Abstract
Electroporation can deliver DNA efficiently and safely to tissues in live animals, including the lung where it causes little inflammation or lung injury. In contrast, cationic lipid mediated gene transfer has been shown to induce an inflammatory response caused by unmethylated plasmid CpG residues which activate the TLR9 signaling pathway. Since TLR9 is located in the endosomal/lysosomal compartment, we hypothesized that plasmids do not activate TLR9 during electroporation because they enter the cytoplasm directly through transient pores in the plasma membrane. To test this, plasmids were transfected into HEK293-TLR9+ and HEK293-TLR9-Null cells. IL-8 expression, an indicator of TLR9 activation, increased more than 10-fold at 24 hrs post-liposome transfection in HEK293-TLR9+ cells, but showed no significant increase in electroporated cells, compared to untransfected cells. In vivo, liposome-mediated gene transfer caused increases in IL-6, IL-12, TNF-α, and IFN-γ in mouse bronchial alveolar lavage fluid, whereas the levels of these cytokines were more than 10-fold lower by comparison following electroporation. Depletion of alveolar macrophages suggested that this inflammatory response is mediated by resident pulmonary epithelial cells. These results suggest that electroporation-mediated gene transfer bypasses the TLR-9 pathway, thus accounting for the low levels of inflammation seen with this approach.
Keywords: electroporation, liposomes, lipoplex, inflammation, gene delivery, nonviral vector
INTRODUCTION, RESULTS, AND DISCUSSION
Toll-like receptors (TLRs) play a critical role in the early innate immune response to invading pathogens by recognizing pathogen-associated molecular patterns. Stimulation of these TLRs activates signaling cascades that lead to induction of a number of pro-inflammatory cytokines, including IL-6, IL-8, IL-12, TNF-α, and IFN-γ.1 Ten TLRs have been identified in humans and 13 have been identified in mice. Among these TLRs, TLR9 recognizes unmethylated cytosine-phosphorothioate-guanine (CpG) motifs in bacterial DNA,2 accounting in large part for the innate immune response to bacterial pathogens and plasmids during gene delivery in vivo.
In non-viral gene therapy, unmethylated bacterial plasmid vectors can potentially activate the TLR9 pathway and cause acute inflammation. Although liposome-mediated gene transfer is an efficient and major method for non-viral gene delivery that can achieve high level gene expression, inflammation and toxicity associated with this approach is of concern.3,4 Encapsulation of CpG oligonucleotide (ODN) in liposomes can increase the inflammatory response to these oligonucleotides,5,6 and the inflammatory response is more severe with lipid-complexed plasmids than with naked DNA,7 suggesting that liposomes can act as an adjuvant for CpG induced inflammation. In another study, the duration of expression of plasmid DNA complexed with liposomes was reduced compared with that of naked DNA,8 which might be related to an increased inflammatory response to the lipoplex. Because these inflammatory responses are caused by unmethylated CpG motifs in plasmids, methylation or elimination of CpG sequences from the DNA can substantially reduce inflammation and increase the duration of gene expression, as has been demonstrated.9-13
For gene therapy to be successful, safe and efficient gene delivery methods must be developed. We have developed a technique to transfer genes to all cell types in the lung, even those “buried” beneath the epithelial layer (i.e., smooth muscle, fibroblasts, and endothelial cells) using electric fields to create transient pores in the plasma membrane that allow the entry of normally impermeable macromolecules into the cytoplasm.14 We have applied this to the mouse and rat lung and obtained high levels of reporter and therapeutic gene expression in all cell types.14,15 Moreover, this gene transfer is very safe, with no apparent trauma, lung injury, or inflammation as assessed histologically and by measurement of pro-inflammaotry cytokines and chemokines in bronchial alveolar lavage fluid (BALF) and lung homogenates.14,15 This lack of inflammatory response is very different to that seen with viral vectors or even liposomes. In this study, we wanted to determine the mechanisms for this lack of inflammation.
While most TLRs are located within the plasma membrane, TLR9 is located in the endoplasmic reticulum (ER) and redistributed upon stimulation to the CpG containing endosomal compartment.16-18 It has been reported that internalization and endosomal maturation is required for CpG activation of TLR9 signaling.19,20 Since electroporation permeabilizes the plasma membrane and allows direct entry of plasmids into the cytoplasm, bypassing endocytosis,21 we hypothesize that electroporation-mediated gene transfer of plasmid DNA bypasses the TLR9 pathway, explaining the relative lack of inflammation seen with this method.
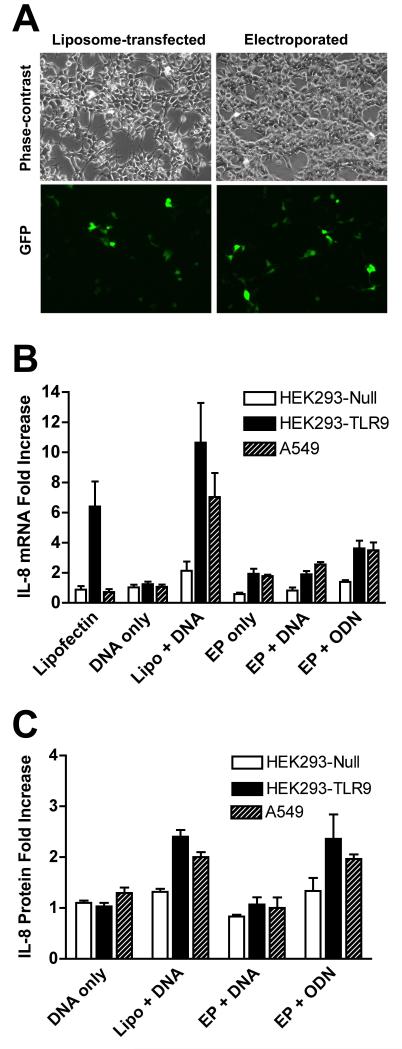
To investigate the role of TLR9 in liposome and electroporation-mediated gene transfer, we first tested the activation of IL-8 in several cell lines that express different levels of TLR9. Wild type Kidney epithelial HEK293 cells and those stably transfected with an empty vector (HEK293-TLR9-null) have very low levels of endogenous TLR9, but when stably transfected with a vector expressing human TLR9, HEK293-TLR9+ cells express 10,000-fold higher levels of TLR9 based on the threshold cycle (Ct) needed to detect product (14 cycles less for HEK293-TLR9+)(Table 1). Human A549 lung adenocarcinoma cells display a moderate level of TLR9 expression (Table 1). Cells were transfected with 5 μg of plasmid expressing GFP using Lipofectin or electroporation and 24 hours later, transfection efficiency was evaluated by the number of GFP expressing cells (Figure 1A). Electroporation parameters were adjusted so that equivalent transfection efficiency was achieved with both methods. Expression of IL-8, as measured at the mRNA and protein levels by real time RT-PCR and ELISA, respectively, was used as an indicator of activation of TLR9 pathway. Neither Lipofectin nor electroporation activated IL-8 expression in HEK 293-TLR9-null cells (Figure 1B and C). By contrast, in TLR9 expressing HEK293-TLR9+ cells, Lipofectin-mediated transfection increased IL-8 mRNA expression more than 10-fold, whereas electroporation caused less than a 2-fold increase. Similar results were seen in A549 cells where IL-8 mRNA expression increased more than 6-fold following liposome-mediated transfection but increased 2.5-fold with electroporation. Although increases in IL-8 protein levels were less pronounced, the same trends were observed. To ensure that electroporation itself did not blunt the TLR9 signaling ability of the cells, a control experiment was also performed in which cells were electroporated in the absence of plasmid and then treated 10 minutes later with a CpG-containing oligonucleotide (ODN M362). As expected, TLR9-expressing cells were still able to respond to the oligonucleotide after being electroporated by producing IL-8.
Table 1.
TLR9 expression level in different cell lines represented by C(t). Total RNA was isolated using the Qiagen RNeasy Mini Kit (Valencia, CA), and 500 ng of the total RNA was used for reverse transcription using the TaqMan Reverse Transcription Reagents Kit (Applied Biosystems, Branchburg, NJ). One-tenth of the cDNA was subjected to quantitative PCR using primers and a TaqMan probe for human TLR9 (Applied Biosystems). GAPDH was used as a loading control.
| HEK293-Null | HEK293-TLR9 | A549 | |
|---|---|---|---|
| TLR9 | 32.8±0.15 | 18.8±0.6 | 27.8±0.5 |
| GAPDH | 16.7±0.06 | 16.5±0.3 | 16.8±0.3 |
Figure 1. Activation of IL-8 expression in cells with different levels of TLR9.
(A) HEK293 cells were transfected with 5 μg pEGFP-N1 (Clontech) by electroporation or Lipofectin (Invitrogen, Carlsbad, CA). For electroporations, 1 × 106 cells were suspended in DMEM containing 10% fetal bovine serum, placed in cuvette with a 4 mm gap, and electroporated at 160V with a capacitance of 950 μF using a Bio-Rad Gene Pulser II. For liposomal gene delivery, DNA was complexed with 10 μl of Lipofectin, incubated for 30 minutes, diluted into DMEM without serum, and added to cells, as described by the manufacturer (Invitrogen). Transfection efficiency was evaluated 24 hours after transfection. (B) HEK293-TLR9-Null, HEK293-TLR9+ cells (InvivoGen, San Diego, CA) and A549 cells (ATCC) were maintained in DMEM supplemented with 10% fetal bovine serum, kanamyacin, antibiotic/antimyotic solution (Invitrogen, Calsbad CA), and 10 μg/ml blasticidin (HEK293 cells only). Cells were transfected with 5 μg pEGFP-N1 using different conditions as indicated. Alternatively, cells were electroporated in the absence of plasmid DNA, and 10 minutes later, the CpG-containing oligonucleotide ODN M362 (InvivoGen) was added to the cells at a final concentration of 10 μg/ml (EP + ODN). Twenty-four hours later, total RNA was isolated and 500 ng was used for reverse transcription. One-tenth of the cDNA was subjected to quantitative PCR. The expression of IL-8 is normalized to the expression of GAPDH. Fold of increase is over the untreated cells. Mean levels ± SEM are shown (n=4). (C) Twenty-four hours after transfection, IL-8 protein levels in cleared supernatant from 1 ml of culture medium were measured using a human IL-8 ELISA kit (BD Biosciences, San Diego, CA). Mean levels ± SEM are shown (n=6).
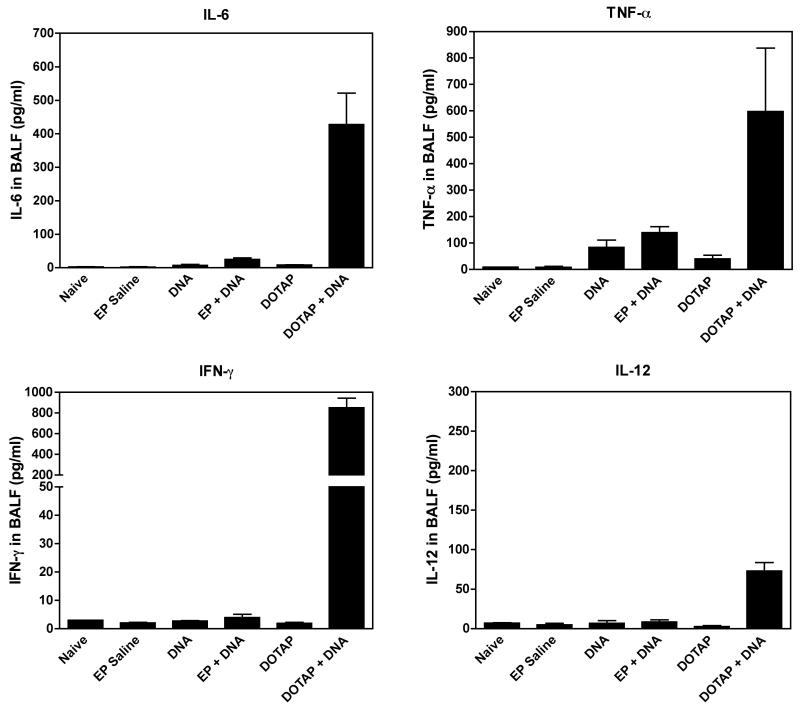
We next studied the inflammatory response in mice following DNA delivery. A single dose of 20 μg luciferase-expressing plasmid DNA was delivered to the mouse lung via the trachea either by electroporation or by liposomes, as described.14,22. One day after gene transfer, at the height of transgene expression, BALF was collected to evaluate the cytokine levels in the mouse lung. While liposome-mediated gene transfer induced high levels of the pro-inflammatory cytokines IL-6, IL-12, TNF-α and IFN-γ, electroporation-mediated gene transfer was accompanied by levels of IL-6, IL-12 and IFN-γ that were only slightly above background (Figure 2). Only TNF-α showed a moderate increase with electroporation, but was only 2.4-fold higher than DNA alone and 3-fold lower than that seen following liposome transfection (Figure 2). These results are consistent with the data from cultured cells, that electroporation-mediated plasmid DNA gene transfer does not induce significant inflammatory response to bacterial CpG containing plasmid DNA.
Figure 2. Cytokines in BAFL 24 hours after gene transfer into mouse lung.
Mice were given 100 μl of plasmid (0.2 mg/ml) in 10 mM Tris, pH 8, 1 mM EDTA, and 140 mM NaCl, delivered by intratracheal injection and immediately electroporated at 200 V/cm using 8 pulses of 10 msec duration each. For liposomal gene delivery, 20 μg plasmid DNA was mixed with 100 μl of DOTAP:DOPE (1:1 w/w; 2 mg/ml) and injected intratracheally. Twenty-four hours later, bronchial alveolar lavage fluid (BALF) was collected and cytokine levels were assayed by Cytometric Bead Array (BD Biosciences). Mean levels ± SEM are shown (n=4).
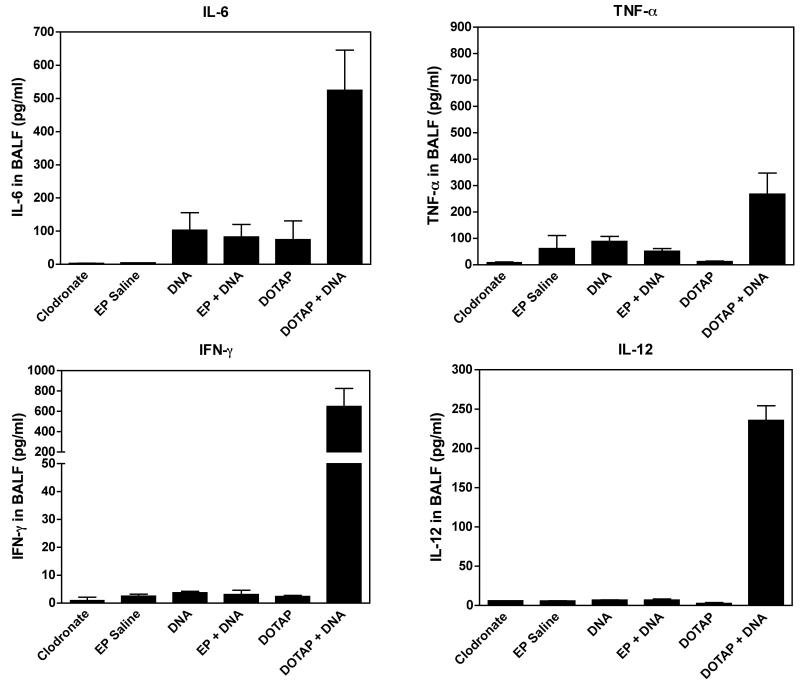
Because alveolar macrophages play an important role in acute lung inflammation, we asked whether the observed inflammation was mediated by alveolar macrophages or resident pulmonary epithelial cells. Two days prior to gene transfer, clodronate was delivered via the trachea into mouse lungs to deplete alveolar macrophages.23 Following the depletion of alveolar macrophages, the profiles of cytokine levels were similar to those without clodronate treatment (Figure 3), suggesting that alveolar macrophages are not responsible for the inflammatory response to liposome-mediated plasmid DNA gene transfer. However, unlike in untreated animals, TNF-α levels failed to increase with electroporation, suggesting that the slight activation of TNF-α seen in macrophage replete mice may be due to macrophage activation, unlike the other cytokines. Further, the fact that alveolar macrophages, at least in mice, do not appear to express TLR9,24 supports our findings that the inflammation observed in liposome-mediated gene transfer is most likely mediated by resident epithelial cells.
Figure 3. After alveolar macrophage depletion, cytokines in BAFL 24 hours after gene transfer into mouse lung.
Mice were given 100 μl clodronate (0.24 mg/ml) via intratracheal intubation to deplete pulmonary macrophages. Two days later, 100 μl of plasmid (0.2 mg/ml) was delivered intratracheally and electroporated or delivered by liposomal gene delivery, as described in Figure 2. Twenty-four hours later, bronchial alveolar lavage fluid (BALF) was collected and cytokine levels were assayed by Cytometric Bead Array (BD Biosciences). Mean levels ± SEM are shown (n=4).
It is well established that the inflammatory response induced by liposome-mediated transfection of plasmids and unmethylated CpG oligonucleotides is mediated by TLR9; when TLR9 is absent, transfection of unmethylated CpG containing DNA does not activate the TLR9 signaling pathway that leads to activation of the inflammatory cytokines expression, such as IL-8.2 Consequently, the lack of a TLR9-mediated immune response following electroporation-mediated DNA delivery was intriguing, given the central role of this pathway in the inflammatory response to non-viral vectors. Our results suggest that this lack of inflammatory response is due to the nature of gene delivery during electroporation. When an external electric field is applied, the plasma membrane is transiently permeabilized, allowing plasmids to translocate directly into the cytoplasm before the electropermeabilized pores reseal,25,26 independent of endocytosis. Since TLR9 is located in endosomal/lysosomal compartment and endosome maturation is required for TLR9 activation by CpG ODN,16,17,19,20 it fails to be activated during electroporation-mediated gene transfer.
Although it may seem counterintuitive that naked DNA in the absence of liposomes or electroporation failed to stimulate TLR9-dependent inflammation in either cultured cells or the lung, despite the presence of multiple CpG motifs in the bacterial DNA, it is not surprising since these plasmids are not taken up by the cells to any great extent. Indeed, naked DNA cannot effectively transfect cells to any great degree in the absence of carriers such as liposomes or polymers due to its relative inability to be endocytosed by the cells. Others have observed a similar lack of inflammation by naked DNA delivered either locally through the airways or systematically through tail vein injection.9,27 Further, it has been shown in macrophages that activation of TLR9 by naked CpG-containing DNA requires endosomal acidification,28,29 supporting the fact that endocytosis of the DNA is a prerequisite for TLR9 pathway activation.
Apart from inducing an acute inflammatory response, TLR9 activation may result in decreased gene expression and duration of expression. In a recent study from the Gill and Hyde groups, it was found that naked plasmid DNA gives a more sustained expression level in the mouse lung than does liposome complexed plasmid.8 When comparing the persistence of plasmid retained in the mouse lung with a previous study, they found no significant loss of naked DNA over 14 days, but the levels of the same plasmid DNA delivered via liposomes decreased significantly.30 The authors speculated that this difference may be cause by differential host inflammatory responses. Thus, delivery by physical methods that prevent this initial inflammatory response may be beneficial for both safety reasons as well as expression profiles.
ACKNOWLEDGEMENTS
We would like to thank Gokhan Mutlu, John Christman, and Amy Bellmeyer for helpful discussions and technical advice. This work was supported in part by grants HL59956, HL71643, and HL81148 from the National Institutes of Health.
LITERATURE CITED
- 1.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- 2.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 3.Audouy SA, de Leij LF, Hoekstra D, Molema G. In vivo characteristics of cationic liposomes as delivery vectors for gene therapy. Pharm Res. 2002;19:1599–1605. doi: 10.1023/a:1020989709019. [DOI] [PubMed] [Google Scholar]
- 4.Yew NS, Scheule RK. Toxicity of Cationic Lipid-DNA Complexes. Adv Genet. 2005;53PA:189–214. doi: 10.1016/S0065-2660(05)53007-4. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki Y, Wakita D, Chamoto K, Narita Y, Tsuji T, Takeshima T, et al. Liposome-encapsulated CpG oligodeoxynucleotides as a potent adjuvant for inducing type 1 innate immunity. Cancer Res. 2004;64:8754–8760. doi: 10.1158/0008-5472.CAN-04-1691. [DOI] [PubMed] [Google Scholar]
- 6.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10:2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 7.Emerson M, Renwick L, Tate S, Rhind S, Milne E, Painter HA, et al. Transfection efficiency and toxicity following delivery of naked plasmid DNA and cationic lipid-DNA complexes to ovine lung segments. Mol Ther. 2003;8:646–653. doi: 10.1016/s1525-0016(03)00233-8. [DOI] [PubMed] [Google Scholar]
- 8.Pringle IA, Raman S, Sharp WW, Cheng SH, Hyde SC, Gill DR. Detection of plasmid DNA vectors following gene transfer to the murine airways. Gene Ther. 2005;12:1206–1214. doi: 10.1038/sj.gt.3302518. [DOI] [PubMed] [Google Scholar]
- 9.McLachlan G, Stevenson BJ, Davidson DJ, Porteous DJ. Bacterial DNA is implicated in the inflammatory response to delivery of DNA/DOTAP to mouse lungs. Gene Ther. 2000;7:384–392. doi: 10.1038/sj.gt.3301097. [DOI] [PubMed] [Google Scholar]
- 10.Yew NS, Cheng SH. Reducing the immunostimulatory activity of CpG-containing plasmid DNA vectors for non-viral gene therapy. Expert Opin Drug Deliv. 2004;1:115–125. doi: 10.1517/17425247.1.1.115. [DOI] [PubMed] [Google Scholar]
- 11.Yew NS, Zhao H, Przybylska M, Wu IH, Tousignant JD, Scheule RK, et al. CpG-Depleted Plasmid DNA Vectors with Enhanced Safety and Long-Term Gene Expression in Vivo. Mol Ther. 2002;5:731–738. doi: 10.1006/mthe.2002.0598. [DOI] [PubMed] [Google Scholar]
- 12.McMahon JM, Wells KE, Bamfo JE, Cartwright MA, Wells DJ. Inflammatory responses following direct injection of plasmid DNA into skeletal muscle. Gene Ther. 1998;5:1283–1290. doi: 10.1038/sj.gt.3300718. [DOI] [PubMed] [Google Scholar]
- 13.Reyes-Sandoval A, Ertl HC. CpG methylation of a plasmid vector results in extended transgene product expression by circumventing induction of immune responses. Mol Ther. 2004;9:249–261. doi: 10.1016/j.ymthe.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level non-viral gene transfer to the lung. Gene Ther. 2003;10:1608–1615. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GRS, Yeldandi A, et al. Gene transfer of the Na+,K+-ATPase β1 subunit using electroporation increases lung liquid clearance in rats. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 17.Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol. 2004;173:1179–1183. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–526. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur J Immunol. 2002;32:1958–1968. doi: 10.1002/1521-4141(200207)32:7<1958::AID-IMMU1958>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 20.Hacker H, Mischak H, Miethke T, Liptay S, Schmid R, Sparwasser T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. Embo J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2:178–187. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 22.Templeton NS, Lasic DD, Frederik PM, Strey HH, Roberts DD, Pavlakis GN. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat. Biotechnol. 1997;15:647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 23.Maus UA, Koay MA, Delbeck T, Mack M, Ermert M, Ermert L, et al. Role of resident alveolar macrophages in leukocyte traffic into the alveolar air space of intact mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1245–1252. doi: 10.1152/ajplung.00453.2001. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki K, Suda T, Naito T, Ide K, Chida K, Nakamura H. Impaired toll-like receptor 9 expression in alveolar macrophages with no sensitivity to CpG DNA. Am J Respir Crit Care Med. 2005;171:707–713. doi: 10.1164/rccm.200408-1078OC. [DOI] [PubMed] [Google Scholar]
- 25.Golzio M, Teissie J, Rols MP. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc Natl Acad Sci U S A. 2002;99:1292–1297. doi: 10.1073/pnas.022646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sukharev SI, Klenchin VA, Serov SM, Chernomordik LV, Chizmadzhev Yu A. Electroporation and electrophoretic DNA transfer into cells. The effect of DNA interaction with electropores. Biophys J. 1992;63:1320–1327. doi: 10.1016/S0006-3495(92)81709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li S, Wu SP, Whitmore M, Loeffert EJ, Wang L, Watkins SC, et al. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol. 1999;276:L796–804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- 28.Yasuda K, Kawano H, Yamane I, Ogawa Y, Yoshinaga T, Nishikawa M, et al. Restricted cytokine production from mouse peritoneal macrophages in culture in spite of extensive uptake of plasmid DNA. Immunology. 2004;111:282–290. doi: 10.1111/j.1365-2567.2004.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuda K, Ogawa Y, Yamane I, Nishikawa M, Takakura Y. Macrophage activation by a DNA/cationic liposome complex requires endosomal acidification and TLR9-dependent and -independent pathways. J Leukoc Biol. 2005;77:71–79. doi: 10.1189/jlb.0204089. [DOI] [PubMed] [Google Scholar]
- 30.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, et al. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]