Abstract
Cancer-associated fibroblasts (CAFs) play an important role in tumor initiation and progression. The aim of this study is to explore the role of 2 CAF markers, fibroblast activated protein (FAP) and α-smooth muscle actin (αSMA), in patients with epithelial ovarian cancer (EOC) post-neoadjuvant chemotherapy. Sixty-six patients with the diagnosis of EOC treated with debulking surgery after neoadjuvant therapy were retrieved from the archives. Immunohistochemistry for FAP and αSMA antibodies were performed on paraffin-embedded tissue. Fisher’s exact test was performed to test the association between FAP and αSMA expression and disease status. Kaplan–Meier method with log-rank test was used to check the survival difference between different FAP tumor/stroma expressions. FAP stromapos. expression was strongly associated with higher recurrences rate [OR: 15.95; 95 % CI: 1.521–835.206; p = 0.0072]. Cases with combined FAP stromapos and FAP tumorneg had higher death rate [OR: 4.845; 95 % CI: 1.53–16.61; p = 0.0046] and higher recurrence rate [OR: 5.12; 95 % CI: 0.91–54.42; p = 0.0487] compared to all the others. Cases with combined FAP stromaneg and FAP tumorneg were more likely to have lower recurrence rates [OR: 0.086; 95 % CI: 0.001–0.997; p = 0.0248]. αSMA was expressed by tumor-associated stroma in 95 % of cases and by tumor cells in 9 % of cases. No statistical power was found for αSMA and disease status. Our data indicate that FAP plays an important role in predicting tumor aggressiveness in patients with EOC post-neoadjuvant therapy, and its frequent expression in this malignancy implicates that FAP targeted therapy could be a very attractive strategy.
Keywords: Cancer-associated fibroblasts (CAF), Epithelial ovarian cancer (EOC), Neoadjuvant therapy, Disease outcome
Introduction
Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer, representing 80–90 % of all malignant ovarian tumors. It is the leading cause of death in the United States in women diagnosed with gynecologic malignancies, with 21,990 new cases and 15,460 women estimated to have died of ovarian cancer in 2011 [1]. The high mortality rate is mainly due to advanced stage disease at initial diagnosis. Primary tumor debulking (cytoreduction) followed by chemotherapy is considered the standard of care for patients with advanced stage epithelial ovarian cancer (EOC) [2]. As an alternative to this practice, some authors have investigated the use of neoadjuvant chemotherapy followed by debulking surgery. Despite continuous debates about the value of this alternative approach, a recent randomized clinical trial has shown that survival after neoadjuvant platinum-based chemotherapy followed by debulking surgery (so-called interval debulking surgery) is similar to survival with the standard approach of primary debulking surgery followed by chemotherapy in women with advanced stage (stage IIIC and IV) EOC [3].
Historically, studies on the underlying biology of EOC have focused on the epithelial component of tumors. However, there is increasing awareness of the necessity to understand a tumor within the context of its surroundings, so called “tumor microenvironment”. The stromal component of tumor usually constitutes a high percentage of tumor volume, especially in malignancies treated with neoadjuvant chemotherapy [4, 5]. The tumor microenvironment is vital for tumor survival, growth, proliferation, metastasis, and even progression. Fibroblast stromal cells acquire perpetually activated phenotypes that are identified by the expression of alpha-smooth muscle actin (α-SMA) and fibroblast activation protein (FAP) [6]. They are also known as tumor-associated fibroblasts (TAF), cancer-associated fibroblasts (CAF) and reactive stroma. Recently, studies showed that CAF contributed to ovarian cancer metastasis by promoting angiogenesis, lymphangiogenesis and tumor invasion [7, 8]. Furthermore, FAP silenced TAFs undergo cell cycle arrest. In consequence, targeting TAFs significantly inhibited the tumorigenicity of breast and prostate xenografts [9], suggesting that FAP is an important regulator of tumor microenvironment and hence tumor formation [10]. Recently, by studying a murine breast cancer model, Liao et al. demonstrated that cancer associated fibroblasts promoted tumor growth and metastasis by modulating of the immune polarization in the tumor immune microenvironment. Therefore, elimination of cancer associated fibroblasts in vivo by a DNA vaccine targeted to FAP results in a shift of the immune microenvironment from Th2 to Th1 polarization The vaccine also improved antimetastatic effects of doxorubin chemotherapy [11].
Residual tumor disease and tumor stage are the two most important factors to predict disease outcome in patients with EOC treated by the conventional tumor debulking followed by chemotherapy [4]. However, this is not the case in patients treated with neoadjuvant therapy. Therefore, in the present study we aimed at exploring the expression of α-SMA and FAP in debulking specimen from patients with EOC after being treated with neoadjuvant chemotherapy and defining their role in predicting disease outcomes.
Materials and Methods
Patient Population
Patients with advanced stage EOC treated with neoadjuvant chemotherapy followed by debulking surgery between January 2002 and December 2011 were retrospectively identified via medical records and pathology reports from the University of Southern California and Oregon Health & Science University. The initial diagnosis of ovarian cancer was made by core biopsy of omental mass or cytology of ascitic fluid. The initial diagnosis only mentioned that tumors are from mullerian origin and high grade, without mentioning the histologic type. Due to the lack of information on histologic subtypes on the initial diagnostic tissue/fluid, histology was not evaluated for its statistical value. All patients had imaging studies and serum cancer antigen-125 (CA-125) levels performed. CA125 levels ranged from 44 to 46,501 U/ml. Once the diagnosis had been made, the patient was given carboplatin AUC 5 and paclitaxel 175 mg/m2 for 3 cycles. Afterwards, the patients were reassessed with CT scan and serum CA-125 levels. If tumor burden was reduced based on the CA-125 levels and imaging, the patient underwent cytoreduction and another 3 or 4 cycles of chemotherapy were administered after surgery. In our series all patients underwent surgery. Hematoxylin and eosin slides from the debulking surgery were retrieved from the pathology archives and the clinical and follow-up data were retrieved form medical records. Because as already known that chemotherapy can deeply affect tumor cell morphology and grading, the histologic subtype and grade of the tumor on the debulking specimen were not recorded for our study [4, 5].
Sixty-six patients were available for evaluation. Pathology archives and medical records were searched for the following; (1) time of initial diagnosis, (2) disease status at last follow-up after last cycle of chemotherapy and (3) disease status [alive with evidence of disease (AWED), alive no evidence of disease (ANED) and dead of disease (DOD)] at last follow-up after the initial diagnosis. The end point of overall survival (OS) was tumor-related death. The OS was calculated from the time of the last chemotherapy cycle to endpoints of time of death at last follow-up, respectively.
Immunohistochemistry
Sixty-six EOC cases were evaluated for immunohistochemical (IHC) analysis. Evaluation of hematoxylin and eosin (H&E) stained sections was performed by light microscopy to evaluate the presence of the tumor. Five normal ovaries were included as controls using examination of H&E stained sections to confirm a normal histology. For immunohistochemical analysis, 4 μm thick sections were deparaffinized with xylene, and washed with ethanol. Sections were cooled for 20 min then incubated 10 min with 3 % H2O2 to quench endogenous peroxidase activity. Blocking was performed using serum-free protein block, Dakocytomation (Carpenteria, CA), for 30 min. The sections were pretreated with an EDTA buffer saline solution, microwaved for 20 min, and then incubated with FAP α (LS-A8023; polyclonal, 1:100 dilution, Life Span BioSciences, WA-USA) and α smooth muscle actin (α-SMA; monoclonal, ready to use, Leica microsystem, Illinois, USA) for 1 h at room temperature. The diaminobenzidine complex was used as a chromogen. Normal skin and colon cancer were used as positive controls for FAP-α and αSMA. Negative control slides lacking primary antibody expression were included in all assays. In addition, 5 normal ovaries and fallopian tubes were stained for both α-SMA and FAP.
Study Design
Two pathologists (PMF, DS) evaluated the FAP and αSMA expression on a whole slide using a double-headed microscope. The cancer associated fibroblasts were defined morphologically as large spindle shaped meschenymal cells [12] and on IHC as positive for FAP-α and αSMA as previously defined [13, 14]. This evaluation was done twice, separated by a 1-month period. The percentage was assessed as follows: 0 %, <10 %, 11–50 %, 51–100 % and the intensity as 0, weak (1+), moderate (2+) and strong (3+). The evaluation was done on 10 fields (40×). Because the percentage did not change between fields, only the intensity was considered for statistical evaluation. The score of the first assessment and the second assessment was reviewed and when there was a discrepancy in scoring, a consensus was reached.
Statistical Analysis
Statistical analyses were performed by R (http://www.r-project.org/). The Cohen’s kappa statistic was used to measure agreement among the mean values of intensity across the two assessments on 10 fields. Kappa values <0.00 indicated no agreement, 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as near perfect agreement. The clinical parameters used for modeling were age, recurrence, and disease status. Overall survival (OS) was measured from the time of last chemotherapy to the time of last visit or time of death. To test the association between FAP as well as αSMA intensity and clinical parameters, Fisher’s exact test was performed for categorical parameters, and logistic regression model was performed for the continuous ones. Kaplan–Meier method with log-rank test was used to calculate the cumulative survival time and survival difference between patients with FAP and αSMA expressions. All reported p values are two sided. P values were considered significant if <0.05.
Results
Patient Population
The clinical and pathological features of the 66 cases are summarized in Table 1. The age of patients ranged from 44 to 87 years (median 57 years). The histologic subytpes were not mentioned in the original surgical or cytologic report and therefore was not taken in consideration in this study. The follow-up period ranged from (0.12 to 9.69), median is 3.4 years. On follow-up, 14/66 (21.21 %) of patients had recurrent disease; 32/66 (48.48 %) had no recurrence, and 20/66 (30.3 %) had persistent disease. At last follow-up 19/66 (28.79 %) were AWED, 19/66 (22.79 %) were ANED and 28/66 (42.42 %) were DOD.
Table 1.
Clinical and pathologic features of 66 patients
| # cases (%) | |
|---|---|
| No. of evaluable patients | 66 |
| Age | |
| Median | 57 |
| Range | (40, 87) |
| Disease status | |
| Alive | 38 (57.58) |
| DOD | 28 (42.42) |
| Recurrence | |
| N | 14 (21.21) |
| Y | 32 (48.48) |
| Persistent | 20 (30.3) |
| FAP tumor | |
| Negative (−) | 33 (50) |
| Positive (+) | 33 (50) |
| FAP stroma | |
| Negative (−) | 9 (13.64) |
| Positive (+) | 57 (86.36) |
| Combination of FAP stroma and tumor | |
| FAP stroma+ / FAP tumor+ | 31 (46.97) |
| FAP stroma− / FAP tumor− | 7 (10.61) |
| FAP stroma+ / FAP tumor− | 26 (39.39) |
| FAP stroma− / FAP tumor+ | 2 (3.03) |
We didn’t find any association between FAP tumor/stroma and CA125 levels. We first performed Pearson Correlation test by treating CA125 and FAP struma as continuous variable. The resulting pvalue is 0.7794. Then we divided CA125 levels into two groups with the cut off of 4,500 [aritis A] and performed fisher’s exact test to check the association between FAP tumor and CA125 level. The resulting pvalue is 0.74.
FAP Expression and Its Association with Disease Outcome
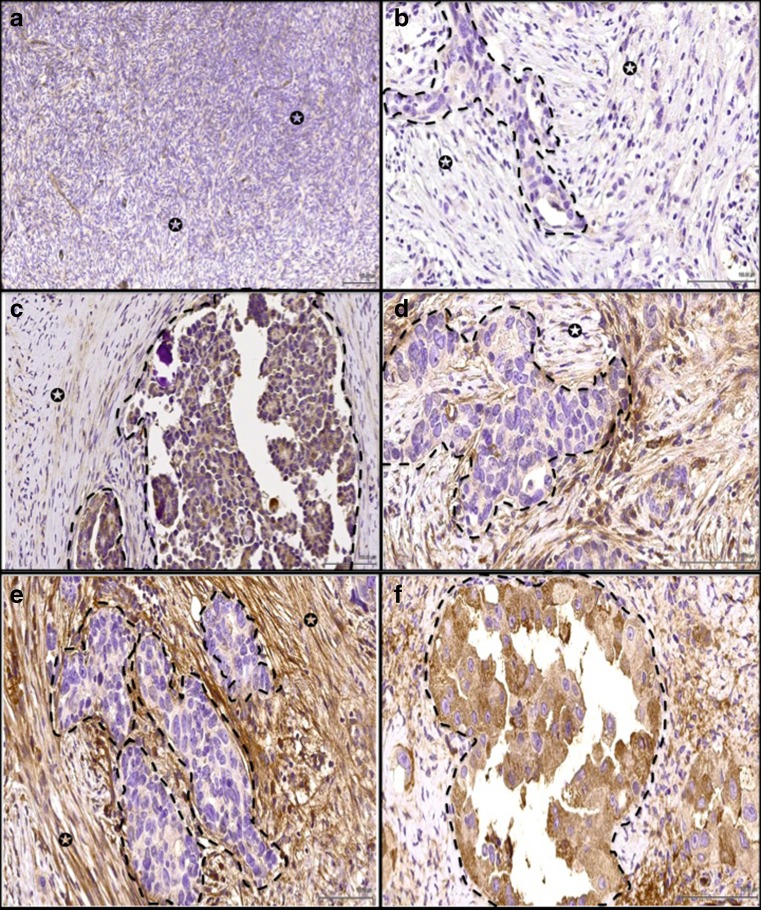
FAP expression was evaluated in normal ovarian and fallopian specimens. FAP was negative in 4/5 normal ovaries and very weakly positive in 1/5 cases (Fig. 1a). The surface epithelium was weakly positive in 1/3 normal ovaries and negative in 2/3 cases. FAP was negative in all epithelial and stromal cells of normal fallopian tube specimens examined (n = 3). FAP was expressed in the tumor-associated stroma of 57/66 (86.36 %) cases and in tumor cells of 33/66 (50 %) cases (Fig. 1b–f). There was a substantial agreement between the first and second assessments (k value of 0.742).
Fig. 1.
a Normal ovary stained with FAP showed stromal cells negative for FAP (arrow indicate stroma). Example of ovarian cancer stained for FAP antibody where (arrow indicates stromal cells, dotted lines highlight the cancer cells). In (b), the stromal cells showed negative expression; c Weak expression. d Moderate expression and e Strong expression. Ovarian cancer cells were strong positive (f). ×40
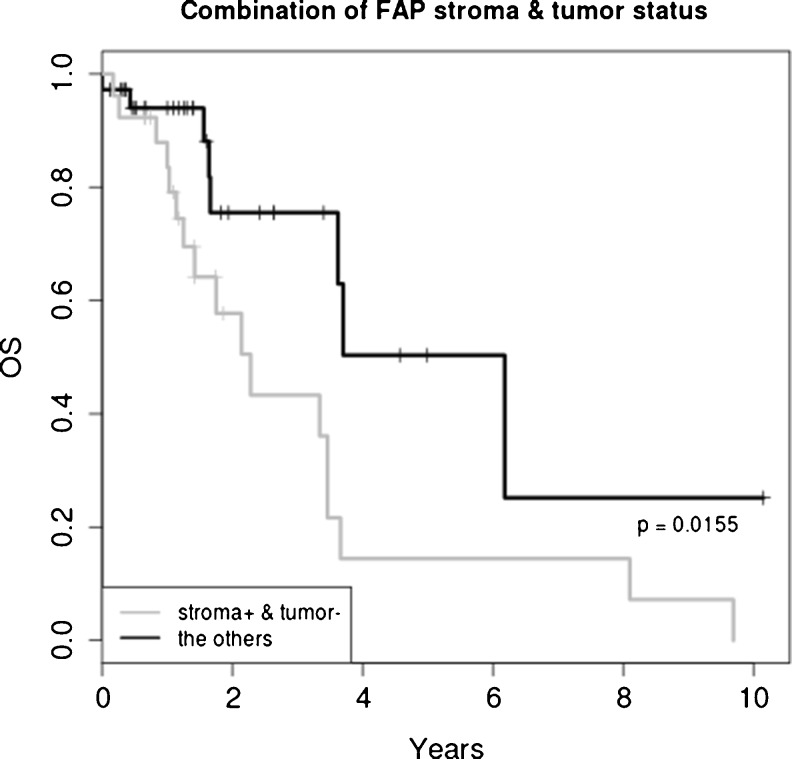
FAP expressions by tumor-associated stroma and by tumor cells themselves are illustrated in Table 2. The recurrence rates were 77.5 %, 66.7 % and 57.8 % for FAP stromapos, FAP tumorpos, and FAP tumorneg respectively. FAP expression in tumor-associated stroma was strongly associated with higher recurrence rates; Cases with FAP stromapos were 15.95 times more likely to recur in comparison to those not expressing FAP [OR: 15.95; 95 % CI: 1.521–835.206; p = 0.0072]. The death rates were 43.9 %, 24.2 % and 65.4 % for FAP stromapos, FAP tumorpos and for FAP stromapos and FAP tumorneg respectively. However, FAP stromapos did not have an association with OS (p = 0.394). When we analyzed the combination of expression of FAP by the stroma and by tumor cells, we found that cases with FAP stromapos and FAP tumorneg had higher death rate [OR: 4.845; 95 % CI: 1.53–16.61; p = 0.0046] and recurrence rate [OR: 5.12; 95 % CI: 0.91–54.42; p = 0.0487] compared to all the others. Also, cases with FAP stromapos and FAP tumorneg were more likely to have shorter OS (p = 0.0155). The 5 year survival probability for FAP stromapos with FAP tumorneg was 14.4%and that of all the others was 50.3 %. Finally, cases with FAP stromaneg and FAP tumorneg were more likely to have lower recurrence rates [OR: 0.086; 95 % CI: 0.001–0.997; p = 0.0248] (Fig. 2).
Table 2.
The association of FAP expression and recurrence/disease status
| P value | Odds ratio | CI for odds ratio | |
|---|---|---|---|
| FAP stroma+ | |||
| Disease statusa | 0.3564 | 2.4870 | 0.288–21.586 |
| Recurrence | 0.0072 | 15.9500 | 1.521–835.206 |
| FAP stroma+ and FAP tumor− | |||
| Disease status | 0.0046 | 4.8450 | 1.525–16.613 |
| Recurrence | 0.0487 | 5.1200 | 0.91–54.415 |
| FAP stroma− and FAP tumor− | |||
| Disease status | 1.0000 | 1.0197 | 0.137–6.624 |
| Recurrence | 0.0248 | 0.0861 | 0.002–0.997 |
aDisease status: alive versus DOD
Fig. 2.
Overall survival analysis showed that cases with FAP stromapos and FAP tumorneg were more likely to have shorter OS (p = 0.0155)
αSMA Expression and Outcome
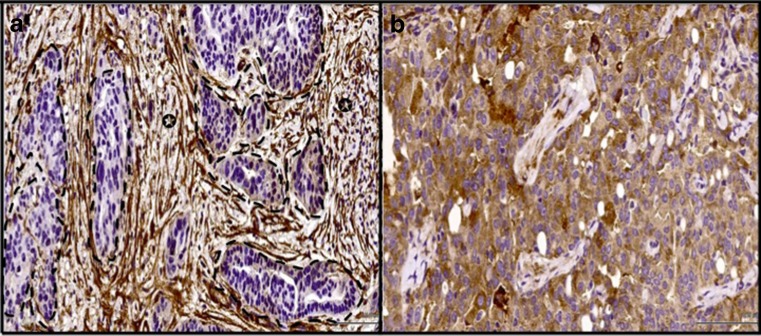
αSMA was negative in all 5/5 normal ovarian stroma and in 3/3 surface ovarian epithelium. All 3 cases of normal fallopian tube epithelium were negative for αSMA. αSMA was expressed by tumor-associated stroma in 63/66 (95.4 %) of cases and by tumor cells in 6/66 (9 %) of cases (Fig. 3a, b). There was a near perfect agreement between the first and second assessments (k value of 0.9). Due to the high percentage of cases with positive stromal expression for αSMA, there was no statistical power for αSMA association with disease outcomes in our analysis.
Fig. 3.
a Example of ovarian cancer stained for αSMA antibody where stromal cells exhibited strong positivity (arrow indicates stromal cells, dotted lines highlight the cancer cells). For b ovarian cancer cells were strong positive for αSMA (×40)
Discussion
Cancer activated fibroblasts (CAFs) can directly promote tumorigenesis through multiple mechanisms, including angiogenesis, proliferation, invasion, survival, and immune suppression [5–8]. These effects are mediated through the expression and secretion of numerous growth factors such as transforming growth factor β (TGFβ), basic fibroblastic growth factor (bFGF), VEGF, and interleukin (IL8) [10, 15]. Also these effects are established trough modulating the rafficking, differentiation status, and function of inflammatory cells in the tumor microenvironment [16, 17]. In addition, CAFs were shown to affect the sensitivity of tumor cells to chemotherapy and radiation therapy [18]. Fibroblast activated protein (FAP) and alpha smooth muscle actin (αSMA) are considered markers for cancer activated fibroblasts (CAFs) in many tumor types. FAP/seprase belongs to the family of plasma membrane-bound serine proteinases [19]. It is located at 2q23 and possesses gelatinolytic and collagenolytic activity as well as dipeptidyl peptidase activity, which have been implicated in matrix digestion and invasion [20]. FAP is expressed in reactive cancer-associated fibrosis and granulation tissue in healing wounds while absent in normal adult tissues, and thus is a highly specific marker of CAFs [10, 19, 20]. Mesenchymal stem cells derived from human bone marrow are a source of TAFs in many tumor models and can express FAP. Moreover, this marker is upregulated when mesenchymal stem cells are recruited into growing tumors [21, 22]. Tumor-associated stromal expression of FAP was found to be associated with more aggressive disease progression, and potential development of metastasis, recurrence, and death in colon and pancreatic cancers [23–25]. Previous studies in ovarian cancers treated with standard surgery followed by chemotherapy showed an association of FAP with advanced stage disease, lymph node metastasis, omental involvement, lymphovascular disease, and increased angiogenesis, but there is lack of data on the association of FAP with patient outcomes [26]. In our series of 66 patients treated with neoadjuvant therapy followed by debulking surgery, FAP was expressed in 86 % of cases, other study showed FAP expression in 91 % of chemonaive ovarian cancer [26]. Furthermore, we found that FAP expression by tumor-associated stroma was associated with higher recurrence rates. Also,FAP stromaneg/ FAP tumorneg cases were less likely to recur and FAP stromapo/FAP tumorng cases were more likely to have shorter OS in comparison to other possible combinations. The significance of our finding is novel and it should be explored further. The origins and function of CAF marker expression in tumor epithelial cells is not clear. It has been proposed that some CAFs may originate from epithelial cells that transdifferentiate to give rise to tumor stroma through an epithelial-to-mesenchymal transition [27]. Whether the positive tumor epithelial cells we observed are CAFs in evolution is unclear, and further in vitro and in vivo studies are warranted.
This is the first study, to our knowledge, to systematically examine the expression of CAF markers, FAP and αSMA, in the tumor and stromal cells of neoadjuvant EOC cases in relation to clinico-pathological characteristics. Despite evidence that FAP expression by tumor-associated stroma indicated worse prognosis in colon, pancreatic and ovarian cancers, it has been shown that FAP expression reduced tumorigenicity in mouse models of melanoma and is also associated with longer survival in patients with invasive breast carcinoma [28, 29]. These conflicting observations suggest that the physiologic responses to FAP may depend not only on the in vivo tumor microenvironment but also on the different microenvironments of FAP expression and even the somatic genetic aberrations found in tumor epithelium.
We also evaluated αSMA expression in this series of EOC cases. This marker has previously been found to be associated with tumor stage, and lymph node and omental metastasis in patients with EOC treated with standard surgery followed by chemotherapy, but again, no data on the association between αSMA expression and tumor outcome has been reported [26]. In our series, the high percentage of cases expressing αSMA limited our ability to perform statistical analyses. Nearly every tumor treated with neoadjuvant chemotherapy expressed αSMA, a much greater frequency of positive cases than a previous report which only found around 20 % of advanced stage, chemonaïve EOCs expressing αSMA [26]. The point of divergence between these two studies is neoadjuvant chemotherapy, either its inclusion or exclusion in patient management. Our result suggests that chemotherapy strongly promotes a reactive CAF phenotype in EOC. This observation is particularly significant as stromal cells can promote resistance to many anti-tumor therapies [30], and chemoresistant recurrent disease is a major clinical challenge for both physicians and patients alike in the management of EOC. Therefore, it is possible that the development of chemoresistance is in part driven by changes in the tumor stroma of EOCs, and that the co-targeting of tumor epithelium and stroma in advanced EOCs could potentially reduce rates of EOC-associated mortality by inhibiting the development of chemoresistance.
The tumor microenvironment has only recently emerged as a novel chemotherapeutic target in the treatment of cancer patients. The absence of FAP in normal ovaries and its presence in ovarian cancer makes FAP a very attractive therapeutic target in EOC. Prior research on small molecular inhibition of FAP seemed ineffective, but recent studies targeting FAP using a novel FAP-activated prodrug, a drug that alters the activation of a cytotoxic compound in the tumor stroma, have reported promising results [10, 31]. Even though extensive preclinical testing is required before FAP targeting can be implemented in clinical trials, early results are extremely encouraging [10, 31].
In summary, as with other malignancies, positive expression of stromal FAP in EOC status post neoadjuvant chemotherapy may serve as a negative prognostic marker for clinical outcome. we believe that FAP inhibitors should be further investigated, especially in light of the limited therapeutic options available in the treatment of epithelial ovarian carcinomas.
Acknowledgments
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.The National Cancer Institute’s Surveillance, Epidemiology and End Results Program. The Center for Disease Control and Prevention’s National Program of Cancer Registries 2012. SEER.cancer.gov
- 2.du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference. Ann Oncol. 2005;16(Suppl 8):viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG, Lyness RW, Atkinson RJ, Dobbs SP, Harley I, McClelland HR, et al. Morphological effects of chemotherapy on ovarian carcinoma. J Clin Pathol. 2002;55:27–31. doi: 10.1136/jcp.55.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petoit JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::AID-CNCR2820731105>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 7.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts are a rate-limiting determinant for tumour progression. Semin Cell Dev Biol. 2012;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otsman A, Augesten M. Cancer-associated fibroblasts and tumor growth-bystanders turning into key players. Curr Opin Genet Dev. 2009;19:67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Lai D, Ma L, Wang F. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int J Oncol. 2012;41:541–550. doi: 10.3892/ijo.2012.1475. [DOI] [PubMed] [Google Scholar]
- 10.Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst. 2012;104:1320–1334. doi: 10.1093/jnci/djs336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 13.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scalan MJ, Raj BKM, Calvo B, Garin-Chelsa P, Sanz-Moncasi MP, Healey JH, et al. Molecular cloning of fibroblast activation protein α, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci U S A. 1994;91:5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller MM, Fusenig NE. Friends or foes-bipolar effects of the tumor stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 16.Servais C, Erez N (2013) From sentinel cells to inflammatory culprits: cancer-associated fibroblasts in tumour-related inflammation. J Pathol 229:198–207 [DOI] [PubMed]
- 17.Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle. 2009;10:1461–1465. doi: 10.4161/cc.8.10.8557. [DOI] [PubMed] [Google Scholar]
- 18.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug update. J Clin Invest. 2006;116:1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien P, O’Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784:1130–1145. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, et al. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010;277:1126–1144. doi: 10.1111/j.1742-4658.2009.07526.x. [DOI] [PubMed] [Google Scholar]
- 21.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge Y, Zhan F, Barlogie B, Epstein J, Shaughnessy J, Jr, Yaccoby S. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 24.Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18:840–846. doi: 10.3748/wjg.v18.i8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13:1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101:830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monsky WL, Lin CY, Aoyama A, Kelly T, Akiyama SK, Mueller SC, et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- 29.Ariga N, Sato E, Ohuchi N, Nagura H, Ohtani H. Stromal expression of fibroblast activation protein/seprase, a cell membrane serine proteinase and gelatinase, is associated with longer survival in patients with invasive ductal carcinoma of breast. Int J Cancer. 2001;95:67–72. doi: 10.1002/1097-0215(20010120)95:1<67::AID-IJC1012>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]