Abstract
New measures are needed to rapidly assess emerging treatments for cystic fibrosis (CF) lung disease. Using an imaging approach, we evaluated the absorptive clearance of the radiolabeled small molecule probe diethylene triamine penta-acetic acid (DTPA) as an in vivo indicator of changes in airway liquid absorption.
DTPA absorption and mucociliary clearance rates were measured in 21 patients with CF (12 adults and nine children) and nine adult controls using nuclear imaging. The effect of hypertonic saline on DTPA absorption was also studied. In addition, in vitro studies were conducted to identify the determinants of transepithelial DTPA absorption.
CF patients had significantly increased rates of DTPA absorption compared with control subjects but had similar mucociliary clearance rates. Treatment with hypertonic saline resulted in a decrease in DTPA absorption and an increase in mucociliary clearance in 11 out of 11 adult CF patients compared with treatment with isotonic saline. In vitro studies revealed that ~50% of DTPA absorption can be attributed to transepithelial fluid transport. Apically applied mucus impedes liquid and DTPA absorption. However, mucus effects become negligible in the presence of an osmotic stimulus.
Functional imaging of DTPA absorption provides a quantifiable marker of immediate response to treatments that promote airway surface liquid hydration.
Introduction
Cystic fibrosis (CF) is a life-shortening autosomal recessive disease that affects >70 000 people worldwide. It is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a protein that functions as a chloride channel found in several organs. Alterations in the CFTR gene markedly impair ion conductance at the epithelial surface, which causes airway surface liquid (ASL) volume depletion and subsequent mucociliary clearance (MCC) dysfunction, infection and premature respiratory failure [1]. Recently, therapies that pharmacologically correct CFTR function within specific genotype-based patient subgroups have emerged [2, 3]. However, quantitative methods for assessing basic lung disease pathophysiology are needed to fully support the development and dissemination of these therapies.
Currently available outcome measures track the downstream effects of CF lung disease and, as such, do not provide a rapid indication of therapeutic response. Biomarkers such as nasal potential difference [4] and sweat chloride concentration can be useful for evaluating the basic efficacy of new treatments, but results obtained from these tests may not correlate with changes occurring in the lung. Pulmonary imaging methods can accelerate therapeutic development by mediating the rapid screening of new therapies and therapy combinations as well as dosing regimens in limited patient populations.
Liquid hyperabsorption is a key element of CF lung pathophysiology that cannot be measured directly through any currently available in vivo method. Aerosol-based pulmonary imaging methods have been developed to measure absorption through the airway epithelium [5] and MCC, the latter of which has been used to demonstrate the efficacy of inhaled osmotic therapies such as hypertonic saline in CF subjects [6, 7]. The present investigation considers the use of a soluble, hydrophilic probe (diethylene triamine penta-acetic acid (DTPA)) as a surrogate marker of liquid absorption in the airways. In vivo, DTPA clearance includes both absorptive and mucociliary components. The latter can be estimated based on the clearance of a nonabsorbable, radiolabelled particle, and DTPA absorption then calculated. The clearance rate of the particle provides an independent measurement of MCC in the lung, a useful second outcome measurement. The rationale for our approach derives from cell culture studies showing that CF human bronchial epithelial (HBE) cells absorb liquid more rapidly than non-CF cells [8, 9]. Previous in vitro results from our laboratory have shown that the absorption rate of radiolabelled DTPA is: 1) increased in CF HBE cell cultures compared with non-CF cell cultures; 2) well correlated with the ASL absorption rate; and 3) influenced by transepithelial osmotic gradients [10]. In vivo, we have previously shown that the absorptive clearance of DTPA is increased in the lungs of CF subjects compared with healthy volunteers [11]. Taken together, these results support the hypothesis that DTPA absorption correlates with transepithelial liquid absorption. The purpose of this study was to measure the absorptive lung clearance of DTPA in adult and paediatric CF and adult control subjects, and to assess DTPA absorption response in vivo following treatment with nebulised hypertonic saline.
Methods
Detailed description of materials and methods can be found in the online supplementary material.
Subjects
Imaging was performed in adult CF (n=20), adult control (n=10) and paediatric CF (n=10) patients. In a pre-determined, randomised order, the adult CF subjects received hypertonic saline (7% sodium chloride) or isotonic saline (0.9% sodium chloride) on separate study days. The paediatric CF and control groups performed only one study day (isotonic saline treatment). The study was not blinded. Written informed consent was obtained from all subjects. This study was approved by the University of Pittsburgh Institutional Review Board (IRB) (Pittsburgh, PA, USA). This study is registered at www.clinicaltrial.gov with identifier numbers NCT01223183 and NCT01486199.
Inhalation of radiopharmaceuticals
Radioaerosol delivery was achieved with a DeVilbiss 646 jet nebuliser (DeVilbiss Healthcare, Somerset, PA, USA) containing 55.5 MBq indium-111–DTPA and 296 MBq technetium-99m–sulfur colloid (SC) in 3–4 mL normal saline with breathing patterns and delivery techniques shown to provide predominantly airway deposition [12, 13].
Imaging protocol
An 80-min image acquisition (1 min per frame) was initiated immediately after aerosol delivery. At the start of the 11th frame (10 min) subjects inhaled either isotonic or hypertonic saline for 10 min. Independent energy windows were used to image technetium-99m and indium-111. The 80-min imaging period included a 10-min baseline measurement, 10 min imaging during saline delivery and then 60 min continuous imaging. The 60-min imaging period is similar to previous measurements of MCC [14, 15].
Image data analysis
Right lung retention curves corrected for decay, background and isotope cross-talk contamination were image-derived for each radioisotope using a semiautomated software program written in house in MATLAB (Mathworks, Natick, MA, USA), which calculated the per cent clearance at 80 min of 99mTc-SC (referred to as MCC) and 111In-DTPA. The difference between MCC and total DTPA clearance is our estimate of DTPA clearance by absorption (ABS). These measures were also obtained from central lung zone retention curves. Additionally, the area above the whole-lung retention curve (AAC) was calculated for 99mTc-SC (AACTc) and 111In-DTPA (AACIn) by integration over all 80 frames. The difference between AACTc and AACIn is our estimate of the AAC for DTPA absorption (AACABS). The study analyst was not blinded but the opportunity for operator bias was negligible due to the automated nature of the analysis software.
Primary HBE cell culture studies
Primary HBE cells were isolated from excess lung airway tissue removed for transplantation under protocols approved by the University of Pittsburgh IRB as previously described [16].
Effects of transepithelial fluid flow
To determine the relative contributions of diffusion and convection on DTPA transport across the epithelium in CF HBE cell cultures, liquid absorption was blocked through the apical delivery of DTPA in a salt-free, iso-osmolar solution of mannitol in water. Unlike salt, HBE cells are unable to actively transport mannitol across their membranes [17], effectively impeding absorption of the liquid bolus. Using three different CF cell lines (six filters per line), radioactive counts and ASL volume [18] were measured over a 10-h period starting at 2 h after the apical addition of 10 μL radiolabelled DTPA in either normal saline or osmolarity-matched mannitol in water.
Effects of mucus
Excessive mucus accumulation is a signature of CF lung disease and previous studies have described DTPA binding to mucus [19]. To study its effects, radioactive counts and ASL volume were measured over 12 h following the addition of 10 μL radiolabelled DTPA in normal saline to CF HBE cell cultures with or without added apical mucus. These measurements were repeated in the presence of a strong transepithelial fluid flow mediated by a hyperosmotic basolateral media (600 mOsm·L−1). Two CF lines were tested (six filters per line).
Statistical analysis
In vivo statistical analysis
Central to peripheral deposition ratio (C/P), MCC, total DTPA clearance, ABS, and AACTc, AACIn and the difference between them were compared between patient groups by t-tests. A paired t-test was used to assess changes between the isotonic and hypertonic saline treatment study days. A series of independent linear regressions was performed to compare image-derived measurements with clinical variables.
In vitro statistical analysis
ASL volume and DTPA absorption rate measurements were compared by t-tests. The Mann–Whitney rank-sum test was used to compare baseline rates of ASL volume and DTPA absorption to rates measured in the presence of mucus, hyperosmotic basolateral media or the combination of both. Sigma-Stat v2.0 (SPSS, Inc., Chicago, IL, USA) was used for all statistical testing.
Results
Subject characteristics
Age and lung function data from the three study groups are presented in table 1. 20 adult CF subjects were enrolled. Four early subjects were excluded due to low deposited doses (technetium-99m <500 counts per min above background). The dosing period was increased at the midpoint of the study and all subsequent deliveries provided sufficient dosing. Two additional adult CF subjects were excluded due to nebuliser or camera malfunctions. Two adult CF patients were G551D heterozygous and on ivacaftor therapy. Because this new therapy is believed to have significant effects on lung physiology, their data are presented but not included in statistical comparisons. Therefore, data from 12 adult CF subjects were included in the primary study analysis with 11 subjects completing both hypertonic and isotonic saline treatment days. 10 of the 12 subjects were homozygous for the F508del mutation, one subject was heterozygous for the G551D mutation (not on ivacaftor) and one subject had multiple polymorphisms (7T/M470V-5T/M470V). 10 paediatric CF subjects and 10 healthy adult control subjects were also enrolled. One subject from each group was excluded due to inadequate lung deposition. Eight of the nine paediatric CF patients were homozygous for the F508del mutation and one patient was heterozygous for the nonsense mutation R1162X. More specific data on subject genotype and age are included in the online supplementary material.
TABLE 1.
Subject pulmonary function and age
| Adult CF | Paediatric CF | Controls | |
|---|---|---|---|
| Subjects n | 14 | 9 | 9 |
| FEV1 % pred | 67.6 ± 16.8 (43–98) | 90.1 ± 14.5 (62–106) | 99.2 ± 8.8 (83–113) |
| FVC % pred | 81.7 ± 18.9 (56–120) | 97.6 ± 8.1 (81–110) | 102.9 ± 6.4 (94–114) |
| FEF25–75% % pred | 43.3 ± 17.3 (20–70) | 79.8 ± 28.9 (45–134) | 86.7 ± 18.3 (60–117) |
| Age years mean ± SD | 23.9 ± 5.5 | 10.7 ± 1.5 | 32.2 ± 15.5 |
Data are presented as mean ± SD (range) unless otherwise stated. CF: cystic fibrosis; FEV1: forced expiratory volume in 1 s; % pred: % predicted; FVC: forced vital capacity; FEF25–75%: forced expiratory flow rate at 25–75% of FVC.
Composite retention data
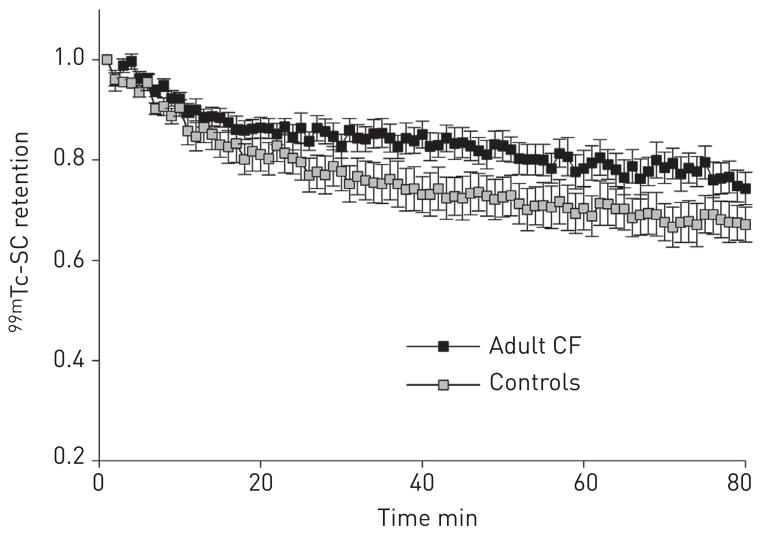
Mean normalised retention curves of indium-111 and technetium-99m in the whole right lung for each study group are shown in figure 1. Qualitatively, the adult CF patients (fig. 1a) demonstrated similar baseline MCC, total DTPA clearance and ABS to that of paediatric CF patients (fig. 1b). Inhaled hypertonic saline treatment increased MCC and total DTPA clearance in the adult CF cohort (fig. 1c) and decreased ABS to a value similar to that observed in control subjects (fig. 1d). Although not statistically significant, a gradually increasing difference in mean normalised 99mTc-SC clearance over 80 min was seen between the control and adult CF group (fig. 2).
FIGURE 1.
Normalised right lung retention of technicium-99m–sulfur colloid (SC) and indium-111–diethylene triamine penta-acetic acid (DTPA) plotted against time for the a) adult cystic fibrosis (CF) group (n=12), b) paediatric CF group (n=9), c) adult CF group with hypertonic saline treatment (n=11) and d) control group (n=9). Data are presented as mean ± SEM.
FIGURE 2.
Comparison of mean ± SEM normalised technetium-99m–sulfur colloid (SC) retention curves in healthy volunteers (n=9) and adult cystic fibrosis (CF) (n=12) subjects following nebulised isotonic saline treatment. The difference in per cent clearance at 80 min did not reach statistical significance (p=0.14).
Image-derived measurements
The average MCC in the adult CF, paediatric CF and control group was 23.6 ± 10.3%, 23.0 ± 13.9% and 31.4 ± 13.1%, respectively (nonsignificant by individual t-test between all groups) (fig. 3a). The mean ± SEM total DTPA clearance in the adult CF, paediatric CF and control group was 55.4 ± 12.3%, 56.6 ± 16.5% and 49.1 ± 13.6%, respectively (nonsignificant between all groups) (fig. 3b). The absorptive component of 111In-DTPA clearance is calculated for each subject by subtracting the 99mTc-SC clearance from the total 111In-DTPA clearance. The average ABS for the adult CF, paediatric CF and control group was 32.0 ± 13.9%, 33.6 ± 9.7% and 17.7 ± 6.9%, respectively (adult CF versus control: p=0.010; paediatric CF versus control: p=0.002; adult CF versus paediatric CF: nonsignificant) (fig. 3c). The two subjects with a G551D mutation on ivacaftor therapy had MCC values of 34.3% and 27.2%, and ABS values of 15.7 and 11.2%.
FIGURE 3.
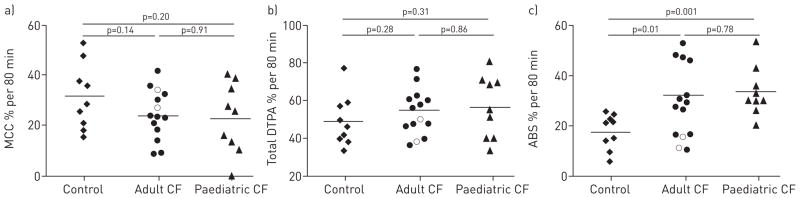
a) Mucociliary clearance (MCC), b) total diethylene triamine penta-acetic acid (DTPA) clearance and c) DTPA absorption (ABS) in healthy volunteers (n=9), and adult (n=12) and paediatric cystic fibrosis (CF) (n=9) subjects following nebulised isotonic saline treatment. Horizontal lines represent the mean clearance rate for each study group. Open circles represent G551D subjects utilizing ivacaftor (not included in statistical analysis).
Central lung zone (large airway) measurements are reported in table 2. Similar to whole-lung analysis, the average MCC and total DTPA clearance were not statistically different between any of the three subject groups. Central lung zone ABS was significantly higher in the adult and paediatric CF groups compared with the control group (adult CF versus control: p=0.014; paediatric CF versus control: p=0.003; adult CF versus paediatric CF: nonsignificant), which mirrored the findings of whole-lung analysis. AAC analysis (table 2) revealed no significant differences in AACTc or AACIn between any of the subject groups. However, AACABS was significantly higher in the adult and paediatric CF groups compared with the control group (adult CF versus control: p=0.010; paediatric CF versus control: p=0.006; adult CF versus paediatric CF: nonsignificant).
TABLE 2.
Central lung zone and area above the curve analysis
| Controls | Paediatric CF | Adult CF
|
||
|---|---|---|---|---|
| IS | HS | |||
| Central MCC % | 36.2 ± 18.0 | 35.2 ± 18.0 | 32.7 ± 13.5 | 45.7 ± 23.7 |
| Central DTPA % | 45.4 ± 16.6 | 57.1 ± 20.6 | 56.0 ± 19.0 | 62.8 ± 21.7 |
| Central ABS % | 9.2 ± 10.0 | 21.9 ± 5.0** | 23.3 ± 13.0* | 17.1 ± 12.2# |
| AACTc | 18.6 ± 9.4 | 14.5 ± 8.8 | 14.3 ± 6.3 | 26.8 ± 10.8## |
| AACIn | 25.9 ± 9.6 | 30.6 ± 11.4 | 30.5 ± 10.3 | 38.8 ± 7.7# |
| AACABS | 7.3 ± 3.5 | 16.1 ± 7.7** | 16.2 ± 8.8** | 12.0 ± 6.8## |
Data are presented as mean ± SD. CF: cystic fibrosis; IS: isotonic saline; HS: hypertonic saline; MCC: mucociliary clearance; DTPA: diethylene triamine penta-acetic acid; ABS: DTPA absorption; AACTc: area above the technetium-99m–sulfur colloid retention curve; AACIn: area above the 111In-DTPA retention curve; AACABS: area above the ABS curve.
: p<0.05 versus controls;
: p<0.01 versus controls;
: p<0.05 versus adult CF subjects with IS treatment;
: p<0.01 versus adult CF subjects with IS treatment.
Mean C/P in the CF adult, CF paediatric and control groups was 2.2 ± 0.5, 2.1 ± 0.4 and 2.4 ± 0.3, respectively (p=0.18 by ANOVA), providing an indication of predominantly bronchial airway deposition and uniformity of deposition pattern across all groups.
Response to osmotic therapy in CF adults
All 11 adult CF subjects showed a decrease in ABS and an increase in MCC following inhalation of hypertonic saline compared with isotonic saline treatment (ABS: 32.0 ± 13.9% versus 22.2 ± 12.8%, p<0.001; MCC: 23.6 ± 10.3% versus 42.4 ± 18.2%, p=0.003) (fig. 4a and b). Six out of 11 subjects showed an increase in total DTPA clearance with hypertonic saline treatment (p=0.118) (fig. 4c). AAC analysis showed that AACTc and AACIn were significantly increased (p=0.001 and p=0.04, respectively), and AACABS was significantly decreased (p=0.008) following hypertonic saline treatment. Baseline deposition pattern (C/P) did not differ between treatment days (2.4 ± 0.5 versus 2.3 ± 0.5; p=0.69) and there was no correlation between the per cent change in ABS and per cent change in MCC between treatment days. Baseline MCC values were increased and ABS values were decreased in the two adult ivacaftor subjects compared with average values in the adult CF group. Following hypertonic saline treatment, one ivacaftor subject showed a strong increase in MCC while the other showed a moderate decrease. Both subjects showed a moderate decrease in ABS with hypertonic saline treatment.
FIGURE 4.
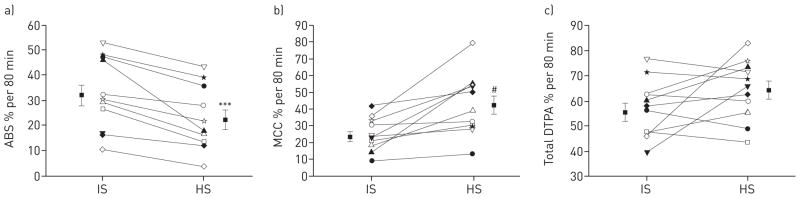
The effects of nebulised hypertonic saline (HS) treatment in individual cystic fibrosis adult subjects. a) Indium-111–diethylene triamine penta-acetic acid (DTPA) absorption (ABS) was decreased in every subject after inhalation of HS treatment. b) Technetium-99m–sulfur colloid clearance was increased by HS treatment in 11 out of 11 subjects. c) Total 111In-DTPA clearance was increased by HS treatment in six out of 11 subjects (p=0.118). Paired t-test was used. Data are presented as mean ± SEM. IS: isotonic saline; MCC: mucociliary clearance. ***: p<0.001 versus IS; #: p=0.003 versus IS.
Relationship of image-derived measurements to clinical variables and deposition pattern
Image-derived retention measurements were compared to pulmonary function data and other clinical variables using linear regression analysis. For all subject groups, neither ABS or AACABS correlated with forced expiratory volume in 1 s (FEV1), forced expiratory flow at 25–75% of forced vital capacity (FEF25–75%), age or sweat chloride concentration with the exception that in the paediatric CF group, ABS and AACABS increased significantly with age (p=0.03 and p=0.05, respectively). Age did not correlate with MCC in the paediatric CF group but MCC did increase with FEV1 (p=0.05) and FEF25–75% (p=0.05). No correlations were found between MCC or AACTc and pulmonary function variables in the adult subject groups.
In all groups combined, MCC correlated with total DTPA clearance (p=0.01) demonstrating the effect of MCC on the small molecule probe. There were no correlations between C/P and ABS, MCC or total DTPA clearance, or with the AAC values for these variables. Initial indium dose did not correlate with MCC, ABS or total DTPA clearance. Similarly, there were no correlations between initial technetium dose and any clearance parameters.
The contribution of transepithelial fluid flow to DTPA absorption
As shown in figure 5a, there was continual liquid absorption from 2 to 12 h following the bolus addition of 10 μL isotonic saline, absorbing at a rate of 20.1 ± 3.6% per 10 h. Conversely, a static ASL volume was observed after the apical addition of the mannitol solution, with a net absorption of −1.2 ± 4.4% per 10 h (p<0.001) (fig. 5b). DTPA was absorbed at a rate of 23.1 ± 2.6% per 10 h when delivered in normal saline and 10.4 ± 1.7% per 10 h when delivered in the iso-osmolar solution of mannitol in water (p<0.001) (fig. 5c), indicating that approximately one-half of DTPA absorption is associated with transepithelial fluid transport with the remainder presumably being associated with diffusion.
FIGURE 5.
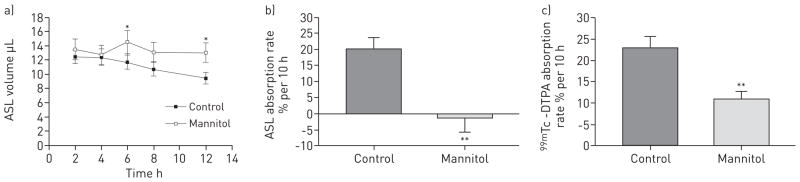
Comparison of airway surface liquid (ASL) volume and diethylene triamine penta-acetic acid (DTPA) absorption after addition of isotonic saline versus iso-osmolar solution of mannitol in water. a) ASL volume following apical volume expansion with either 10 μL isotonic saline (control) or 300 mM mannitol in water on differentiated cystic fibrosis human bronchial epithelial cell cultures. b) The per cent ASL absorption rate from 2 to 12 h after apical addition of isotonic saline or mannitol in water. c) The per cent 99mTc-DTPA absorption rate over the same 10-h period after apical addition of isotonic saline or mannitol in water. Data are presented as mean ± SEM of 18 cultures from three different tissue donors. *: p<0.05 versus control; **: p<0.01 versus control.
The effect of accumulated mucus on ASL volume and DTPA absorption
As shown in figure 6a, the rate of ASL volume absorption was significantly decreased when exogenous mucus was applied to the apical cell surface (8.6 ± 3.8% versus 35.3 ± 2.4% per 12 h for control, p<0.001) and significantly increased by a transepithelial osmotic gradient generated by basolateral mannitol (54.4 ± 2.6% per 12 h, p<0.001 versus control). In the presence of both exogenous mucus and the transepithelial osmotic gradient, the rate of ASL volume absorption remained significantly elevated (49.3 ± 1.5% per 12 h, p<0.03 versus control). DTPA absorption mirrored the response of ASL volume absorption for each of the conditions described above. The rate of DTPA absorption was significantly decreased by exogenous mucus (13.3 ± 3.1% versus 37.7 ± 2.6% per 12 h, p<0.001) and was significantly increased by the transepithelial osmotic gradient (93.7 ± 0.6% per 12 h, p<0.001 versus control). DTPA absorption in the presence of both mucus and the osmotic gradient was 90.6 ± 1.2% per 12 h (p<0.03 versus control). These data reveal that the impeding effects of apical mucus on the rates of both DTPA and ASL volume absorption become negligible in the presence of an osmotic gradient.
FIGURE 6.
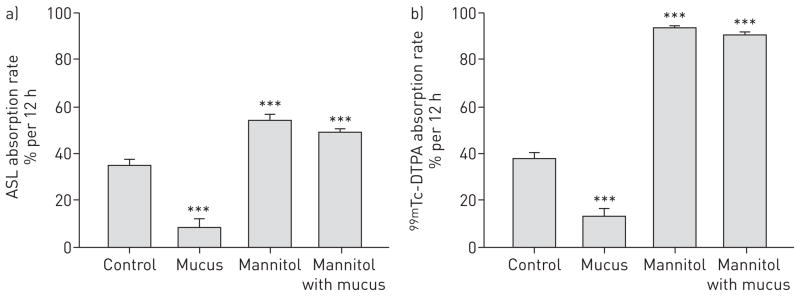
The effect of accumulated mucus on airway surface liquid (ASL) and diethylene triamine penta-acetic acid (DTPA) absorption in cystic fibrosis (CF) human bronchial epithelial (HBE) cell cultures. a) ASL volume absorption represented as a per cent change at 12 h in the absence or presence of mucus (10 μL, sampled from non-CF HBE cells), hyperosmotic basolateral growth medium (normal medium plus 300 mM mannitol) or the combination of both. b) 99mTc-DTPA absorption represented as a per cent change at 12 h in the absence or presence of mucus, hyperosmotic basolateral media or both. Data are presented as mean ± SEM of 12 cultures from two different tissue donors. ***: p<0.001 versus control.
Discussion
Airway liquid hyperabsorption is a key contributor to the impairment of MCC in CF. Measuring changes in airway liquid absorption through functional imaging methods may allow for rapid screening of medications designed to restore ASL volume, such as inhaled osmotics, sodium channel blockers, and CFTR correctors and potentiators. In the current study, MCC and DTPA absorption were measured in vivo using dual-isotope (99mTc-SC and 111In-DTPA) imaging. While statistically significant differences in baseline MCC were not detected between study groups, DTPA absorption was found to be increased in CF patients, consistent with our previous in vivo [11] and in vitro [10] results. These findings were similar in central zone measurements and when data were analysed using the AAC. Interestingly, the majority of paediatric CF patients who demonstrated airway hyperabsorption of DTPA had well-maintained pulmonary function, implying that DTPA absorption is linked to a basic defect of CF lung disease that precedes the decline in FEV1. We also demonstrated the acute effects of inhaled hypertonic saline by showing that it significantly increases MCC and decreases DTPA absorption in adult CF patients, a result consistent with its mechanism of action of hydrating the airway surface.
The underlying mechanisms contributing to airway DTPA absorption rates probably involve the net transport of sodium across the epithelium mediated by the epithelial sodium channel (ENaC) [16, 20]. In CF, ENaC becomes dysregulated, leading to the hyperabsorption of sodium [20]. The transepithelial movement of water that occurs in response to the skewed sodium gradient is a likely driving force for the heightened DTPA absorption measurement in our CF cohorts. Future studies could investigate changes in DTPA absorption with direct pharmacological blockers of ENaC to begin to probe this mechanism.
Baseline DTPA absorption measurements in adult CF subjects demonstrated significant intersubject variability compared with paediatric CF subjects and healthy controls. In light of previous work that showed that mucus possesses binding sites for 99mTc-DTPA [19], we posited that the variability observed in the adult subjects may be partially attributable to different volumes of accumulated mucus between subjects. This is supported by the finding that apical mucus reduced the rate of ASL and DTPA absorption in cultured primary HBE cells. However, the rate of ASL volume and DTPA absorption increased in response to a basolateral osmotic gradient in the presence or absence of apical mucus. This finding argues against DTPA binding to mucus, instead favouring a mechanism whereby mucus acts as a resistor to transepithelial fluid flow. We conclude that the intersubject variability in baseline DTPA absorption among the adult CF subjects may be at least partly attributable to differential amounts of accumulated mucus.
While the two-dimensional planar imaging used in this study is able to provide high temporal resolution, it does not allow for discrimination between airway and alveolar compartments. In the peripheral lung, DTPA clearance is highly sensitive to changes in permeability mediated by inflammatory stimuli such as cigarette smoke [21]. In CF, lung disease typically progresses from the airways; however, inflammation in alveoli has been described [22], as have CFTR-related differences in alveolar fluid transport [23]. Measurements of absorption made in this study will probably include some contribution from alveoli; however, we do not anticipate that these effects will dominate the measurement due to the delivery techniques used to target aerosol deposition to the airways. The high C/P dose ratios and MCC rates measured here indicate general success in this strategy.
DTPA absorption measurements will probably be a function of both transepithelial liquid transport and paracellular permeability associated with physical properties of airway epithelial cells. Our in vitro studies demonstrated that liquid absorbing through the epithelium accounted for about half of the total DTPA absorbed, with simple diffusion probably accounting for the rest (fig. 5). It has been previously documented that CF HBE cell cultures exhibited lower transepithelial resistance (TER) values, a measurement related to paracellular permeability [24]. However, studies performed in our laboratory demonstrated no relationship between TER values and DTPA absorption rates in CF and non-CF HBE cell cultures [10]. While we cannot exclude the possibility that differences in baseline absorption between CF and non-CF subjects may be the result of differences in airway epithelial integrity [25, 26], the consistent and significant effects of osmotic therapies on DTPA absorption demonstrated both in vitro and in vivo favour a mechanism linked to liquid transport.
Some previous imaging studies have shown significant differences in whole-lung MCC between CF and non-CF subjects [15, 27], while others have not [6]. Our results showed that baseline MCC in CF subjects was not significantly decreased compared with control subjects. It is possible that the aerosol distribution pattern may not be optimised to expose MCC deficiencies between non-CF and CF subjects during nonexacerbation periods, or that the duration of the measurement was too short to detect a difference. Additionally, our study was powered primarily to detect therapy-associated changes in measured values through paired comparisons. We estimate that we could detect an ~8% therapy-associated change in MCC with 12 subjects, for example. However, our study was probably not sufficiently powered to detect a difference between baseline measurements of MCC through unpaired comparisons between the CF and non-CF groups. We estimate that only baseline differences ≥17% would be detectable with 12 subjects.
Three CF patients with the G551D mutation were included in our study, two of whom were on ivacaftor therapy. DTPA absorption rates in the treated subjects were substantially lower than the CF average, while the untreated subject demonstrated absorption above the mean rate of the CF group (52.9% per 80 min). The rate of MCC in the untreated subject was within 2% of the average of the CF group, while the treated subjects had MCC rates substantially higher than the CF average (34.3% and 27.2% per 80 min). While this observation is interesting, it is impossible to link ivacaftor treatment with DTPA absorption response without establishing a baseline and studying more subjects.
In conclusion, DTPA absorption provides a quantifiable marker of immediate response to treatments that influence airway hydration in CF patients and may accelerate CF therapy development. DTPA absorption was increased in paediatric CF patients with normal spirometry values, suggesting that our imaging test elucidates the basic defect in airway hydration prior to the development of abnormal spirometry. We also demonstrated significant decreases in DTPA absorption rate after treatment with inhaled hypertonic saline. Though multiple factors may affect DTPA absorption across airway epithelia, our in vitro and in vivo results demonstrate a significant dependence on transepithelial fluid movement.
Supplementary Material
Acknowledgments
The authors wish to thank Adrienne DeRicco, Elizabeth Hartigan and Sandra Hurban (all Children’s Hospital of Pittsburgh, University of Pittsburgh Medical Center, Pittsburgh, PA, USA) for their assistance in performing these studies.
Support statement: This project was supported by US National Institutes of Health grants NIH K25 HL08153, NIH R01 HL112863, NIH P30 DK072506 and NIH R01 HL108929, and a Cystic Fibrosis Foundation RDP to the University of Pittsburgh.
Footnotes
Conflict of interest: Disclosures can be found alongside the online version of this article at www.erj.ersjournals.com
This article has supplementary material available from www.erj.ersjournals.com
References
- 1.Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- 2.Eckford PD, Li C, Ramjeesingh M, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem. 2012;287:36639–36649. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPhail GL, Clancy JP. Ivacaftor: the first therapy acting on the primary cause of cystic fibrosis. Drugs Today (Barc) 2013;49:253–260. doi: 10.1358/dot.2013.49.4.1940984. [DOI] [PubMed] [Google Scholar]
- 4.Fajac I, Hubert D, Bienvenu T, et al. Relationships between nasal potential difference and respiratory function in adults with cystic fibrosis. Eur Respir J. 1998;12:1295–1300. doi: 10.1183/09031936.98.12061295. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WD, Ilowite JS. Dual pathway clearance of 99mTc-DTPA from the bronchial mucosa. Am Rev Respir Dis. 1989;139:1132–1138. doi: 10.1164/ajrccm/139.5.1132. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SH, Bennett WD, Zeman KL, et al. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 7.Robinson M, Regnis JA, Bailey DL, et al. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1503–1509. doi: 10.1164/ajrccm.153.5.8630593. [DOI] [PubMed] [Google Scholar]
- 8.Blouquit S, Regnier A, Dannhoffer L, et al. Ion and fluid transport properties of small airways in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:299–305. doi: 10.1164/rccm.200506-987OC. [DOI] [PubMed] [Google Scholar]
- 9.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159:256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corcoran TE, Thomas KM, Brown S, et al. Liquid hyper-absorption as a cause of increased DTPA clearance in the cystic fibrosis airway. EJNMMI Res. 2013;3:14. doi: 10.1186/2191-219X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran TE, Thomas KM, Myerburg MM, et al. Absorptive clearance of DTPA as an aerosol-based biomarker in the cystic fibrosis airway. Eur Respir J. 2010;35:781–786. doi: 10.1183/09031936.00059009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donaldson SH, Corcoran TE, Laube BL, et al. Mucociliary clearance as an outcome measure for cystic fibrosis clinical research. Proc Am Thorac Soc. 2007;4:399–405. doi: 10.1513/pats.200703-042BR. [DOI] [PubMed] [Google Scholar]
- 13.Bennett WD, Laube BL, Corcoran T, et al. Multisite comparison of mucociliary and cough clearance measures using standardized methods. J Aerosol Med Pulm Drug Deliv. 2013;26:157–164. doi: 10.1089/jamp.2011.0909. [DOI] [PubMed] [Google Scholar]
- 14.Robinson M, Daviskas E, Eberl S, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Eur Respir J. 1999;14:678–685. doi: 10.1034/j.1399-3003.1999.14c30.x. [DOI] [PubMed] [Google Scholar]
- 15.Regnis JA, Robinson M, Bailey DL, et al. Mucociliary clearance in patients with cystic fibrosis and in normal subjects. Am J Respir Crit Care Med. 1994;150:66–71. doi: 10.1164/ajrccm.150.1.8025774. [DOI] [PubMed] [Google Scholar]
- 16.Myerburg MM, Harvey PR, Heidrich EM, et al. Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol. 2010;43:712–719. doi: 10.1165/rcmb.2009-0348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurt K, Bilton D. Inhaled mannitol for the treatment of cystic fibrosis. Exp Rev Respir Med. 2012;6:19–26. doi: 10.1586/ers.11.87. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PR, Tarran R, Garoff S, et al. Measurement of the airway surface liquid volume with simple light refraction microscopy. Am J Respir Cell Mol Biol. 2011;45:592–599. doi: 10.1165/rcmb.2010-0484OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheema MS, Groth S, Marriott C. Binding and diffusion characteristics of 14C EDTA and 99mTc DTPA in respiratory tract mucus glycoprotein from patients with chronic bronchitis. Thorax. 1988;43:669–673. doi: 10.1136/thx.43.9.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 21.Nolop KB, Maxwell DL, Fleming JS, et al. A comparison of 99mTc-DTPA and 113mIn-DTPA aerosol clearances in humans. Effects of smoking, hyperinflation, and in vitro oxidation. Am Rev Respir Dis. 1987;136:1112–1116. doi: 10.1164/ajrccm/136.5.1112. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich M, Worlitzsch D, Viglio S, et al. Alveolar inflammation in cystic fibrosis. J Cyst Fibros. 2010;9:217–227. doi: 10.1016/j.jcf.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Comellas AP, Karp PH, et al. CFTR is required for maximal transepithelial liquid transport in pig alveolar epithelia. AJP Lung Cell Mol Physiol. 2012;303:L152–L160. doi: 10.1152/ajplung.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devor DC, Pilewski JM. UTP inhibits Na+ absorption in wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am J Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson HE, Dragomir A, Lazorova L, et al. CFTR and tight junctions in cultured bronchial epithelial cells. Exp Mol Pathol. 2010;88:118–127. doi: 10.1016/j.yexmp.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 26.LeSimple P, Liao J, Robert R, et al. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol. 2010;588:1195–1209. doi: 10.1113/jphysiol.2009.182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson M, Eberl S, Tomlinson C, et al. Regional mucociliary clearance in patients with cystic fibrosis. J Aerosol Med. 2000;13:73–86. doi: 10.1089/089426800418604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.