Abstract
Studies on interaction of tumor cells with extracellular matrix (ECM) components showed increased extracellular protease activity mediated by the family of matrix metalloproteinases (MMPs). Here we studied the effect of human breast cancer cell line MCF-7-laminin (LM) interaction on MMPs and the underlying signaling pathways. Culturing of MCF-7 cells on LM coated surface upregulated MMP-9 expression as well as reduced tissue inhibitor of metalloproteinases-1 (TIMP-1) expression. LM induced MMP-9 expression is abrogated by the blockade of α2 integrin. Inhibitor studies indicate possible involvement of phosphatidyl-inositol-3-kinase (PI3K), extracellular signal regulated kinase (ERK) and nuclear factor-kappaB (NF-κB) in LM induced signaling. LM treatment also enhanced phosphorylation of FAK (focal adhesion kinase), PI3K, ERK; nuclear translocation of ERK, pERK, NF-κB and cell migration. Our findings indicate that, binding of MCF-7 cells to LM, possibly via α2β1 integrin, induces signaling involving FAK, PI3K, ERK, NF-κB followed by upregulation of MMP-9 and cell migration.
Keywords: MCF-7, Signaling, ECM, Laminin, Integrin, MMP-9
Introduction
Breast cancer is the most commonly occurring cancer in women, comprising almost one third of all malignancies in females [1]. In most cases, it is not the primary tumor, but its metastases at distant sites are the main cause of death [2]. A major requirement of malignant tumor cells is the ability to invade host tissues and establish distant metastatic foci [3]. For this purpose malignant cells have to penetrate vascular basement membrane several times, involving three major steps in each occasion: i) attachment of tumor cells to basement membrane through laminin, ii) production of matrix degrading proteolytic enzymes, iii) migration through the basement membrane [4]. The major constituents of all basement membranes are collagen IV and laminin, which both exist as multiple isoforms and self assemble to form irregular network. Basement membranes are connected to cells by several cell surface receptors of the integrin family, which bind preferentially to laminins and collagen IV, and via some lectin-type interactions [5]. Previous studies suggest that interaction of tumor cells to laminin can induce the collagenolytic dissolution of the basement membrane [4].
Basement membrane degradation is facilitated by the family of enzymes known as matrix metalloproteinases (MMPs), which are Zn+2 ion dependent endopeptidases [6]. This family comprises 25 related, yet distinct vertebrate gene products, of which 24 are found in mammals [6]. Although MMPs differ in their substrate specificity, some of them show overlapping specificity [7]. Gelatinase A (MMP-2) and gelatinase B (MMP-9) readily digest the denatured collagens, gelatins. Both MMP-2 and MMP-9 expression and activity are important for experimental metastasis [8]. Culturing of cells in presence of intact matrix proteins such as fibronectin or laminin or matrix-derived peptides was shown to influence metalloproteinase expression [9, 10]. However, some of our previous works have showed that, different cancer cells exhibit more enhanced gelatinolytic activity upon interaction with intact matrix proteins rather than with their small peptide derivative indicating multivalent ligand receptor interaction is more effective [8, 11, 12].
Activation of signal transduction pathways induced by matrix proteins may be altered when normal tissue becomes neoplastic. Laminin exerts its signaling via a non-integrin 67 kDa laminin receptor and via integrins [13]. Out of the several laminin isoforms described in mammals, laminin-1 expression seems to be largely limited to epithelial basement membranes [14]. The classic laminin-1, which is also called laminin-111 or LM-111, is a cross-shaped glycoprotein comprising α1, β1 and γ1 chains and is the most important isoform in early development, yet remains present as a major epithelial laminin in some adult tissues [14, 15]. Culture of human cervical cancer cells SiHa on laminin-1 coated surface has been reported to express and activate MMP-9 by possible involvement of α2β1 integrin receptor and participation of focal adhesion kinase (FAK), integrin linked kinase (ILK), phosphatidylinositol-3-kinase (PI3K), extracellular signal regulated kinase (ERK) followed by increased DNA-binding activity of NF-κB and Ap1 and subsequent stimulation of MMP-9 gene expression [16]. In the present communication we studied the response of human breast cancer cell line MCF-7 on laminin-111 coated surface and the underlying signaling mechanism.
Materials
MCF-7 cell line was purchased from National Centre for Cell Sciences (NCCS), Pune, India. Minimum essential medium (MEM), Trypsin, Gentamycin, Fetal Bovine Serum (FBS) were purchased from GIBCO™-Invitrogen. Laminin-111 (900 kDa), Protease Inhibitor Cocktail Tablets (complete, mini, EDTA-free) were purchased from Roche, Germany. Gelatin Sepharose 4B beads was purchased from GE Healthcare Biosciences AB, Uppsala, Sweden. Gelatin powder was purchased from Sigma-Aldrich. All integrin blocking antibodies were purchased from Gibco-BRL, except anti-integrin α5, which was from Santa Cruz Biotechnology, Inc. Primary and secondary antibodies were also purchased from Santa Cruz Biotechnology, Inc. Chemiluminescent substrate SuperSignal West Femto was purchased from Pierce, Thermo Fisher Scientific Inc. Inhibitors of ERK (PD 98059), PI3K (LY 294002), MEK (U0126), p38 (SB 203580) were purchased from Promega. NF-κB inhibitor (BAY-11-7085) was purchased from Alexis Biochemicals, Lausen, Switzerland. Primers (MMP-9 and G3PDH) were synthesized by Operon, Germany. RNAqueous 4 PCR (Total RNA isolation kit) was purchased from Ambion, Austin, TX, USA. SYBR Green JumpStart™Taq Readymix™ was purchased from Sigma, USA and qPCR 96-well plate was purchased from Eurogentec. Coomassie brilliant blue was purchased from Merck. Immobilon-P Membrane (PVDF) was purchased from Millipore, USA.
Methods
Cell Culture
MCF-7 (human breast cancer cell line) was obtained from National Centre for Cell Sciences (NCCS), Pune. This cell line was grown and maintained in MEM (Minimum Essential Medium), containing 10 % FBS in a 5 % CO2 incubator at 37 °C.
Treatment of Cells With Laminin
MCF-7 cells (300,000 cells/ml) were grown on laminin coated 35 mm petridishes in MEM (Serum Free Culture Media) for overnight (18 h) at 37 °C, 5 % CO2.
Cell Adhesion Assay
To assay the binding capacity of MCF-7 cells to LM, microtitre plate wells were coated with the ligand (5, 10, 20 μg/ml) in triplicate. Rests of the steps were performed as previously described [8].
Gelatin Zymography
The gelatinases were separated from the culture supernatants of control and experimental sets using gelatin sepharose 4B beads with shaking for 2 h at 4 ° C. To assay the gelatinase activity, Gelatin Zymography was performed using a 7.5 % SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) co-polymerized with 0.1 % gelatin as described in earlier report [17] with only modification of 44 h incubation in reaction buffer.
Study the Effects of Cell Signaling Inhibition on MMPs
Initially cells were untreated or treated with anti-α2, anti-α3, anti-α4, anti-α5 integrin antibodies (1 μg/ml) or with chemical inhibitors of PI3K, ERK, MEK, p38 (LY294002, PD98059, U0126, SB203580 respectively, conc. of 50 μM each) in SFCM [8] for 1 h at 37 °C, 5 % CO2. Bay 117085, the NF-κB inhibitor, was treated in 5 and 10 μM concentration [18] for 24 h at 37 °C, 5 % CO2. Then both the control and experimental sets were grown on coated LM (20 μg/ml) surface for overnight in SFCM. The culture supernatant was subjected to gelatin zymography to visualize gelatinase activity.
Whole Cell Extraction
Control and treated cells were collected, washed with ice cold PBS (phosphate buffered saline) and were extracted in lysis buffer (50 mM Tris pH 7.5, 150 mM NaCl, 1 % NP40, 0.1 % SDS, 0.5 % Sodium Deoxycholate, Protease inhibitor cocktail tablets, 1 mM sodium orthovanadate and 1 mM Sodium fluoride) on ice and clarified by centrifugation [19]. The protein concentrations were estimated by Lowry method and equal amount of proteins were loaded for western blot assay.
Immunoblot Assay of Cell Signaling Proteins
Equal amount (100 μg) of protein from whole cell extracts of control and experimental sets were subjected to western blot analysis using primary antibodies [anti-FAK, anti-phospho-FAK (Tyr-397), anti-PI3K (p85), anti-phospho-PI3K (Tyr-508), anti-ERK, anti-phospho-ERK (Thr 202/Tyr 204), anti-TIMP-1; 1 μg/2 ml dilution for each] followed by the respective secondary antibodies (1 μg/200 ml dilution) using methods discussed previously [19]. Blots were reprobed with anti β-tubulin antibody as internal loading control. [ERK: Extracellular signal regulated kinase, TIMP-1: Tissue inhibitor of metalloproteinases-1].
Immunocytochemistry
MCF-7 cells were grown on coverslips in absence and presence of 20 μg/ml laminin for overnight. After PBS wash, cells were fixed with 3.5 % formaldehyde and treated with 0.5 % Triton X-100. 1 % BSA solution was used for blocking non specific sites. Cells were then incubated with primary antibodies against ERK, phospho-ERK, NF-κB (1 μg/ml dilution), washed thrice with PBS and incubated with respective FITC coupled secondary antibody (1 μg/ml dilution in humid chamber). Cells were washed six times with PBS and coverslips were mounted on glass slides and observed under fluorescence microscope (400X).
Wound Healing Assay
MCF-7 cells (300,000 cells/ml) were cultured in a monolayer in absence (−LM) and presence (+LM) of laminin (20 μg/ml, overnight). The monolayer was then scratched with a sterile pipette tip, followed by washing with SFCM to remove cellular debris. The cells were maintained in fresh SFCM and cell migration was observed under microscope and photographed at successive time intervals (0, 6, 22 and 30 h).
Real Time PCR
RNA was extracted from MCF-7 cells, grown with or without coated laminin (20 μg/ml for overnight), using kit of RNAqueous, Ambion, USA. Real-time quantitative RT-PCR, using relative quantitation by the comparative CT method, was used to determine mRNA expression level. Two microliter of cDNA was subjected to real-time quantitative RT-PCR by the real time PCR (ABI-7500, USA) with SYBR Green as a fluorescent reporter using the SYBR Green JumpStart™Taq Readymix™ (Sigma, USA). The specific gene primers (MMP-9 and the internal control gene GAPDH) were used to amplify the target genes in separate reaction tubes. Threshold cycle numbers (CT), of triplicate reactions, were determined using the ABI-7500 software and the mean CT of triplicate reactions was determined. The levels of specific gene expression were normalized to GAPDH levels. The expression level of the target gene modulated can be expressed as 2ΔΔCT fold, where ΔΔCT = ΔCT(sample) − ΔCT(calibrator) and ΔCT is the CT of the housekeeping gene (GAPDH) subtracted from the CT of the target gene. The calibrator used in our experiments is the control MCF-7 cells and the samples are the LM treated MCF-7 cells. The ΔCT value is inversely proportional to the mRNA expression of the samples in this formula. No primer dimers were obtained for either the target genes or GAPDH as assessed by melt curve analysis. The specificity of the products was also confirmed by melt curve analysis. . The PCR cycles in all cases were started with Taq activation at 94 °C for 5 min and followed by final extension of 72 °C for 7 min. The primer sequences are:
hMMP-9: 5′-CGCTACCACCTCGAACTTTG-3′ (forward),
5′GCCATTCACGTCGTCCTTAT-3′-(reverse);
GAPDH: 5′-CGGAGTCAACGGATTTGGTCGTAT- 3′ (forward)
5′-AGCCTTCTCCATGGTGGTGAAGAC- 3′ (reverse).
Conditions used for PCR consisted of 40 cycles for MMP-9 at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 90 s in thermal cycler [19].
Results
LM Enhances Cell Migration, Upregulates MMP-9, Downregulates TIMP-1 in MCF-7 Cells
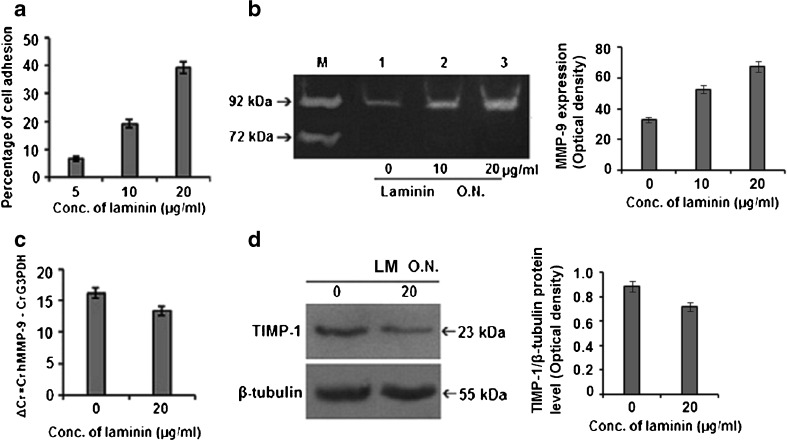
Cell adhesion assay showed that, MCF-7 cells bind efficiently to laminin (LM-111) and percentage of adhesion increases with the increase in concentration of LM (Fig. 1a). Culture of MCF-7 cells in presence of LM showed increase in MMP-9 gelatinolytic activity dose dependently with appreciable upregulation at 20 μg/ml for overnight (O.N.) in the culture supernatant without any appreciable change in MMP-2 activity (Fig. 1b). Quantitative Real-Time RT-PCR analysis of LM treated MCF-7 cells (20 μg/ml for O.N.) indicates increase in expression of MMP-9 mRNA by 2ΔΔCT = 22.809 = 7 folds [where ΔΔCT = ΔCT(sample) − ΔCT(calibrator) = (35.220 − 19.004) − (29.996 − 16.589) = 16.216 − 13.407 = 2.809] than the untreated cells (Fig. 1c). LM at 20 μg/ml for O.N. was shown to downregulate TIMP-1 protein expression in whole cell extracts by western blot assay (Fig. 1d).
Fig. 1.
Effect of laminin on cell adhesion, MMP-9 and TIMP-1 expression: a Graphical representation of cell adhesion assay. b MCF-7 cells were grown in SFCM in absence (lane 1) and presence of 10 μg/ml (lane 2) and 20 μg/ml (lane 3) LM for overnight (O.N.). Culture supernatants were subjected to gelatin zymography. Culture supernatant of HT-1080 cells grown in SFCM for 24 h was used as MMP-9 (92 kDa)/MMP-2 (72 kDa) marker (lane M). c MCF-7 cells were grown in absence (0) and in presence of 20 μg/ml LM coated dish and total RNA of each set was subjected to real time RT-PCR. In the given graph the CT value is inversely proportional to the mRNA expression of the samples. d Untreated and LM-treated cells were extracted and subjected to western blot using primary antibodies against TIMP-1. β-tubulin was used as internal control
LM Enhances Motility of MCF-7 Cells
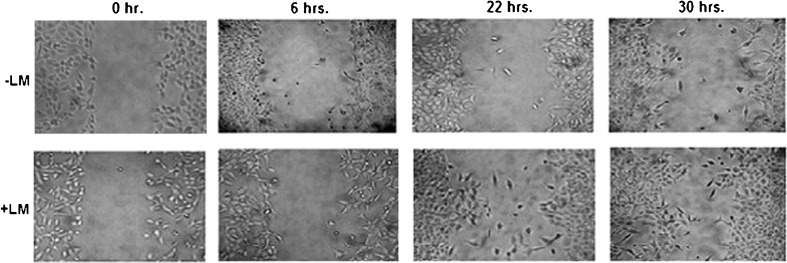
Photographs, taken at different time intervals during wound healing assay, indicate that MCF-7 cells grown on LM coated surface are able to migrate and heal experimentally scratched wounds more efficiently than the cells grown on LM free surface (Fig. 2).
Fig. 2.
Effect of laminin on cell migration: MCF-7 cells were (300,000 cells/ml) treated without (−LM) or with 20 μg/ml LM for overnight (+LM) before creating the scratch wound. Photographs were taken just after the scratch (0 h), and then at 6, 22 and 30 h intervals
Involvement of Integrin in LM Mediated Upregulation of MMP-9
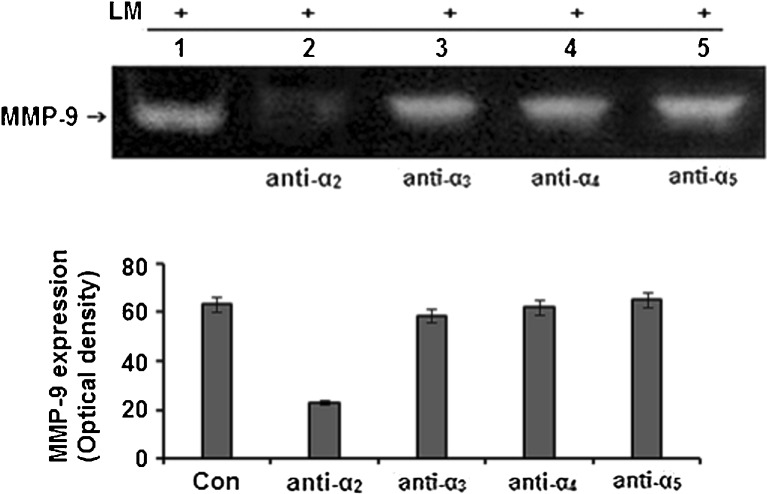
MCF-7 cells, masked with antibodies specific for α2 integrin receptors, showed appreciable downregulation of LM-induced MMP-9 activity when the culture supernatant was subjected to gelatin zymography (Fig. 3).
Fig. 3.
Effect of integrin masking on LM induced MMP-9 secretion: MCF-7 cells (300,000 cells/ml) were treated without (lane 1) or with anti-α2 (lane 2), anti-α3 (lane 3), anti-α4 (lane 4) and anti-α5 (lane 5) integrin antibodies for 1 h and then allowed to grow in presence of 20 μg/ml LM for overnight. The culture supernatants were subjected to gelatin zymography
Involvement of Cell Signaling Molecules in LM Mediated Upregulation of MMP-9
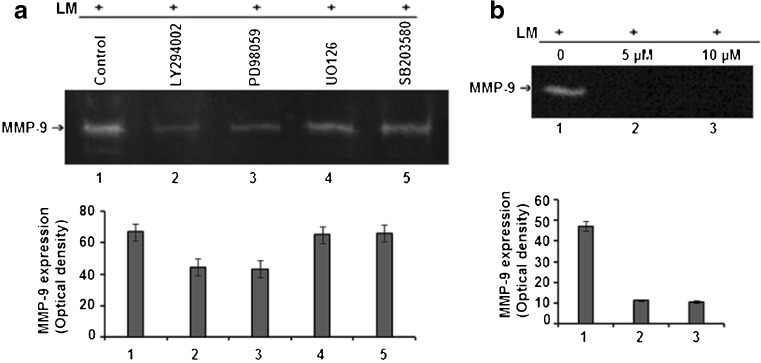
The LM induced gelatinolytic activity of MMP-9 was found to be appreciably reduced by PI3K, ERK inhibitors (Fig. 4a) and NF-κB inhibitor (Fig. 4b), rather than the MEK, p38 inhibitors.
Fig. 4.
Effect of cell signaling inhibitors on LM induced MMP-9 secretion: a MCF-7 cells, untreated (lane 1) or treated with PI3K inhibitor (lane 2), ERK inhibitor (lane 3), MEK inhibitor (lane 4), p38 inhibitor (lane 5) (50 μM each) in SFCM for 1 h, were cultured (300,000 cells/ml) in SFCM in presence of 20 μg/ml LM for overnight. b MCF-7 cells (300,000 cells/ml), untreated (lane 1) or treated with 5 μM (lane 2), 10 μM (lane 3) NF-κB inhibitor BAY-11-7085 (24 h each), were allowed to grow on LM (20 μg/ml) for overnight in SFCM. The culture supernatants in all the above cases were subjected to gelatin zymography
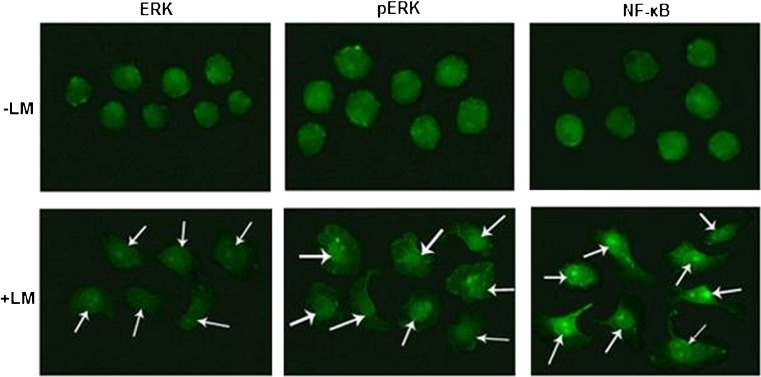
Immunocytochemical Study of Cell Signaling Proteins
LM treatment on MCF-7 cells for overnight induces nuclear translocation of ERK, pERK and NF-κB as visualized by immunocytochemistry (Fig. 5).
Fig. 5.
Immunocytochemical study: MCF-7 cells were treated without (−LM) or with 20 μg/ml laminin (+LM) in SFCM for overnight on coverslips. The cells were then subjected to immunocytochemical analysis with anti-ERK, anti-pERK, anti-NF-κB primary antibodies and respective FITC-coupled secondary antibodies
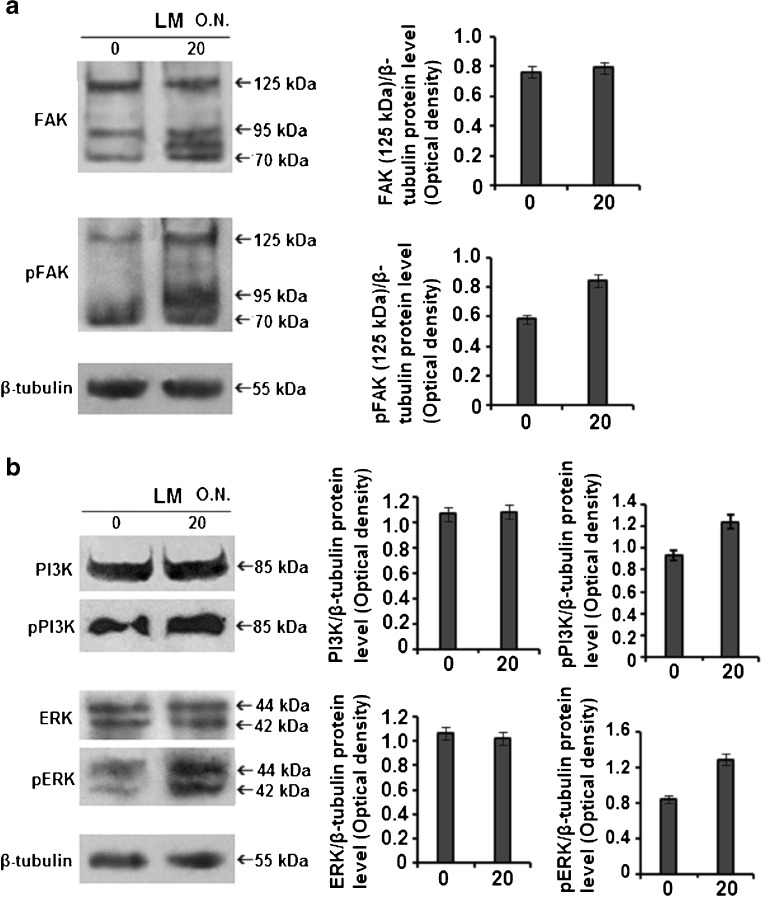
Laminin Mediated Modulation of FAK, PI3K, ERK in MCF-7 Cells
Comparative western blot from whole cell extracts of MCF-7 cells shows that, laminin treatment causes upregulation and phosphorylation, hence activation of FAK (Fig. 6a). When the expression status of ERK, PI3K were studied using same technique, laminin treatment was not found to appreciably change their total protein expression, but considerably enhanced their phosphorylation, hence activation (Fig. 6b).
Fig. 6.
Comparative immunoblot: MCF-7 cells (300,000 cells/ml) were cultured in SFCM in absence (0) and presence of 20 μg/ml LM for overnight (O.N.). Cells were collected, extracted and equal protein (100 μg/lane) was subjected to western blot for FAK, pFAK (a) and PI3K, pPI3K, ERK, pERK (b) expression. β-tubulin was used as internal control
Discussion
Previous studies from our lab on effects of different ECM components on MMP-2 and MMP-9 activities in cancer cell lines indicate that, such responses and the underlying signaling vary according to the type of cancer cells and the type of ECM components used [8, 11, 12]. Human breast cancer cell line MCF-7, which normally secretes pro-MMP-2, was found to exhibit pro-MMP-9 activity and activation of MMP-2 in presence of soluble fibronectin [8]. Upregulation of MMP-9 was observed when human cervical cell line SiHa was cultured on laminin coated petridish [16]. In the present communication, we report that culture of MCF-7 cell line on laminin-coated surface enhances cell adhesion, induces pro-MMP-9 gene transcription and gelatinolysis. Downregulation of TIMP-1 in presence of laminin may contribute to enhanced MMP-9 activity.
To understand the signaling pathway, we initially studied the possible cell surface receptor. Laminin-1 has attachment sites for several integrins including α2β1 integrins [16]. α2 masked MCF-7 cells in our study showed appreciable downregulation of laminin induced MMP-9 expression indicating possible role of α2β1 integrin in mediating this signal. FAK, the intracellular non-receptor protein tyrosine kinase and the key component of focal adhesion complex, was reported to be autophosphorylated in response to binding of β1 integrin to ECM and play critical roles in β1 integrin-dependent signaling [20, 19]. FAK is also a potent regulator of MMP-9 expression and activity [19, 21]. Our study showed laminin-induced phosphorylation of 125 kDa form of FAK (Tyr-397), indicating possible involvement of FAK in LM-mediated signaling cascade in MCF-7 cells. Activation of FAK is coupled to assembly of focal adhesions, which plays major role in cell attachment and migration [22, 23]. In our study, LM treated cells showed enhanced migration in wound healing assay. LM induced enhanced cell migration was reported to involve activation of various signaling molecules such as PI3K, FAK, ERK, NF-κB in different model system [24, 16]. Henceforth we studied these molecules in our system.
Our experiments showed that laminin-induced MMP-9 expression was appreciably decreased by inhibition of ERK and PI3K, both of which are downstream targets of FAK [7]. PI3K signaling cascade has been linked to proliferation, cell survival, cytoskeletal rearrangement etc. [25] all of which regulate cancer progression. In a different carcinoma model PI3K was reported to play pivotal role in upregulation of MMP-9 [26], which seems to be consistent in our system as well. The major autophosphorylation site of FAK (Tyr-397) is one of the binding sites for the SH2 domains of p85 regulatory subunit of PI3K and is responsible for the in vivo association of FAK with PI3K [27]. In resting stage its p85 catalytic subunit binds and inactivates kinase activity of p110, the catalytic subunit of PI3K. Tyr phosphorylation of p85 is very important in relieving inhibitory effect of p85 on p110 [25]. PI3K was found to be phosphorylated at its p85 regulatory subunit hence activated in response to LM treatment in our study. MAPK/ERK pathway is one of the possible downstream targets of PI3K [16]. MAP family kinases are likely to play vital role in signaling processes that regulate several MMPs, including MMP-9 by modulating several transcription factors [28]. Laminin derived peptides have been reported to enhance MMP-9 secretion via ERK pathway in human salivary gland adenoid cystic carcinoma [29]. Laminin-1 treatment was found to enhance ERK phosphorylation and nuclear translocation in MCF-7 cells in our study. The exact function of nuclear ERK itself is not fully clear here, but it can be predicted that nuclear ERK may facilitate the transactivation of MMP-9.
PI3K can also activate IκB kinases (IKKs), which in turn phosphorylate IκB, the NF-κB inhibitor, targeting it for proteosomal degradation. NF-κB, which remains in cytoplasm in IκB bound form, gets activated and enters nucleus to act as transcription factor of several genes [30]. In human cancer cells, NF-κB positively regulates cell survival, proliferation, chemoresistance, angiogenesis, cellular invasion, oncogenesis etc. [31, 32]. In our study appreciable nuclear localization of NF-κB was observed in MCF-7 cells in presence of LM. Previous literatures indicate that, in several cell lines MMP-9 upregulation is dependent on transcriptional modulation of MMP-9 gene, requiring involvement of NF-κB [16, 33, 12, 34]. This is consistent with our finding that LM induced MMP-9 activity level goes down below detection limit in presence of NF-κB inhibitor. Taken together these informations indicate that, NF-κB may be another essential component in LM mediated signaling in MCF-7 cells and possibly involved in upregulating MMP-9 level. Increase in MMP-9 expression and decrease in TIMP-1 expression, which is a negative regulator of MMP-9, in LM-111 treated MCF-7 cells in our study may cumulatively exhibit enhanced gelatinolytic activity of MMP-9.
Acknowledgments
The authors wish to express their thanks to Director, Chittaranjan National Cancer Institute, for academic, financial and infrastructural support.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Richie RC, Swanson JO. Breast cancer: a review of the literature. J Insur Med. 2003;35(2):85–101. [PubMed] [Google Scholar]
- 2.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25(1):45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 4.Turpeenniemi-Hujanen T, Thorgeirsson UP, Rao CN, Liotta LA. Laminin increases the release of type IV collagenase from malignant cells. J Biol Chem. 1986;261(4):1883–1889. [PubMed] [Google Scholar]
- 5.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18(2):123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 6.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26(8):587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal S, Ganguly KK, Moulik S, Chatterjee A. Modulation of MMPs by cell surface integrin receptor alpha5beta1. Anticancer Agents Med Chem. 2012;12(7):726–732. doi: 10.2174/187152012802650183. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci. 2008;82(9–10):467–476. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Stack S, Gray RD, Pizzo SV. Modulation of plasminogen activation and type IV collagenase activity by a synthetic peptide derived from the laminin A chain. Biochemistry. 1991;30(8):2073–2077. doi: 10.1021/bi00222a011. [DOI] [PubMed] [Google Scholar]
- 10.Terranova VP, Williams JE, Liotta LA, Martin GR. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- 11.Maity G, Fahreen S, Banerji A, Roy Choudhury P, Sen T, Dutta A, Chatterjee A. Fibronectin-integrin mediated signaling in human cervical cancer cells (SiHa) Mol Cell Biochem. 2010;336(1–2):65–74. doi: 10.1007/s11010-009-0256-5. [DOI] [PubMed] [Google Scholar]
- 12.Sen T, Dutta A, Maity G, Chatterjee A. Fibronectin induces matrix metalloproteinase-9 (MMP-9) in human laryngeal carcinoma cells by involving multiple signaling pathways. Biochimie. 2010;92(10):1422–1434. doi: 10.1016/j.biochi.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells. Cancer Lett. 2005;223(1):1–10. doi: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Ekblom P, Lonai P, Talts JF. Expression and biological role of laminin-1. Matrix Biol. 2003;22(1):35–47. doi: 10.1016/S0945-053X(03)00015-5. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Fassler R, Hohenester E. Laminin: the crux of basement membrane assembly. J Cell Biol. 2004;164(7):959–963. doi: 10.1083/jcb.200401058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maity G, Sen T, Chatterjee A. Laminin induces matrix metalloproteinase-9 expression and activation in human cervical cancer cell line (SiHa) J Cancer Res Clin Oncol. 2011;137(2):347–357. doi: 10.1007/s00432-010-0892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen T, Moulik S, Dutta A, Choudhury PR, Banerji A, Das S, Roy M, Chatterjee A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009;84(7–8):194–204. doi: 10.1016/j.lfs.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Dutta A, Sen T, Chatterjee A. Culture of K562 human myeloid leukemia cells in presence of fibronectin expresses and secretes MMP-9 in serum-free culture medium. Int J Clin Exp Pathol. 2010;3(3):288–302. [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguly KK, Sen T, Pal S, Biswas J, Chatterjee A. Studies on Focal Adhesion Kinase in human breast cancer cell MDA-MB-231. Adv Biol Chem. 2012;2:29–42. doi: 10.4236/abc.2012.21004. [DOI] [Google Scholar]
- 20.Chen LM, Bailey D, Fernandez-Valle C. Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci. 2000;20(10):3776–3784. doi: 10.1523/JNEUROSCI.20-10-03776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sein TT, Thant AA, Hiraiwa Y, Amin AR, Sohara Y, Liu Y, Matsuda S, Yamamoto T, Hamaguchi M. A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and −9. Oncogene. 2000;19(48):5539–5542. doi: 10.1038/sj.onc.1203932. [DOI] [PubMed] [Google Scholar]
- 22.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7(8):1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 24.Ganguly KK, Pal S, Moulik S, Chatterjee A. Integrins and metastasis. Cell Adh Migr. 2013;7(3):251–261. doi: 10.4161/cam.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, Mills GB. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276(29):27455–27461. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- 26.Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, Chen LZ, Tan HX, Li W, Bi J, Zhang LJ. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol Res. 2009;39(2):177–186. doi: 10.1111/j.1872-034X.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271(42):26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 28.O-charoenrat P, Wongkajornsilp A, Rhys-Evans PH, Eccles SA. Signaling pathways required for matrix metalloproteinase-9 induction by betacellulin in head-and-neck squamous carcinoma cells. Int J Cancer. 2004;111(2):174–183. doi: 10.1002/ijc.20228. [DOI] [PubMed] [Google Scholar]
- 29.Freitas VM, Vilas-Boas VF, Pimenta DC, Loureiro V, Juliano MA, Carvalho MR, Pinheiro JJ, Camargo AC, Moriscot AS, Hoffman MP, Jaeger RG. SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol. 2007;171(1):124–138. doi: 10.2353/ajpath.2007.051264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9(11):601–604. doi: 10.1016/S0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5(4):297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 33.Pal S, Ganguly KK, Chatterjee A. Extracellular matrix protein fibronectin induces matrix metalloproteinases in human prostate adenocarcinoma cells PC-3. Cell Commun Adhes. 2013;20(5):105–114. doi: 10.3109/15419061.2013.833193. [DOI] [PubMed] [Google Scholar]
- 34.Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene. 2008;27(29):4044–4055. doi: 10.1038/onc.2008.57. [DOI] [PubMed] [Google Scholar]