Abstract
A wide spectrum of both normal and diseased cell types shed extracellular vesicles that facilitate intercellular communication without direct cell-to-cell contact. Microparticles (MPs) are a subtype of extracellular vesicles that participate in multiple biological processes. They carry abundant bioactive molecules including different forms of nucleic acids and proteins that can markedly modulate cellular behavior. MPs are involved in several hallmarks of cancer such as drug resistance, thrombosis, immune evasion, angiogenesis, tumor invasion and metastasis. Such MPs originate from either cancer or other host cells. As MPs are secreted and can be detected in various body fluids, they can be used as potential diagnostic and prognostic biomarkers as well as vehicles for delivery of cytotoxic drugs. This review summarizes accumulating evidence on the biological properties of MPs in cancer, with reference to their potential usage in clinical settings.
Keywords: Biomarkers, Metastasis, Angiogenesis, Cancer therapy, Bone marrow derived cells, Tumor microenvironment
Introduction
Body fluids and interstitial spaces contain extracellular vesicles (EVs)—also called microvesicles—which are derived from normal and diseased cells upon activation or apoptosis [1–4]. Such EVs are released from the cell surface as bilayered membrane structures and can be categorized and subdivided according to their size, mechanism of cellular release, content, surface markers, cellular origin and physiological role. The major types of EVs are exosomes, ectosomes, oncosomes, and apoptotic bodies [5].
Exosomes originate from endosomal cell compartments in the cytoplasm, known as multivesicular bodies, that fuse with the cell membrane to form 30–120 nm diameter phospholipid bilayer secreted vesicles [6–8]. In contrast, ectosomes which are often called microparticles (MPs), are 0.1–1.0 μm in diameter, and they are formed directly from activated or early apoptotic cell membranes by a blebbing or shedding process. Thus, their surface markers resemble that of the parental cell enabling identification of their cellular origin [9]. Oncosomes are large vesicles, 1–10 μm in diameter, which are originated from migrating tumor cells undergoing amoeboid movement [10]. They are known to transfer oncogenic materials between cells [11, 12]. Similarly to MPs, apoptotic bodies are also released as blebs from cells undergoing late apoptosis and are approximately 1–5 μm in diameter [13].
All EVs are characterized by the presence of phosphatidylserine (PS) on their outer leaflet, although to a lower extent in exosomes and some subsets of MPs [14, 13, 15, 16]. Size parameters determined by scattering flow cytometry and PS detection by Annexin V or lactadherin are only a few of the methods available today for EV detection and are reviewed elsewhere [17–20].
In vivo, apoptotic bodies are rapidly recognized and engulfed by neighboring phagocytic cells, limiting their identification and characterization to in vitro studies performed in cell cultures [21, 22]. Exosomes and MPs, on the other hand, are detectable in in vivo systems, which enable the characterization of their role in biological and pathological settings, although their presence in the circulation may vary between different animal models [23]. Numerous studies have demonstrated their contribution to pathophysiological processes, highlighting the importance of understanding their properties and mechanisms of action [24–33].
In cancer, MPs and exosomes were shown to facilitate tumor progression by various mechanisms, (see reviews [34, 35]). A growing body of evidence suggests that MPs may play a pivotal role in the promotion of tumor growth and in supporting metastasis spread. They do so by transferring microRNAs (miRNAs), mRNA, genomic DNA, retrotransposons, proteins and lipid components between cells, which are not usually located in close proximity. In this mini-review we focus on the autocrine, paracrine and endocrine properties of tumor- and stroma- derived MPs, and their regulation of malignant progression.
Platelet-Derived Microparticles
EV formation was first documented by Wolf in 1967, where he noticed the presence of procoagulant particulate matter, which he referred to as “platelet dust”, surrounding activated blood platelets [36]. Over the past few decades, “platelet dust”, which was subsequently replaced by the term Platelet MPs (PMPs), served as a notable subject in cancer related research, in which the effects of PMPs on tumor angiogenesis and metastasis were studied [37].
Characteristics of Tumor Derived Microparticles
Tumor-derived MPs (TMPs) exhibit a molecular signature determined by the cancer cells from which they are derived. For example, gliomas often express the oncogenic form of the epidermal growth factor receptor, also known as EGFRvIII, that induces MAPK and Akt pathways to promote anchorage-independent cell growth. Al-Nedawi et al. observed that when cancer cells lacking EGFRvIII expression were exposed to MPs obtained from aggressive EGFRvIII-expressing glioma, recipient cells became positive for EGFRvIII [11]. Another study by Al-Nedawi et al. demonstrated that MPs produced by human cancer cells expressing activated EGFR can elicit EGFR-dependent responses paracrinically, as described above, in cultured endothelial cells [38].
Other receptors such as the pro-coagulant transmembrane receptor, tissue factor (TF), were shown to play a role in TMP-mediated cancer associated thrombosis, which is one of the most common complications leading to cancer patient mortality [39–41]. In a study by Davila et al. injection of TMPs derived from breast and pancreatic cancer cell lines into mice resulted in strong, TF-dependent, procoagulation activity [42]. In another study, the expression of TF on TMPs from human colorectal carcinoma cells were implicated as an important effector of K-ras-dependent tumor progression [43]. In addition to TF, P-selectin, a glycoprotein ligand-1 (PSGL-1) usually expressed by platelets, was also shown to modulate thrombus formation via association with TMPs [44]. An interesting research direction for the role of TF in cancer progression has been recently suggested in the context of cancer stem cells (CSCs). CSCs are a subset of tumor cells with stem cell characteristics, mainly due to their ability to self-renew and replicate limitlessly. CSCs are considered as the primary tumor cells with tumor initiating capacity. They have been shown to resist many treatments, and even metastasize [45, 46]. As TF is associated with oncogenic events in cancer and in angiogenesis, it may also accompany characteristics of tumor cell aggressiveness and markers of CSCs (e.g., CD133 in glioma tumors). It has been demonstrated that the blockade of TF in host cells perturbs tumor initiation and therefore can be used as a therapeutic intervention for cancer [47, 48]. Overall, it has been proposed that TF affects the CSC properties and tumor growth due to its wide range of tumorigenic activities.
TMPs can also promote tumor resistance to therapy. A study by Bebawy et al. showed that MPs isolated from drug-resistant cancer cells transfer the plasma membrane multidrug efflux transporter P-glycoprotein (P-gp) to drug sensitive cells, thus facilitating ‘non-genetic’ tumor cell resistance [49]. Similar results were recently reported by Pasquier et al. where intercellular transfer of functional P-gp occurred between drug resistant donor and drug sensitive recipient cells in the absence of drug selection pressure [50]. Jaiswal et al. suggested that TMP-dependent P-gp transfer is a selective process based on specific recognition between the TMPs and target cells [51]. In an earlier study by the same group, the authors showed that TMPs consist of regulatory microRNA (miRNA) as well as ABCB1 and ABCC1 gene transcripts encoding P-gp and multi-drug resistance-associated protein 1 (MRP1), respectively. These TMPs promoted the transfer of nucleic acids between drug-sensitive cells to multi-drug resistant cells [52].
The transfer of nucleic acids through MPs was first documented by Ratajczak et al. who showed that MPs from embryonic stem cells can deliver mRNA to target cells which can be later translated into proteins [53]. Subsequently, exosomes from human and mouse mast cell lines were undergo microarray assessments and found to contain mRNA of approximately 1,300 genes which could not be found in the cytoplasm of the donor cells, suggesting that mRNA can be delivered to other cells via exosomes [54]. In cancer, Balaj et al. suggested that some nucleic acids transferred via TMPs promote tumor growth. In their study, TMPs were shown to carry retrotransposon elements that are known to promote genome instability and subsequent tumorigenesis [55]. As such, medulloblastoma cells, which bear frequent amplification of the c-Myc oncogene, shed TMPs with higher DNA/RNA levels of c-Myc compared to cells without c-Myc amplification, thus inducing an aggressive tumor type [55]. However, the transfer of functional properties between cells using MPs through DNA/RNA needs further validation.
The impact of MPs and TMPs on growing tumors has been studied in additional directions. For example, the ability of tumor cells to overcome and escape the immune system has been recently studied in the context of TMPs. Poutsiaka et al. showed that membrane vesicles shed from tumor cells cause a reduction in macrophage immune region-associated antigen expression, which is essential for early phases of the immune response towards the growing tumor [56]. In another study, membrane vesicles extracted from human breast carcinoma, but not human fibrosarcoma, were shown to dramatically inhibit 3H-thymidine incorporation by peripheral blood lymphocytes, therefore suggesting a role of TMPs in the escape of tumor cells from immunological surveillance [57]. The activity of immune cells against tumor cells was also studied. Fas receptor and Fas ligand (FasL) are expressed on T lymphocytes and NK cells in order to promote interactions with their target cells. It has been shown that TMPs shed from human colorectal carcinoma cell lines also express biologically active Fas or FasL, which in turn protect the cells from FasL-mediated apoptosis [58]. Overall, these findings suggest that TMPs aid tumor cells to escape the immune system.
Another interaction between TMPs and bone marrow derived cells (BMDCs) has been studied in the context of chronic lymphocytic leukemia (CLL). B-cell derived MPs were shown to deliver the phospho-receptor tyrosine kinase, Axl, to CLL bone marrow stromal cells, thus activating PI3K/AKT which further activates mTOR/p70S6K/HIF-1α axis to produce vascular endothelial growth factor (VEGF) that supports leukemic disease progression [59]. Taken together, TMPs act by different ways to promote both solid tumors and hematological malignancies.
Microparticles and Angiogenesis
One important mechanism by which MPs contribute to tumor progression is by supporting angiogenesis—the formation of new blood vessels in tumors that supply nutrients and oxygen critical for sustaining tumor growth [60]. Tumor angiogenesis is mediated locally, by rapidly proliferating mature endothelial cells, as well as systemically, by the mobilization of bone marrow derived endothelial precursor cells [61]. The latter process supports systemic de novo angiogenesis, and is also called vasculogenesis. Various studies demonstrated that MPs from tumor cells, platelets and endothelial cells express adhesion molecules, growth factors and matrix metalloproteinases (MMPs) that are essential for local and systemic angiogenesis. The specific mechanisms by which MPs affect angiogenesis largely depend on their cell of origin, how they were generated, and the microenvironment. For example, Taraboletti et al. showed that MPs shed by human ovarian carcinoma cell lines contain VEGF and two MMPs—MMP-2 and MMP-9—that can stimulate the motility and invasiveness of endothelial cells in vitro [62]. The authors suggested that higher stimulation of endothelial cell motility was achieved only following vesicle burst induced by acidic pH which resembles that of the tumor microenvironment.
Other studies have also attributed the involvement of TMPs in tumor angiogenesis to different mediators. Mutated EGFR transferring TMPs, as described above, were shown to induce the expression of VEGF and VEGF receptor in endothelial cells [38]. In another study it was shown that TMPs shed from human ovarian carcinoma cell lines that express the extracellular MMP inducer, CD147, promote the angiogenic properties of human umbilical vein endothelial cells (HUVECs) in a CD147-dependent manner [63]. In another study, Taverna et al. suggested that TMPs shed from a human hepatoma cell line carry three Fibroblast Growth Factor-2 (FGF-2) isoforms that promote migration of endothelial cells [64]. Not only angiogenic factors per se contribute to tumor angiogenesis. TF expressing TMPs were also found to contribute to tumor angiogenesis in addition to their known role in thrombosis. Yu et al. suggested that tumor and host compartments mutually transfer TF-containing MPs that contribute to pro-coagulant and pro-angiogenic modulation of endothelial cells [65]. Furthermore, the cross-talk between TMPs and endothelial MPs (EMPs) was shown to promote a pro-tumoral vascular niche by altering the activation of endothelial cells. In turn, such MPs induce tumor cell invasion, proliferation and stem cell phenotype [66]. Thus TMPs shed from different tumor cell types can promote angiogenesis and the activity of endothelial cells by altering the expression of angiogenic factors or other angiogenic mediators.
In addition to various proteins expressed on TMPs, lipid components have also been shown to play a role in TMP-mediated angiogenesis. Fibrosarcoma TMPs containing sphingomyelin were shown to be involved in neovascularization by inducing endothelial cell migration [67].
In terms of differences between local and systemic angiogenesis, TMPs were also shown to act endocrinically on proangiogenic BMDCs to promote their mobilization in an osteopontin (OPN) dependent manner. In a recent study by Fremder et al. the authors suggest a mechanism by which TMPs shed from chemotherapy-treated cells express high levels of OPN that triggers the mobilization and tumor homing of different types of BMDCs known to support tumor angiogenesis. TMPs from OPN depleted tumors, however, did not show the same pattern of BMDC recruitment to tumors suggesting that OPN plays an important role in this process. Importantly, the authors also showed that the mobilization and tumor homing of BMDCs mediated by TMPs does not induce their activation at the tumor site, only their recruitment to this site, suggesting that TMPs act as a messenger rather than a cell activator [68].
TMPs are not the only MPs which support tumor angiogenesis. PMPs display angiogenic properties partially due to the expression of proangiogenic factors that can promote the growth of capillary-like structures [69]. PMPs were shown to stimulate proliferation and tube formation of HUVECs and protect them from apoptosis [70]. They have also been shown to induce chemotactic events and invasion in vitro in Matrigel containing endothelial cells, human lung cancer cells, or breast cancer cells [71, 72]. Other studies have demonstrated that monocyte-derived TF-bearing MPs also induce tube formation by endothelial cells [73]. In addition, MPs derived from endothelial cells (EMPs) have been described as proangiogenic mainly because they contain MMPs (largely MMP-2 and MMP-9). Since MMPs are involved in endothelial cell invasion and new capillary formation, EMPs can promote new vessel sprouting. This is an example of an autocrine pathway by which endothelial cells can also contribute to their own angiogenic process through MPs [74]. Lastly, MPs derived from endothelial precursor cells have been shown to transfer mRNA associated with signaling pathways that activate angiogenesis [75].
Interestingly, MPs were also described to have anti-angiogenic activity. Yang et al. showed that lymphocyte-derived MPs strongly suppressed aortic ring vessel sprouting in vitro. This effect was found to be associated with down-regulation of VEGFR2, one of the main receptors for VEGF-A that mediates angiogenesis and increases reactive oxygen species [76]. Taken together, MPs originating from tumor cells are mostly known for their pro-angiogenic activities, while those originating from other cell types may possess different angiogenic properties.
Microparticles and Metastasis
Metastasis is a cascade of molecular and cellular events involving tumor cell dissemination from the primary tumor, systemic circulation, arrest at a secondary site, and subsequent colonization and growth [77]. Proteolytic enzymes are thought to play a major role in the promotion of tumor invasion and metastasis explaining why MP-associated proteolytic activity was studied in depth in the past years. In a study by Muralidharan-Chari et al. the authors suggest a mechanism by which the ADP-ribosylation factor 6 (ARF6) GTP/GDP cycle regulates the release of MPs containing protease cargo from tumor cells, thus facilitating extracellular matrix degradation and promotion of the invasive phenotype in these cells [78].
Proteolytic enzymes are an important component promoting tumor cell dissemination and metastatic activities. Studies have shown that MPs shed by breast and ovarian carcinoma and fibrosarcoma cell lines contain proteases such as MMP-2 and MMP-9 that are known to play important roles in tumor invasion and metastasis [79–82]. In addition, lung cancer cells secrete large quantities of TMPs that have a potential role in the recruitment of stromal fibroblasts and endothelial cells to tumors. These TMPs were shown to stimulate the secretion of MMP-9 from fibroblasts enhancing the metastatic potential of lung cancer cells in an in vivo metastasis assay [83]. Furthermore, MMP2 has been shown to be transferred to tumor cells by PMPs [84]. Thus, proteolytic enzymes use MPs or TMPs as vehicles for their transfer to tumor cells in order to promote the metastatic process.
However, not only active proteolytic molecules stored in TMPs and MPs promote metastasis. Other components stored in MPs may alter the tumor microenvironment to become more prone to metastasis. For example, TMPs were shown to express the serine protease urokinase-type plasminogen activator (uPA) that enables the conversion of MMPs zymogens to their active form [81, 85]. In addition, low pH is a characteristic of the tumor microenvironment. It has been shown that increased activity of MMP2 and MMP9 was induced upon exposure of ovarian carcinoma TMPs to an acidic environment (pH 5.6). The authors suggested that the cysteine protease cathepsin B might play a role in the pH-dependent activation of MMPs, as these conditions are considered within the pH optimum for their activity. The authors demonstrated that inhibiting Cathepsin B expression or its activity abolished TMP-induced metalloproteinase activity at low pH. These results further suggest that the acidic microenvironment in tumors promotes tumor cell invasion and metastasis via TMPs secreting cathepsin-B [86]. Overall, mediators that are stored in TMPs may activate MMPs, thus increasing metastatic potential.
Tumor-stroma interactions are considered a fundamental necessity for tumor development and progression. Interplay between tumor and non-malignant host cells (e.g., stromal cells, immune cells, activated endothelium) have also been described in the context of MPs. In a study by Castellana et al. TMPs derived from the metastatic PC3 prostate carcinoma cell line were shown to trigger ERK1/2 phosphorylation, increase active MMP-9 expression, and promote migratory and therapy resistant traits in fibroblasts. In response, TMP-stimulated fibroblasts were shown to secrete their own MPs which further promoted the migratory and invasive properties of the PC3 tumor cells [87]. A more recent study also describes possible MP transfer between immune and cancer cells, resulting in an increase in tumor cell migratory and metastatic phenotype. The transfer of integrin α(M)β2 (CD11b/CD18) via immune cell-derived MPs was suggested by the authors to serve as a key element in inducing cell migration [88]. Although this study was conducted in in vitro settings, it is possible that such interactions may occur in vivo where tumor cells could utilize immune cell phenotypes transferred via shed MPs in order to enhance their metastatic potential.
It should be noted that in addition to MPs, the contribution of exosomes to metastasis has also been investigated. It was reported that exosomes derived from the highly metastatic B16-F10 melanoma cells can fuse or be uptaken by the B16-F1 cells which possess low-metastatic potential, and thus induce their metastatic properties [89, 90]. Exosomes may also contribute to metastasis by altering stromal cells’ properties at the primary tumor microenvironment or supporting cells at the pre-metastatic site. For example, exosomes from melanoma cells may ‘condition’ the lymphnodes for metastatic cell seeding by a synchronized molecular signaling which affects melanoma cell recruitment to metastatic sites [91]. Also, miRNA from tumor-derived exosomes have been shown to affect stromal cells at the pre-metastatic sites, by altering the expression of pro-metastatic molecules, thus promoting tumor cell seeding and spread of metastasis [92]. In addition, exosomes can shift the characteristics of BMDCs towards a tumor pro-invasive phenotype. Peinado et al. recently demonstrated that exosomes from highly metastatic melanomas educate BMDCs through the receptor tyrosine kinase MET, to become pro-invasive, and to acquire vasculogenic phenotypes—properties critical for metastatic spread [93]. Stromal cells, on the other hand, can also affect the metastatic properties of tumor cells via microvesicles, as exosomes secreted from fibroblasts have been shown to induce an autocrine Wnt-planar cell polarity (PCP) signaling in tumor cells which in turn, promotes breast carcinoma cell invasive properties [94]. In patients, it has been demonstrated that exosomes proteomic profiling is different between the primary tumor and the metastatic sites of colorectal cancer patients. Exosomes of metastatic sites are enriched with metastatic factors and signaling pathways which can contribute to the cross-talk between tumor cells and stromal cells at the metastatic microenvironment [95]. Overall, TMPs and exosomes not only affect tumor cell proliferation, growth, and angiogenesis, but also the metastasis cascade by virtue of enhancing metalloproteinase activities via various independent mechanisms, and by altering the pre-metastatic sites to the tumor advantage.
Microparticles as Potential Biomarkers in Cancer
The notion that the levels of MPs in the body fluids of cancer patients are significantly higher than those of healthy individuals could provide vast diagnostic and prognostic opportunities. The possibility that MPs contain information about tumor cells or their activity, combined with the fact that they can be easily detected in the circulation, allows for the use of MPs as a remarkable biomarker tool. This research direction has been extensively studied in recent years, and some studies are summarized here. For example, the number and proteolytic content of MPs shed by ovarian cancer cells were found to correlate with the invasive phenotype of the tumor cells, suggesting that the metastatic potential of tumor cells can be evaluated by proteolytic enzyme activity stored in MPs [80]. Indeed, the levels of MPs and their MMP-2 activity derived from benign and malignant serous cyst fluid of ovarian cancer patients positively correlated with tumor aggressiveness and stage [81]. In addition, as stated above, tumor cells may shed TMPs containing oncogenic receptors such as EGFRvIII, which can transfer to other cell types in the tumor bed [38]. Indeed, EGFRvIII was detected in MPs of glioblastoma patients, suggesting that TMPs detected in peripheral blood may be used as a diagnostic biomarker for therapeutic purposes [96]. Thus, parameters on the quantity and quality of MPs could provide valuable information on malignant progression. In other studies, MPs were evaluated as a biomarker for tumor progression in breast cancer patients. Circulating levels of both leukocyte-derived MPs (LMPs) (CD45+) and endothelial MPs (EMPs) were analyzed and compared to the profiles of the most abundantly used breast cancer biomarkers, CEA and CA15-3. LMP levels in breast cancer patients were significantly different from those of controls, and correlated with tumor size, with similar sensitivity to that observed when using the marker CA15-3. However, such correlation was not found with EMPs, suggesting that CD45+LMPs, but not EMPs, could serve as a diagnostic tool for breast cancer staging [97]. Furthermore, gastric cancer patients were also shown to present elevated TMP plasma levels, which increased in correlation with disease progression [98]. Similarly to TMPs, PMPs were recently described as potential gastric cancer staging biomarker candidates, as their levels in the plasma were found to correlate with disease progression [99].
PMPs were also suggested as predictive biomarkers for treatment outcome. In a study by Helley et al. whole blood PMPs were quantified in hormone-refractory prostate cancer patients (HRPC) prior to treatment with docetaxel-based chemotherapy. High levels of PMPs were shown to be in significant correlation with shorter overall survival of patients following therapy [100]. However, in non-small cell lung cancer patients, high levels of MPs in peripheral blood both before and after treatment, correlated with increased progression-free survival and overall survival [101]. In a recent study by Reynés et al. peripheral blood MP levels were shown to be significantly higher in patients with glioblastoma. Following treatment, MP levels were shown to decrease, however, no data was reported with respect to the correlation with overall survival, probably due to the aggressive phenotype of such tumors [102]. Similar results were also reported by Shao et al. who described that exosomes and MPs from tumor origin were elevated in glioblastoma patients, but their levels markedly decreased upon treatment. Again, whether lowered levels of MPs following treatment is an indication for successful therapy is yet to be established [103]. In addition to the aforementioned studies, TMPs have been reviewed as a biomarker for thrombosis, an important clinical condition in cancer and other pathologies. The role of TMPs as thrombotic biomarkers was recently reviewed by Geddings and Mackman, who suggested that TF-positive MPs are an indication of increased risk of thrombosis in cancer patients [104]. Interestingly, it has been demonstrated that the activity of MPs expressing TF in pancreatic cancer patients was correlated with poorly differentiated and invasive tumors indicating poor survival [105]. Overall, these examples stress the diagnostic and prognostic potential of MPs in the clinical settings. In addition, they also demonstrate the different MP patterns following therapy, suggesting that each cancer type manifests a unique pattern of MPs that should be addressed separately in the prediction of therapy outcome.
Future Research
A growing body of literature suggests that tumors recruit many types of immune cells which in turn assist in tumor progression and growth. In this regard, PMPs, for example, have been shown to participate in the trafficking of immune cells as well as in the promotion of hematopoiesis [106]. They do so by expressing several chemokine and cytokine receptors that upon binding to normal and malignant hematopoietic cells, promote their activation, mobilization, proliferation and adhesion properties [107, 106, 108]. The high concentration of PMPs in inflammatory areas, could recruit immune cells to the pro-inflammatory sites. It is therefore plausible that the same mechanism occurs in the recruitment of immune cells to the tumor microenvironment, thus facilitating tumor progression. In this regard, another intriguing aspect is related to the specific properties of immune cell derived MPs. Mesri and Altieri exhibited that MVs shed from stimulated polymorphonuclear leukocytes induce endothelial cells to secrete the cytokines IL-6 and IL-8 and to enhance the expression of leukocyte-endothelial cell adhesion molecules [109]. The authors suggested that this could be a general mechanism in inflammatory responses. Given the inflammatory nature of tumor development, it would be interesting to explore whether such mechanisms are also involved in the recruitment of leukocytes to the tumor milieu, and to study possible MP-mediated reciprocal communication between cancer cells and their microenvironment stromal cells.
A fascinating possibility would be to exploit MPs as vehicles for anti-cancer drug delivery. A study by Shedden et al. demonstrated that gene expression associated with vesicle shedding can be enhanced in tumor cells, consequently resulting in drug efflux achieved by shedding of microvesicles (a mixture of exosomes and MPs) containing the cytotoxic drug [110]. In a recent study by Tang et al. the authors induced tumor cells to shed cytotoxic drug-containing microvesicles that were then used for therapy [111]. Although in this particular study injection of cytotoxic drug-containing MPs resulted in inhibition of ovarian cancer growth, it is still crucial to consider the possibility of undesired transfer of other MP content, such as oncogenic receptors that might accelerate malignancy. It should be noted that synthetic MPs have been studied as therapeutic vehicles for more than a decade. The use of liposomes containing drugs, such as liposomal doxorubicine, has been shown to improve treatment efficacy of various solid tumors by different mechanisms [112, 113]. Therapeutic approaches in this direction were also explored for synthetic lyposomes containing tumor cell binding factors on their surface designed for use as a vehicle of toxic drugs [114]. In this regard, Toledano-Furman et al. used surface molecules of mesenchymal stem cells known to home to tumors as nano-ghost vehicles containing anti-cancer drugs as a new treatment modality for cancer [115]. The use of MPs as an alternative biological source to synthetic lyposomes or nano-ghosts would probably be of a better nature and even more effective in targeting the tumor microenvironment. This treatment direction should be further elucidated in the future.
On a more technical note, standardized written protocols for MP purification are currently missing and should be carefully delineated in order to utilize MPs for clinical purposes. In this respect, future efforts should be made to maximize the resolution of distinction between MPs and other types of vesicles. This could be achieved by nanoparticle tracking analysis and atomic force microscopy, methods that are likely to provide more accurate sizing of MPs compared with conventional flow cytometry [19]. However, it is also important to note that these particular techniques are less feasible than flow cytometry, as their accessibility is lower and they are considered less ‘user-friendly’. Therefore, the right balance between accurate sizing of MPs and technical feasibility is needed in order to enable the integration of MP based procedures in the clinic.
Concluding Remarks
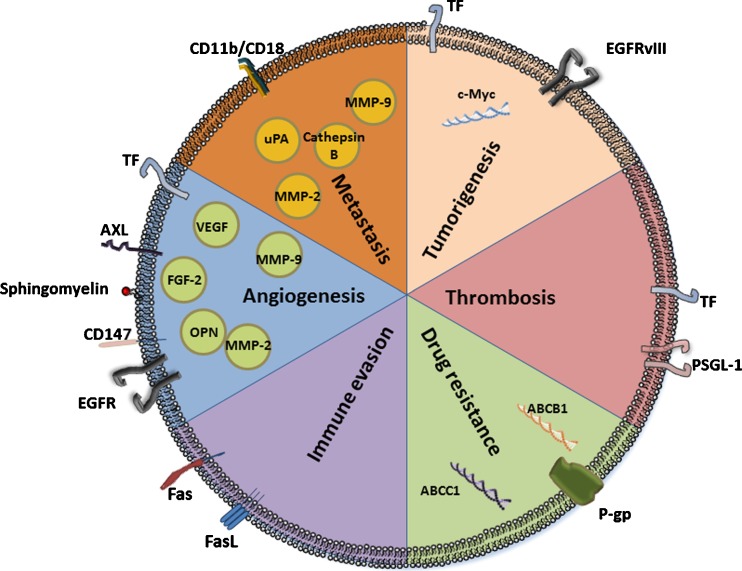
Vesicle shedding is a normal biological process that occurs both in physiological and pathological conditions. MPs are formed directly from the cell surface by the outward budding and fission of the cell membrane. They were shown to contain cell surface receptors as well as soluble mediators such as growth factors and cytokines, thus comprising an ensemble of intercellular communication possibilities. As illustrated in Fig. 1, tumor associated MPs, PMPs and other stroma-derived MPs (e.g. fibroblasts, endothelial and immune cells) can actively alter the genomic stability in normal cells and further promote malignant transformation. Furthermore, matrix degrading enzymes released by shed MPs can facilitate the promotion of tumor angiogenesis and metastasis. Expanding the current knowledge of the biology, properties and mechanisms of action of tumor associated MPs would benefit the development of potential diagnostic, prognostic and therapeutic targets.
Fig. 1.
The roles of MPs in tumorigenesis: the effect of MPs on various hallmarks of cancer is presented in this illustration. MPs contain DNA fragments with oncogenic capacity that can be transferred between cells. They express factors related to the coagulation system, and as such promote tumorigenesis and various aspects in the pathology of cancer. MPs promote tumor resistance by transferring molecules associated with multi-drug resistance. They express factors that inhibit immune cell activity against cancer cells. MPs contain factors related to the extracellular matrix and the proliferation of endothelial cells, and as such they promote angiogenesis and metastatic spread. Studying MPs in cancer may provide a new research direction on the cross-talk between the tumor and its stroma. In addition, MPs can be used as a diagnostic tool for cancer intervention due to their presence in the circulation. Abbreviations: EGFR epidermal growth factor receptor, FasL Fas ligand, FGF Fibroblast Growth Factor, MMP matrix metalloproteinase, OPN Osteopontin, P-gp P-glycoprotein, PSGL-1 P-selectin glycoprotein ligand 1, TF tissue factor, VEGF vascular endothelial growth factor, uPA urokinase-type plasminogen activator
Acknowledgments
This review was supported by the European Research Council (under the FP-7 program –260633), Israel Cancer Research Fund (708-12), Israel Science Foundation (490/12), and Rappaport research fund to YS.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Smalley DM, Sheman NE, Nelson K, Theodorescu D. Isolation and identification of potential urinary microparticle biomarkers of bladder cancer. J Proteome Res. 2008;7(5):2088–2096. doi: 10.1021/pr700775x. [DOI] [PubMed] [Google Scholar]
- 2.Burnier L, Fontana P, Kwak BR, Angelillo-Scherrer A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb Haemost. 2009;101(3):439–451. [PubMed] [Google Scholar]
- 3.Zachau AC, Landen M, Mobarrez F, Nybom R, Wallen H, Wetterberg L. Leukocyte-derived microparticles and scanning electron microscopic structures in two fractions of fresh cerebrospinal fluid in amyotrophic lateral sclerosis: a case report. J Med Case Rep. 2012;6(1):274. doi: 10.1186/1752-1947-6-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 5.El Andaloussi S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 6.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6(3):267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 8.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6(8):607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer–the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33(5):455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 13.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6(1):21–29. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 14.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 15.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21(3):157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Connor DE, Exner T, Ma DD, Joseph JE. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost. 2010;103(5):1044–1052. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta SK, Guchhait P, Thiagarajan P. Lactadherin binding and phosphatidylserine expression on cell surface-comparison with annexin A5. Transl Res. 2006;148(1):19–25. doi: 10.1016/j.lab.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 19.van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8(12):2596–2607. doi: 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- 20.Orozco AF, Lewis DE. Flow cytometric analysis of circulating microparticles in plasma. Cytometry A. 2010;77(6):502–514. doi: 10.1002/cyto.a.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler U, Groscurth P. Morphological features of cell death. News Physiol Sci. 2004;19:124–128. doi: 10.1152/nips.01519.2004. [DOI] [PubMed] [Google Scholar]
- 22.Hengartner MO. Apoptosis: corralling the corpses. Cell. 2001;104(3):325–328. doi: 10.1016/s0092-8674(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 23.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119(23):5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skeppholm M, Mobarrez F, Malmqvist K, Wallen H. Platelet-derived microparticles during and after acute coronary syndrome. Thromb Haemost. 2012;107(6):1122–1129. doi: 10.1160/TH11-11-0779. [DOI] [PubMed] [Google Scholar]
- 25.Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J. 2003;145(6):962–970. doi: 10.1016/S0002-8703(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 26.Garcia S, Chirinos J, Jimenez J, Del Carpio MF, Canoniero M, Jy W, Horstman L, Ahn Y. Phenotypic assessment of endothelial microparticles in patients with heart failure and after heart transplantation: switch from cell activation to apoptosis. J Heart Lung Transplant. 2005;24(12):2184–2189. doi: 10.1016/j.healun.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Simak J, Gelderman MP, Yu H, Wright V, Baird AE. Circulating endothelial microparticles in acute ischemic stroke: a link to severity, lesion volume and outcome. J Thromb Haemost. 2006;4(6):1296–1302. doi: 10.1111/j.1538-7836.2006.01911.x. [DOI] [PubMed] [Google Scholar]
- 28.Sabatier F, Darmon P, Hugel B, Combes V, Sanmarco M, Velut JG, Arnoux D, Charpiot P, Freyssinet JM, Oliver C, Sampol J, Dignat-George F. Type 1 and type 2 diabetic patients display different patterns of cellular microparticles. Diabetes. 2002;51(9):2840–2845. doi: 10.2337/diabetes.51.9.2840. [DOI] [PubMed] [Google Scholar]
- 29.Chirinos JA, Heresi GA, Velasquez H, Jy W, Jimenez JJ, Ahn E, Horstman LL, Soriano AO, Zambrano JP, Ahn YS. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45(9):1467–1471. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 30.Alijotas-Reig J, Palacio-Garcia C, Llurba E, Vilardell-Tarres M. Cell-derived microparticles and vascular pregnancy complications: a systematic and comprehensive review. Fertil Steril. 2013;99(2):441–449. doi: 10.1016/j.fertnstert.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Pereira J, Alfaro G, Goycoolea M, Quiroga T, Ocqueteau M, Massardo L, Perez C, Saez C, Panes O, Matus V, Mezzano D. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb Haemost. 2006;95(1):94–99. [PubMed] [Google Scholar]
- 32.Mobarrez F, Nybom R, Johansson V, Hultman CM, Wallen H, Landen M, Wetterberg L. Microparticles and microscopic structures in three fractions of fresh cerebrospinal fluid in schizophrenia: case report of twins. Schizophr Res. 2013;143(1):192–197. doi: 10.1016/j.schres.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103(30):11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang HG, Grizzle WE. Exosomes: a novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32(3–4):623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. doi: 10.1111/j.1365-2141.1967.tb08741.x. [DOI] [PubMed] [Google Scholar]
- 37.Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8(43):237–241. [PubMed] [Google Scholar]
- 38.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119(1):60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 40.Amer MH. Cancer-associated thrombosis: clinical presentation and survival. Cancer Manag Res. 2013;5:165–178. doi: 10.2147/CMAR.S47094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2(11):2065–2067. doi: 10.1111/j.1538-7836.2004.00972.x. [DOI] [PubMed] [Google Scholar]
- 42.Davila M, Amirkhosravi A, Coll E, Desai H, Robles L, Colon J, Baker CH, Francis JL. Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6(9):1517–1524. doi: 10.1111/j.1538-7836.2008.02987.x. [DOI] [PubMed] [Google Scholar]
- 43.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 44.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206(9):1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 46.Wicha MS. Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res. 2006;12(19):5606–5607. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 47.Milsom C, Magnus N, Meehan B, Al-Nedawi K, Garnier D, Rak J. Tissue factor and cancer stem cells: is there a linkage? Arterioscler Thromb Vasc Biol. 2009;29(12):2005–2014. doi: 10.1161/ATVBAHA.108.177444. [DOI] [PubMed] [Google Scholar]
- 48.Garnier D, Milsom C, Magnus N, Meehan B, Weitz J, Yu J, Rak J. Role of the tissue factor pathway in the biology of tumor initiating cells. Thromb Res. 2010;125(Suppl 2):S44–S50. doi: 10.1016/S0049-3848(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 49.Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23(9):1643–1649. doi: 10.1038/leu.2009.76. [DOI] [PubMed] [Google Scholar]
- 50.Pasquier J, Galas L, Boulange-Lecomte C, Rioult D, Bultelle F, Magal P, Webb G, Le Foll F. Different modalities of intercellular membrane exchanges mediate cell-to-cell p-glycoprotein transfers in MCF-7 breast cancer cells. J Biol Chem. 2012;287(10):7374–7387. doi: 10.1074/jbc.M111.312157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaiswal R, Luk F, Dalla PV, Grau GE, Bebawy M. Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PLoS One. 2013;8(4):e61515. doi: 10.1371/journal.pone.0061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaiswal R, Gong J, Sambasivam S, Combes V, Mathys JM, Davey R, Grau GE, Bebawy M. Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB J. 2012;26(1):420–429. doi: 10.1096/fj.11-186817. [DOI] [PubMed] [Google Scholar]
- 53.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20(5):847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 54.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 55.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poutsiaka DD, Taylor DD, Levy EM, Black PH. Inhibition of recombinant interferon-gamma-induced Ia antigen expression by shed B16 F10 melanoma cell membrane vesicles. J Immunol. 1985;134(1):145–150. [PubMed] [Google Scholar]
- 57.Dolo V, Pizzurro P, Ginestra A, Vittorelli ML. Inhibitory effects of vesicles shed by human breast carcinoma cells on lymphocyte 3H-thymidine incorporation, are neutralised by anti TGF-beta antibodies. J Submicrosc Cytol Pathol. 1995;27(4):535–541. [PubMed] [Google Scholar]
- 58.Albanese J, Meterissian S, Kontogiannea M, Dubreuil C, Hand A, Sorba S, Dainiak N. Biologically active Fas antigen and its cognate ligand are expressed on plasma membrane-derived extracellular vesicles. Blood. 1998;91(10):3862–3874. [PubMed] [Google Scholar]
- 59.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115(9):1755–1764. doi: 10.1182/blood-2009-09-242719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 61.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 62.Taraboletti G, D’Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D, Giavazzi R, Pavan A, Dolo V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8(2):96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Millimaggi D, Mari M, D’Ascenzo S, Carosa E, Jannini EA, Zucker S, Carta G, Pavan A, Dolo V. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia. 2007;9(4):349–357. doi: 10.1593/neo.07133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taverna S, Ghersi G, Ginestra A, Rigogliuso S, Pecorella S, Alaimo G, Saladino F, Dolo V, Dell’Era P, Pavan A, Pizzolanti G, Mignatti P, Presta M, Vittorelli ML. Shedding of membrane vesicles mediates fibroblast growth factor-2 release from cells. J Biol Chem. 2003;278(51):51911–51919. doi: 10.1074/jbc.M304192200. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, May L, Milsom C, Anderson GM, Weitz JI, Luyendyk JP, Broze G, Mackman N, Rak J. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol. 2008;28(11):1975–1981. doi: 10.1161/ATVBAHA.108.175083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pasquier J, Thawadi HA, Ghiabi P, Abu-Kaoud N, Maleki M, Guerrouahen BS, Vidal F, Courderc B, Ferron G, Martinez A, Al Sulaiti H, Gupta R, Rafii S, Rafii A. Microparticles mediated cross-talk between tumoral and endothelial cells promote the constitution of a pro-metastatic vascular niche through Arf6 up regulation. Cancer Microenviron. 2014 doi: 10.1007/s12307-013-0142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim CW, Lee HM, Lee TH, Kang C, Kleinman HK, Gho YS. Extracellular membrane vesicles from tumor cells promote angiogenesis via sphingomyelin. Cancer Res. 2002;62(21):6312–6317. [PubMed] [Google Scholar]
- 68.Fremder E, Munster M, Aharon A, Miller V, Gingis-Velitski S, Voloshin T, Alishekevitz D, Bril R, Scherer SJ, Loven D, Brenner B, Shaked Y. Tumor-derived microparticles induce bone marrow-derived cell mobilization and tumor homing: A process regulated by osteopontin. Int J Cancer. 2013 doi: 10.1002/ijc.28678. [DOI] [PubMed] [Google Scholar]
- 69.Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67(1):30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Kim HK, Song KS, Chung JH, Lee KR, Lee SN. Platelet microparticles induce angiogenesis in vitro. Br J Haematol. 2004;124(3):376–384. doi: 10.1046/j.1365-2141.2003.04773.x. [DOI] [PubMed] [Google Scholar]
- 71.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113(5):752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 72.Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46(7):1199–1209. doi: 10.1111/j.1537-2995.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 73.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100(5):878–885. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 74.Taraboletti G, D’Ascenzo S, Borsotti P, Giavazzi R, Pavan A, Dolo V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002;160(2):673–680. doi: 10.1016/S0002-9440(10)64887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110(7):2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 76.Yang C, Mwaikambo BR, Zhu T, Gagnon C, Lafleur J, Seshadri S, Lachapelle P, Lavoie JC, Chemtob S, Hardy P. Lymphocytic microparticles inhibit angiogenesis by stimulating oxidative stress and negatively regulating VEGF-induced pathways. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R467–R476. doi: 10.1152/ajpregu.00432.2007. [DOI] [PubMed] [Google Scholar]
- 77.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80(8 Suppl):1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 78.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dolo V, Ginestra A, Cassara D, Violini S, Lucania G, Torrisi MR, Nagase H, Canevari S, Pavan A, Vittorelli ML. Selective localization of matrix metalloproteinase 9, beta1 integrins, and human lymphocyte antigen class I molecules on membrane vesicles shed by 8701-BC breast carcinoma cells. Cancer Res. 1998;58(19):4468–4474. [PubMed] [Google Scholar]
- 80.Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17(2):131–140. doi: 10.1023/a:1006500406240. [DOI] [PubMed] [Google Scholar]
- 81.Ginestra A, La Placa MD, Saladino F, Cassara D, Nagase H, Vittorelli ML. The amount and proteolytic content of vesicles shed by human cancer cell lines correlates with their in vitro invasiveness. Anticancer Res. 1998;18(5A):3433–3437. [PubMed] [Google Scholar]
- 82.Dolo V, Ginestra A, Ghersi G, Nagase H, Vittorelli ML. Human breast carcinoma cells cultured in the presence of serum shed membrane vesicles rich in gelatinolytic activities. J Submicrosc Cytol Pathol. 1994;26(2):173–180. [PubMed] [Google Scholar]
- 83.Wysoczynski M, Ratajczak MZ. Lung cancer secreted microvesicles: underappreciated modulators of microenvironment in expanding tumors. Int J Cancer. 2009;125(7):1595–1603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cells via upregulation of MMP-2 production. Int J Cancer. 2009;124(8):1773–1777. doi: 10.1002/ijc.24016. [DOI] [PubMed] [Google Scholar]
- 85.Angelucci A, D’Ascenzo S, Festuccia C, Gravina GL, Bologna M, Dolo V, Pavan A. Vesicle-associated urokinase plasminogen activator promotes invasion in prostate cancer cell lines. Clin Exp Metastasis. 2000;18(2):163–170. doi: 10.1023/a:1006778000173. [DOI] [PubMed] [Google Scholar]
- 86.Giusti I, D’Ascenzo S, Millimaggi D, Taraboletti G, Carta G, Franceschini N, Pavan A, Dolo V. Cathepsin B mediates the pH-dependent proinvasive activity of tumor-shed microvesicles. Neoplasia. 2008;10(5):481–488. doi: 10.1593/neo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castellana D, Zobairi F, Martinez MC, Panaro MA, Mitolo V, Freyssinet JM, Kunzelmann C. Membrane microvesicles as actors in the establishment of a favorable prostatic tumoral niche: a role for activated fibroblasts and CX3CL1-CX3CR1 axis. Cancer Res. 2009;69(3):785–793. doi: 10.1158/0008-5472.CAN-08-1946. [DOI] [PubMed] [Google Scholar]
- 88.Ma J, Cai W, Zhang Y, Huang C, Zhang H, Liu J, Tang K, Xu P, Katirai F, Zhang J, He W, Ye D, Shen GX, Huang B. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin alpha(M)beta(2) to tumor cells. J Immunol. 2013;191(6):3453–3461. doi: 10.4049/jimmunol.1300171. [DOI] [PubMed] [Google Scholar]
- 89.Poste G, Nicolson GL. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci U S A. 1980;77(1):399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hao S, Ye Z, Li F, Meng Q, Qureshi M, Yang J, Xiang J. Epigenetic transfer of metastatic activity by uptake of highly metastatic B16 melanoma cell-released exosomes. Exp Oncol. 2006;28(2):126–131. [PubMed] [Google Scholar]
- 91.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 92.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 95.Ji H, Greening DW, Barnes TW, Lim JW, Tauro BJ, Rai A, Xu R, Adda C, Mathivanan S, Zhao W, Xue Y, Xu T, Zhu HJ, Simpson RJ. Proteome profiling of exosomes derived from human primary and metastatic colorectal cancer cells reveal differential expression of key metastatic factors and signal transduction components. Proteomics. 2013;13(10–11):1672–1686. doi: 10.1002/pmic.201200562. [DOI] [PubMed] [Google Scholar]
- 96.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toth B, Nieuwland R, Liebhardt S, Ditsch N, Steinig K, Stieber P, Rank A, Gohring P, Thaler CJ, Friese K, Bauerfeind I. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008;28(2A):1107–1112. [PubMed] [Google Scholar]
- 98.Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, Czupryna A, Szczepanik A. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer Immunol Immunother. 2010;59(6):841–850. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim HK, Song KS, Park YS, Kang YH, Lee YJ, Lee KR, Ryu KW, Bae JM, Kim S. Elevated levels of circulating platelet microparticles, VEGF, IL-6 and RANTES in patients with gastric cancer: possible role of a metastasis predictor. Eur J Cancer. 2003;39(2):184–191. doi: 10.1016/s0959-8049(02)00596-8. [DOI] [PubMed] [Google Scholar]
- 100.Helley D, Banu E, Bouziane A, Banu A, Scotte F, Fischer AM, Oudard S. Platelet microparticles: a potential predictive factor of survival in hormone-refractory prostate cancer patients treated with docetaxel-based chemotherapy. Eur Urol. 2009;56(3):479–484. doi: 10.1016/j.eururo.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 101.Fleitas T, Martinez-Sales V, Vila V, Reganon E, Mesado D, Martin M, Gomez-Codina J, Montalar J, Reynes G. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7(10):e47365. doi: 10.1371/journal.pone.0047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reynes G, Vila V, Fleitas T, Reganon E, Font de Mora J, Jorda M, Martinez-Sales V. Circulating endothelial cells and procoagulant microparticles in patients with glioblastoma: prognostic value. PLoS One. 2013;8(7):e69034. doi: 10.1371/journal.pone.0069034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shao H, Chung J, Balaj L, Charest A, Bigner DD, Carter BS, Hochberg FH, Breakefield XO, Weissleder R, Lee H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18(12):1835–1840. doi: 10.1038/nm.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thaler J, Ay C, Mackman N, Metz-Schimmerl S, Stift J, Kaider A, Mullauer L, Gnant M, Scheithauer W, Pabinger I. Microparticle-associated tissue factor activity in patients with pancreatic cancer: correlation with clinicopathological features. Eur J Clin Investig. 2013;43(3):277–285. doi: 10.1111/eci.12042. [DOI] [PubMed] [Google Scholar]
- 106.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 107.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30(5):450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 108.Janowska-Wieczorek A, Majka M, Kijowski J, Baj-Krzyworzeka M, Reca R, Turner AR, Ratajczak J, Emerson SG, Kowalska MA, Ratajczak MZ. Platelet-derived microparticles bind to hematopoietic stem/progenitor cells and enhance their engraftment. Blood. 2001;98(10):3143–3149. doi: 10.1182/blood.v98.10.3143. [DOI] [PubMed] [Google Scholar]
- 109.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161(8):4382–4387. [PubMed] [Google Scholar]
- 110.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63(15):4331–4337. [PubMed] [Google Scholar]
- 111.Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, Zhang G, Feng ZH, Ye D, Huang B. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 112.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51(4):691–743. [PubMed] [Google Scholar]
- 113.Gabizon A, Tzemach D, Mak L, Bronstein M, Horowitz AT. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J Drug Target. 2002;10(7):539–548. doi: 10.1080/1061186021000072447. [DOI] [PubMed] [Google Scholar]
- 114.Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31(6 Suppl 13):196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 115.Toledano Furman NE, Lupu Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, Baruch L, Machluf M. Reconstructed stem cell nano-ghosts: a natural tumor targeting platform. Nano Lett. 2013 doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]