Abstract
Thrombospondins (TSP) are multifunctional proteins that are deposited in the extracellular matrix where they directly affect the function of vascular and other cell types. TSP-4, one of the 5 TSP family members, is expressed abundantly in tendon and muscle. We have examined the effect of TSP-4 deficiency on tendon collagen and skeletal muscle morphology and function. In Thbs4−/− mice, tendon collagen fibrils are significantly larger than in wild-type mice, and there is no compensatory over-expression of TSP-3 and TSP-5, the two TSPs most highly homologous to TSP-4, in the deficient mice. TSP-4 is expressed in skeletal muscle, and higher levels of TSP-4 protein are associated with the microvasculature of red skeletal muscle with high oxidative metabolism. Lack of TSP-4 in Medial soleus, red skeletal muscle with predominant oxidative metabolism, is associated with decreased levels of several specific glycosaminoglycan modifications, decreased expression of a TGFβ receptor beta-glycan, decreased activity of lipoprotein lipase, which associates with vascular cell surfaces by binding to glycosaminoglycans, and decreased uptake of VLDL. The soleus muscle is smaller and hind- and fore-limb grip strength is reduced in Thbs4−/− mice compared to wild-type mice. These observations suggest that TSP-4 regulates the composition of the ECM at major sites of its deposition, tendon and muscle, and the absence of TSP-4 alters the organization, composition and physiological functions of these tissues.
Keywords: thrombospondin-4, skeletal muscle, tendon, glycosaminoglycans
1. INTRODUCTION
Thrombospondins (TSPs) are matricellular glycoproteins (Adams, 2001; Adams and Lawler, 2004; Bornstein, 1995, 2001; Mosher and Adams, 2012; Murphy-Ullrich and Iozzo, 2012). TSPs have not been shown to provide structure to the extracellular matrix (ECM), as do the collagens or elastins, but they are known to regulate many of the protein-protein and protein-cell interactions within the ECM (Adams, 2001; Bornstein, 2001). The functions of the TSPs depend upon their interactions with other ECM constituents, i.e., other proteins, proteoglycans, glycosaminoglycans (GAGs) and cell-surface receptors, including multiple integrins, CD36 and CD47 (IAP) (Adams, 2001; Chen et al., 2000; Stenina et al., 2007). These interactions allow the TSPs to influence diverse cellular responses, such as attachment, spreading, motility, proliferation, apoptosis, and responses to proteases, growth factors and cytokines (Adams, 2001). TSPs are synthesized by many different cell types, and their expression is often regulated, allowing them to exert control over many different cellular responses (Adams and Lawler, 2004; Roberts et al., 2012; Sweetwyne and Murphy-Ullrich, 2012). The five TSP family members are each encoded by a different gene. They all carry a common signature, a highly homologous C-terminal part that includes calcium-binding (type 3) repeats and EGF-like domains (type 2 repeats) (Misenheimer and Mosher, 2005). The N-terminal part of each TSP is distinct (Adams, 2001; Chen et al., 2000). Based on their domain structure and oligomerization status, the TSPs can be divided into two groups: members of subgroup A (TSP-1, TSP-2) form trimers, and members of subgroup B (TSP-3, TSP-4, TSP-5) form pentamers (Engel, 2004). Despite the high degree of homology among the TSP family members, the promoters of the TSP genes, the regulatory regions of their mRNAs and, as a result, their tissue expression patterns are quite distinct, suggesting that each TSP may have unique functions.
Thrombospondin-4 (TSP-4), a member of subgroup B, was first described in 1993 by Lawler et al based on detection of its mRNA in Xenopus laevis (Lawler et al., 1993). Subsequent studies have indicated that TSP-4 mRNA is expressed at high levels in tendon and muscle as well as in neural and osteogenic tissues (Arber and Caroni, 1995; Hauser et al., 1995). TSP-4 protein has also been detected in brain capillaries (Stenina et al., 2003). Recent interest in TSP-4 has been stimulated by the demonstration that the human gene (THBS4) encoding for TSP-4 contains a single nucleotide polymorphism, which is expressed at high frequency in Caucasians and is associated with a significantly increased risk of premature myocardial infarction (Cui et al., 2006; Cui et al., 2004; Kato T, 2003; McCarthy et al., 2004; Stenina et al., 2004; Topol et al., 2001; Walton B, 2003; Wessel et al., 2004; Yamada et al., 2002).
Mice with inactivated genes for TSP-1, TSP-2, TSP-3 and TSP-5 (also known as cartilage oligomeric matrix protein or COMP) have been described (Hankenson et al., 2005; Kyriakides et al., 1998; Lawler et al., 1998; Svensson et al., 2002). Inactivation of these individual genes is not embryonically lethal, and the spontaneous phenotype of the adult mice is largely unremarkable. However, when each of the TSP knock-out (KO) mice is challenged, specific abnormal responses have been noted. We recently reported that TSP-4 regulates local vascular inflammation, and the lack of TSP-4 delays the development of atherosclerotic lesions in a mouse model and results in fewer inflammatory lesion (Frolova et al., 2010). We and others reported that in myocardium, TSP-4 regulates ECM production, and the deficiency in TSP-4 causes increased fibrosis in response to pressure overload and negatively affects the cardiomyocytes function (Cingolani et al., 2011; Frolova et al., 2012; Lynch et al., 2012). TSP-4 controls important physiological and pathological processes in nervous system (Risher and Eroglu, 2012).
In the present study, we have focused on the distribution and function of TSP-4 in two major tissues of its deposition, tendon and muscle, and have examined the effects of its deficiency on the tendon and muscle structure and function. These tissues represent ~40% of human body weight, are a rich source of TSP-4 in humans (Arber and Caroni, 1995; Lawler et al., 1993), and deficiencies of TSPs have significant functional consequences in muscle and tendon (Adams and Lawler, 1994; Gilsohn and Volk; Kyriakides et al., 1998). Our results suggest that TSP-4 is critically required for the maintenance of ECM composition, structure and function at the cellular level and at the level of the whole limb.
2. RESULTS
2.1. Thbs4−/− mice
The TSP-4 deficient mice appeared normal at birth and displayed no obvious phenotype during their early and adult development. Their lifespan was similar to that of WT mice. There was a slight decrease (10–15%) in body weight of the Thbs4−/− mice with age although the mice appeared to be healthy. The skeletal systems of the Thbs4−/− and WT control mice were imaged using a custom-built micro-computed tomography system (Cleveland Clinic, Cleveland, OH) at 30μm voxel resolution and visualized using the VolSuite 3D rendering software (Ohio SuperComputer Center, Columbus, OH). The resulting data suggested that the abnormality in bone density and structure observed in the Thbs3−/− mice (Hankenson et al., 2005) was not present in the Thbs4−/− mice. Furthermore, skeletal anatomic aberrations were absent by visual inspection. In this work, we focused our analyses on the two tissues, tendon and muscle, in which abundant TSP-4 expression had been reported (Arber and Caroni, 1995; Hauser et al., 1995).
2.2. TSP-4 expression in tendon
TSP-4 shares up to 82% homology with TSP-3 and TSP-5 in the highly conserved carboxy-terminal region (Adams, 2004). We previously reported that the levels of other TSP-3 and TSP-5 were not changed in the blood vessel wall of Thbs4−/− mice (Frolova et al., 2010). We examined the expression patterns of TSP-3 and TSP-5 in tendon and skeletal muscle of WT and Thbs4−/− mice and found that their expression is not changed, and there is no compensation for the deficiency in TSP-4. In tendons of the WT triceps muscle, TSP-4 and TSP-3 are expressed in a non-overlapping fashion intersecting each other at an angle in WT mice (Fig. 1, A). The pattern and intensity of TSP-3 staining in muscle was unaltered in Thbs4−/− mice (not shown). In tendons, TSP-4 and TSP-3 are associated with distinct fibrillar structures. Similar to TSP-4, TSP-5 is found in the perimysium of muscle and in tendon (Fig. 1, B–D). The staining of TSP-5 overlaps extensively with TSP-4 staining (Fig. 1, B, C) in WT mice. However, the intensity of TSP-5 staining in tendon was not changed in Thbs4−/− mice (Fig. 1, D). Western blotting of extracts of skeletal muscle (soleus) revealed that both TSP-3 and TSP-5 are present in the skeletal muscle tissue at low levels, and their expression is not altered (Suppl. Fig. 1). TSP-1 and TSP-2 were also expressed at the levels comparable to the expression levels in WT mice.
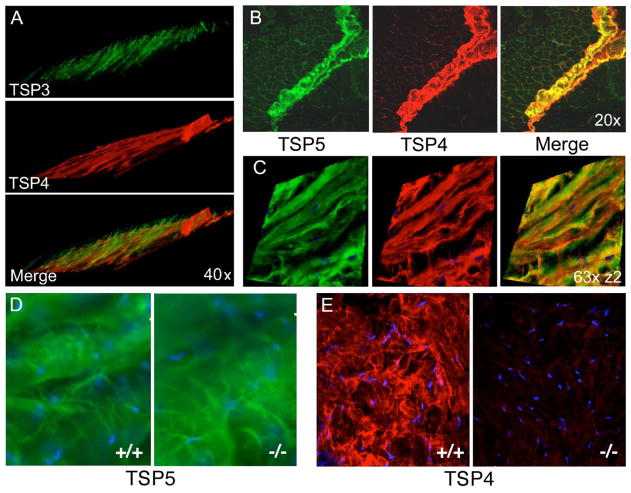
Figure 1. Expression of subgroup B thrombospondins in tendon.
(A) 3-D reconstruction of TSP-4 and TSP-3 expression in WT triceps tendon showing different orientations of the two molecules as determined by immunohistochemistry. (B) Colocalization of TSP-4 and TSP-5 in perimysium and (C) in patellar tendon; immunohistochemical 3-D reconstruction. (D) Expression of TSP-5 does not change in Thbs4−/− tendon compared to WT. (E) Lack of TSP4 expression in Thbs4−/− patellar tendon. Nuclei (blue) were visualized by DAPI staining.
Immunohistochemical analysis of transverse sections of the patellar tendon demonstrated that TSP-4 is abundant (Fig. 1E, left panel). Specificity of the anti-TSP-4 antibody was demonstrated by the absence of any reactivity in the patellar tendon of Thbs4−/− mice (Fig. 1E, right panel) or in any other tissue derived from these animals (Frolova et al., 2010; Frolova et al., 2012), and the absence of the protein as detected by Western blotting (Fig. 6A and 8C). The abundant expression of TSP-4 in the tendon suggests its potential importance in the organization of this tissue.
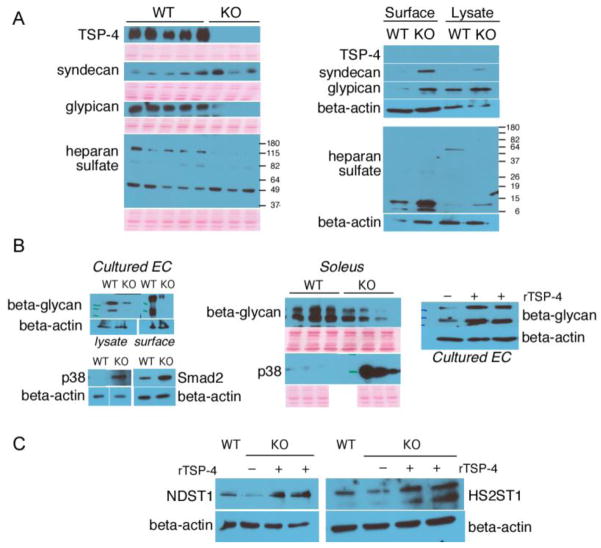
Figure 6. Levels of Heparan Sulfate core proteins.
(A, left panel) soleus protein extracts; (A, right panel) cultured mouse EC; (B) levels of beta-glycan and activation of intracellular signaling mediators in cultured EC and muscle tissue extracts; (C) levels of key enzymes controlling the synthesis and modifications of HS in cultured EC by Western blotting.
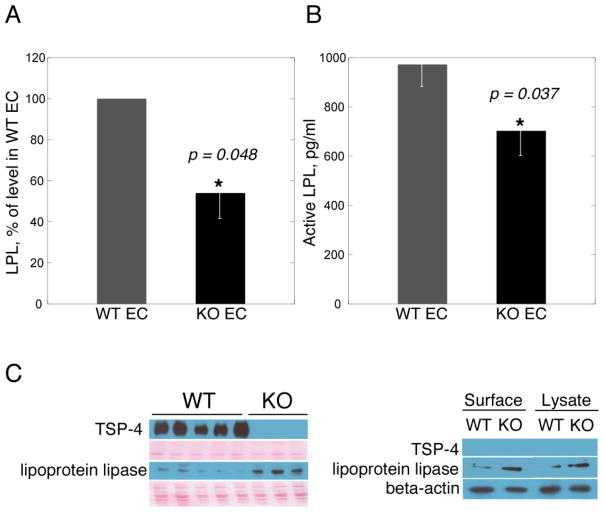
Figure 8. Activity and expression of endothelial LPL in Thbs4−/− mice.
(A) activity in cultured EC; (B) activity of LPL in vivo in plasma of Thbs4−/− and WT mice calculated as a difference between the activity before and after heparin injection; (C) Levels of LPL in cultured mouse EC: left panel – soleus extracts from Thbs4−/− (KO) and WT (WT) mice with loading control (membrane stained with Ponceau S to visualize total protein); right panel – lysates of cultured EC from Thbs4−/− (KO) and WT (WT) mice with loading control (beta-actin).
2.3. TSP-4 regulates the structure of the collagen fibrils in tendon
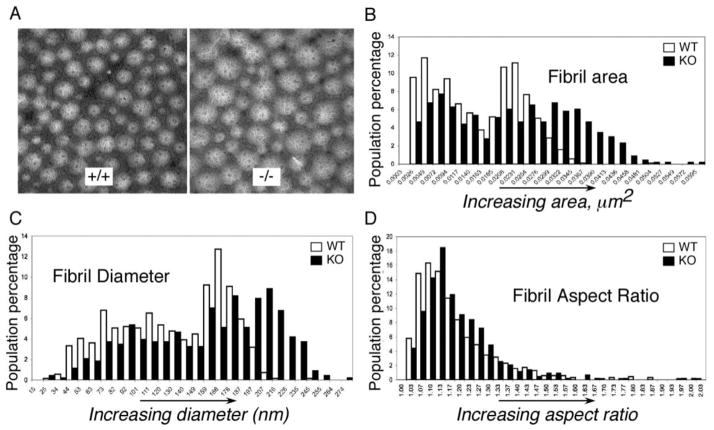
Patellar tendons were harvested, and collagen fibers were imaged by transmission electron microscopy (TEM). The morphological analysis of transverse sections of patellar tendons demonstrated that the distribution of the areas and diameters of the collagen fibrils were different in WT and Thbs4−/− mice (Fig. 2, A). In the Thbs4−/− mice, fibril areas (Fig. 2, B) and fibril diameters (Fig. 2, C) were more variable, and larger fibrils were over-represented (Fig. 2, B, C). Fibril area distribution in WT tendon has two readily discernable peaks centered at ~ 0.0050 μm2 and ~0.0230 μm2. The two peaks of fibril area in Thbs4−/− tendon were less pronounced, broader and shifted toward larger fibril areas with peaks at ~ 0.0072 μm2 and at 0.028 μm2. (Fig. 2, B). The population of fibers with areas greater than 0.035 μm2 comprised 16% of all fibers in Thbs4−/− tendon, but less then 1% in the WT mice. In Thbs4−/− tendon, the larger population of fibers had diameters centered around ~200 nm, whereas in the WT tendons, the larger population of fibers had diameters centered around ~160 nm (Fig. 2, C). All the differences were statistically significant (p<0.05, n≥5). Despite this shift in size distribution, the shapes of fibrils were not altered in tendons of Thbs4−/− mice; WT and Thbs4−/− fibrils were similar in aspect ratios (Fig. 2, D).
Figure 2. Collagen fibrils in tendon of Thbs4−/− mice.
(A) Transmission electron micrographs (TEMS) of collagen fibrils in patellar tendon of Thbs4−/− (right) and WT (left) mice. Scale bars = 0.5 μm. Histograms of collagen fibril area (B), fibril diameter (C) and fibril aspect ratio (D) in WT mice (open bars, n = 5) and Thbs4−/− mice (black bars, n =5). Fibril areas and fibril diameters are more variable, and larger fibrils are more prevalent in patellar tendon of Thbs4−/− mice but the shapes of fibrils, assessed from the aspect ratio, are not altered.
2.4. Distribution of TSP-4 in WT muscle
TSP-4 expression was reported in myotendinous junctions and in the perimysium of muscles(Arber and Caroni, 1995). We found that TSP-4 expression is not limited to the perimysium but is also abundant in the endomysium of muscles (Fig. 3, A–E). TSP-4 was detected in a close proximity to every fiber in the soleus, where the fibers are predominantly type I (slow oxidative) (Fig. 3, B). TSP-4 was also found around some but not all fibers in the gastrocnemius. These fibers are type I (slow oxidative) and type IIa (fast oxidative), as revealed by comparison with parallel muscle sections stained for the activity of succinate dehydrogenase, an enzyme involved in oxidative phosphorylation and immunostaining for the myosin type (not shown). Immunostaining revealed only modest staining for TSP-4 in a punctate pattern in the white portion of the muscle, which consists of type IIb fibers (Fig. 3, C, E).
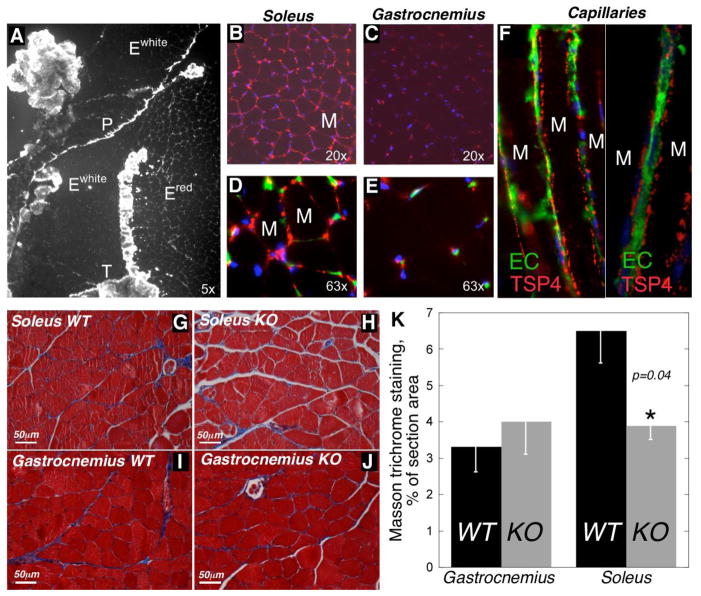
Figure 3. Expression of TSP4 in the skeletal muscle.
(A) Expression of TSP-4 (white color) in triceps. TSP-4 is abundant in tendon (T), perimysium (P) and endomysium of soleus (Ered, also shown in B, D), whereas its amount is lower in the endomysium of gastrocnemius and plantaris (Ewhite, also shown in C, E). TSP-4 (red color) is seen close to endothelial cells, which are visualized by FITC-conjugated lectin staining (green) (D, E). M = myocyte in B and D. (F) Capillaries between soleus muscle fibers are surrounded by globular aggregates of TSP-4. M = myocyte. Nuclei (blue) are detected with DAPI (B – F). (G–J) ECM staining with Masson trichrome (ECM = blue color) in soleus (G, H) and gastrocnemius (I, J) of WT (G, I) and Thbs4−/− mice (H, J). (K) Quantification of ECM in the skeletal muscles of WT and Thbs4−/− mice (% of area stained with Masson trichrome).
Previously, we reported that TSP-4 is expressed by endothelial cells and smooth muscle cells in the walls of blood vessels (Stenina et al., 2003). To determine whether TSP-4 in skeletal muscle tissue is also found in association with the microvessels adjacent to the muscle fibers, we injected FITC-conjugated Lycopersicon esculentum lectin, which binds specifically to endothelial cell membranes (Hansen-Smith et al., 1992), into WT and Thbs4−/− mice and co-stained the sections with anti-TSP-4 antibodies. TSP-4 localized in close proximity to endothelial cells on the basal side of endothelium (Fig. 3, F).
2.5. Extracellular matrix in soleus of Thbs4−/− mice
ECM was visualized by Masson trichrome staining of soleus sections (Fig. 3G–J). The area of deposition of ECM was quantified in the muscle of WT and Thbs4−/− mice using ImagePro 6.1 as described in our previous reports (Frolova et al., 2010; Frolova et al., 2012). There was no change in gastrocnemius, where TSP-4 was poorly expressed. However, a significant decrease of ECM was detected in soleus, where TSP-4 is abundant (Fig. 3K).
2.6. Comparison of TSP-4 to TSP-3 and TSP-5 expression in blood vessels of muscle
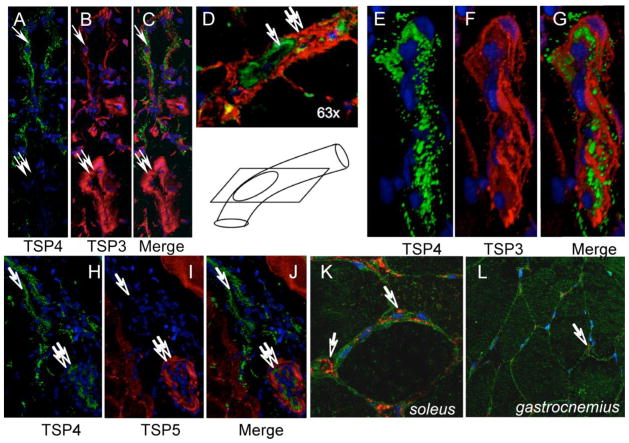
We have visualized blood vessels of muscle by staining endothelial cells using FITC-conjugated lectin from Lycopersicon esculentum or anti-vWF antibody. TSP-4 was detected in arteries, veins and capillaries but its expression was discontinuous: regions rich in TSP-4 and region lacking TSP-4 were present in each type of vessel. To compare the distribution and localization of all the homologous TSPs of subgroup B, we stained blood vessels with antibodies against TSP-3 and TSP-5. While all three TSPs were detected in small arteries, they did not co-localize (Fig. 4, A–J). TSP-4 was abundant in capillaries, but there was no TSP-5 or TSP-3 expression in the capillaries. Thus, while all three TSPs can be present in arterial wall and both TSP-4 and TSP-3 are found in veins, they do not co-localize and there is no evidence for compensation of TSP-4 deficiency. TSP-5 expression was observed along the basal membrane of the muscle fibers (Fig. 4, K and L), whereas TSP-4 expression was detected in the space between fibers, its expression was restricted to the capillaries.
Figure 4. Non-overlapping localization of TSP-3, TSP-5 and TSP-4 in blood vessels.
(A) Expression of TSP-3 (red) and TSP-4 (green) in vascular wall: TSP-3 is present in both vein (single arrow) and artery (double arrow). TSP-4 was not detected in all the arteries, while TSP-3 was always found in tunica media of arteries (double arrow). (D) Non-overlapping expression of TSP-3 (green, single arrow) and TSP-4 (red, double arrow) in the vascular wall. The vessel was sectioned in the direction shown in the cartoon, lower panel. (E–G) In the wall of veins, both TSP-4 and TSP-3 are detected in non-overlapping staining pattern. (H–J) TSP-4 (green) is consistently expressed in the vein wall, while no TSP-5 (red) expression is detected in veins (single arrow). In arteries, TSP-5 is expressed in tunica media. (K,L) TSP-5 (green) is expressed around muscle fibers, whereas TSP-4 (red) is found close to capillaries (arrow).
2.7. TSP-4 and heparan sulfates in the muscle
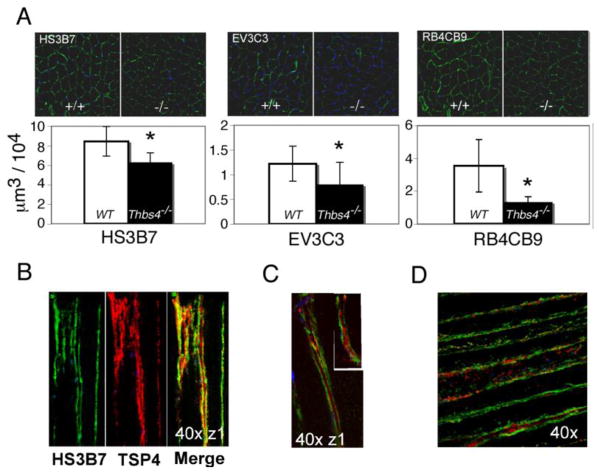
All TSP family members have heparin-binding sites (Adams, 2001; Chen et al., 2007), and certain functions of the TSPs may be dependent on their interactions with glycosaminoglycans (GAGs). Heparan Sulfates (HS) are present in the ECM, in the basal lamina and on the cell membranes, and are known to be involved in wide variety of cellular functions (Jenniskens et al., 2006). On this basis, we postulated that the absence of TSP-4 might affect the structure of specific HS in Thbs4−/− mice. Accordingly, immunostaining was performed with antibodies to six distinct HS epitopes (Dennissen et al., 2002; Jenniskens et al., 2000; van Kuppevelt et al., 1998) that report on different unidentified modifications of HS chains (Fig. 5 and Suppl. Fig. 2). Of the six HS epitopes analyzed, the three recognized by antibodies HS3B7, RB4CB9, and EV3C3, showed decreased presence in the soleus of Thbs4−/− mice compared to WT (Fig. 5, A). In WT mice, these three epitopes either overlapped with TSP-4 staining or were detected in close proximity to TSP-4 (Fig. 5B–D). The effect of TSP-4 deficiency on the presence of GAG epitopes was selective as no difference in the expression of three other HS epitopes, A4BO8, HS4C3, RB4CB12 (Suppl. Fig. 2), was observed.
Figure 5. Differential expression of specific heparan sulfate epitopes in soleus of Thbs4−/− mice.
(A) Expression of HS epitopes (green) recognized by HS3B7, EV3C3, and RB4CB9 antibodies in soleus. Upper panels – immunohistochemistry, representative images. Lower panels: Reduced amount of the epitopes was recognized by HS3B7, EV3C3, and RB4CB9 antibodies in soleus of Thbs4−/− mice (shaded bars) compared to WT mice (open bars). (B–D) HS are localized on the surface or in basal lamina of myofibers. TSP-4 (red) colocalizes with the (B) HS3B7 (green) epitope and locates between the GAGs in close proximity to (C) EV3C3 (green) and (D) RB4CB9 (green) epitopes.
2.8. HS core proteins in soleus and cultured microvascular endothelial cells (EC) from TSP-4−/− mice
Specific HS core proteins (syndecan, glypican, perlican) as well as total Heparan Sulfates were detected in the soleus tissue and in microvascular EC from WT and Thbs4−/− mice by Western blotting (Fig. 6). Equal loading was routinely monitored by staining the membranes with Ponceau Red before immediately after the transfer (before incubations with primary antibodies) and by staining the membranes with Coommassie Blue after completion of the experiment. Perlican could not be detected (not shown). Syndecan levels were not changed in the tissue, but more of this protein was detected on the surface of the EC from Thbs4−/− mice and in whole EC lysates. Glypican was remarkably absent for the muscle tissues of Thbs4−/− mice, but was expressed at the higher levels on the surface of cultured EC cells. In addition to glypican, another HS protein of MW ~130 kD was completely missing from the muscle tissues of Thbs4−/− mice. Based on the MW, we have analyzed the expression of beta-glycan, a receptor for TGFβ, and found that beta-glycan was indeed significantly downregulated in muscle tissue and cultured EC from Thbs4−/− mice (Fig. 6B). Addition of 10 μm rTSP-4 to the culture media increased the production of beta-glycan in cultured microvascular EC. To understand how the decreased expression of beta-glycan affects TGFβ-related signaling, we examined phosphorylation of p38 and smad2, two main intracellular mediators of TGFβ signaling. Both pathways were activated more in cells and tissues from Thbs4−/− mice, suggesting that beta-glycan normally plays inhibitory role, and this function is lost in Thbs4−/− mice.
2.9. Levels of HS-synthesizing enzymes in cultured microvascular EC from TSP-4−/− mice
Changes in expression of HS core proteins both in the lysates and on the surface of cultured EC suggested that TSP-4 influences their expression rather than their retention in tissues. Western blotting of lysates of microvascular EC isolated from WT and TSP-4−/− mice with antibodies against heparan sulfate 2-O-sulfotransferase 1 and N-heparan sulfate sulfotransferase 3, two enzymes controlling key steps in synthesis of HS (the initial chemical modifications required for the biosynthesis of the functional oligosaccharide sequences that define the specific ligand binding activities of HS), revealed the decreased levels of both enzymes in EC from TSP-4−/− mice and stimulation of their expression by rTSP-4 added to the cell culture media (Fig. 6C).
2.10. Uptake of very low density lipoproteins (VLDL) by endothelial cells of TSP-4−/− mice
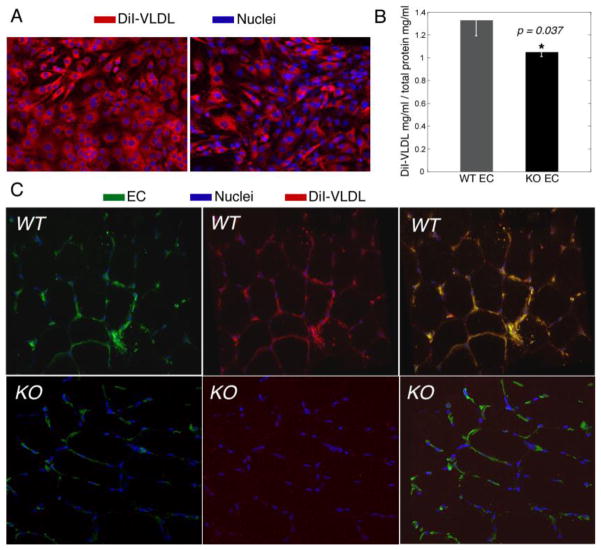
The binding and activity of many proteins and lipoproteins depends on surface of EC depends on the composition of GAGs (Kolset and Salmivirta, 1999). Specific modifications of GAGs are recognized by antithrombin (De Agostini et al., 1990; Naimy et al., 2008; Rosenberg and de Agostini, 1992), Fibroblasts growth factor 2 (FGF2)(Ye et al., 2001) and lipoprotein lipase (LPL)(Misra et al., 1994; Parthasarathy et al., 1994; Shimada et al., 1981). Furthermore, LPL is active only when bound to the cell surface GAGs (Berryman and Bensadoun, 1995; Eisenberg et al., 1992; Fuki et al., 2003; Rumsey et al., 1992; Williams et al., 1992). We have evaluated the uptake of VLDL, which is dependent on lipoprotein lipase activity (the rate limiting enzyme for hydrolysis of lipoprotein triglyceride that also mediates nonenzymatic interactions between lipoproteins and heparan sulfate proteoglycans), in EC derived from Thbs4−/− and WT mice and in vivo in soleus of Thbs4−/− and WT mice. The uptake of DiI-VLDL was significantly reduced in cultured EC of Thbs4−/− mice (Fig. 7, A and B) and in the skeletal muscle of Thbs4−/− mice (Fig. 7C). The decreased uptake of VLDL (Fig. 7) and the decreased levels of GAGs (Fig. 5) suggests that TSP-4 may regulate lipoprotein lipase activity by affecting the composition of ECM and cell surface GAGs.
Figure 7. Uptake of VLDL by EC of Thbs4−/− and WT mice.
(A, B) Uptake of DiI-VLDL by cultured EC: (A) representative image by immunofluorescence, confocal microscopy; (B) quantification of fluorescence. (C) Uptake of DiI-VLDL by EC in soleus of Thbs4−/− (KO) and WT (WT) mice.
2.11. LPL levels and activity in Thbs4−/− mice
LPL activity was measured in cultured EC isolated from Thbs4−/− and WT mice (Fig. 8A). The activity of endothelial LPL was also evaluated in vivo in Thbs4−/− and WT mice (Fig. 8B). In both models, the activity of LPL was reduced in the Thbs4−/− mice. The expression of LPL was analyzed by Western blotting (Fig. 8C). More LPL protein was detected both in the extracts of muscle tissues from TSP-4−/− mice and in the lysates and on the surface of EC from TSP-4−/− mice, suggesting that the loss of activity could not be explained solely by the decreased levels of LPL.
2.12. Metabolic changes in Thbs4−/− mice
Impaired release of free fatty acids from VLDL may lead to global metabolic changes associated with the decreased uptake of free fatty acids by skeletal muscles (e.g., the metabolism of energy in the skeletal muscle may rely more on glucose uptake and metabolism leading to global changes in metabolic indeces). We have measured blood glucose, insulin, LDL, HDL, non-esterified fatty acids (NEFA, free fatty acids), and VLDL in WT and Thbs4−/− mice at 8, 20 and 35 weeks (Table 1). No difference was detected in levels of LDL, HDL, NEFA or cholesterol between two strains of mice. However, glucose levels in the blood of Thbs4−/− mice were altered as early as 8 weeks and it remained consistently lower in fasting mice of older ages. Difference in insulin levels (lower levels in Thbs4−/− mice) became statistically significant at 20 weeks of age. At 8 weeks, difference in NEFA levels was detected in mice that received oil gavage: the blood levels of NEFA were lower in Thbs4−/− mice. Significantly lower levels of NEFA were detected in older mice of 55 weeks of age without oil gavage. To test whether the subtle alteration may become overt in mice on the high fat/high carbohydrate content, we kept groups of mice on the high fat/high carbohydrate diet starting at age 4 weeks. In addition to differences in blood glucose and insulin, mice on the high fat/high carbohydrate content diet developed higher blood levels of total cholesterol and VLDL, consistent with the inability to process VLDL dues to the impaired function of endothelial LPL.
Table 1. Metabolic changes in Thbs4−/− mice.
Glucose, insulin, non-esterified fatty acids (NEFA), total cholesterol, VLDL, LDL (not shown – no difference), and HDL (not shown – no difference) were measured in WT and Thbs4−/− mice at age 8 weeks, 20 weeks, and 35 weeks. Blood from mice on normal chow diet and from mice on high carbohydrate diet (started at 4 weeks of age) was used. In mice on chow diet, NEFA were measured in fasted mice and in mice that received oil gavage. NEFA were also measured at 55 weeks of age and were found significantly different between WT and Thbs4−/− mice. Only statistically significant changes (p < 0.05)are shown in the table. Data from fasted mice on chow diet without further manipulation are not shaded, data from mice on high carbohydrate content diet and mice after the oil gavage are shaded.
| Glc, mg/dL | Glc, mg/dL, high carb | Insulin, ug/L | Insulin, Ug/L, high carb | NEFA | NEFA, mmol/L oil gavage | Cholester, mg/dL high carb | VLDL, mg/dL, high carb | |
|---|---|---|---|---|---|---|---|---|
| 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks | 8 weeks |
| KO, n=5 | 74.8±14 | 0.44±0.08 | ||||||
| WT, n=4 | 96.5±18 | 0.61 ±0.03 | ||||||
| p | 0.018 | 0.006 | ||||||
| 20 weeks | 20 weeks | 20 weeks | 20 weeks | 20 weeks | 20 weeks | 20 weeks | 20 weeks | 20 weeks |
| KO, n=11 | 0.19±0.09 | |||||||
| WT, n=12 | 0.36 ±0.23 | |||||||
| p | 0.028 | |||||||
| 35 weeks | 35 weeks | 35 weeks | 35 weeks | 35 weeks | 35 weeks | 35 weeks | 35 weeks | 35 weeks |
| KO, n=5–10 | 106±9.9 | 152±23 | 1.68±0.41 | 144±35 | 14±3.6 | |||
| WT, n=5–12 | 124±12.8 | 177±27.5 | 3.01±1.02 | 100±9.5 | 9.3±3.25 | |||
| p | 0.014 | 0.021 | 0.0012 | 0.049 | 0.04 | |||
| 55 weeks | 55 weeks | 55 weeks | 55 weeks | 55 weeks | 55 weeks | 55 weeks | 55 weeks | 55 weeks |
| KO, n=6 | 0.67±0.09 | |||||||
| WT, n=10 | 0.85 ±0.1 | |||||||
| p | 0.015 |
2.13. Altered mass of skeletal muscle of Thbs4−/− mice
Muscles from male and female WT and Thbs4−/− mice were dissected and weighed. There was no difference in the weights of gastrocnemius of WT and Thbs4−/− mice: e.g., at 29 weeks, the weight of gastrocnemius was 75±6 mg/cm in Thbs4−/− mice vs 73±3 mg/cm in WT males (n=8, p=0.28). However, the weight of the soleus was altered in Thbs4−/− mice starting as early as at 8 weeks of age.
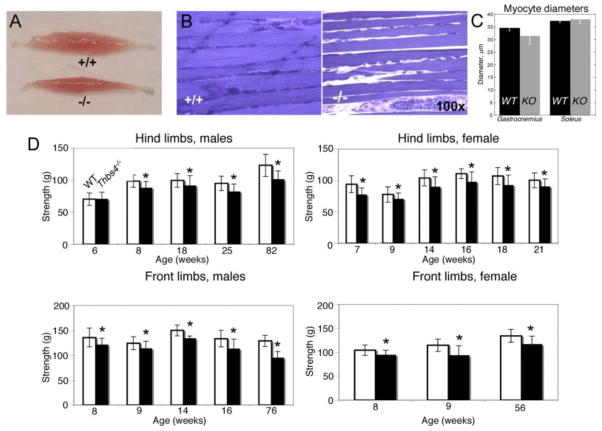
At 8–14 weeks of age, the soleus from Thbs4−/− animals had ~ 30% less mass compared to WT mice (2.75±0.37 mg/cm in Thbs4−/−, n =10, versus 3.89±0.27 mg/cm in WT, n=4, p<0.05, males, weight normalized per tibia length) (Fig. 9, A). Differences in the weight of the soleus were sustained from 29 to 50 weeks and continued in older mice [75 weeks of age (2.21±0.5 mg/cm in Thbs4−/− mice, n =4, versus 4.1±0.65 mg/cm in WT, n = 5, p<0.05)]. The soleus of female mice tended to be smaller in Thbs4−/− versus WT as well; at 72 weeks of age, 4.1±0.5 mg/cm in Thbs4−/− mice versus 5.34±0.39 mg/cm in WT, n = 4 for both groups, p<0.05. Examination of the microstructure of the soleus from Thbs4−/− mice revealed that the individual the lack of adhesion between the fibers (Fig. 9, B), consistent with the loss of ECM (Fig. 3H–K). While anecdotal, we found that the soleus muscles from Thbs4−/− mice were more fragile and difficult to section than the same tissue derived from WT mice. The size of myocytes was not changed, suggesting that the altered weight and function of the muscles were due to the loss of ECM and resulting functional changes.
Figure 9. Muscle morphology and function are changed in Thbs4−/− mice.
(A) Lower soleus muscle mass in WT and Thbs4−/− mice. (B) Muscle fibers of WT and Thbs4−/− soleus (note additional space between fibers that appeared during sample preparation). (C) Myocyte diameter in soleus and gastrocnemius of WT and Thbs4−/− mice. (D) Muscle strength of hind limbs (top panels) and front limbs (bottom panels) of males (left panels) and females (right panels) are decreased in Thbs4−/− as compared to WT mice (mean value ± standard deviations, *p<0.05, 3≤n≤13 for each age group, 6–8 trials were performed with each mouse in recording limb strength).
2.14. Altered function of limbs in Thbs4−/− mice
To determine whether the abnormal tendon structure and the biochemical changes in skeletal muscle translate into a dysfunction of the whole limb, we examined whether the lack of TSP-4 and its influence on muscle mass affected the strength of Thbs4−/− mice. The strength of the front and hind limbs of mice of both genders at different ages was measured using a dynamometer. The average grip strength in younger mice (<6 week old) was nearly identical in both groups of animals in both genders (Fig. 9, C; p = 0.86). Average strength decreased modestly in Thbs4−/− mice starting at the 7th week of age, and continued to decrease markedly by 76–82 weeks compared to WT mice (Fig. 9, C). This observation suggests that, compared to WT mice, Thbs4−/− mice are less capable of maintaining grip strength with age thereby implying possible impairment of muscle function.
3. DISCUSSION
In this work, we have examined the effect of TSP-4 deletion on the structure of tendon and muscle and on the performance of the whole limb in Thbs4−/− mice. We found profound changes in the structure of tendon and in the composition of red muscle ECM, which translated into the impaired metabolism of the main source of energy of the red muscle (VLDL) and in altered intracellular signaling. The major findings of our study are: 1) TSP-4 controls the deposition of ECM in tendon and skeletal muscle; 2) TSP-4 is necessary for the correct organization of collagen fibrils in tendon; 3) The expression of TSP-4 in skeletal muscle is associated with fibers relying on oxidative metabolism, and TSP-4 is necessary to maintain the uptake of fatty acids from VLDL; 4) The effects of TSP-4 on skeletal muscle structure and metabolism are, at least in part, mediated by controlling the correct HS modification; and 5) The function of the whole limb is affected by the changes in tendon and skeletal muscle structure and function.
Similar to mice with deficiencies of the other two members of TSP subgroup B (Hankenson et al., 2005; Svensson et al., 2002), Thbs4−/− mice do not show obvious anatomical or functional differences compared to WT mice. However, when Thbs4−/− mice are challenged in disease models that affect the organs with the highest level of TSP-4 expression (e.g., heart (Cingolani et al., 2011; Frolova et al., 2012; Lynch et al., 2012) and blood vessels (Frolova et al., 2010; Mustonen et al., 2008), the role of TSP-4 as regulator of ECM and cell-matrix interactions are readily discerned. In the models of pressure overload, TSP-4 was necessary to control fibrosis of myocardium and to prevent excessive ECM deposition leading to heart hypertrophy and abnormal function of cardiomyocytes and the whole myocardium.
On closer examination of Thbs4−/− mice, structural and/or functional abnormalities were found in tendon and skeletal muscle, two tissues that display abundant TSP-4 expression in WT mice. Tendon was known as a tissues with abundant expression of TSP-4 (Hauser et al., 1995; Hecht et al., 1998; Sodersten et al., 2006), TSP-1 (Kannus et al., 1998), TSP-2 (Kyriakides et al., 1998) and TSP-5 (Hecht et al., 1998). Our data add TSP-3 to this list. Thus, mouse tendon expresses all five TSP family members. In tendon of Thbs4−/− mice, collagen fibers are abnormal in size and organization. TEM analysis of patellar tendon revealed alterations in the size of collagen fibers. The most noticeable change was the increased number of larger fibers in Thbs4−/− patellar tendon. Deficiencies of several other matrix proteins, including thrombospondin-2 (Kyriakides et al., 1998), decorin (Zhang et al., 2006), lumican (Jepsen et al., 2002), collagen type V (Marchant et al., 1996), fibromodulin (Ezura et al., 2000) and biglycan (Ameye et al., 2002; Corsi et al., 2002) are associated with alterations in the diameter of collagen fibrils in various tissues, including patellar tendon. The alteration in fiber diameter in Thbs4−/− patellar tendon is similar to that observed in the FDL (flexor digitorum longus) tendon in decorin-deficient mice, where an increased number of larger diameter fibers was observed. Also, of note, larger collagen fibrils were observed in the patellar tendon of Thbs2−/− mice. However, in Thbs2−/− mice, tail tendons were lax, and both ligaments and skin were fragile (Kyriakides et al., 1998). Such manifestations of collagen disorganization were not observed in the Thbs4−/− mice, although the changes in fibril size and in their organization clearly indicate that TSP-4 is required for the correct fibril assembly and likely for their interaction with other ECM proteins and/or the maintenance of the correct composition of the ECM in tendon.
In WT muscle, TSP-4 expression is detected in the perimysium and in the endomysium and is especially abundant in red muscles. ECM levels are affected by TSP-4 deficiency in soleus (red muscle): the amount of ECM is decreased ~40% in soleus of Thbs4−/− mice, but not in gastrocnemius. This decrease in ECM production or retention is in contrast with the effect of TSP-4 on ECM in myocardium, where the deficiency in TSP-4 results in increased production of ECM and increased size of the heart. Although the mechanisms of ECM regulation in both organs are unknown, it is clear that the effects of TSP-4 on ECM production and composition are organ-specific. Our results demonstrate that TSP-4 is required for the correct modification, production and/or retention of several heparan sulfate proteoglycans in skeletal muscle. TSP-4 expression is associated with different types and sizes of blood vessels in the muscle. When present, it is localized primarily to the tunica adventitia in larger vessels or on the basal side of endothelium in capillaries.
TSP-4 shares up to 82% homology with TSP-3 and TSP-5 in C-terminal part and may be able to form heteromers with TSP-5 (Hecht et al., 1998; Sodersten et al., 2006). Therefore, we considered whether another subgroup B family member might substitute for TSP-4. Such substitution and compensation of functions would be suggested by the similarity in localization and increase in levels of TSP3 and/or TSP5 in Thbs4−/− mice. All three TSP subgroup B members are expressed in tendon, skeletal muscle and blood vessels, but the patterns of their distributions in these tissues are distinct. TSP-5 had been previously demonstrated to be present in blood vessels (Riessen et al., 2001), but this localization had not been reported for TSP-3. The absence of TSP-4 in Thbs4−/− mice did not alter the intensity or distribution of other two highly homologous family members of subgroup B in blood vessels, suggesting that the functions of TSP-4 are different from the functions of TSP-3 and TSP-5, and TSP-4 deficiency is not compensated. Although we found TSP-3 to be expressed in tendon, it is associated with different tendon structures than TSP-4 or TSP-5. TSP-5 is also expressed in tendon, and its expression overlaps with that of TSP-4. However, based on our careful examination of the two molecules using immunohistochemistry/confocal microscopy and Western blotting of the extracts from soleus, we did not detect any increase in the expression of TSP-5 or TSP-3 in tendon of Thbs4−/− mice. Based on the detection in Western blotting, there was no compensatory overexpression of any of four other TSPs in skeletal muscle tissues.
All TSPs, including TSP-4, have heparin-binding sites (Chen et al., 2007; Narouz-Ott et al., 2000) and interact with HS, which are ubiquitously distributed in the ECM and on cell surfaces, and are involved in the structural integrity of skeletal muscle ECM, the continuity between muscle ECM and tendon and, as a result, directly affect the function of tendons and muscles and the limb in whole. Using unique antibodies that recognize distinct but unidentified epitopes of GAGs (Dennissen et al., 2002; Jenniskens et al., 2000; van Kuppevelt et al., 2001), we found that TSP-4 influences the distribution of specific GAG epitopes in muscle. Our findings point to a selective interrelationship between TSP-4 and HS. TSP-4 co-localized with HS modifications recognized by the antibodies EV3C3, HS3B7, RB4CD9, but did not change the distribution of HS modifications recognized by the antibodies A4BO8, HS4C3V, RB4CB12. HSPGs are important for the structural integrity and proper function of the endothelium. HSPGs located on the basal side of vessels interact with a variety of basement membrane proteins (Hayashi et al., 1992). Basal HSPGs inhibit smooth muscle cell proliferation and migration (Castellot et al., 1981) and regulate processes of filtration in the endothelium (Kanwar and Farquhar, 1979). Thus, processes of nutrient exchange between the endothelium and muscle cells could be impaired with the loss of TSP-4 because of changes in the composition and/or amount of HS. We have identified altered levels of two HS core proteins in the muscle tissues of TSP-4−/− mice: loss of glypican and beta-glycan. Loss of beta-glycan was confirmed using cultured microvascular EC from TSP-4−/− mice and was associated with increased activity of two mediators of main pathways of TGFβ signaling (p38 and smad2) both in the tissues and in cultured EC. The role of beta-glycan as a TGFβ receptor is controversial: both activating and inhibiting activities on TGFβ have been ascribed (Bilandzic and Stenvers, 2011; Gatza et al., 2010). Our data suggest that beta-glycan has inhibiting effect on TGFβ signaling in skeletal muscle, and TSP-4 is required for its production/retention in the ECM. The decreased expression of two key enzymes controlling HS synthesis and modification and the restoration of their expression by rTSP4 suggests that TSP-4 affects the intracellular synthesis and correct modification of HS and, as a result, the levels of specific HS proteoglycans, including beta-glycan.
Many plasma proteins bind to the cell surface using distinct specific modifications and combinations of surface GAGs (De Agostini et al., 1990; Kolset and Salmivirta, 1999; Misra et al., 1994; Naimy et al., 2008; Parthasarathy et al., 1994; Rosenberg and de Agostini, 1992; Shimada et al., 1981; Ye et al., 2001). Based on our observation that TSP-4 is highly expressed in red muscle that utilizes primarily oxidative metabolism and that in Thbs4−/− mice these muscles are affected more than white muscles that utilize primarily glycolytic metabolism, we tested the activity of LPL, an endothelial enzyme responsible for the uptake and hydrolysis of lipoproteins (Berryman and Bensadoun, 1995; Eisenberg et al., 1992; Fuki et al., 2003; Rumsey et al., 1992; Williams et al., 1992). LPL becomes active upon binding to the EC surface by interacting with GAGs. We found that the uptake of VLDL and the activity of endothelial LPL were decreased in Thbs4−/− mice. Although the activity of LPL was reduced in both the tissues of soleus and in cultured EC, the levels of production of LPL were not decreased but rather increased in Thbs4−/− mice, and more LPL was associated with the cell surface. These observations suggest that LPL in Thbs4−/− mice is less active, probably as a result of incorrect binding to the surface due to the changes in HS composition, which does not support the enzyme activity. These relationships suggest a role of TSP-4 in maintenance of LPL activity by supporting production and modification of specific HS and, as a result, in the metabolism of red muscle.
The weight of the soleus muscle was decreased in Thbs4−/− mice as compared to WT. The loss in weight of older Thbs4−/− mice may be due to the reduction of red muscle mass in these animals. We also noticed that Thbs4−/− mice have higher blood levels of lactate, especially in older mice (data not shown), which suggests a perturbation of energy metabolism in muscle. Microscopic examination revealed alterations of structure consistent with altered ECM: lack of ECM or loss of ECM during the preparation of the section and loss of attachment between individual muscle fibers in Thbs4−/− mice. We attributed these changes to the loss of ECM, because the diameter of myocytes was not changed, and the staining of ECM revealed lower levels of ECM in Thbs4−/− mice.
Based on these structural changes in the skeletal muscle, abnormal tendon structure and decreased availability of energy supply from lipoproteins, we expected to find a decline in muscle mass and performance of the whole limb. Indeed, Thbs4−/− mice have less muscle strength than WT mice.
Taken together, these observations indicate a role of TSP-4 in the organization and functions of tendons, muscles and blood vessels, which translate into functional differences at the cellular level and differences in performance of the whole limb between Thbs4−/− and WT mice.
4. EXPERIMENTAL PROCEDURES
4.1
Thbs4−/− mice have been described previously (Frolova et al., 2010; Frolova et al., 2012). In this study, we used mice that had been backcrossed for at least 8 generations with C57BL/6 mice. As controls, wild-type (WT) littermates or WT age-matched litters of brother and sister matings were used. Genotyping was verified by PCR with primers that distinguish between the wild-type and the mutant allele. The primers used are:
GGAGAGAGAATAGCAAGATCAGCTC: gene-specific endogenous;
AACAAGCAATGGAAGGCAGACCCTG: gene-specific, endogenous and targeted;
GGGTGGGATTAGATAAATGCCTGCTCT:Neo, targeted.
The absence of TSP-4 mRNA and protein was confirmed by Northern and Western blots as well as by specific immunostaining of multiple tissues with anti-TSP-4 antibody (Frolova et al., 2010; Frolova et al., 2012). The mice were housed in the Biological Resource Unit in a temperature-controlled environment with a 12:12-h light-dark cycle and were fed a standard chow diet ad libitum. All animal procedures were performed according to NIH guidelines under protocols approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
4.2. Antibodies
The TSP-4 antibody used (R&D, Minneapolis, MN) was raised in a goat using a part of the TSP-4 molecule that is homologous between human and mouse (Ala22-Asn961), and it recognized both human and mouse TSP-4. Rabbit anti-TSP-3 IgG was from Santa Cruz (Santa Cruz, CA). Rat anti-COMP (TSP-5) IgG was from Affinity BioReagents (Golden, CO). Antibodies to specific GAG modifications have been previously described (Dennissen et al., 2002; Jenniskens et al., 2000; van Kuppevelt et al., 2001). For immunohistochemistry, the periplasmic fractions containing VSV-tagged single chain antibodies, designated HS3B7 (epitope preference not known), RB4CB9 (IdoA, NS (inhibitory: 2OS, 6OS)), EV3C3 (epitope preference not known), RB4CD12 (NS, 2OS, 6OS [IdoA2S-GlcNS6S]), AO4BO8 (IdoA, NS, 2OS, 6OS [IdoA2S-GlcNS6S]), HS4C3 (NS, 3OS, 6OS [IdoA2S-GlcNS3S6S]) were used. Polyclonal rabbit anti-VSV IgG from MBL (Woburn, MA) and Alexa 488-labeled anti-rabbit IgG (Molecular Probes, Eugene, OR) were used for their detection. Other secondary antibodies, Rhodamine Red-X anti-goat IgG, Rhodamine Red-X anti-rabbit IgG, Rhodamine Red-X anti-rat IgG, FITC anti-rabbit IgG were from Jackson ImmunoResearch (West Grove, PA), Alexa 488 anti-goat IgG was from Invitrogen/Life technologies (Grand Island, NY). Anti-CD31 antibody was from BD Biosciences (San Jose, CA). Anti-beta-glycan, anti-phospho-SMAD2, and anti-phospho-p38 antibodies were from Cell Signaling Technology (Beverly, MA), anti-NDST1 and anti-HS2ST1 antibodies were from MyBiosource (San-Diego, CA), anti-LPL antibody was from LSBio (Seattle, WA), anti-TSP1 was from LabVision/Neo Markers (mouse ab-4, Fremont, CA), anti-TSP2 antibody was from R&D Systems (Minneapolis, MN), anti-glypican antibody was from Proteintech (Chicago, IL), anti-perlican antibody was from Acris Antibodies (San Diego, CA), anti-syndican antibody was from Abcam (Cambridge, MA), and anti-Heparan Sulfate antibody was from AMSBio (Lake Forest, CA).
4.3. Immunohistochemistry
Mice were sacrificed by CO2 inhalation followed by cervical dislocation; triceps were harvested and placed on 7% Gum Tragacanth (Sigma, St. Louis, MO), frozen in liquid nitrogen-cooled isopentane (Sigma, St. Louis, MO) and stored at −80°C until processing. Frozen sections (10–12 μm) were cut in a cryostat (Leica, Wetzlar, Germany) and placed on microscope slides (Superfrost; Fisher Scientific, Waltham, MA). Sections were immediately blocked by incubation in PBS containing 5% BSA (MB Biomedicals, OH) and donkey IgG (1:50) for 30 min at 4°C. Following this treatment, the slides were incubated with primary antibodies for 2 h at 4°C. The concentrations of the primary antibodies used were as follows: rabbit anti-TSP-3: 1:50; goat anti-TSP-4: 1: 200; anti-TSP-5: 1: 10. After washing with PBS/BSA (3x10 min), primary antibodies were detected by incubating sections in Rhodamine Red-X (1:200) or FITC (1:100)-conjugated secondary antibodies for 45 min at 4°C. For double immunofluorescence labeling using antibodies to TSP-4 and TSP-3 or TSP-5, the two primary and secondary antibodies were applied to the sections simultaneously. For double-labeling sections for TSP-4 and GAG epitopes, cryosections of transverse triceps were first blocked and then incubated with the periplasmic fraction containing the selected single chain antibody (1:1–1:5) for 1.5 h. After three washes in PBS/BSA, the anti-VSV antibody (1:100–1:500 diluted with PBS/BSA) and anti-TSP-4 was added for 1 h followed by detection with FITC-conjugated anti-rabbit IgG (1:200) and Rhodamine Red-X anti-goat IgG. Sections were mounted in Vectashield with DAPI (Vector Lab. Inc., Burlingame, CA).
4.4. Confocal Microscopy
Z-stacks of images were collected using a Leica TCS-SP3-AOBS Laser Scanning Confocal Microscope (Leica Microsystems Inc., Bannockburn, IL) with an HCX Plan Apo 40x/1.25 NA oil immersion objective. All z-series were collected using the same number of slices, step size (0.4 μm), and collection parameters including brightness, contrast and pinhole. Image stacks from the z-series were reconstructed and analyzed using Volocity 4.1.0 software (Improvision Inc., Lexington, MA). Matching areas from WT and Thbs4−/− samples (WT, n=5; Thbs4−/−, n=5), which excluded tendons and blood vessels, were selected for analysis. The mean integrated optical density (IOD) was calculated from selected areas for comparison between WT and Thbs4−/− samples.
4.5. Transmission Electron Microscopy
Three male and three female mice of WT (C57/BL6) or Thbs4−/− genotypes were analyzed. Following sacrifice ~ 2 × 3 mm of the central region of the patellar tendons was dissected and cleaned of loose connective tissue, the paratendon and epitendon. Tissues were immediately fixed in 2.5% glutaraldehyde/4% paraformaldehyde, pH 7.3, for 24 h, followed by postfixation with 1% osmium tetroxide for 1h. After en bloc staining and dehydration with a series of graded ethanol, the samples were embedded with eponate 12, polymerized at 68 °C for 48 h, trimmed, sectioned at 70–90 nm using a diamond knife on a Leica Ultramicrotome, and poststained in 50% saturated uranyl acetate and 0.2% lead citrate. The sections were examined and photographed at 60 kV using a Philips CM12 transmission electron microscope (FEI, Hillsboro, OR). Film images of the central region of the tendon of each mouse were taken (one per mouse), digitized on a MicroTek flatbed scanner. Morphometric fibril analysis was performed using Image Pro Plus 6.1 (Media Cybernetics, Silver Spring, MD). In total, 429 to 693 collagen fibers per group were analyzed to measure the frequency of differences in fibril diameters.
4.6. Morphometric Analysis of Collagen Fibrils
Fibril morphometric parameters including area, number, roundness, radius-ratio, aspect ratio (the ratio between the major axis and the minor axis of the ellipse equivalent to the object), perimeter, and mean separation were extracted from EM images using a semi-automated batch-processing routine generated for Image Pro Plus 6.1 (Media Cybernetics, Silver Springs, MD). For accurate segmentation of fibrils, image noise was removed using a median filter and local equalization, and rank filters were applied to enhance fibril contrast. Subsequently, touching fibrils were separated using modified watershed and seed-fill algorithms, and detected fibrils were thresholded using various size and shape delimiters to produce a binary fibril mask. To confirm whether fibrils were correctly segmented, pseudo-colored fibril outlines (generated from the fibril mask) were superimposed upon the original EM image allowing the software user (blinded observer) to add missing fibrils or remove incorrectly segmented fibrils. Fibril morphometric parameters were calculated in the final (user-modified) fibril mask and exported to Excel. Lastly, for analysis of fibril separation, Euclidean Distance Map (EDM) and skeletonized representations of the inverted fibril mask were multiplied together to produce an image containing medial separation lines (1 pixel wide) between adjacent fibrils with pixel gray values corresponding to fibril separation (distance of medial pixel to fibril border). These pixel values were summed, divided by the total number of skeletal pixels, and multiplied by 2 to report mean fibril separation. With all parameters exported, customized Excel scripts were used to pool morphometric indices for Thbs4−/− and WT groups, calculate ranges, means, and standard errors, and to create histograms for each parameter to examine population distributions within each group.
4.7. Measurement of grip muscle strength
The front limb grip strength was measured using a dynamometer (Chatillon Digital Force Gauge, DFIS 2, Columbus Instruments, Columbus, OH). The mouse was allowed to grasp a horizontal fore limb pull bar with both front paws, and gentle traction was applied to the tail; at the point when the mouse released the bar, grip strength was recorded (Smith et al., 1995). The mean grip strength from between six to eight trials per mouse was analyzed. To measure the hind limb grip strength, the forepaws were supported for stability with a horizontal fore limb pull bar while the hind limbs gripped an angled wire mesh. The mouse was pulled by its tail toward the mesh steadily until it let go (~3 sec). The mean pulling force from six to eight trials per mouse was analyzed.
4.8. Staining and analysis of capillaries
Mice were anesthetized with 100 μl of ketamine/xylazine solution (100 mg/kg and 20 mg/kg respectively), followed by injection into the heart through the diaphragm of FITC-conjugated lectin from Lycopersicon esculentum (Sigma, St. Louis, MO), 60 μg/25 g of body weight in 150–200 μl of PBS. Anesthetized mice were sacrificed by cervical dislocation in 10 min. Triceps were isolated, frozen, sectioned, and stained for TSP-4 as described above.
In order to detect the changes in the vascularity of the soleus and the white part of the gastrocnemius muscles between WT and Thbs4−/− mice, z-series were collected from the same location in the muscles with a Leica TCS-SP3-AOBS Laser Scanning Confocal Microscope using an HC Plan Apo 20x/0.7 NA oil immersion objective. Image stacks from the z-series were reconstructed using Volocity software (Improvision Inc., Lexington, MA, USA). Matching areas from WT and Thbs4−/− two-dimensional images of the reconstructions were selected for analysis using Image Pro Plus 6.1 (Media Cybernetics, Inc., Silver Spring, MD, USA). Grayscale images of the lectin labeling were extracted from each reconstructed image and analyzed using Image Pro Plus 6.1 to determine the area of lectin labeling per total selected area.
4.9. Uptake of DiI-VLDL in vivo
Male WT and Thbs4−/− mice, 24-weeks of age, were injected with 100 μl (0.5 mg/ml) of DiI-VLDL (Kalen Biomedical, Montgomery Village, MD), in the retro-orbital sinus. After 30 min, the mice were sacrificed by isoflurane overdose and perfused with 10 ml PBS injected in the left heart ventricle. Tissues were collected and frozen in O.C.T. and processed for immunohistochemistry as described above to visualize TSP-4, CD31 and DiI-VLDL by microscopy.
4.10. Uptake of DiI-VLDL by cultured endothelial cells (EC)
Mouse lung EC were isolated from WT and Thbs4−/− mice as described (Mahabeleshwar et al., 2006). After EC reached confluency, they were incubated in EGM-2 medium without heparin or serum for 72 hours, washed twice with PBS, and DiI-VLDL (6 mg/ml in 0.5 ml of the medium supplemented with serum and heparin) was added. After 3 hours at 37°C, cells were washed twice with PBS containing 0.4% BSA, followed by three washes in PBS. The cells were lysed with 1% SDS in 0.1M NaOH for 1 hour with gentle shaking. Fluorescence was measured at an excitation wave length 520nm and an emission wave length of 580 nm. Each experiment was performed in triplicate. To visualize the uptake of DiI-labeled VLDL, EC were seeded onto chamber slides. When cells reached confluency, DiI-VLDL (6 mg/ml) was added under the conditions described above. At the end of the experiment, the cells were washed 3 times with PBS and fixed in 4% paraformaldehyde.
4.11. Measurement of Lipoprotein Lipase (LPL) activity
Confluent cultures of EC from WT and Thbs4−/− mice were incubated overnight with 60 μg/ml VLDL (Kalen Biomedical, Montgomery Village, MD) in serum- and heparin-free medium. After removing the medium, medium containing 10 U/ml heparin and 2% BSA was added for 40 min to induce release of cell-surface-bound LPL. The cells were lysed by sonication, and LPL in the cells treated with heparin and in untreated cells was compared.
To measure active endothelial LPL in vivo, blood plasma samples were collected from WT and Thbs4−/− mice before and 10 min after the injection of 20U of heparin. The amount of active LPL was calculated as a difference between the activity before and after the heparin injection. LPL activity in culture supernatants and plasma samples was measured using Roar LPL Activity kit (Roar Biomedical, Inc., New York, NY) according to the manufacturer’s instructions.
4.12
Metabolic indeces (blood glucose, insulin, lipoproteins, cholesterol, non-esterified fatty acids) were measured as was previously described (Frolova et al., 2010).
4.12. Statistical analysis
All data are represented as means ± SE or SD as indicated. Significant differences between two groups were determined with using an unpaired Student’s t-test. The significance level (p) was set at 0.05. *p < 0.05 versus control; and **p < 0.001 versus control are distinguished by the number of asterisks.
Supplementary Material
Levels of TSP-1, TSP-2, TSP-3, and TSP-5 in extracts from soleus tissues of WT and Thbs4−/− mice with the loading control (Ponceau S staining of membrane to visualize total protein).
Unchanged levels of HS epitopes (green) recognized by A4BO8, HS4C3V, RB4CD12 antibodies in the soleus of WT (left panels) and Thbs4−/− (right panels) mice.
Highlights.
TSP-4 controls the deposition of ECM in tendon and skeletal muscle.
In tendon, TSP-4 deficiency results in altered structure of collagen fibrils.
TSP-4 deficiency results in impaired metabolism in red skeletal muscle.
TSP-4 deficiency causes incorrect modification of heparan sulfates and, as a result, decreased activity of lipoprotein lipase and loss of betaglycan (TGF® receptor III).
These changes result in decreased strength of the whole limb.
Acknowledgments
We thank John Peterson, Mei Yin (Imaging Core, LRI, Cleveland Clinic) for excellent technical assistance, Suneel Apte, (Department of Biomedical Engineering) for helpful discussions and Quteba Ebrahem (Department of Ophthalmic Research, Cole Eye Institute, Cleveland Clinic) for help with the technique and pilot experiments with lectin injections.
This work was supported by National Institutes of Health Grants 1R01 DK067532-01 (OSA) and 1R01HL117216 (EFP and OSA).
Abbreviations
- TSP
thrombospondin
- EGF
epidermal growth factor like domains
- SNP
single nucleotide polymorphism
- EC
endothelial cells
- ECM
extracellular matrix
- ES cell line
embryonic stem cell line
- COMP
cartilage oligomeric matrix protein
- GAG
glycosaminoglycan
- HS
heparan sulfates
- IOD
integrated optical density
- TEM
transmission electron microscopy
- FITC
fluorescein isothiocyanate
- DAPI
4′,6-diamidino-2-phenylindole
- LPL
lipoprotein lipase
- VLDL
very low density lipoproteins
- DiI-VLDL
1,1′-dioctadecyl- 3,3,3′,3′-tetramethylindocarbocyanine perchlorate-labeled VLDL
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- Adams JC. Functions of the conserved thrombospondin carboxy-terminal cassette in cell-extracellular matrix interactions and signaling. Int J Biochem Cell Biol. 2004;36:1102–1114. doi: 10.1016/j.biocel.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Adams JC, Lawler J. Cell-type specific adhesive interactions of skeletal myoblasts with thrombospondin-1. Mol Biol Cell. 1994;5:423–437. doi: 10.1091/mbc.5.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF. Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J. 2002;16:673–680. doi: 10.1096/fj.01-0848com. [DOI] [PubMed] [Google Scholar]
- Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J Cell Biol. 1995;131:1083–1094. doi: 10.1083/jcb.131.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, Bensadoun A. Heparan sulfate proteoglycans are primarily responsible for the maintenance of enzyme activity, binding, and degradation of lipoprotein lipase in Chinese hamster ovary cells. J Biol Chem. 1995;270:24525–24531. doi: 10.1074/jbc.270.41.24525. [DOI] [PubMed] [Google Scholar]
- Bilandzic M, Stenvers KL. Betaglycan: a multifunctional accessory. Mol Cell Endocrinol. 2011;339:180–189. doi: 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot JJ, Jr, Addonizio ML, Rosenberg R, Karnovsky MJ. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981;90:372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FH, Herndon ME, Patel N, Hecht JT, Tuan RS, Lawler J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J Biol Chem. 2007;282:24591–24598. doi: 10.1074/jbc.M611390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, Bedja D, Barth AS, Moens AL, Kass DA. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109:1410–1414. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Cui J, Randell E, Renouf J, Sun G, Green R, Han FY, Xie YG. Thrombospondin-4 1186G>C (A387P) is a sex-dependent risk factor for myocardial infarction: a large replication study with increased sample size from the same population. Am Heart J. 2006;152:543, e541–545. doi: 10.1016/j.ahj.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Cui J, Randell E, Renouf J, Sun G, Han FY, Younghusband B, Xie YG. Gender dependent association of thrombospondin-4 A387P polymorphism with myocardial infarction. Arterioscler Thromb Vasc Biol. 2004;24:e183–184. doi: 10.1161/01.ATV.0000147304.67100.ee. [DOI] [PubMed] [Google Scholar]
- De Agostini AL, Lau HK, Leone C, Youssoufian H, Rosenberg RD. Cell mutants defective in synthesizing a heparan sulfate proteoglycan with regions of defined monosaccharide sequence. Proc Natl Acad Sci U S A. 1990;87:9784–9788. doi: 10.1073/pnas.87.24.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennissen MA, Jenniskens GJ, Pieffers M, Versteeg EM, Petitou M, Veerkamp JH, van Kuppevelt TH. Large, tissue-regulated domain diversity of heparan sulfates demonstrated by phage display antibodies. J Biol Chem. 2002;277:10982–10986. doi: 10.1074/jbc.M104852200. [DOI] [PubMed] [Google Scholar]
- Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest. 1992;90:2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Role of oligomerization domains in thrombospondins and other extracellular matrix proteins. Int J Biochem Cell Biol. 2004;36:997–1004. doi: 10.1016/j.biocel.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, Blech L, Febbraio M, Bornstein P, Plow EF, Stenina OI. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ Res. 2010;107:1313–1325. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova EG, Sopko N, Blech L, Popović ZB, Li J, Vasanji A, Drumm C, Krukovets I, Jain MK, Penn MS, Plow EF, Stenina OI. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012 doi: 10.1096/fj.11-190728. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuki IV, Blanchard N, Jin W, Marchadier DH, Millar JS, Glick JM, Rader DJ. Endogenously produced endothelial lipase enhances binding and cellular processing of plasma lipoproteins via heparan sulfate proteoglycan-mediated pathway. J Biol Chem. 2003;278:34331–34338. doi: 10.1074/jbc.M302181200. [DOI] [PubMed] [Google Scholar]
- Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsohn E, Volk T. Slowdown promotes muscle integrity by modulating integrin-mediated adhesion at the myotendinous junction. Development. 137:785–794. doi: 10.1242/dev.043703. [DOI] [PubMed] [Google Scholar]
- Hankenson KD, Hormuzdi SG, Meganck JA, Bornstein P. Mice with a disruption of the thrombospondin 3 gene differ in geometric and biomechanical properties of bone and have accelerated development of the femoral head. Mol Cell Biol. 2005;25:5599–5606. doi: 10.1128/MCB.25.13.5599-5606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Smith F, Banker K, Morris L, Joswiak G. Alternative histochemical markers for skeletal muscle capillaries: a statistical comparison among three muscles. Microvasc Res. 1992;44:112–116. doi: 10.1016/0026-2862(92)90105-x. [DOI] [PubMed] [Google Scholar]
- Hauser N, Paulsson M, Kale AA, DiCesare PE. Tendon extracellular matrix contains pentameric thrombospondin-4 (TSP-4) FEBS Lett. 1995;368:307–310. doi: 10.1016/0014-5793(95)00675-y. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Madri JA, Yurchenco PD. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992;119:945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JT, Deere M, Putnam E, Cole W, Vertel B, Chen H, Lawler J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998;17:269–278. doi: 10.1016/s0945-053x(98)90080-4. [DOI] [PubMed] [Google Scholar]
- Jenniskens GJ, Oosterhof A, Brandwijk R, Veerkamp JH, van Kuppevelt TH. Heparan sulfate heterogeneity in skeletal muscle basal lamina: demonstration by phage display-derived antibodies. J Neurosci. 2000;20:4099–4111. doi: 10.1523/JNEUROSCI.20-11-04099.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenniskens GJ, Veerkamp JH, van Kuppevelt TH. Heparan sulfates in skeletal muscle development and physiology. J Cell Physiol. 2006;206:283–294. doi: 10.1002/jcp.20450. [DOI] [PubMed] [Google Scholar]
- Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. J Biol Chem. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L, Jarvinen TA, Jarvinen TL, Kvist M, Natri A, Jarvinen M. Location and distribution of non-collagenous matrix proteins in musculoskeletal tissues of rat. Histochem J. 1998;30:799–810. doi: 10.1023/a:1003448106673. [DOI] [PubMed] [Google Scholar]
- Kanwar YS, Farquhar MG. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979;76:1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TYA, Murase Y, Hirashiki A, Noda A, Yamada Y. Specific gene polymorphisms could be risk factors for coronary artery disease in individuals with or without hypertention. Circulation (Suppl) 2003;108:IV–712. [Google Scholar]
- Kolset SO, Salmivirta M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell Mol Life Sci. 1999;56:857–870. doi: 10.1007/s000180050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Duquette M, Whittaker CA, Adams JC, McHenry K, DeSimone DW. Identification and characterization of thrombospondin-4, a new member of the thrombospondin gene family. J Cell Biol. 1993;120:1059–1067. doi: 10.1083/jcb.120.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, Lynch KA, Okada T, Aronow BJ, Osinska H, Prywes R, Lorenz JN, Mori K, Lawler J, Robbins J, Molkentin JD. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149:1257–1268. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Somanath PR, Byzova TV. Methods for isolation of endothelial and smooth muscle cells and in vitro proliferation assays. Methods Mol Med. 2006;129:197–208. doi: 10.1385/1-59745-213-0:197. [DOI] [PubMed] [Google Scholar]
- Marchant JK, Hahn RA, Linsenmayer TF, Birk DE. Reduction of type V collagen using a dominant-negative strategy alters the regulation of fibrillogenesis and results in the loss of corneal-specific fibril morphology. J Cell Biol. 1996;135:1415–1426. doi: 10.1083/jcb.135.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Parker A, Salem R, Moliterno DJ, Wang Q, Plow EF, Rao S, Shen G, Rogers WJ, Newby LK, Cannata R, Glatt K, Topol EJ. Large scale association analysis for identification of genes underlying premature coronary heart disease: cumulative perspective from analysis of 111 candidate genes. J Med Genet. 2004;41:334–341. doi: 10.1136/jmg.2003.016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misenheimer TM, Mosher DF. Biophysical characterization of the signature domains of thrombospondin-4 and thrombospondin-2. J Biol Chem. 2005;280:41229–41235. doi: 10.1074/jbc.M504696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra KB, Kim KC, Cho S, Low MG, Bensadoun A. Purification and characterization of adipocyte heparan sulfate proteoglycans with affinity for lipoprotein lipase. J Biol Chem. 1994;269:23838–23844. [PubMed] [Google Scholar]
- Mosher DF, Adams JC. Adhesion-modulating/matricellular ECM protein families: a structural, functional and evolutionary appraisal. Matrix Biol. 2012;31:155–161. doi: 10.1016/j.matbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Murphy-Ullrich JE, Iozzo RV. Thrombospondins in physiology and disease: new tricks for old dogs. Matrix Biol. 2012;31:152–154. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, Ruskoaho H, Rysa J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem Biophys Res Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- Naimy H, Leymarie N, Bowman MJ, Zaia J. Characterization of heparin oligosaccharides binding specifically to antithrombin III using mass spectrometry. Biochemistry. 2008;47:3155–3161. doi: 10.1021/bi702043e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, Paulsson M. Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. J Biol Chem. 2000;275:37110–37117. doi: 10.1074/jbc.M007223200. [DOI] [PubMed] [Google Scholar]
- Parthasarathy N, Goldberg IJ, Sivaram P, Mulloy B, Flory DM, Wagner WD. Oligosaccharide sequences of endothelial cell surface heparan sulfate proteoglycan with affinity for lipoprotein lipase. J Biol Chem. 1994;269:22391–22396. [PubMed] [Google Scholar]
- Riessen R, Fenchel M, Chen H, Axel DI, Karsch KR, Lawler J. Cartilage oligomeric matrix protein (thrombospondin-5) is expressed by human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:47–54. doi: 10.1161/01.atv.21.1.47. [DOI] [PubMed] [Google Scholar]
- Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DD, Miller TW, Rogers NM, Yao M, Isenberg JS. The matricellular protein thrombospondin-1 globally regulates cardiovascular function and responses to stress via CD47. Matrix Biol. 2012;31:162–169. doi: 10.1016/j.matbio.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RD, de Agostini AI. New approaches for defining sequence specific synthesis of heparan sulfate chains. Adv Exp Med Biol. 1992;313:307–316. doi: 10.1007/978-1-4899-2444-5_30. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Obunike JC, Arad Y, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase-mediated uptake and degradation of low density lipoproteins by fibroblasts and macrophages. J Clin Invest. 1992;90:1504–1512. doi: 10.1172/JCI116018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Gill PJ, Silbert JE, Douglas WH, Fanburg BL. Involvement of cell surface heparin sulfate in the binding of lipoprotein lipase to cultured bovine endothelial cells. J Clin Invest. 1981;68:995–1002. doi: 10.1172/JCI110354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Hicks PS, Ortiz LR, Martinez MJ, Mandler RN. Quantitative measurement of muscle strength in the mouse. J Neurosci Methods. 1995;62:15–19. doi: 10.1016/0165-0270(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Sodersten F, Ekman S, Schmitz M, Paulsson M, Zaucke F. Thrombospondin-4 and cartilage oligomeric matrix protein form heterooligomers in equine tendon. Connect Tissue Res. 2006;47:85–91. doi: 10.1080/03008200600584124. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. Int J Biochem Cell Biol. 2004;36:1013–1030. doi: 10.1016/j.biocel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Desai SY, Krukovets I, Kight K, Janigro D, Topol EJ, Plow EF. Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation. 2003;108:1514–1519. doi: 10.1161/01.CIR.0000089085.76320.4E. [DOI] [PubMed] [Google Scholar]
- Stenina OI, Topol EJ, Plow EF. Thrombospondins, their polymorphisms, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:1886–1894. doi: 10.1161/ATVBAHA.107.141713. [DOI] [PubMed] [Google Scholar]
- Svensson L, Aszodi A, Heinegard D, Hunziker EB, Reinholt FP, Fassler R, Oldberg A. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol Cell Biol. 2002;22:4366–4371. doi: 10.1128/MCB.22.12.4366-4371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina O, Daley GQ. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–2644. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- van Kuppevelt TH, Dennissen MA, van Venrooij WJ, Hoet RM, Veerkamp JH. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J Biol Chem. 1998;273:12960–12966. doi: 10.1074/jbc.273.21.12960. [DOI] [PubMed] [Google Scholar]
- van Kuppevelt TH, Jenniskens GJ, Veerkamp JH, ten Dam GB, Dennissen MA. Phage display technology to obtain antiheparan sulfate antibodies. Methods Mol Biol. 2001;171:519–534. doi: 10.1385/1-59259-209-0:519. [DOI] [PubMed] [Google Scholar]
- Walton BWJ, Coresh J, Boerwinkle E. Thrombospondin-2 and Lymphotoxin-a gene variations predict coronary heart disease in a large prospective study. Circulation (Suppl) 2003;108:IV–771. [Google Scholar]
- Wessel J, Topol EJ, Ji M, Meyer J, McCarthy JJ. Replication of the association between the thrombospondin-4 A387P polymorphism and myocardial infarction. Am Heart J. 2004;147:905–909. doi: 10.1016/j.ahj.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Williams KJ, Fless GM, Petrie KA, Snyder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267:13284–13292. [PubMed] [Google Scholar]
- Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med. 2002;347:1916–1923. doi: 10.1056/NEJMoa021445. [DOI] [PubMed] [Google Scholar]
- Ye S, Luo Y, Lu W, Jones RB, Linhardt RJ, Capila I, Toida T, Kan M, Pelletier H, McKeehan WL. Structural basis for interaction of FGF-1, FGF-2, and FGF-7 with different heparan sulfate motifs. Biochemistry. 2001;40:14429–14439. doi: 10.1021/bi011000u. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of TSP-1, TSP-2, TSP-3, and TSP-5 in extracts from soleus tissues of WT and Thbs4−/− mice with the loading control (Ponceau S staining of membrane to visualize total protein).
Unchanged levels of HS epitopes (green) recognized by A4BO8, HS4C3V, RB4CD12 antibodies in the soleus of WT (left panels) and Thbs4−/− (right panels) mice.