Abstract
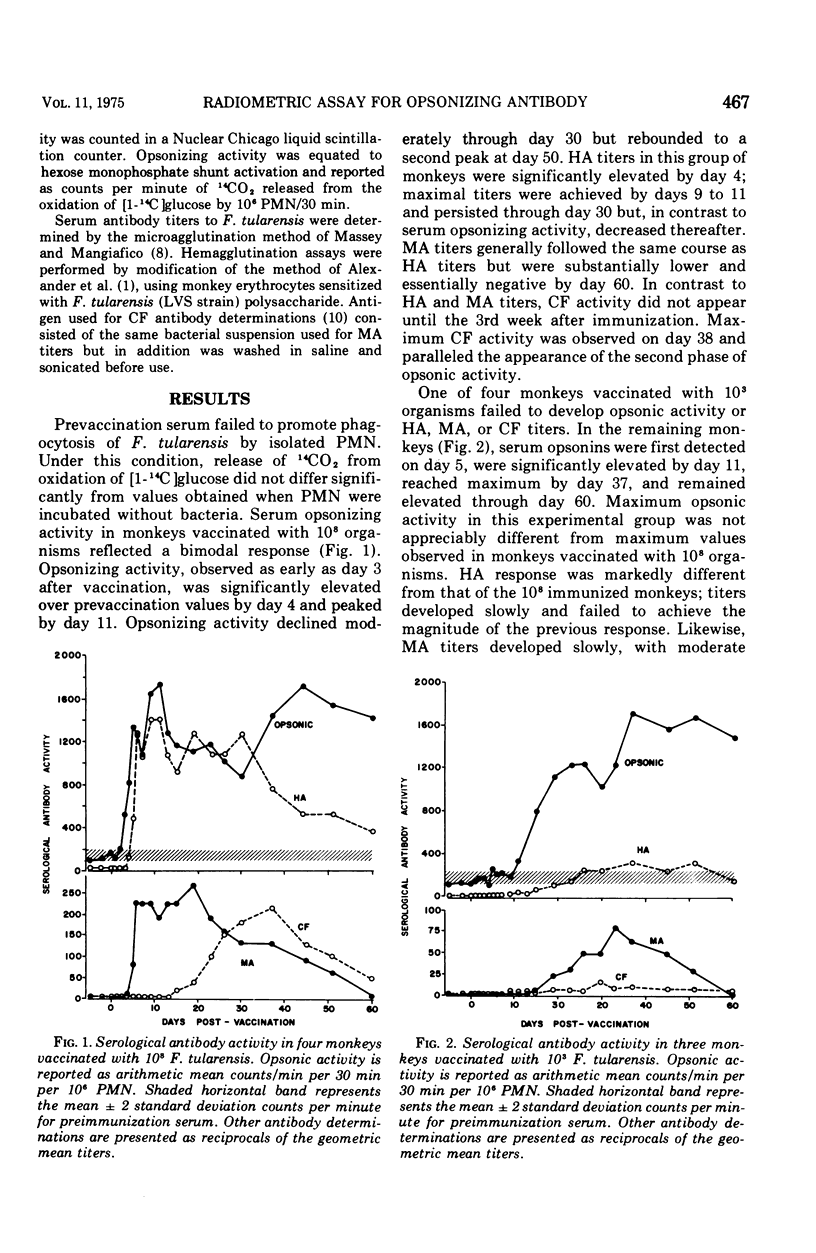
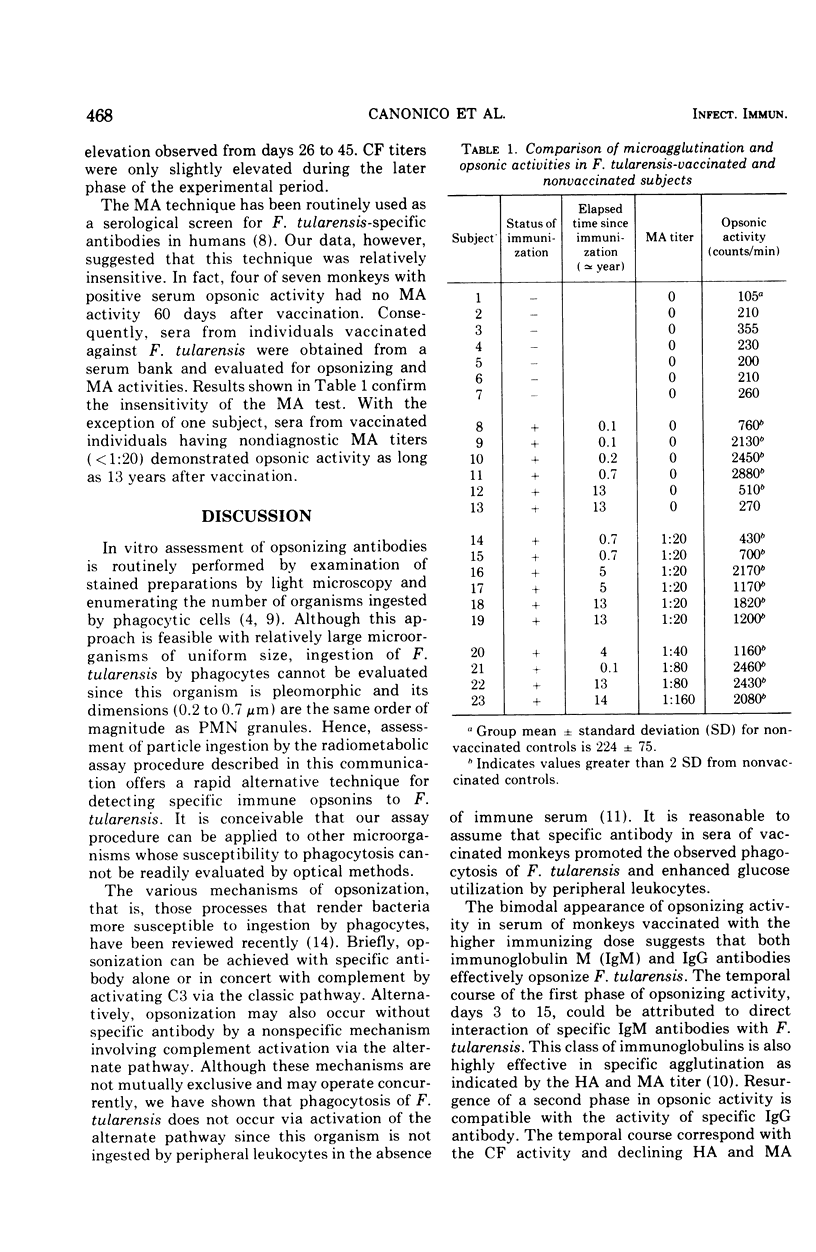
The burst in oxidative metabolism that is mediated through activation of the hexose monophosphate shunt and accompanies particle ingestion by polymorphonuclear leukocytes was used as the indicator in an in vitro radiometabolic assay for detection of specific opsonizing antibody to Francisella tularensis. Release of 14CO2 from radiolabeled glucose was increased significantly when specific immune serum added to suspensions of monkey polymorphonuclear leukocytes and F. tularensis. With this method, opsonizing antibodies to F. tularensis were detected in monkey serum 3 days after vaccination. Significantly increased opsonic activity in these monkeys preceded the appearance of, and persisted longer than, antibody activity as determined by conventional serological techniques. In addition, sera from 11 of 12 humans that were immunized 1 month to 13 years previously and had nondiagnostic agglutinating antibody titers demonstrated significant opsonizing activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER M. M., WRIGHT G. G., BALDWIN A. C. Observations on the agglutination of polysaccharide-treated erythrocytes by tularemia antisera. J Exp Med. 1950 Jun 1;91(6):561–566. doi: 10.1084/jem.91.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Wang P., McCall C. E. Hexose monophosphate shunt activity and oxygen consumption during phagocytosis: temporal sequence. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1434–1436. doi: 10.3181/00379727-140-36690. [DOI] [PubMed] [Google Scholar]
- Forman M. L., Stiehm E. R. Impaired opsonic activity but normal phagocytosis in low-birth-weight infants. N Engl J Med. 1969 Oct 23;281(17):926–931. doi: 10.1056/NEJM196910232811704. [DOI] [PubMed] [Google Scholar]
- Foshay L., Ruchman I., Nicholes P. S. ANTITULARENSE SERUM: CORRELATION BETWEEN PROTECTIVE CAPACITY FOR WHITE RATS AND PRECIPITABLE ANTIBODY CONTENT. J Clin Invest. 1947 Jul;26(4):756–760. doi: 10.1172/JCI101858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRENIS L. J., STRAUSS B. Effect of size and concentration of latex particles on respiration of human blood leucocytes. Proc Soc Exp Biol Med. 1961 Aug-Sep;107:748–750. doi: 10.3181/00379727-107-26743. [DOI] [PubMed] [Google Scholar]
- Kvarstein B. Oxygen consumption during the initial stage of human leucocyte phagocytosis of polystyrene latex particles. Scand J Clin Lab Invest. 1970 Jun;25(4):337–348. doi: 10.3109/00365517009046214. [DOI] [PubMed] [Google Scholar]
- Massey E. D., Mangiafico J. A. Microagglutination test for detecting and measuring serum agglutinins of Francisella tularensis. Appl Microbiol. 1974 Jan;27(1):25–27. doi: 10.1128/am.27.1.25-27.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Eichenwald H. F. Leukocyte function and the development of opsonic and complement activity in the neonate. Am J Dis Child. 1971 Feb;121(2):120–126. doi: 10.1001/archpedi.1971.02100130074008. [DOI] [PubMed] [Google Scholar]
- Pike R. M. Antibody heterogeneity and serological reactions. Bacteriol Rev. 1967 Jun;31(2):157–174. doi: 10.1128/br.31.2.157-174.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor R. A., White J. D., Ayala E., Canonico P. G. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect Immun. 1975 Jan;11(1):146–151. doi: 10.1128/iai.11.1.146-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS J., QUASTEL J. H. PARTICLE UPTAKE BY POLYMORPHONUCLEAR LEUCOCYTES AND EHRLICH ASCITES-CARCINOMA CELLS. Biochem J. 1963 Oct;89:150–156. doi: 10.1042/bj0890150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. II. Contact angles and phagocytosis of encapsulated bacteria before and after opsonization by specific antiserum and complement. J Reticuloendothel Soc. 1972 Nov;12(5):497–502. [PubMed] [Google Scholar]
- Winkelstein J. A. Opsonins: their function, identity, and clinical significance. J Pediatr. 1973 May;82(5):747–753. doi: 10.1016/s0022-3476(73)80062-9. [DOI] [PubMed] [Google Scholar]



