Abstract
A cue associated with a rewarding event can trigger behavior towards the cue itself due to the cue acquiring incentive value through its pairing with the rewarding outcome (i.e., sign-tracking). For example, rats will approach, press, and attempt to “consume” a retractable lever conditioned stimulus (CS) that signals delivery of a food unconditioned stimulus (US). Attending to food-predictive CSs is important when seeking out food, and it is just as important to be able to modify one’s behavior when the relationships between CSs and USs are changed. Using a discriminative autoshaping procedure with lever CSs, the present study investigated the effects of orbitofrontal cortex (OFC) lesions on sign-tracking and reversal learning. Insertion of one lever was followed by sucrose delivery upon retraction, and insertion of another lever was followed by nothing. After the acquisition phase, the contingencies between the levers and outcomes were reversed. Bilateral OFC lesions had no effect on the acquisition of sign-tracking. However, OFC-lesioned rats showed substantial deficits in acquiring sign-tracking compared to sham-lesioned rats once the stimulus-outcome contingencies were reversed. Over the course of reversal learning, OFC-lesioned rats were able to reach comparable levels of sign-tracking as sham-lesioned rats. These findings suggest that OFC is not necessary for the ability of a CS to acquire incentive value and provide more evidence that OFC is critical for modifying behavior appropriately following a change in stimulus-outcome contingencies
Keywords: Sign-tracking, Goal-tracking, Autoshaping, Incentive salience, Orbitofrontal cortex, Reversal learning
The ability to attend to cues that predict rewarding outcomes and ignore cues that have no consequences is critical for navigating one’s environment. Additionally, it is just as important to be able to modify one’s behavior when the contingencies between cues and outcomes are altered. Cues paired with rewarding events can capture attention and trigger behavior towards the cue (i.e., sign-tracking, also called autoshaping; [2]) rather than the goal (i.e., goal-tracking; [3]). Many researchers have argued that these cue-directed behaviors are the result of the cue acquiring incentive salience, in which the incentive motivational value of the rewarding outcome is transferred to the cue [1]. Sign-tracking has been frequently observed using autoshaping, a Pavlovian conditioning paradigm in which rats will approach, press, and attempt to “consume” a retractable lever conditioned stimulus (CS) that signals the delivery of a food unconditioned stimulus (US) (e.g., [10,18]). While some rats may direct their behavior towards the CS at cue onset (sign-trackers), other rats may direct their behavior towards the site of US delivery (goal-trackers) (e.g., [10]). Although sign-tracking and goal-tracking are considered conditioned responses (CRs), only with sign-tracking does the CS become attributed with incentive salience [12].
In terms of the neural circuitry mediating sign-tracking, Flagel et al. [9] observed increased c-fos mRNA expression in the orbitofrontal cortex (OFC) in sign-trackers compared to goal-trackers and rats that received unpaired presentations of the lever CS and food US. Previous studies have demonstrated OFC’s importance in encoding stimulus-outcome relationships in several behavioral paradigms including outcome-specific Pavlovian-instrumental transfer (PIT) [13,14], devaluation of the US following Pavlovian conditioning [11,19], and reversals of cue-outcome contingencies [20-22].
The present study investigated the effects of OFC lesions on sign-tracking using a discriminative autoshaping procedure with lever CSs (CS+ and CS-). Following pretraining lesions, rats underwent 12 days of autoshaping. After the initial 12 days of autoshaping, the contingencies of the levers were reversed, and rats were run through another 12 days of autoshaping.
The subjects were male Long-Evans rats (n = 20; Charles River Laboratories, Raleigh, NC), which weighed 300-325 g on arrival. Rats were individually housed in a climate-controlled colony room that was illuminated from 7:00 AM to 7:00 PM. Before and two weeks after surgery, rats were given ad libitum access to food and water. Rats were then placed on a food restriction schedule and maintained at 85% of their ad libitum weights throughout the duration of the experiment. Surgery was performed under aseptic conditions with isoflurane anesthesia using procedures based on Stalnaker et al. [23]. Injections were made with a glass micropipette attached by plastic tubing to a picospritzer (General Valve Corporation, Fairfield, NJ). OFC lesions (n = 12) were made with 4 infusions of N-methyl-D-aspartic acid (NMDA; Sigma, St. Louis, MO) at a concentration of 12.5 mg/ml in PBS. Two injections (0.1 μl) were made at 4.0 mm anterior to bregma and 3.8 mm ventral to the skull surface, with one injection at 2.2 mm and the other injection at 3.7 mm lateral to the midline. The other two injections were made at 3.0 mm anterior to bregma and 5.2 mm ventral to the skull surface, with one injection (0.1 μl) at 4.2 mm lateral to the midline and the other injection (0.05 μl) at 3.2 mm lateral to the midline. Sham-lesioned rats (n = 8) received identical surgical procedures as OFC-lesioned rats, but no injections were given. The behavioral training apparatus consisted of eight individual chambers (20.5 cm × 22.0 cm × 22.5 cm) with stainless steel front and back walls, clear acrylic sides, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. An illuminated clear acrylic food cup was recessed in an opening of the front wall, and photocells at the front of the food cup recorded entries and time spent in the cup. Locally-manufactured retractable levers were located on either side of the cup. Each chamber was enclosed inside a sound attenuating shell. Sucrose was delivered via a pump located outside the sound attenuating shell through a hole that was located at the bottom of the food cup. An infrared light was located outside of each chamber, and cameras mounted within the shell allowed for television viewing.
Rats received two 64-min magazine training sessions, in which rats were given sixteen 0.1-ml deliveries of 8% (w/v) sucrose solution, with a mean intertrial interval (ITI) of 240 s. Rats then underwent 12 sessions of autoshaping. Within each 64-min session, 25 CS+ and 25 CS-trials (mean ITI = 77 s) were presented in random order. On CS+ trials, one lever was extended for 10 s followed by 0.1 ml delivery of 8% sucrose upon retraction. On CS- trials, the other lever was extended for 10 s, but no sucrose was delivered. For half the rats, the CS+ lever was the left lever and the CS- lever was the right lever. For the other half of rats, the sides of the CS+ and CS- levers were reversed. Rats then underwent another 12 days of autoshaping in which the contingencies of the lever CSs were reversed (i.e., CS+ became CS- and vice versa). Rate of lever pressing and the percentage of trials in which at least one lever press occurred were the primary measures (sign-tracking). Additionally, the percentage of time rats spent with their heads in the food cup was also reported (goal-tracking).
For both the acquisition and reversal phases, the data was initially analyzed using 4-way mixed ANOVAs with 4 factors: Lesion (OFC, Sham), Cue (CS+, CS-), Interval of cue (1st 5 s, 2nd 5 s), and Session (days 1-12). As in previous autoshaping studies [5,6], peak lever press responding was observed during the 2nd 5 s of CS presentations. Table 1 presents the mean lever press rate and percentage of trials with a lever press of OFC- and sham-lesioned rats during the first and second 5 s intervals of CS+ and CS- presentations, averaged over the entire training period. Peak levels of sign-tracking for both lever press measures occurred within the second 5 s of CS presentations during the acquisition and reversal learning phases. 4-way ANOVAs confirmed a main effect of Interval for both lever press measures during the acquisition phase (Fs1,16 > 9.5, p < 0.01) and reversal phase (Fs1,16 > 9.7, p < 0.01) of training. In contrast, there were no Lesion × Interval interactions for either lever press measure during the acquisition (Fs1,16 < 3.2, p > 0.09) or reversal (Fs1,16 < 0.91, p > 0.35) phases of training. Thus, data analysis was restricted to the 2nd 5 s of CS presentations using 3-way mixed ANOVAs with 3 factors: Lesion, Cue, and Session.
Table.
| Measure | OFC (acq) | Sham (acq) | OFC (rev) | Sham (rev) |
|---|---|---|---|---|
| Rate CS+ 1st 5 s | 13.1 ± 3.3 | 17.5 ± 3.6 | 16.2 ± 4.3 | 22.1 ± 3.4 |
| Rate CS+ 2nd 5 s | 18.0 ± 4.4 | 19.3 ± 4.8 | 20.0 ± 4.9 | 23.3 ± 3.5 |
| Rate CS- 1st 5 s | 1.1 ± 0.5 | 1.5 ± 0.6 | 4.6 ± 1.1 | 5.1 ± 1.1 |
| Rate CS- 2nd 5 s | 2.3 ± 0.8 | 2.4 ± 0.8 | 6.1 ± 1.4 | 6.9 ± 1.5 |
| Percent trials CS+ 1st 5 s | 45.2 ± 7.5 | 57.7 ± 7.8 | 51.5 ± 8.3 | 72.8 ± 6.6 |
| Percent trials CS+ 2nd 5 s | 55.1 ± 8.0 | 61.5 ± 8.8 | 57.0 ± 8.9 | 73.3 ± 5.8 |
| Percent trials CS- 1st 5 s | 4.9 ± 1.8 | 7.0 ± 2.5 | 20.2 ± 4.0 | 19.2 ± 3.3 |
| Percent trials CS- 2nd 5 s | 10.0 ± 2.9 | 9.5 ± 2.5 | 23.5 ± 4.2 | 24.2 ± 3.1 |
Notes. Rate = lever presses/min over all trials; % trials = percentage of trials on which at least one lever press response occurred; OFC = orbitofrontal cortex; acq = acquisition phase (sessions A1-A12); rev = reversal phase (sessions R1-R12). Entries are mean ± SEM, averaged over all sessions of each phase.
After behavioral testing, rats were anesthetized with sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.9% saline, followed by 10% (v/v) Formalin in 0.1M PBS. Brains were removed and stored in 0.1M PBS and 20% (w/v) sucrose. Forty-μm slices were collected and Nissl stained to verify lesion placements. To determine the percentage of damage of each lesion, a matrix of dots was digitally placed over the targeted regions of OFC (lateral OFC and dorsolateral OFC/agranular insular regions) on figures 5-11 of the Paxinos and Watson [17] atlas. After viewing each brain slice under a microscope, lesions were drawn on the appropriate figure. The percentage of damage to each region of OFC was calculated by dividing the number of dots within the damaged area over the total number of dots within each region. Rats with less than 50% total damage to OFC were removed from the data analysis. Figure 1 presents a schematic representation of neuronal damage in OFC-lesioned rats (n = 10). On average, 85.1 ± 2.9% (mean ± SEM) of OFC was eliminated (ranging from 68.1% to 95%), consisting of 77.6 ± 6.6% damage to lateral OFC and 89.5 ± 1.4% damage to dorsolateral OFC/agranular insular regions. Sham-lesioned rats (n = 8) had no observable damage other than near the micropipette track.
Figure 1.
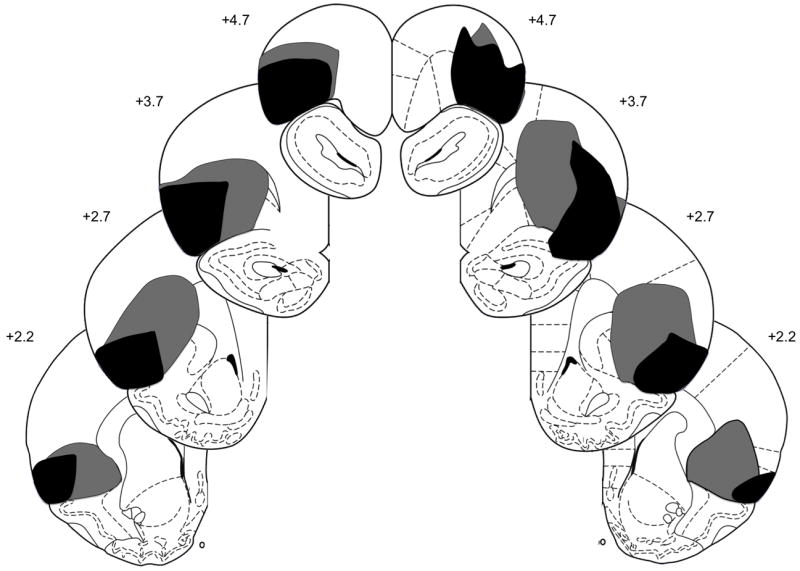
Schematic representation of orbitofrontal cortex (OFC) lesions showing the minimum (black) and maximum (gray) amount of neuronal damage. Coronal sections are 4.70 mm to 2.20 mm relative to bregma. Diagrams from Paxinos and Watson [19].
Figure 2A presents the number of lever presses per minute during the last 5 s CS interval over the course of training. During the acquisition phase, OFC- and sham-lesioned rats acquired sign-tracking at comparable rates, pressing more to the CS+ than the CS-. A 3-way ANOVA confirmed a main effect of Cue (F1,16 = 37.1, p < 0.01) and Session (F11,176 = 10.3, p < 0.01), but no effect of Lesion (F1,16 = 0.05, p = 0.84). In addition, there was no Lesion × Cue (F1,16 = 0.06, p = 0.81) or Lesion × Cue × Session (F11,176 = 0.66, p = 0.77) interactions. Similarly, both groups of rats showed no differences in this measure following reversal of the CS-US contingencies, pressing more to the CS+ than the CS-. A 3-way ANOVA confirmed a main effect of Cue (F1,16 = 35.3, p < 0.01) but no effect of Lesion (F1,16 = 0.43, p = 0.52) or Session (F11,176 = 0.79, p = 0.65). Additionally, there were no Lesion × Cue (F1,16 = 0.23, p = 0.64) or Lesion × Cue × Session (F11,176 = 1.17, p = 0.31) interactions.
Figure 2.
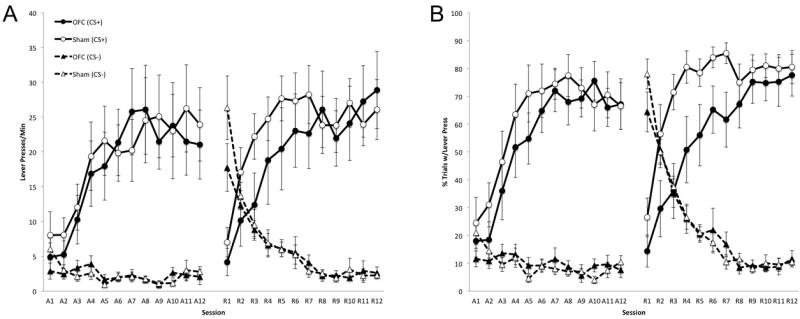
Effects of orbitofrontal cortex (OFC) lesions on sign-tracking during the last 5 s of CS presentations as measured by lever press rate (A) and percentage of trials with a lever press (B). Sessions A1-A12 correspond to the acquisition phase, and sessions R1-R12 correspond to the reversal phase. Error bars represent ± SEM.
Figure 2B presents the percentage of trials with a lever press during the last 5 s CS interval over the course of training. During the acquisition phase, OFC- and sham-lesioned rats showed comparable rates of sign-tracking. A 3-way ANOVA confirmed main effects of Cue (F1,16 = 122.97, p < 0.01) and Session (F11,176 = 12.96, p < 0.01) but no effect of Lesion (F1,16 = 0.28, p = 0.60). Additionally, there was no Lesion × Cue (F1,16 = 0.60, p = 0.45) or Lesion × Cue × Session (F11,176 = 1.06, p = 0.40) interaction. During the reversal phase, OFC and shamlesioned rats quickly reduced responding to the former CS+ that became the CS-. However, OFC-lesioned rats showed substantial deficits in responding to the new CS+ compared to shamlesioned rats over the first half of the reversal phase. Over the second half of reversal training, OFC-lesioned rats reached comparable levels of responding to the CS+ as sham-lesioned rats. A 3-way ANOVA confirmed a main effect of Cue (F1,16 = 91.89, p < 0.01) but no effect of Session (F11,176 = 0.70, p = 0.74) or Lesion (F1,16 = 2.67, p = 0.12). Although there was no Lesion × Cue (F1,16 = 3.27, p = 0.09) interaction, there was a Lesion × Cue × Session (F11,176 = 1.87, p = 0.046) interaction. Separate 2-way ANOVAs with Lesion and Session as factors within each cue confirmed a Lesion × Session interaction for the CS+ (F11,176 = 2.3, p = 0.01) but not for the CS- (F11,176 = 0.85, p = 0.59). Finally, separate ANOVAs over the first and second halves of reversal learning with Lesion as a factor confirmed a main effect of Lesion for the first half of training (F1,16 = 5.3, p = 0.035) but not the second half of training (F1,16 = 0.85, p = 0.37).
Figure 3 presents the percentage of time spent in the food cup during the last 5 s CS interval over the course of training. During the acquisition phase, OFC- and sham-lesioned rats showed highly variable levels of food cup responding (i.e., goal-tracking). A 3-way ANOVA confirmed a main effect of Session (F11,176 = 4.52, p < 0.01), but no effect of Lesion (F1,16 = 0.04, p = 0.85) or Cue (F1,16 = 4.27, p = 0.055). Additionally, there was no Lesion × Cue (F1,16 = 2.04, p = 0.17) or Lesion × Cue × Session (F11,176 = 1.26, p = 0.25) interaction. During the reversal phase, OFC- and sham-lesioned rats spent more time in the food cup during CS- than CS+ presentations. Additionally, OFC-lesioned rats spent more time overall in the food cup during CS presentations than sham-lesioned rats. A 3-way ANOVA confirmed a main effect of Lesion (F1,16 = 4.80, p = 0.044), Cue (F1,16 = 11.60, p < 0.01) and Session (F11,176 = 10.25, p < 0.01). However, there was no Lesion × Cue (F1,16 = 0.0, p = 0.99) or Lesion × Cue × Session (F11,176 = 0.86, p = 0.58) interaction.
Figure 3.
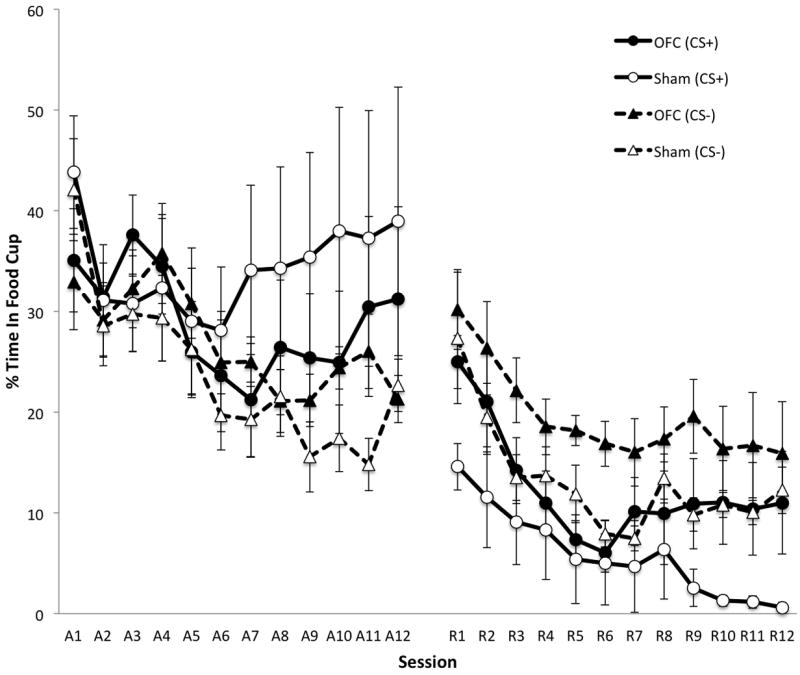
Effects of orbitofrontal cortex (OFC) lesions on food cup behavior during the last 5 s of CS presentations as measured by percentage of time spent in the food cup. Sessions A1-A12 correspond to the acquisition phase, and sessions R1-R12 correspond to the reversal phase. Error bars represent ± SEM.
The results of the present study suggest that OFC is not necessary for the acquisition of sign-tracking to a lever CS, but OFC is needed for normal reversal learning following reversal of stimulus-outcome contingencies. Deficits in reversal learning were observed in terms of percentage of trials with a lever press and not lever press rate. These conflicting results can be accounted for by a select few of OFC-lesioned rats that showed high levels of responding early during reversal learning, which has less of an effect on the percentage of trials with a lever press than lever press rate. Although activation of OFC has been observed in sign-trackers to presentations of the lever CS [9], observing activation of a particular brain region does not necessarily mean that region is required for acquisition or expression of the behavior of interest. In contrast to the present study, OFC lesions have been found to disrupt sign-tracking to a visual CS (white rectangle on a monitor) [7]. However, the neural circuitry underlying sign-tracking has been shown to differ based on the nature of the CS [4-6,15,16]. Thus, it may not be surprising that OFC lesions have different effects on sign-tracking to lever and visual CSs. Additionally, the OFC lesions in Chudasama and Robbins’ [7] study included the present study’s targeted regions but also the medial and ventral regions of OFC, which may be another reason why contrasting results were observed. Interestingly, Chudasama and Robbins [7] found deficits in OFC-lesioned rats in reversal learning when the stimulus-outcome relationships were reversed. However, the nature of the deficit in OFC-lesioned rats in Chudasama and Robbins’ [7] study was due to perseverative errors (continuing to sign-track to the former CS+), whereas OFC-lesioned rats in the present study reduced responding to the former CS+ but were slower to acquire responding to the new CS+. Notably, OFC lesions have previously been shown to produce deficits in response inhibition [8] and in the number of trials to reach criterion responding [21] following reversals using a 2-odor go, no-go design. Together, these findings suggest that OFC is important for response inhibition and acquisition following reversals of stimulus-outcome contingencies.
For future investigations, it would be interesting if OFC lesions would continue to impair reversal learning of sign-tracking after subsequent reversals. Perhaps, OFC-lesioned rats and sham-lesioned rats would acquire sign-tracking at comparable rates. In contrast, OFC-lesioned rats may continue to show deficits compared to sham-lesioned rats despite having acquired signtracking under either contingency. Additionally, future sign-tracking studies investigating the effects of OFC lesions following devaluation of the US would bring novel insights into the nature of the learning processes involved in sign-tracking. Previous investigations have shown that OFC lesions impair the normal reduction in responding to a CS following devaluation of its associated US using a Pavlovian procedure [11,19], but OFC lesions have no effect on devaluation using an instrumental procedure [13,14]. Although autoshaping is a Pavlovian conditioning paradigm by design, it has been argued that sign-tracking is a product of adventitious reinforcement. Both Pavlovian and instrumental learning processes may be involved in sign-tracking, as previous investigations have shown that responding is maintained (Pavlovian) but reduced (instrumental) when responding to the CS cancels delivery of the rewarding outcome (also known as negative auto-maintenance; [24,25]). Investigating the effects of OFC lesions on devaluation of an autoshaped CS would elucidate the extent to which Pavlovian and instrumental learning processes are involved in sign-tracking.
In conclusion, the present study found that OFC is not necessary for the ability of a CS to acquire incentive value. However, OFC is needed for updating stimulus-outcome contingencies during reversal learning, providing further support that OFC is critical for modifying behavior following changes in the environment.
Research Highlights.
Orbitofrontal cortex lesions had no effect on initial acquisition of sign-tracking.
Orbitofrontal cortex lesions impaired sign-tracking during reversal learning.
Orbitofrontal cortex is critical for modifying previously learned behaviors.
Acknowledgments
I would like to thank Peter Holland for his guidance and support for this project, Weidong Hu for assistance with histology, and Thomas Stalnaker for technical support with surgery. This work was supported by NIH grant MH53667.
Abbreviations
- CS
conditioned stimulus
- US
unconditioned stimulus
- OFC
orbitofrontal cortex
- CR
conditioned response
- PIT
Pavlovian-instrumental transfer
- PBS
phosphate buffered saline
Footnotes
Conflict of interest: The author declares no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Brown PL, Jenkins HM. Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Hillsdale, NJ: Lawrence Erlbaum Associates; 1977. pp. 67–97. [Google Scholar]
- 4.Chang SE, Holland PC. Effects of nucleus accumbens core and shell lesions on autoshaped lever-pressing. Behav Brain Res. 2013;256:36–42. doi: 10.1016/j.bbr.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SE, Wheeler DS, Holland PC. Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Res. 2012a;1450:49–56. doi: 10.1016/j.brainres.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem. 2012b;97:441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal-thalamocortical pathway on odor reversal learning: Inability to extinguish an incorrect response. Exp Brain Res. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- 9.Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE. A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience. 2011a;196:80–96. doi: 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011b;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007a;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostlund SB, Balleine BW. The contribution of orbitofrontal cortex to action selection. Ann NY Acad Sci. 2007b;1121:174–192. doi: 10.1196/annals.1401.033. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson JA, Robbins TW, Everitt BJ. Dissociable roles of the central and basolateral amygdala in appetitive emotional learning. Eur J Neurosci. 2000a;12:405–413. doi: 10.1046/j.1460-9568.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulated cortex and nucleus accumbens core impairs Pavlovian approach behavior: Further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000b;114:42–63. [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 18.Peterson GB, Ackilt JE, Frommer GP, Hearst ES. Condtioned approach and contact behavior toward signals for food or brain-stimulation reinforcement. Science. 1972;177:1009–1011. doi: 10.1126/science.177.4053.1009. [DOI] [PubMed] [Google Scholar]
- 19.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 21.Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learn Mem. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003b;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- 23.Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Stiers M, Silberberg A. Lever-contact responses in rats: Automaintenance with and without a negative response-reinforcer dependency. J Exp Anal Behav. 1974;22:497–506. doi: 10.1901/jeab.1974.22-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams DR, Williams H. Auto-maintenance in the pigeon: Sustained pecking despite contingent non-reinforcement. J Exp Anal Behav. 1969;12:511–520. doi: 10.1901/jeab.1969.12-511. [DOI] [PMC free article] [PubMed] [Google Scholar]


