording included telemetry nodes and leads, oximetry nodes and leads, mechanical ventilators, mobile computer units (utilized for bedside recording into the electronic medical record), sequential compression devices, and hemodialysis units (non-continuous).
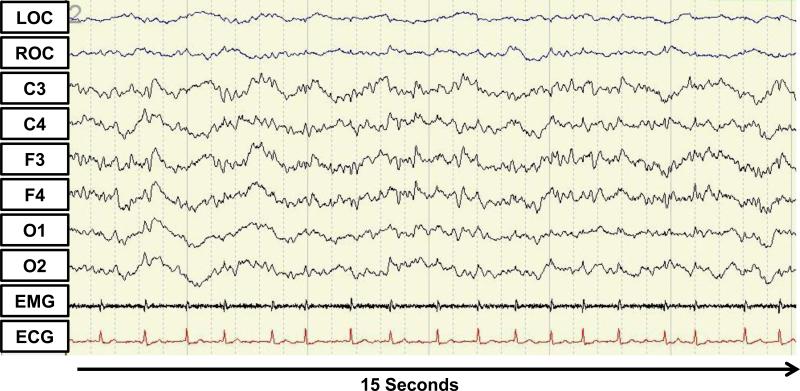
The proportion of epochs in all interpreted studies that was scored as unsure was 9.5%. Re-evaluation of these studies, using the criteria of Cooper et al.35 and Drouot et al.39 for atypical ICU sleep revealed that 9 of 26 (35%) patients with interpretable EEG and at least one epoch of sleep present during their study had atypical critical care sleep patterns; of the 27 patients with interpretable sleep data, 1 patient with a short PSG had only wake recorded and could therefore not be assessed for atypical sleep. Figure 2 presents a sample EEG tracing of atypical sleep. Based on CAM-ICU and chart review, 4 of 9 (44%) patients with atypical sleep had clinical evidence of delirium as compared to 3 of 17 (18%) of patients with typical sleep; this difference did not reach statistical significance.
Figure 2.
Electroencephalography tracing from an atypical sleep patient. LOC and ROC indicate left and right electrooculogram respectively. C3, C4, F3, F4, O1, O2 indicate EEG lead locations. EMG indicates electromyogram and ECG indicates electrocardiogram.
Atypical sleepers received benzodiazepines 11% (1/9) of the time, opioids 56% (5/9) of the time and on sleep aids 11% (1/9) of the time. Forty-four percent (4/9) of atypical sleepers received none of these three classes of medication. Typical sleep patients received benzodiazepines 50% (7/14) of time, opioids 71% (10/14) and sleep aids 7% of the time (1/14). Twenty-one percent (3/14) of typical sleepers received none of these three classes of medication (see Table 3).
For typical sleep patients, repeat analysis of epochs marked as “unsure” by the sleep technician allowed classification of most epochs as either wake or stage N1 sleep. The majority of the uncertainty reflected difficulty in identifying the transition from wake to sleep in patients with frequent variation in sleep wake status (i.e. micro-sleeps). Following review of all sleep studies by sleep medicine physicians (MPK and HKY), and, considering atypical sleep, the proportional amount of scoring uncertainty was reduced to 0.3%.
PSG study duration
Nocturnal recordings of at least four hours in length were accomplished for 23 of 29 patients. For all initiated studies, median sleep study duration was 15.1 hours (Table 4A). Reasons for unattended PSG discontinuation are delineated in Table 4B. Patient request for discontinuation was the most common reason for incomplete studies with 9 of 29 patients (31%) requesting leads to be removed before completing 24 hours of monitoring; typically these patients requested lead removal in the early morning upon “waking from sleep.” Qualitative comments from patients requesting lead removal reflected patient discomfort with leads and a desire to move from bed to chair. Two patients requested lead removal at or before four hours of data acquisition which resulted in exclusion of their PSG data from sleep architecture analysis; both of these patients named lead discomfort and inability to sleep as the reason for their request. Patient transfer out of the MICU to the floor was also common (8 of 29 studies, 28%).
Table 4.
PSG duration and reasons for discontinuation prior to 24 hours.
| A. PSG recording time in hours. | ||
|---|---|---|
| All Patients n=28a | Ventilated Patients n=7 | |
| Minimum Value | 1.4 | 6.3 |
| 1st Quartile | 11.0 | 9.9 |
| Median | 15.1 | 16.9 |
| 3rd Quartile | 19.0 | 21.6 |
| Maximum Value | 24.0 | 21.8 |
| B. PSG reason for discontinuation prior to 24 hour goal. | ||
| All Patients N=29 | Ventilated Patients n=7 | |
| Patient request | 9 (31%) | 1 (14%) |
| Patient transfer | 8 (28%) | 1 (14%) |
| PSG technical failure | 5 (17% | 3 (43%) |
| Other | 3 (10%) | 1 (14%) |
| Patient condition deterioration | 2 (7%) | 1 (14%) |
| 24 hours completed | 2 (7%) | 0 (0%) |
No data recorded on one patient; length of study unknown
Patients who were mechanically ventilated (n=7) had a median (interquartile range) study duration in hours of 16.9 (11.7). There was no significant difference in study duration compared with non-ventilated patients. As might be expected, these patients were more severely ill than the non-ventilated study sample (mean APACHE II score = 20.7, p = 0.01). Mechanically ventilated patients requested discontinuation (1 of 7, 14%) and were transferred (1 of 7, 14%) less frequently than the general study population (see above). There were trends suggesting that the patients in the most severely ill quartile (APACHE scores 19-33) had longer studies with a mean (SD) duration in hours of 14.0 (6.3) versus those in the least severely ill quartile (APACHE scores 2-8), with mean (SD) duration in hours of 7.9 (5.7) (p = 0.09).
Technical limitations
Technical difficulties occurred in 8 of 29 PSG studies. Technical difficulties were significant enough to be the primary reason for PSG discontinuation for 5 of them. In 3 cases, these technical problems led to complete PSG data exclusion. One excluded patient had significant scalp edema and although leads remained in place for the full 24-hours, EEG recording quality was severely limited and therefore uninterpretable. A second patient experienced equipment failure with no data recorded on the Safiro device; leads remained in place without difficulty throughout this study. Unexplained data truncation occurred in 3 of 29 patients, although in only one case did this cause significant shortening of the study (PSG length 12 hours or less) and resultant data exclusion. Unintended lead removal occurred in 3 of 29 patients (10%) and, in two cases, this caused significant shortening of the study (PSG length 12 hours or less). For the three patients with lead removal, continuous nebulization treatment and patient delirium were factors that contributed to difficulty with lead maintenance.
Patient sleep characteristics
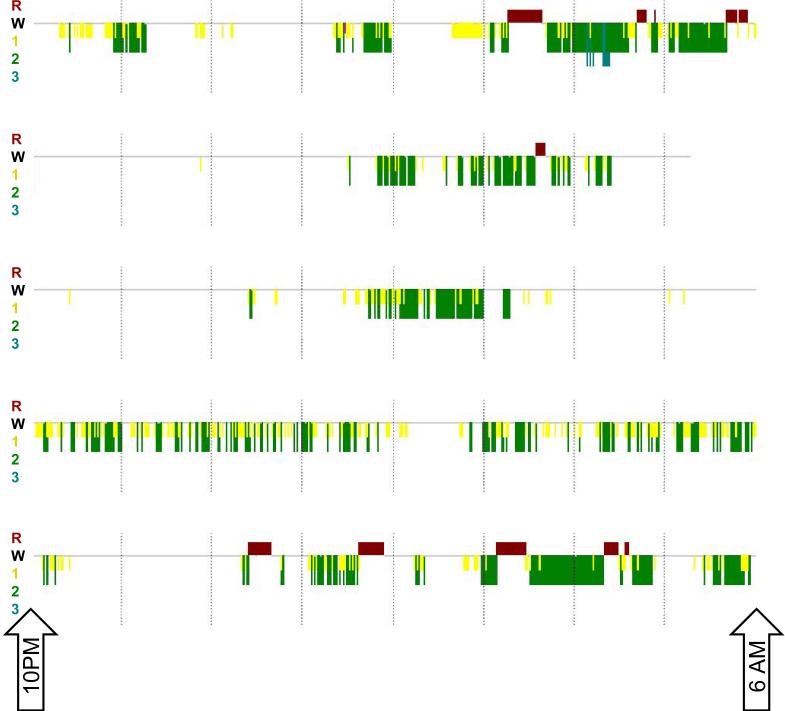
For all patients, the overall mean (SD) sleep efficiency was 38.0% (16.6) for the entire period of PSG recording. Analysis of sleep between 10:00 PM and 6:00 AM revealed an overnight mean (SD) sleep efficiency of 48.8% (20.5). During daytime recording hours (6:00 AM to 10:00 PM), patients were asleep 29.2% (SD 23.3) of the time. Mean (SD) wake after sleep onset in the overnight period was 155 (85.6) minutes. Representative 10:00 PM to 6:00 AM hypnograms reveal the highly fragmented overnight sleep of study patients (Figure 3). Review of the sleep architecture demonstrated a paucity of N3 slow wave sleep (3.9% of total sleep time) and REM sleep (10.5% of total sleep time). Stage N1 sleep comprised 25.8% of all sleep and stage N2 sleep comprised 59.7% of all sleep. Stage distribution did not vary significantly in overnight versus daytime sleep; there was a trend towards increased REM proportion during daytime versus overnight hours. Patients experienced frequent arousals during their sleep with an overall NREM arousal index of 30.4 per hour and a REM arousal index of 16.4 per hour. This rate of arousal did not vary significantly between overnight and daytime sleep. Table 5A and B present sleep parameters for typical and atypical patient subsets. Patients with typical and atypical sleep patterns had similar sleep efficiencies, sleep architecture and arousal indices.
Figure 3.
Representative hypnograms between 10:00PM and 6:00AM reflecting patient sleep fragmentation in 5 typical sleep patients. R (bar above horizontal line) indicates REM sleep. W (gray line without bars above or below horizontal line) indicates wake state. 1, 2, and 3 (with bars of increasing depth below horizontal line) indicate stage N1, N2 and N3 sleep respectively.
Table 5.
Sleep duration, architecture and arousal indices.
| A. Sleep characteristics of MICU patients with Typical Sleep (n=14). | |||
|---|---|---|---|
| Whole Study Mean (SD) | Overnight Mean (SD) | Daytime Mean (SD) | |
| Sleep Quantity | |||
| Hours Available for Sleep | 14.8 (4.1) | 7.6 (1.2) | 7.2 (3.4) |
| Hours of Total Sleep Time | 6.2 (1.5) | 4.2 (1.6) | 2.0 (1.1) |
| Sleep Efficiency (%) | 44.1 (13.7) | 54.3 (18.2) | 35.2 (25.5) |
| Sleep Architecture | |||
| Proportion REM (%) | 12.9 (14.2) | 11.8 (12.2) | 18.5 (24.3) |
| Proportion N1 (%) | 22.7 (13.4) | 23.4 (13.8) | 24.3 (13.3) |
| Proportion N2 (%) | 60.4 (12.3) | 60.7 (11.4) | 54.6 (20.5) |
| Proportion N3 (%) | 3.9 (5.9) | 4.1 (6.8) | 2.4 (4.9) |
| Arousals | |||
| NREM Arousal Index | 33.0 (13.3) | 32.3 (13.8) | 34.6 (14.1) |
| REM Arousal Index | 18.7 (16.1) | 9.2 (7.3) | 23.0 (17.5) |
| B. Sleep characteristics MICU patients with Atypical Sleep (n=9). | |||
| Whole Study Mean (SD) | Overnight Mean (SD) | Daytime Mean (SD) | |
| Sleep Quantity | |||
| Hours Available for Sleep | 17.2 (5.3) | 7.7 (0.9) | 9.5 (4.7) |
| Hours of Total Sleep Time | 4.8 (3.4) | 3.0 (1.7) | 1.7 (2.2) |
| Sleep Efficiency (%) | 28.5 (16.8) | 40.1 (21.7) | 18.7 (14.8) |
| Sleep Architecture | |||
| Proportion REM (%) | 6.8 (6.0) | 7.5 (10.5) | 9.4 (10.0) |
| Arousals | |||
| NREM Arousal Index (per hour) | 26.5 (6.0) | 27.4 (9.0) | 23.4 (4.4) |
| REM Arousal Index (per hour) | 12.4 (4.9) | 21.4 (8.6) | 4.0 (5.6) |
DISCUSSION
We have demonstrated that it is technically feasible to obtain high quality unattended polysomnographic data among critically ill patients. However, several patients tolerated the PSG procedure poorly. ICU patient tolerance of PSG has not been reported in the literature and may be related to the inclusion of non-ventilated patients in our study population. We felt this was an important group to study as this group may be more easily targeted for sleep improvement interventions.
Readily interpretable sleep EEG data was recorded in 93% (27/29) patients. Consistent with past studies, patient sleep in the MICU was highly fragmented and was distributed across day and night time periods.38, 48 Sleep efficiencies overnight were low, and there was an abnormally high amount of sleep during the daytime period. Total sleep time of 5.6 hours indicates generalized sleep deprivation, and an evaluation of the distribution of the sleep stages specifically indicates N3 and REM specific sleep deprivation. Additionally, approximately one third of patients had evidence of atypical sleep.
Technical issues were encountered during our unattended PSG studies. Data truncation and data loss were the most significant technical issues and did significantly shorten studies (PSG time less than 12 hours). This likely could have been avoided had the studies been attended. Lead instability contributed to shortening of several studies, though this caused significant shortening (study length less than 12 hours) of the study in only 2 cases. Despite data and lead issues, ICU-based use of unattended PSG conveys a cost and personnel savings in comparison to fully attended monitoring, and, therefore, is a more efficient strategy overall. In future studies nurse training could be performed to minimize some but not all of the lead complications. There was no ICU equipment interference with our EEG recordings, despite previous reports of this problem in the literature.31 Overall, we judge unattended PSG in the MICU to be technically feasible.
Notably, patient, family and caregiver tolerance or perceived tolerance of PSG is poor. This is reflected in the large number of patients deemed “inappropriate” by a member of their caregiving team, the low screen to enrollment ratio and the frequent request by patients to have their studies discontinued. Patients specifically described the leads as limiting mobility and their ability to sleep. Severity of illness did not appear to influence enrollment, and the more severely ill patients tended to have longer unattended PSG recording times. The fact that these patients were unlikely to be transferred and / or possibly unable to request study discontinuation likely contributed to the longer recording times. Patients undergoing mechanical ventilation were less likely to request lead discontinuation than non-ventilated patients perhaps due to the severity of their illness and / or related to their inability to communicate.
Half of our patients had an APACHE II score less than or equal to 14 (estimated hospital mortality 15% or less).44 Among these patients with a lower severity of illness, turnover of patients in the MICU played a role in PSG study length. About one third of patients had their studies stopped in anticipation of transfer to another ward. While this does not directly inform issues of PSG feasibility, this does highlight the fast turnover of today's MICU admissions. Because critically ill patients move to different physical locations and are cared for by multiple teams in rapid succession, sleep improvement interventions should be standardized and integrated across levels of inpatient care. These results regarding enrollment barriers, patient intolerance and patient turnover were echoed in a recent study by Elliot et al. which enrolled 57 out of 266 eligible patients; of the 209 non-enrolled patients, impending discharge from the ICU prevented enrollment in 135 cases and patient refusal prevented enrollment in 74 cases.38
Review of the primary PSG data allowed identification of an important subclass of sleep patients – critically ill patients with atypical sleep. Inclusion of atypical sleep during EEG interpretation reduced scoring uncertainty to virtually zero. Cooper et al.35 originally identified atypical sleep in 5 ICU patients (out of 20 total patients) and described it as sleep with characteristics intermediate between sleep and coma. Drouot et al.39 further described atypical sleep in a cohort of 16 ICU patients (out of 57 total patients) who also demonstrated a lack of K complexes and sleep spindles in epochs that were otherwise consistent with N2 sleep. Atypical sleep seems to occur in a significant fraction of critically ill patients and may clarify prior difficulties in ICU PSG scoring.49 Although atypical sleep may signal pathophysiology related to delirium, medication use or severity of illness, no direct correlation among these clinical factors and atypical sleep was found in our cohort or of those previously described.35, 39 Notably, Drouot et al. saw this atypical pattern in non-sedated patients who were in respiratory failure, and we note that 44% of the atypical sleep patients in our study were on none of the following medications: benzodiazepines, opioids or sleep aids. This suggests that medication effects may not explain atypical sleep. Total sleep time, sleep architecture and arousal indices did not differ between typical and atypical sleepers in our study. Potentially owing to the limited power provided by our small sample, we did not observe a statistically significant relationship between atypical sleep and delirium. There was a trend towards higher rates of delirium in atypical sleepers. Further characterization of atypical ICU sleep and its correlates and consequences is needed to advance the field.
Finally, our data highlight the importance of measuring sleep in critically ill, non-ventilated patients. Seventy-six percent of our enrolled and 87% of our non-enrolled patients were not ventilated. Our work is unique compared to the published literature that has focused on studying sleep in mechanically ventilated patients. As indicated by our PSG studies and prior studies with healthy volunteers in simulated ICU environments25, 26, 50 and non-ventilated ICU patients,38, 48 these patients also experience severe sleep disruption and circadian rhythm loss similar to that of their ventilated counterparts. They are similarly at risk for ICU delirium and its downstream morbidity and mortality. In addition it may be easier to improve upon their sleep quality without the significant barrier of overcoming mechanical ventilation related sleep disturbance. A challenge to further study of these patients will be improving patient, family and caregiver acceptance of PSG and patient tolerance of the PSG lead application and monitoring.
Study Limitations
This study was focused upon assessment of the technical feasibility of unattended PSG. Acceptability by patients, family and clinical staff emerged as a limitation to PSG use. The somewhat low screen to enrollment rate reflected a significant lack of acceptance by clinical staff who indicated that their patients were not appropriate for study participation. In addition, patient discomfort and patient transfer during PSG recording time significantly truncated many studies. As these were not anticipated barriers, they were not investigated with formal instruments. It is somewhat reassuring that patient demographic profiles, reason for ICU admission, severity of illness (APACHE II) and ventilator status were similar between enrolled and unenrolled patient groups. It is notable that less severely ill patients and non-ventilated patients had generally shorter recording times which reflected a higher rate of patient transfer and the ability to communicate and therefore requests lead removal. Despite these limitations, our study demonstrates sleep architecture and arousal indices in-line with other ICU studies. Our primary goal of investigating the technical feasibility of unattended PSG use was accomplished.
Implications for Practice
The findings of this study, in conjunction with other ICU studies of sleep deprivation, suggest that patient sleep in the ICU is profoundly disrupted. Clinical providers need to invest greater attention to sleep promotion during day and night periods. Night staff should avoid unnecessary overnight disturbance of patients, and day staff should encourage wakefulness during the day time period. Less severely ill and non-ventilated ICU patients experience severe sleep loss, along with their more ill and / or ventilated counterparts, and may be an ideal target population for sleep improvement interventions.
Implications for future research
PSG provides unique and important data to ICU sleep deprivation studies. For example, future explorations of atypical sleep would require sleep monitoring that includes EEG leads. The successful use of an unattended PSG inclusive of EEG without electrical interference or severe data loss indicates that this is an accurate, cost effective modality for use in ICU research studies. There are issues with patient, family and staff acceptability that pose a barrier to patient enrollment.
CONCLUSIONS
Unattended PSG with full sleep EEG montage is a technically feasible methodology for the study of sleep in the ICU. Technical limitations were acceptably low and data produced from these studies was generally of high quality. Patient intolerance of the PSG procedure was largely due to discomfort with leads. MICU sleep was highly disrupted with prominent loss of REM and N3 sleep. Critically ill patients had deranged circadian sleep patterns with abnormally high amounts of sleep occurring during the day. About one third of patients demonstrated atypical sleep. Recognition of this unusual sleep pattern clarified prior difficulties in scoring the sleep of ICU patients.
ACKNOWLEDGEMENTS
We appreciate the assistance of our RPSGT colleagues Elizabeth Lowe, Vincent McClain and Rebecca Khozein who executed the polysomnography studies.
Sources of Funding: This work was supported by a grant to Dr. Pisani 5R21NR11066, by a grant to Drs. Knauert, Yaggi and Redeker 5P20NR014126, and by a grant to Dr. Murphy and Ms. Araujo P30AG21342.
List of abbreviations
- AASM
American Academy of Sleep Medicine
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- CAM-ICU
Confusion assessment method for the ICU
- ECG
electrocardiogram
- EEG
electroencephalography
- EMG
electromyogram
- EOC
electrooculogram
- Hz
hertz
- ICU
Intensive care unit (specialty not designated)
- MICU
Medical intensive care unit
- NREM
Non-rapid eye movement sleep
- PSG
Polysomnography
- RASS
Richmond agitation sedation scale
- REM
Rapid eye movement sleep
- RPSGT
Board certified sleep technician
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that they have no conflict of interests.
REFERENCES
- 1.Kamdar BB, Needham DM, Collop NA. Sleep deprivation in critical illness: its role in physical and psychological recovery. J Intensive Care Med. 2012;27:97–111. doi: 10.1177/0885066610394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 3.Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102–12. quiz 14-5. [PubMed] [Google Scholar]
- 4.Celik S, Oztekin D, Akyolcu N, Issever H. Sleep disturbance: the patient care activities applied at the night shift in the intensive care unit. J Clin Nurs. 2005;14:102–6. doi: 10.1111/j.1365-2702.2004.01010.x. [DOI] [PubMed] [Google Scholar]
- 5.Le A, Friese RS, Hsu CH, Wynne JL, Rhee P, O'Keeffe T. Sleep disruptions and nocturnal nursing interactions in the intensive care unit. J Surg Res. 2012;177:310–4. doi: 10.1016/j.jss.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, et al. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–12. [PubMed] [Google Scholar]
- 7.Oto J, Yamamoto K, Koike S, Imanaka H, Nishimura M. Effect of daily sedative interruption on sleep stages of mechanically ventilated patients receiving midazolam by infusion. Anaesth Intensive Care. 2011;39:392–400. doi: 10.1177/0310057X1103900309. [DOI] [PubMed] [Google Scholar]
- 8.Gehlbach BK, Chapotot F, Leproult R, Whitmore H, Poston J, Pohlman M, et al. Temporal disorganization of circadian rhythmicity and sleep-wake regulation in mechanically ventilated patients receiving continuous intravenous sedation. Sleep. 2012;35:1105–14. doi: 10.5665/sleep.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, et al. Bench-to-bedside review: delirium in ICU patients - importance of sleep deprivation. Crit Care. 2009;13:234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, et al. The Effect of a Quality Improvement Intervention on Perceived Sleep Quality and Cognition in a Medical ICU*. Crit Care Med. 41:800–9. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard TD, Jackson JC, Pandharipande PP, Pun BT, Thompson JL, Shintani AK, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38:1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–81. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollinger T, Bollinger A, Oster H, Solbach W. Sleep, immunity, and circadian clocks: a mechanistic model. Gerontology. 2010;56:574–80. doi: 10.1159/000281827. [DOI] [PubMed] [Google Scholar]
- 15.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faraut B, Touchette E, Gamble H, Royant-Parola S, Safar ME, Varsat B, et al. Short sleep duration and increased risk of hypertension: a primary care medicine investigation. J Hypertens. 2012;30:1354–63. doi: 10.1097/HJH.0b013e32835465e5. [DOI] [PubMed] [Google Scholar]
- 19.Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson ML, Gunzelmann G, Whitney P, Hinson JM, Belenky G, Rabat A, et al. Deconstructing and reconstructing cognitive performance in sleep deprivation. Sleep Med Rev. 2013;17:215–25. doi: 10.1016/j.smrv.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro-Costa E, Dewey ME, Ferri CP, Uchoa E, Firmo JO, Rocha FL, et al. Association between sleep duration and all-cause mortality in old age: 9-year follow-up of the Bambui Cohort Study, Brazil. J Sleep Res. 2011;20:303–10. doi: 10.1111/j.1365-2869.2010.00884.x. [DOI] [PubMed] [Google Scholar]
- 22.Parthasarathy S, Tobin MJ. Sleep in the intensive care unit. Intensive Care Med. 2004;30:197–206. doi: 10.1007/s00134-003-2030-6. [DOI] [PubMed] [Google Scholar]
- 23.Ambrogio C, Koebnick J, Quan SF, Ranieri M, Parthasarathy S. Assessment of sleep in ventilator-supported critically III patients. Sleep. 2008;31:1559–68. doi: 10.1093/sleep/31.11.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topf M, Davis JE. Critical care unit noise and rapid eye movement (REM) sleep. Heart Lung. 1993;22:252–8. [PubMed] [Google Scholar]
- 25.Topf M, Bookman M, Arand D. Effects of critical care unit noise on the subjective quality of sleep. J Adv Nurs. 1996;24:545–51. doi: 10.1046/j.1365-2648.1996.22315.x. [DOI] [PubMed] [Google Scholar]
- 26.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 14:R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buxton OM, Ellenbogen JM, Wang W, Carballeira A, O'Connor S, Cooper D, et al. Sleep Disruption due to Hospital Noises: A Prospective Evaluation. Ann Intern Med. 2012;157:170–9. doi: 10.7326/0003-4819-157-3-201208070-00472. [DOI] [PubMed] [Google Scholar]
- 28.Redeker NS. Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32:31–8. doi: 10.1111/j.1547-5069.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 29.Drouot X, Cabello B, d'Ortho MP, Brochard L. Sleep in the intensive care unit. Sleep Med Rev. 2008;12:391–403. doi: 10.1016/j.smrv.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, Margarit L, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. 2012;13:7–14. doi: 10.1016/j.sleep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: Sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11:226. doi: 10.1186/cc5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson PL. Measuring sleep in critically ill patients: beware the pitfalls. Crit Care. 2007;11:159. doi: 10.1186/cc6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topf M. Effects of personal control over hospital noise on sleep. Res Nurs Health. 1992;15:19–28. doi: 10.1002/nur.4770150105. [DOI] [PubMed] [Google Scholar]
- 34.Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d'Ortho MP, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36:1749–55. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 35.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 36.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166:1423–9. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 37.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 38.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 17:R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, Margarit L, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. 13:7–14. doi: 10.1016/j.sleep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, et al. Atypical Sleep in Ventilated Patients: Empirical Electroencephalography Findings and the Path Toward Revised ICU Sleep Scoring Criteria. Crit Care Med. 2013 doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 42.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 44.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 45.Pisani MA, Araujo KL, Van Ness PH, Zhang Y, Ely EW, Inouye SK. A research algorithm to improve detection of delirium in the intensive care unit. Crit Care. 2006;10:R121. doi: 10.1186/cc5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SFftAAoSM. The AASM Manual for the Scoring of Sleep and Associated Events: Rulles, Terminology and Technical Specifications. 1st ed. American Acadmeny of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 47.Drouot X, Roche-Campo F, Thille AW, Cabello B, Galia F, Margarit L, et al. A new classification for sleep analysis in critically ill patients. Sleep Med. 2011;13:7–14. doi: 10.1016/j.sleep.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63:1210–4. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- 49.Watson PL, Pandharipande P, Gehlbach BK, Thompson JL, Shintani AK, Dittus BS, et al. Atypical sleep in ventilated patients: empirical electroencephalography findings and the path toward revised ICU sleep scoring criteria. Crit Care Med. 2013;41:1958–67. doi: 10.1097/CCM.0b013e31828a3f75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallace CJ, Robins J, Alvord LS, Walker JM. The effect of earplugs on sleep measures during exposure to simulated intensive care unit noise. Am J Crit Care. 1999;8:210–9. [PubMed] [Google Scholar]
- 51.Chesson AL, Jr., Berry RB, Pack A. American Academy of Sleep M, American Thoracic S, American College of Chest P. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26:907–13. doi: 10.1093/sleep/26.7.907. [DOI] [PubMed] [Google Scholar]
- 52.Carskadon MA, Dement WC. Normal Human Sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Saunders/Elsevier; Philadelphia, PA: 2011. [Google Scholar]