Abstract
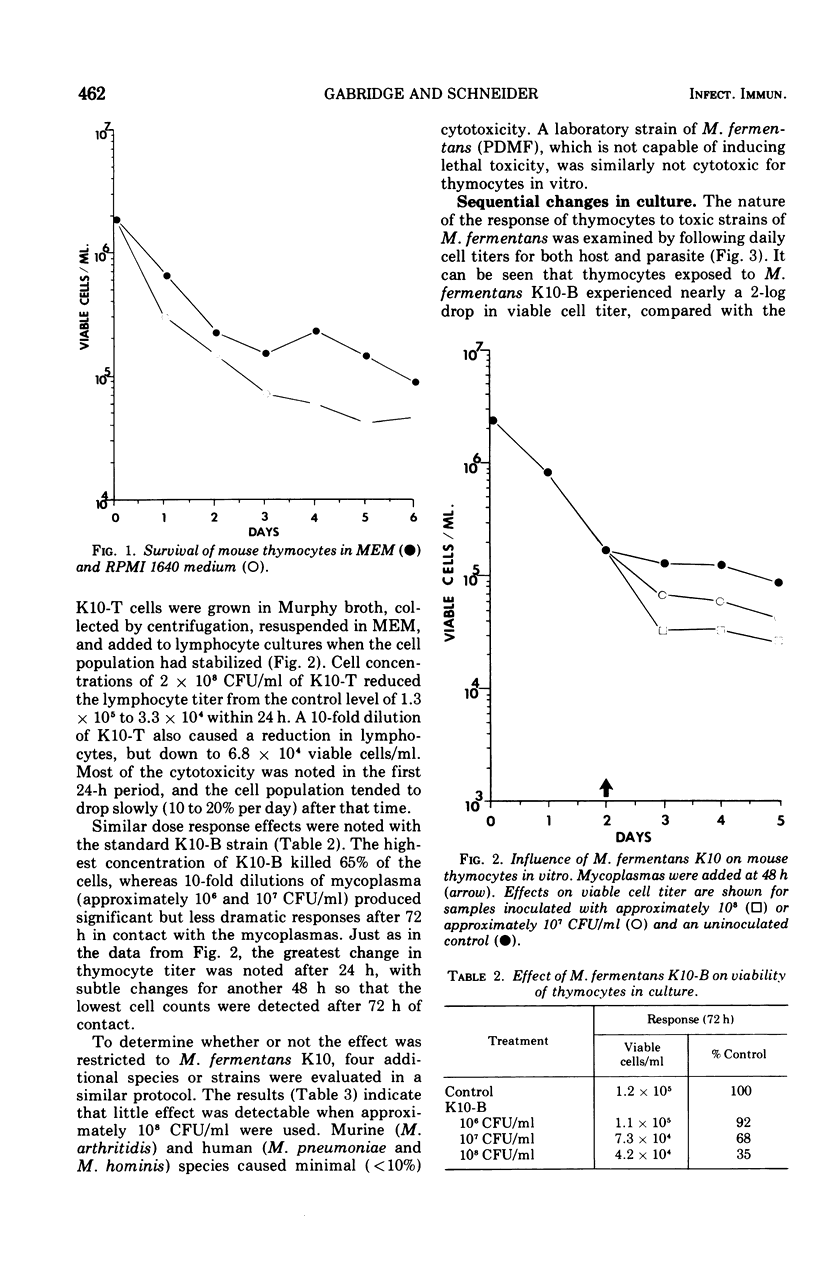
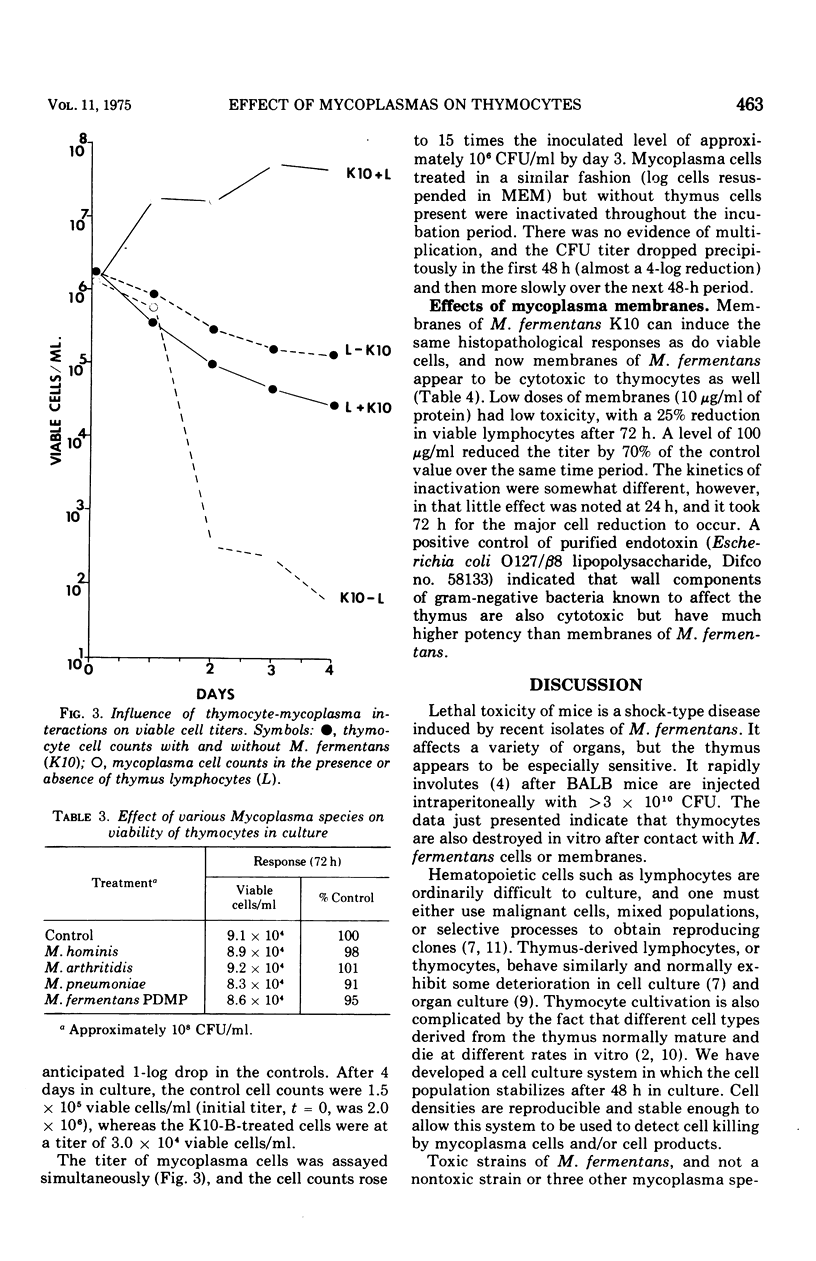
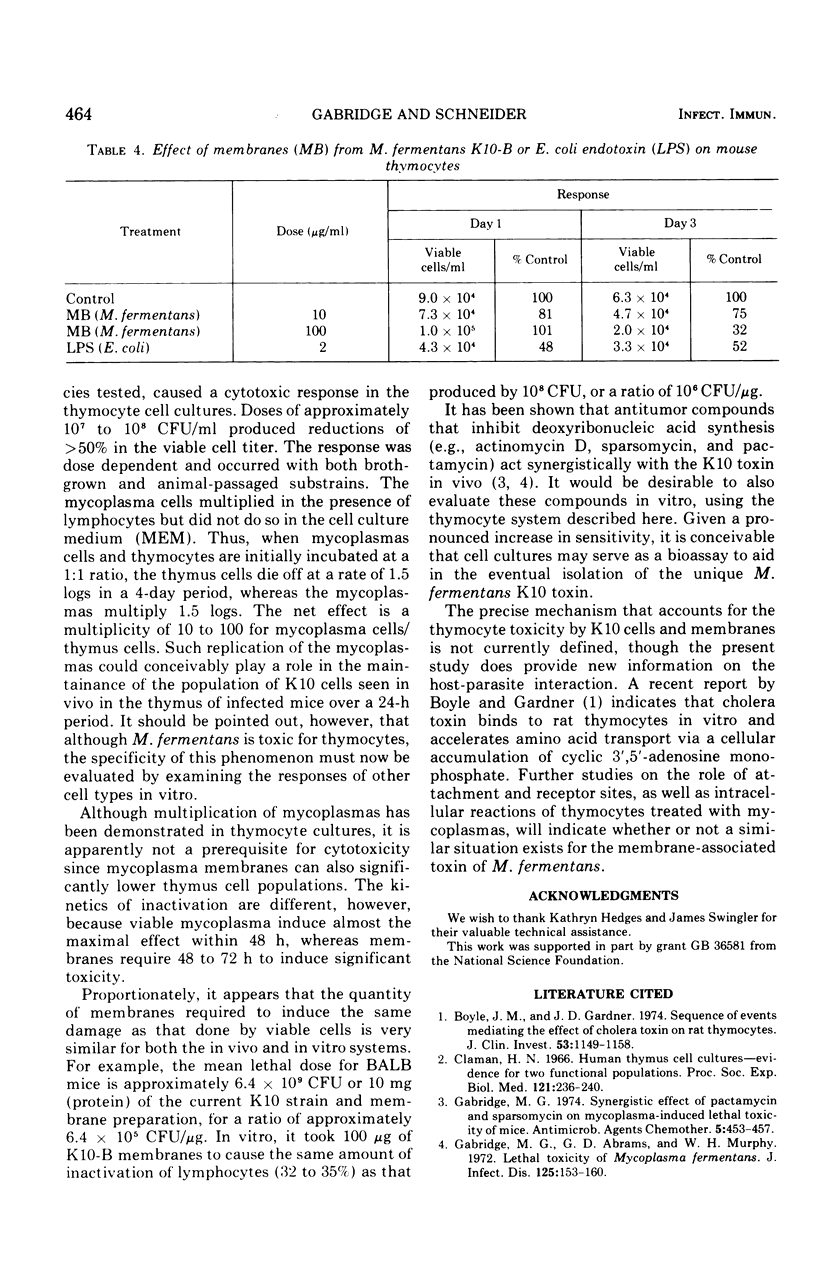
The in vitro response of mouse thymocytes to various mycoplasmas was evaluated. Cultures of thymus cells from BALB mice were prepared in Earle minimal essential medium with 10 per cent fetal calf serum. After an initial drop in viability, cell populations stabilized at approximately 10-5 viable cells/ml for 3 to 5 days. Concentration of 10-6 to 10-8 colony-forming units of toxic isolates of Mycoplasma fermentans per ml killed over 50 per cent of these cells in a dose-dependent fashion. Four other mycoplasmas (M. pneumoniae, M. hominis, M. arthritidis and a nontoxic strain of M. fermentans) did not induce cytotoxicity of mouse thymocytes. Toxic isolates of M. fermentans multiplied in the presence of thymus cells as they were being inactivated. However, nonviable membrane preparations of these mycoplasma were also toxic, indicating that growth of the organisms is not a prerequisite for the toxic effect. The relevance of these findings for the isolation and identification of the membrane-associated toxic factor is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle J. M., Gardner J. D. Sequence of events mediating the effect of cholera toxin on rat thymocytes. J Clin Invest. 1974 Apr;53(4):1149–1158. doi: 10.1172/JCI107653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Human thymus cell cultures-evidence for two functional populations. Proc Soc Exp Biol Med. 1966 Jan;121(1):236–240. doi: 10.3181/00379727-121-30746. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Abrams G. D., Murphy W. H. Lethal toxicity of Mycoplasma fermentans for mice. J Infect Dis. 1972 Feb;125(2):153–160. doi: 10.1093/infdis/125.2.153. [DOI] [PubMed] [Google Scholar]
- Gabridge M. G., Murphy W. H. Toxic membrane fractions from Mycoplasma fermentans. Infect Immun. 1971 Dec;4(6):678–682. doi: 10.1128/iai.4.6.678-682.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G. Synergistic effect of pactamycin and sparsomycin on Mycoplasma-induced lethal toxicity of mice. Antimicrob Agents Chemother. 1974 May;5(5):453–457. doi: 10.1128/aac.5.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber B., Kollar E. J., Moscona A. A. Aggregation in vivo of dissociated cells. 3. Effect of state of differentiation of cells on feather development in hybrid aggregates of embryonic mouse and chick skin cells. J Exp Zool. 1968 Aug;168(4):455–472. doi: 10.1002/jez.1401680406. [DOI] [PubMed] [Google Scholar]
- Howe M. L., Goldstein A. L., Battisto J. R. Isogeneic lymphocyte interaction: recognition of self antigens by cells of the neonatal thymus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):613–619. doi: 10.1073/pnas.67.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel T., Russell P. J. Differentation of foetal mouse thymus. Ultrastructure of organ cultures and of subcapsular grafts. Immunology. 1971 Oct;21(4):659–674. [PMC free article] [PubMed] [Google Scholar]
- Moore G. E., Gerner R. E., Franklin H. A. Culture of normal human leukocytes. JAMA. 1967 Feb 20;199(8):519–524. [PubMed] [Google Scholar]
- Shelton E. Differentiation of mouse thymus cultured in diffusion chambers. Am J Anat. 1966 Nov;119(3):341–357. doi: 10.1002/aja.1001190302. [DOI] [PubMed] [Google Scholar]
- Wagner H., Harris A. W., Feldmann M. Cell-mediated immune response in vitro. II. The role of thymus and thymus-derived lymphocytes. Cell Immunol. 1972 May;4(1):39–50. doi: 10.1016/0008-8749(72)90004-4. [DOI] [PubMed] [Google Scholar]
- Weber W. T. Qualitative and quantitative studies on mixed homologous chicken thymus cell cultures. Clin Exp Immunol. 1970 Jun;6(6):919–940. [PMC free article] [PubMed] [Google Scholar]



