Abstract
Skeletal muscle satellite cells (SCs) have been shown to be instrumental in the muscle adaptive response to exercise. The present study determines age-related differences in SC content and activation status following a single bout of exercise. Ten young (22 ± 1 years) and 10 elderly (73 ± 1 years) men performed a single bout of resistance-type exercise. Muscle biopsies were collected before and 12, 24, 48, and 72 h after exercise. SC content and activation status were assessed in type I and type II muscle fibers by immunohistochemistry. Myostatin and MyoD protein and messenger RNA (mRNA) expression were determined by Western blotting and rtPCR, respectively. In response to exercise, it took 48 h (young) and 72 h (elderly) for type II muscle fiber SC content to exceed baseline values (P < 0.01). The number of myostatin + SC in type I and II muscle fibers was significantly reduced after 12, 24, and 48 h of post-exercise recovery in both groups (P < 0.01), with a greater reduction observed at 24 and 48 h in the young compared with that in the elderly men (P < 0.01). In conclusion, the increase in type II muscle fiber SC content during post-exercise recovery is delayed with aging and is accompanied by a blunted SC activation response.
Keywords: Muscle stem cells, Myostatin, MyoD, Myogenin
Introduction
The age-related loss of skeletal muscle mass is characterized by a reduction in muscle fiber cross-sectional area and number, and specific type II muscle fiber atrophy (Lexell et al. 1988; Verdijk et al. 2007). This decline in type II muscle fiber cross-sectional area is accompanied by a muscle fiber type–specific decline in the number of skeletal muscle satellite cells (SCs) (Verdijk et al. 2007). These SCs represent a small pool of myogenic precursor cells, which are essential for muscle fiber maintenance, repair, and growth. Although SCs typically reside in a quiescent state, they can become activated following exercise and/or skeletal muscle fiber damage (O’Reilly et al. 2008; McKay et al. 2009). Following activation, SCs can proliferate and subsequently differentiate to form new myonuclei, or return to a quiescent state to replenish the resident pool of SCs.
Physical activity has been shown to stimulate muscle protein synthesis, resulting in net muscle protein accretion (Witard et al. 2009; Phillips et al. 1997). However, it has been suggested that adequate SC activation, proliferation, and differentiation are required to allow substantial muscle fiber hypertrophy during more prolonged exercise training (Kadi et al. 2004; Petrella et al. 2008). The progression of SCs through the various stages of activation, proliferation, and/or differentiation is mediated by the expression of different myogenic regulatory factors, or MRFs (MyoD, Myf-5, Myogenin, and Mrf4) (Yin et al. 2013). In addition, myostatin has been suggested to be of key importance in SC function during muscle fiber growth (Langley et al. 2002; Siriett et al. 2006). As a member of the transforming growth factor-β (TGF-β) family, myostatin acts as a strong negative regulator of skeletal myogenesis (McPherron and Lee 1997; Lee 2004). It has been suggested that myostatin may be a crucial mediator in the age-related loss of muscle mass (Siriett et al. 2006). An inability to properly downregulate myostatin may be of significant importance in the reduced myogenic capacity typically observed in aged human skeletal muscle in response to anabolic stimuli (McKay et al. 2012; Leger et al. 2008). Likewise, the upregulation and/or downregulation of messenger RNA (mRNA) expression of the MRF proteins in response to a single bout of resistance-type exercise has been shown to be altered in elderly (Hameed et al. 2003; Drummond et al. 2009), which might be indicative of impairments in SC function in senescent muscle.
In support of a reduced SC responsiveness with aging, previous studies have indicated that the exercise-induced increase in SC content may be attenuated in the elderly. Dreyer et al. (2006) showed that 24 h following a single bout of eccentric exercise, the increase in SC content was greater in young (+141 %) when compared with that in elderly (+51 %) men. In a more recent study, McKay et al. (2012) assessed changes in type I and type II muscle fiber SC content during 48 h of post-exercise recovery in young and elderly men. This study showed that type II muscle fiber SC content increased in the young but not in the elderly men during the first 48 h of post-exercise recovery (McKay et al. 2012). The inability to properly increase the SC pool size in response to exercise might contribute to the reduced skeletal muscle adaptive response to exercise training that has typically been observed in the elderly (Kosek et al. 2006; Petrella et al. 2006) and may predispose to the development of sarcopenia. Even though McKay et al. (2012) did not show any detectable increases in type II muscle fiber SC content following a single bout of exercise, it has been well established that in response to prolonged resistance-type exercise training, type II muscle fiber hypertrophy is accompanied by a concomitant increase in type II muscle fiber SC content in healthy elderly (Leenders et al. 2013; Verdijk et al. 2009a). So far, only limited data exist on the time-dependent changes in SC content and activation status during post-exercise recovery in human skeletal muscle. A more comprehensive understanding of the timeline of the changes in SC activation, proliferation, and differentiation during post-exercise recovery in both young and elderly adults is required to understand the potential underlying mechanisms responsible for the blunted increase in muscle mass and strength observed in senescent muscle. Therefore, in the present study, we assessed type I and type II muscle fiber SC content and activation status following 12, 24, 48, and 72 h of recovery from a single bout of resistance-type exercise in both young and elderly men. This is the first study to show that the increase in type II muscle fiber SC content is delayed and accompanied by a blunted SC activation response in healthy elderly men.
Methods
Participants
Ten healthy young (22 ± 1 years) and 10 healthy elderly (73 ± 1 years) men were recruited to participate in this study. Participants were informed about the nature and risks of the experimental procedures before their written consent was obtained. The study was approved by the Medical Ethical Committee of the Maastricht University Medical Centre+ and complied with the guidelines set out in the Declaration of Helsinki. Medical history of all participants was evaluated and a resting electrocardiogram was performed before selection. Exclusion criteria were defined that would preclude successful participation in the exercise session, which included (silent) cardiac or peripheral vascular disease and orthopedic limitations. Body composition (fat and fat-free mass) was determined by Dual Energy X-Ray Absorptiometry scan (DEXA; Hologic Inc., Bedford, USA). Lean mass and percentage body fat were determined on a whole body level and for specific regions (e.g., trunk and legs). Next, all eligible men participated in an orientation trial to become familiarized with the resistance-type exercise protocol and the equipment. Proper lifting technique was demonstrated and then practiced by the participants for each of the two lower limb exercises (leg press and leg extension). Subsequently, maximal strength (one-repetition maximum, or 1-RM) was determined by using the multiple repetitions testing procedure during two separate visits (Mayhew et al. 1995).
Protocol
At 08:00 h, following 24 h of a controlled diet, participants reported to the lab after an overnight fast. Following 30 min of supine rest, a muscle biopsy was taken from the M. vastus lateralis. Thereafter, participants were provided with a standardized breakfast. After breakfast, participants rested for 30 min after which they performed a single bout of exercise. The single bout of resistance-type exercise consisted of two different exercises. Participants performed six sets of 10 repetitions at 75 % 1-RM on the horizontal leg press machine (Technogym BV, Rotterdam, The Netherlands) and six sets of 10 repetitions at 75 % 1-RM on the leg extension machine (Technogym BV). A resting period of 2 min between sets was allowed. The entire protocol required approximately 40 min to complete. All participants were verbally encouraged during the test to complete the whole protocol. Before and after the exercise session, a 5–10-min warm up/cooling down at low intensity was performed on a cycle ergometer. At the end of the exercise protocol, the participants rested for 3 h in a supine position. At 12:30 h, participants received a standardized lunch, after which participants were provided with a standardized dinner for the same evening. At 20:30 h, participants reported back to the laboratory for collection of a muscle biopsy and blood sample (t = 12 h). Exactly 24, 48, and 72 h after the start of the exercise, a third, fourth, and fifth muscle biopsy and blood sample were collected.
Diet and physical activity standardization
During the entire 4-day intervention period, all participants consumed a controlled diet. Participants’ energy requirements were calculated using the Harris and Benedict equations with a physical activity index of 1.4 (Harris and Benedict 1918). On average, the young men consumed 150 ± 6 kJ · kg bw−1 · d−1, consisting of 70 ± 1 En % carbohydrate, 13 ± 1 En % protein (equal to 1.16 ± 0.03 g protein · kg bw−1·d−1), and 20 ± 1 En % fat. Elderly men consumed 125 ± 4 kJ · kg bw−1 · d−1, consisting of 65 ± 1 En % carbohydrate, 15 ± 1 En % protein (equal to 1.11 ± 0.04 g protein · kg bw−1·d−1), and 22 ± 1 En % fat. All volunteers were instructed to refrain from any sort of heavy physical exercise 3 days before the first test day until the day of the last blood and muscle biopsy sampling.
Muscle biopsy
Muscle biopsies were obtained from the middle region of the vastus lateralis muscle, ∼15 cm above the patella and approximately 2 cm away from the fascia by means of the percutaneous needle biopsy technique described by Bergström et al. (1975). Muscle biopsies were carefully freed from any visible fat and blood, with one part embedded in Tissue-Tek (Sakura Finetek Europe B.V., Zoeterwoude, Netherlands) and rapidly frozen in liquid nitrogen cooled isopentane, while another part was directly frozen in liquid nitrogen and stored at −80 °C for subsequent histochemical and biochemical analysis, respectively.
Immunohistochemical analysis
From all muscle biopsy samples, 5-μm-thick cross sections were cut at −20 °C using a cryostat. Muscle samples collected before and after 12, 24, 48, and 72 h of post-exercise recovery from each individual participant were mounted together on uncoated glass slides and air-dried for 3 h at room temperature before being stored at −20 °C for subsequent analyses. All muscle cross sections were stained with antibodies against Pax7 (supernatant from growing cells; neat; DSHB), myostatin (near C-terminus; 1:100; AB3239; Millipore, Etobicoke, ON, Canada) previously validated to detect the C-terminal (active) myostatin (Bish et al. 2010; Jespersen et al. 2011), MyoD1 (anti-MyoD1; clone 5.8A; Dako, Burlington, ON, Canada), A4.951 [myosin heavy chain type I (MHCI; slow isoform); supernatant; neat; DSHB], and laminin (1:1000; L8271, Sigma-Aldrich, Oakville, ON, Canada, and Abcam ab11575, Abcam, Cambridge, MA, USA). Appropriate secondary antibodies were applied: Dylight 488 (1:500, Thermo Scientific, Ontario, ON, Canada), Alexa Fluor 594 (1:500, Invitrogen, Molecular Probes, Carlsbad, CA, USA), biotinylated secondary antibody (1:200; Vector Canada, Burlington, ON, Canada), streptavidin-594 fluorochrome (1:500; Invitrogen, Molecular Probes), Alexa Fluor 488 (1:500), Alexa Fluor 594 (1:500), Alexa Fluor 647 (1:500); Alexa Fluor 350 (1:20), Alexa Fluor 488 (1:500), and Alexa Fluor 647 (1:500). The immunohistochemical staining procedures were adapted from previously published methods (McKay et al. 2012; Snijders et al. 2014). All stained muscle samples were viewed with the Nikon Eclipse 90i microscope outfitted with a high-resolution QImaging camera for fluorescence detection (Nikon Instruments, Melville, NY, USA), and images were captured with a ×40 objective and analyzed using the Nikon NIS Elements AR 3.0 software (Nikon Instruments). Fiber circularity was calculated as (4π•cross-sectional area (CSA))/(perimeter)2. For each subject, fiber type distribution (fiber%: number of type I fibers/total fiber number), fiber CSA, fiber area percentage (CSA%: the area percentage occupied by type I fibers, calculated by multiplying type I fiber% and type I CSA and then dividing by total area), number of SCs per fiber, and number of SC per square millimeters of muscle fiber area were determined for the type I and type II muscle fibers, separately. In addition, we determined the number of SC stained positive for MyoD or Myostatin (see Figs. 1 and 2 for representative images of the immunohistological analyses, respectively). As described previously (Mackey et al. 2009), at least 75 type I and 75 type II muscle fibers were included to make a reliable estimation of SC content in human muscle biopsy samples (Table 1). Slides were masked for both groups and time points. Due to technical difficulties, MyoD + SCs were only assessed in mixed muscle fiber in 14 out of 20 participants (6 young and 8 elderly men), whereas the number of myostatin + SCs was determined in type I and type II muscle fibers separately in all participants (10 young and 10 elderly men). Representative images of the immunohistological analyses are provided in Figs. 1 and 2.
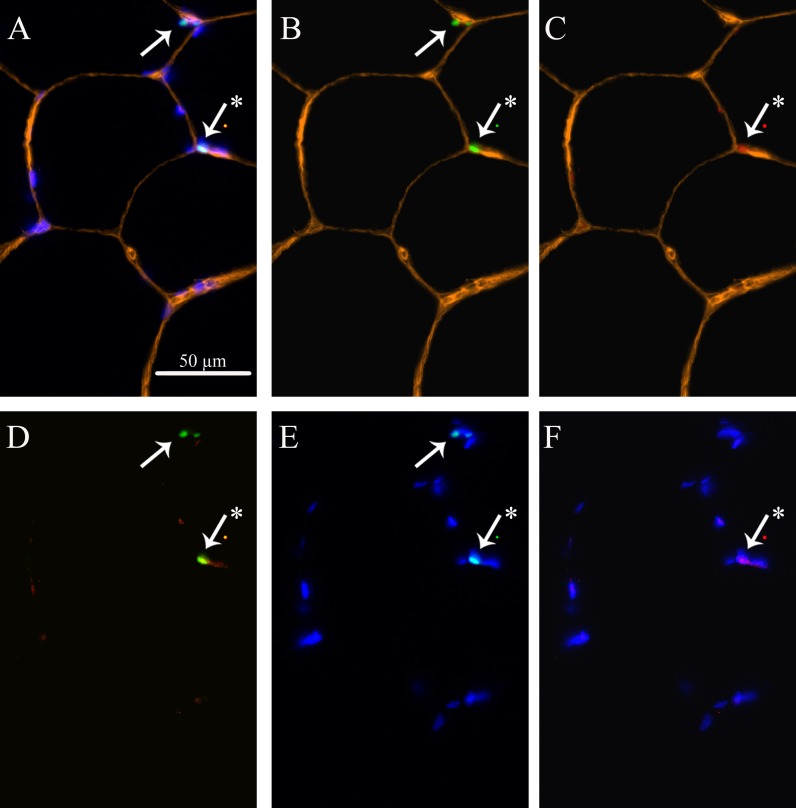
Fig. 1.
Representation of skeletal muscle satellite cells (SC) and MyoD in mixed muscle fibers. a Laminin (yellow) + Dapi (blue) + Pax7 (green) + MyoD (red). b Laminin + Pax7. c Laminin + MyoD. d Pax7 + MyoD. e Dapi + Pax7. f Dapi + MyoD. Arrows point at SCs. Asterisk indicates an activated SC
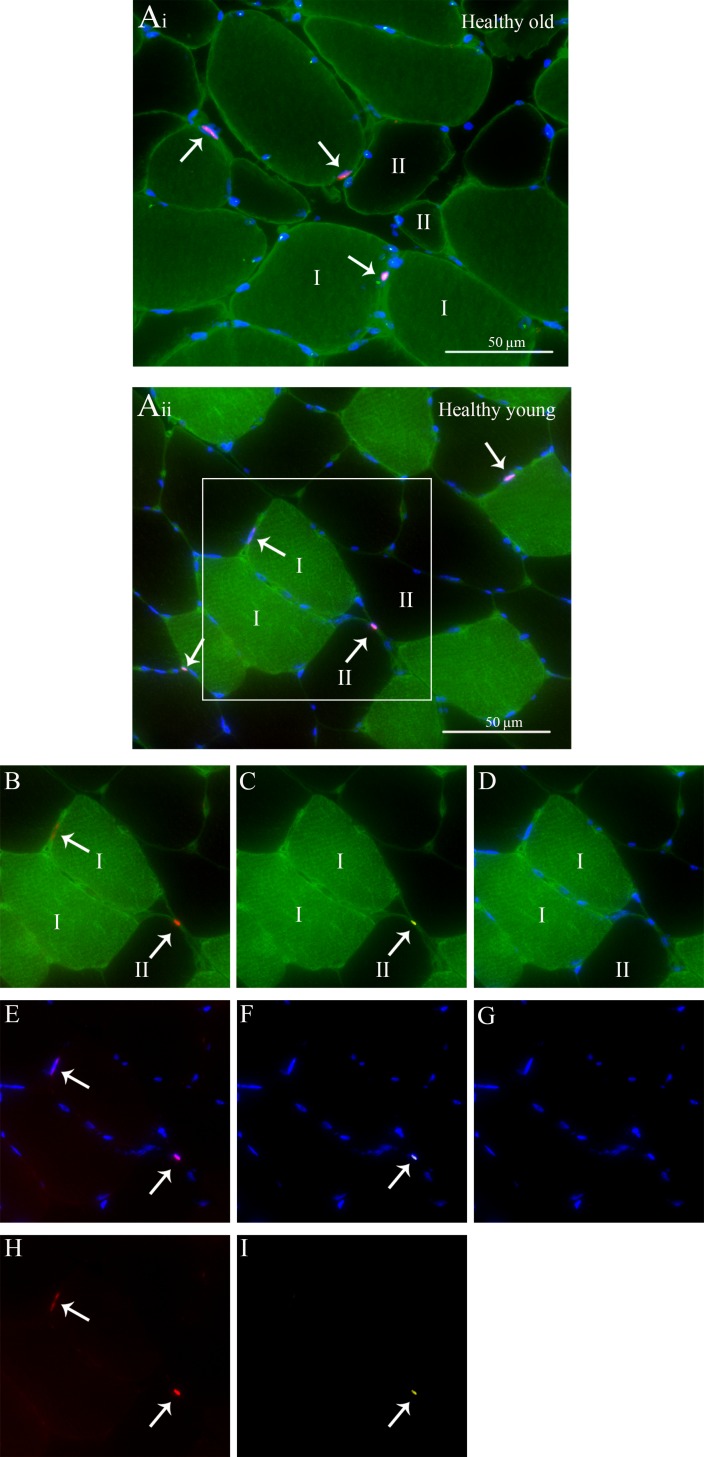
Fig. 2.
Representation of fiber-type-specific analyses of skeletal muscle satellite cell (SC) content and myostatin in both a healthy old (a i) and young (a ii) man. a i-ii MHCI (green) + laminin (green) + Dapi (blue) + Pax7 (red) + myostatin (yellow) staining. b MHCI + laminin + Pax7. c MHCI + laminin + myostatin. d MHCI + laminin + Dapi. e Dapi + Pax7. f Dapi + myostatin. g Dapi only. h Pax7 only. i Myostatin only. Arrows point at the SCs
Table 1.
Characteristic of immunohistological analyses
| Young | Old | ||||
|---|---|---|---|---|---|
| Type I | Type II | Type I | Type II | ||
| Fiber number | Pre- | 112 ± 10 | 109 ± 8 | 103 ± 18 | 87 ± 11 |
| 12 h | 114 ± 17 | 120 ± 14 | 116 ± 9 | 101 ± 9 | |
| 24 h | 105 ± 13 | 104 ± 12 | 86 ± 9 | 97 ± 12 | |
| 48 h | 94 ± 11 | 86 ± 6 | 99 ± 12 | 97 ± 11 | |
| 72 h | 96 ± 9 | 102 ± 7 | 99 ± 13 | 91 ± 8 | |
Values are means ± SEM; fiber number: the number of fibers included in the fiber-type-specific immunohistological analyses of satellite cell content
Quantitative rtPCR
Total RNA was isolated from 10–20 mg of frozen muscle tissue using Trizol® Reagent (Invitrogen), according to the manufacturer’s protocol. Total RNA quantification was carried out spectrophotometrically at 260 nm (NanoDrop ND-1000 Spectrophotometer, Thermo Fisher Scientific, USA), and RNA purity was determined as the ratio of readings at 260/280 nm. The first-strand complementary DNA (cDNA) was synthesized from 1-μg RNA sample using iScriptTM cDNA synthesis kit (BIO-RAD). Taqman PCR was carried out using 7300 Real-Time PCR system (Applied BioSystems, USA) with 2 μL of cDNA (diluted five times), Mastermix: Taqman® Gene Expresseion Mastermix (Applied Biosytems) was added resulting in a total volume of 25 μL. Taqman primer/probe sets were obtained from Applied Biosystems (Taqman® Gene Expression Assays): myogenin, MyoD, and myostatin; primers are listed in Table 2. Each sample was run in duplicate. The housekeeping gene hydroxymethylbilane synthase (HMBS) was used as an internal control. The thermal cycling conditions used were 2 min at 50 °C, 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Ct values of the target gene were normalized to Ct values of the internal control HMBS, and final results were calculated according to the 2−∆∆Ct method. The muscle biopsy attained before the single bout of exercise was used as a reference and given a value of 1, and fold changes at 12, 24, 48, and 72 h of post-exercise recovery were calculated.
Table 2.
qRT-PCR probes
| Gene name | Probe number | NCBI gene |
|---|---|---|
| ID number | ||
| Myostatin | Hs 00193363 | 2660 |
| MyoD | Hs 00159528 | 4654 |
| Myogenin | Hs 00231167 | 4656 |
| HMBS | Hs 00609297 | 3145 |
MyoD myogenic determination factor, HMBS hydroxymethylbilane synthase
Western blotting
From each muscle sample frozen for biochemical analyses, 30–40 mg was homogenized in Tris buffer (50 mM, 1 mM EDTA, 1 mM EGTA, 0.1 % Nonidet P40, 0.1 % 2-mercaptoethanol pH 7.4) supplemented with the following protease and phosphatase inhibitors: aprotinin 2 mg/mL, leupeptin 2 mg/mL, benzamidine 300 mM, and PMSF 100 mM. After homogenization, each muscle extract was centrifuged for 5 min at 10,000g (4 °C) and supernatant was used as nuclear fraction. Sample buffer was added to the supernatant to a final concentration of 60 mM Tris, 5 % glycerol, 20 mg/mL SDS, 0.1 mM DTT, and 20 μg/mL bromophenol blue and was then boiled for 5 min at 100 °C and subsequently cooled on ice. Immediately before analyses, the muscle extraction sample was warmed to 50 °C and centrifuged for 1 min at 3,000g (RT). Total amount of sample loaded on the gel was based on weight (3.125 mg muscle per lane). Protein samples were run on a 4–15 % Criterion Tris-HCl gel (Biorad Order No. 3450027) for 10 min at 50 V (constant voltage) and 1 h 40 min at 120 V (constant voltage) and transferred onto a polyvinylidene difluoride membrane (PVDF) in 1 h 30 min at constant 500 mA. Specific proteins were detected by incubation with specific antibodies in 1 % bovine serum albumin (BSA) in 0.1 % phosphate-buffered saline (PBS)-Tween 20 after blocking in 1 % BSA in 0.1 % PBS-Tween (Sigma Order No. A6003-25G). The antibodies used in this study were anti MyoD (37 KD; rabbit polyclonal IgG, Santa Cruz sc-760), anti-Myostatin (50 KD; rabbit polyclonal IgG; Santa Cruz sc-6885-R), and anti-α-actin (42 KD; mouse monoclonal IgM; Sigma A2172). Following incubation, membranes were washed (3 × 5 min) in 0.1 % PBS-Tween 20 and incubated (1 h at RT) with the appropriate secondary antibody, polyclonal rabbit anti-mouse IgG-HRP (Dako, P0161), and polyclonal swine anti-rabbit IgG-HRP (Dako, P0399) dissolved in 1 % BSA 0.1 % PBS-Tween 20. After a final wash step (3 × 5 min) in 0.1 % Tween 20-PBS, the membranes were incubated with Supersignal West Dura Extended Duration Substrate (Thermo Scientific, No. 340760) for 1 min. Visualization and quantification of the protein bands were enabled by Biorad universal hood II and Chemidoc XRS camera and Quantity One 4.6.5 software, respectively.
Statistics
All data are expressed as means ± SEM. An independent samples Student’s t test was used to compare differences between groups at baseline. Data were analyzed using a two-way repeated measures ANOVA with group (young vs old) as between-subject factor and time (before exercise (pre-) vs 12, 24, 48, or 72 h post-exercise recovery) and fiber type (type I vs II) as within-subject factors. In the two-way repeated measures ANOVA design, post-exercise time points were compared with baseline values only and Bonferroni corrections were applied to account for multiple comparisons to baseline. Separate analyses (within groups or within fiber type) were performed in case significant interactions and Bonferroni corrections were applied to correct for multiple testing. Statistical significance was set at P < 0.05. All calculations were performed using SPSS 19.0 (Chicago, IL).
Results
Subject characteristics
Characteristics of both groups are presented in Table 3. Muscle strength as assessed by 1-RM; both leg press and leg extension were significantly lower in the elderly (168 ± 8 and 84 ± 3 kg, respectively) compared with the young men (214 ± 7 and 137 ± 4 kg, respectively; P < 0.001). In accordance, leg lean mass was significantly lower in the older compared with that in the young men (18.4 ± 0.5 vs 21.1 ± 0.4 kg, respectively; P < 0.001).
Table 3.
Subjects’ characteristics
| Young | Old | |
|---|---|---|
| Age (years) | 22 ± 1 | 73 ± 1* |
| Height (m) | 1.82 ± 0.02 | 1.75 ± 0.02* |
| Weight (kg) | 76 ± 2 | 79 ± 3 |
| BMI (kg/m2) | 23.0 ± 0.6 | 25.7 ± 0.8* |
| Whole body lean mass (kg) | 58.4 ± 1.2 | 55.9 ± 1.9 |
| Leg lean mass (kg) | 21.1 ± 0.4 | 18.4 ± 0.5* |
| Whole body fat (%) | 15 ± 2 | 22 ± 1* |
| 1-RM leg press (kg) | 214 ± 7 | 168 ± 8* |
| 1-RM leg extension (kg) | 137 ± 4 | 84 ± 3* |
Values are means ± SEM; n = 10 per group
BMI body mass index, 1-RM one-repetition maximum
*Significantly different compared with young men
Muscle fiber cross-sectional area and fiber type distribution
No significant differences were observed in type I muscle fiber cross-sectional area between groups. In contrast, type II muscle fiber cross-sectional area was significantly smaller in the older compared with that in the young men (5,574 ± 371 vs 7,584 ± 390 μm2, respectively; P < 0.01). Furthermore, in the healthy young men, muscle fiber cross-sectional area was significantly greater in type II compared with type I fibers (P < 0.01; Table 4). In contrast, type II muscle fiber cross-sectional area was significantly smaller than type I muscle fiber cross-sectional in the elderly men (P < 0.01; Table 4). No differences in fiber type distribution (fiber% and/or CSA%) nor in fiber circularity were observed between young and elderly men (Table 4). In addition, no changes were observed in muscle fiber cross-sectional area, fiber type distribution, and/or fiber circularity within the 72 h of post-exercise recovery (data not shown).
Table 4.
Muscle fiber distribution and fiber area
| Fiber type | Young | Old | |
|---|---|---|---|
| Fiber circularity | I | 0.77 ± 0.01 | 0.75 ± 0.01 |
| II | 0.77 ± 0.01 | 0.72 ± 0.01 | |
| Fiber type distribution (fiber%) | I | 51 ± 3 | 56 ± 5 |
| II | 49 ± 3 | 44 ± 5 | |
| Fiber type distribution (CSA%) | I | 47 ± 4 | 57 ± 4 |
| II | 53 ± 4 | 43 ± 4 | |
| Muscle fiber CSA (μm2) | I | 6316 ± 486 | 6566 ± 364 |
| II | 7584 ± 390# | 5574 ± 371#* |
Data represent means ± SEM
CSA cross-sectional area. #: Significantly different compared with type I muscle fibers (P < 0.05). *: Significantly different compared with young (P < 0.05)
Satellite cell content
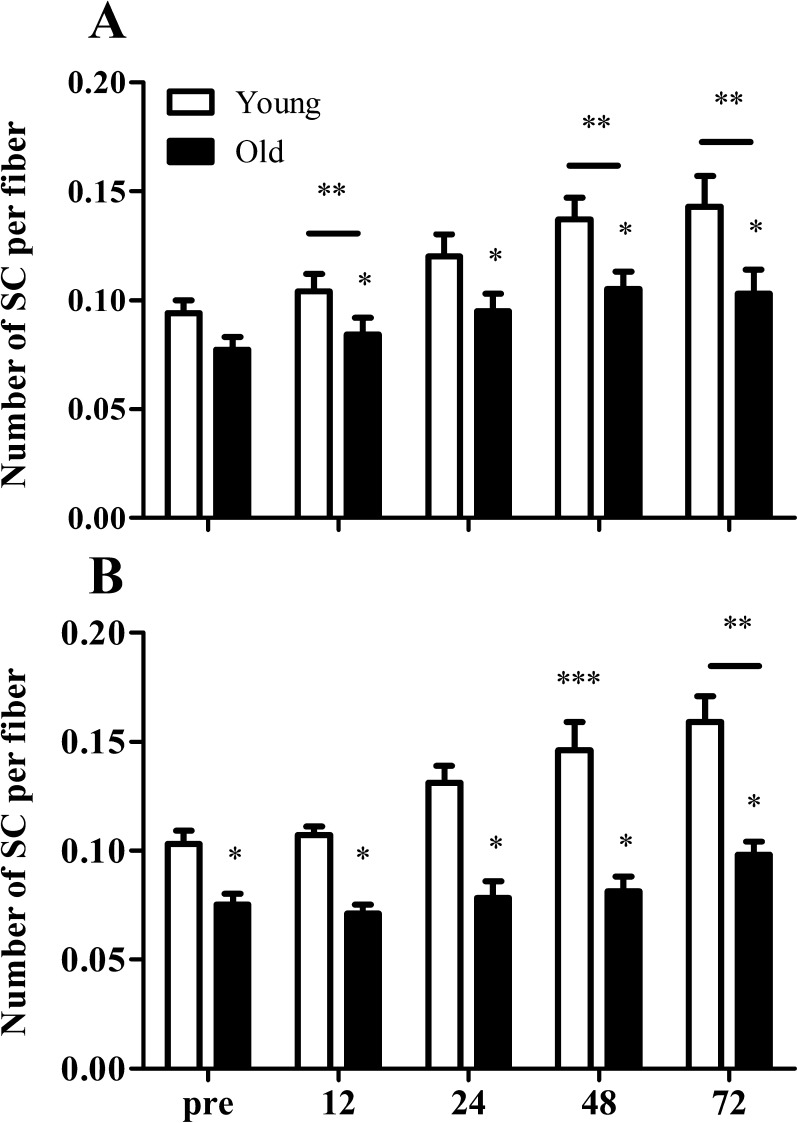
At baseline, the number of SCs in the type II muscle fibers was significantly lower in the elderly compared with that in the young men (0.077 ± 0.005 vs 0.102 ± 0.007 SCs per muscle fiber, respectively; P < 0.01). The number of SC per type I muscle fiber had significantly increased after 12 (+14 ± 5 and +10 ± 7 %; P < 0.01), 48 (+42 ± 12 and +40 ± 8 %; P < 0.001), and 72 h (+49 ± 18 and +45 ± 19 %; P < 0.001) of recovery when compared with pre-exercise values in both the young and the elderly men, respectively (Fig. 3a). At 24 h post-exercise, type I muscle fiber SC content tended to be greater compared to baseline values in both groups (Fig. 3a; P = 0.052). Type II muscle fiber SC content was significantly increased at 48 (+34 ± 11 %; P < 0.001) and 72 h (+48 ± 15 %; P < 0.001) after the single bout of exercise in the healthy young men, with a non-significant increase also observed at 24 h (P = 0.052). In contrast, type II muscle fiber SC content was only increased after 72 h of post-exercise recovery (+34 ± 11 %) in the older men (Fig. 3b; P < 0.001). When expressed per millimeter squared, we observed similar changes in SC contents during post-exercise recovery in both the young and older men. The number of SCs per millimeter squared in type I muscle fibers had increased significantly after 24 (P < 0.01), 48 (P < 0.001), and 72 h (P < 0.001) of post-exercise recovery when compared with baseline values in both the young and older men (Table 5). Whereas the number of SCs per millimeter squared in type II muscle fiber was significantly increased at 24 (P < 0.01), 48(P < 0.001), and 72 h (P < 0.001) after cessation of exercise in the healthy young men, changes in type II muscle fiber SC content in the older men were not observed until after 72 h of post-exercise recovery (Table 5).
Fig. 3.
Mean number of satellite cells (SCs) per muscle fiber in both type I (a) and type II (b) muscle fibers before (pre-) and 12, 24, 48, and 72 h after a single bout of resistance in young (n = 10) and old (n = 10) men. Values represent means ± SEM. Single asterisk indicates significant group effect (P < 0.01). Double asterisk indicates significant effect of time compared with pre-exercise value (P < 0.01). Triple asterisk indicates significant effect of time compared with pre-exercise value in young men only (P < 0.01). Bar indicates that the effect of time is present in both groups
Table 5.
Type I and II muscle fiber satellite cell content
| Group | Type | Pre | 12 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|---|---|---|
| Nr of SC/mm2 | Young | I | 16.6 ± 1.7 | 18.4 ± 2.9 | 20.0 ± 2.7* | 22.0 ± 1.9* | 23.4 ± 3.0* |
| Old | I | 11.6 ± 1.2 | 13.1 ± 1.4 | 16.1 ± 1.3* | 16.7 ± 2.0* | 16.5 ± 3.0* | |
| Young | II | 14.0 ± 1.0 | 13.7 ± 1.0 | 17.7 ± 1.4* | 18.5 ± 1.3* | 20.3 ± 2.0* | |
| Old | II | 14.4 ± 1.9 | 15.7 ± 1.5 | 14.8 ± 1.3 | 15.4 ± 1.3 | 19.4 ± 2.0* |
Data represent means ± SEM
SC satellite cell. * Significantly different compared with pre-exercise values
*Significantly different compared with pre-exercise values
MyoD + satellite cells
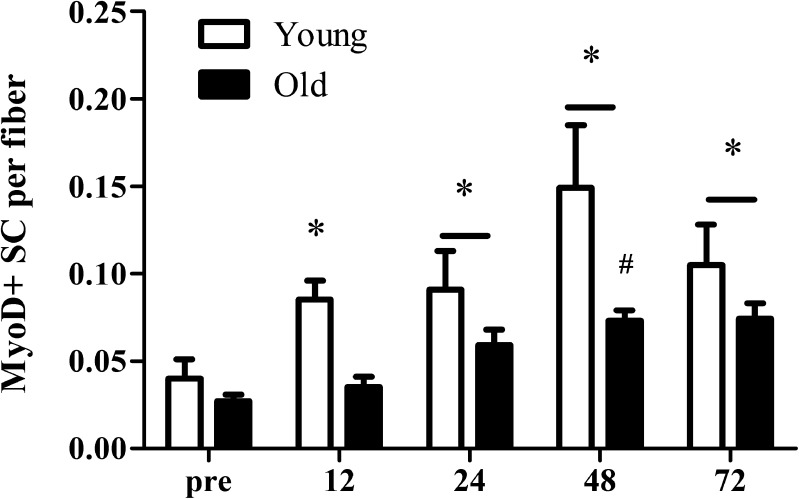
At baseline, MyoD was expressed in 25–30 % of all SCs with no significant differences between groups (see Fig. 1 for representative image of the immunohistological staining). In response to the single bout of resistance-type exercise, the number of mixed muscle MyoD + SCs per fiber was significantly increased at all time points when compared with pre-exercise values (Fig. 4; P < 0.01) in the healthy young men. In contrast, mixed muscle MyoD + SCs per fiber was increased after 24 (P < 0.001), 48 (P < 0.001), and 72 h (P < 0.001) of post-exercise recovery in the elderly men. In addition, we observed a significantly greater increase in the number of MyoD + SCs in the young compared with the elderly men at 48 h after a single bout of exercise (time × group interaction, P = 0.027; Fig. 5).
Fig. 4.
Changes in mixed muscle MyoD + satellite cells (SC) per muscle fiber before and 12, 24, 48, and 72 h after a single bout of resistance-type exercise in young (n = 6) and old (n = 8) men. Values represent means ± SEM. Asterisk indicates significant effect of time compared with pre-exercise value (P < 0.01). Number sign indicates significant time × group interaction; increase from pre-exercise value is smaller in old compared with young men (P < 0.01). Bar indicates that the effect of time is present in both groups
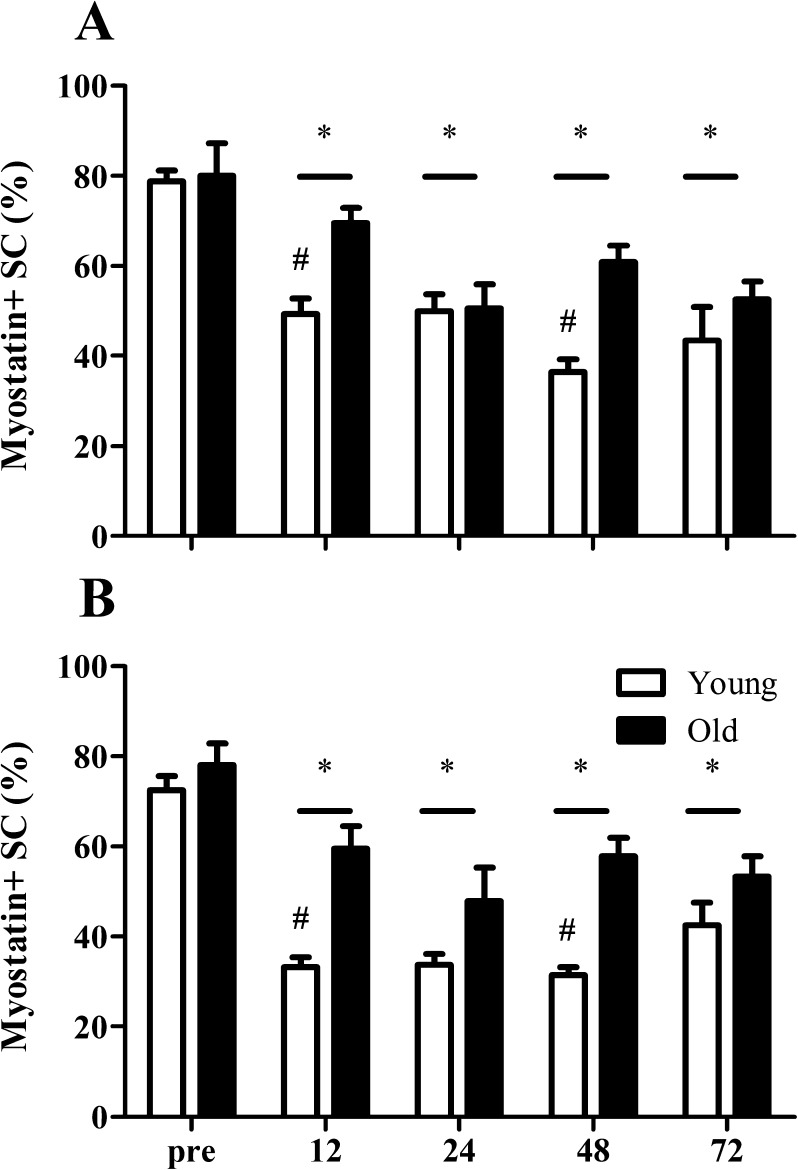
Fig. 5.
Proportion of myostatin + satellite cells (SC) in both type I (a) and type II (b) muscle fibers before (pre-) and 12, 24, 48, and 72 h after a single bout of resistance exercise in young (n = 10) and old (n = 10 ) men. Values represent means ± SEM. Asterisk indicates significant effect of time compared with pre-exercise value (P < 0.01). Number sign indicates significant time × group interaction; decrease from pre-exercise value is larger in young compared with old men (P < 0.01). Bar indicates that the effect of time is present in both groups
Myostatin + satellite cells
At baseline, myostatin was expressed in 70–80 % of all SCs with no differences between type I and type II muscle fibers (see Fig. 2 for representative image of the immunohistological staining). In addition, before exercise, the proportion of myostatin + SCs did not differ between groups. In response to the single bout of resistance-type exercise, both groups showed a substantial decline in the percentage of myostatin + SCs at all time points when compared to pre-exercise values (Fig. 5a, b; P < 0.001). However, at 12 (P < 0.01) and 48 h (P < 0.01) after exercise cessation, the reduction in the percentage of myostatin + SCs was significantly higher in the young compared with that in the elderly men (Fig. 5a, b). Furthermore, both groups showed a larger decrease in the proportion of myostatin + SCs in type II compared to type I muscle fibers at 12 and 24 h after the single bout of resistance-type exercise.
mRNA expression
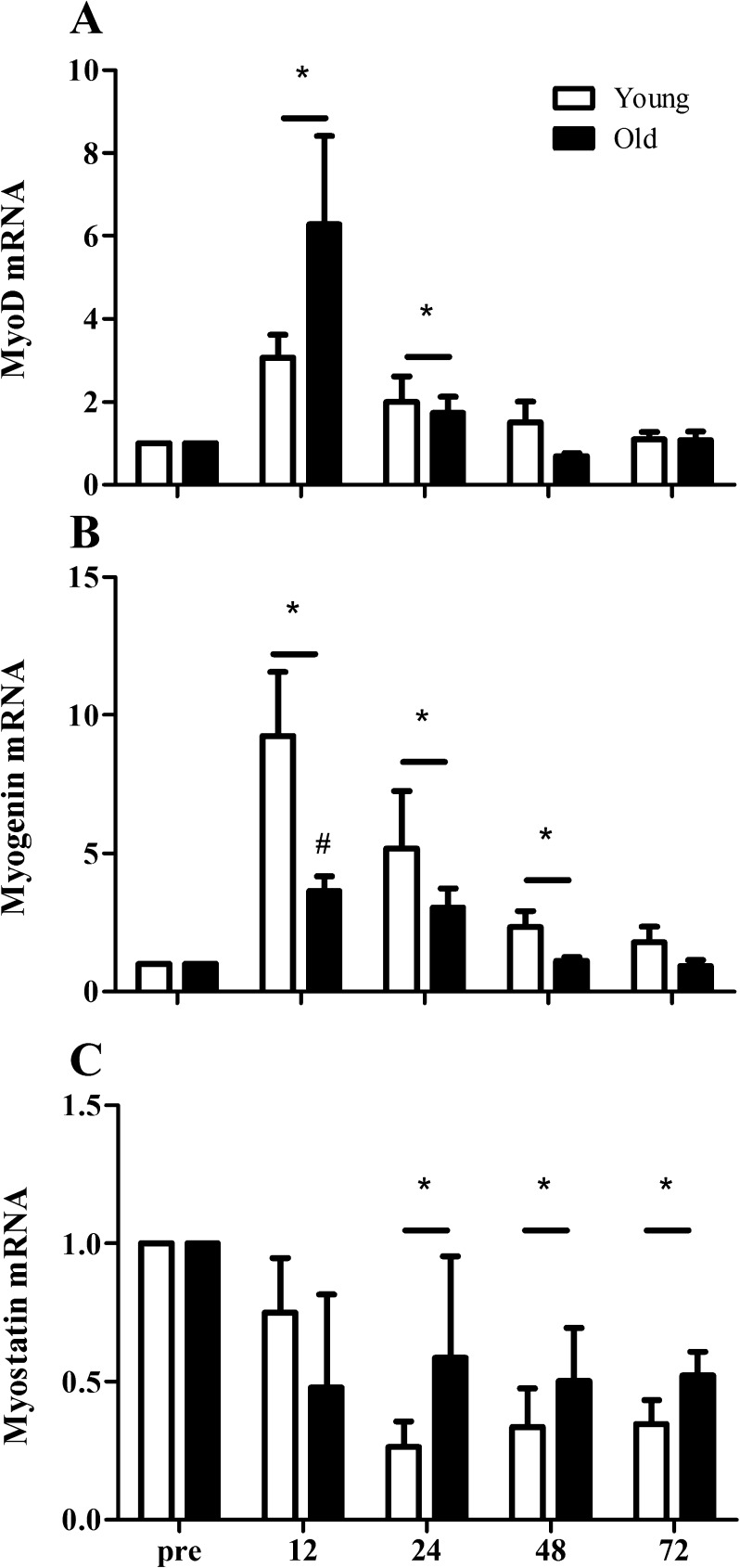
MyoD mRNA expression increased significantly at 12 (∼3- and ∼6-fold, respectively; P < 0.01) and 24 h (∼2- and ∼2-fold, respectively; P = 0.03) after the single bout of resistance-type exercise in both young and elderly men (Fig. 6a). The increase in MyoD mRNA expression over time did not differ between groups. In contrast, myogenin mRNA expression revealed a larger increase in the young (9.3 ± 2.3-fold) compared with the elderly men (3.6 ± 0.5-fold) at 12 h after the single exercise bout (Fig. 6b; time × group interaction P = 0.04). At 24 (P = 0.02) and 48 h (P = 0.03), myogenin mRNA expression was increased in both groups, with a tendency for a larger increase in the young compared with the elderly men at 48 h post-exercise recovery (time × group interaction, P = 0.056). Myostatin mRNA expression was substantially lowered at 24 (P < 0.01), 48 (P < 0.001), and 72 h (P < 0.001) after the single exercise bout, with no differences between groups (Fig. 6c).
Fig. 6.
Changes in MyoD (a), myogenin (b), and myostatin (c) mRNA expression before (pre-) and 12, 24, 48, and 72 h after a single bout of resistance exercise in young (n = 10) and old (n = 10) men. Fold changes for mRNA expression were calculated using 2−∆∆Ct method with genes of interest normalized to HMBS. Asterisk indicates significant effect of time compared with pre-exercise value (P < 0.01). Number sign indicates significant time × group interaction; increase from pre-exercise value is smaller in old compared with young men (P < 0.01). Bar indicates that the effect of time is present in both groups
Protein expression
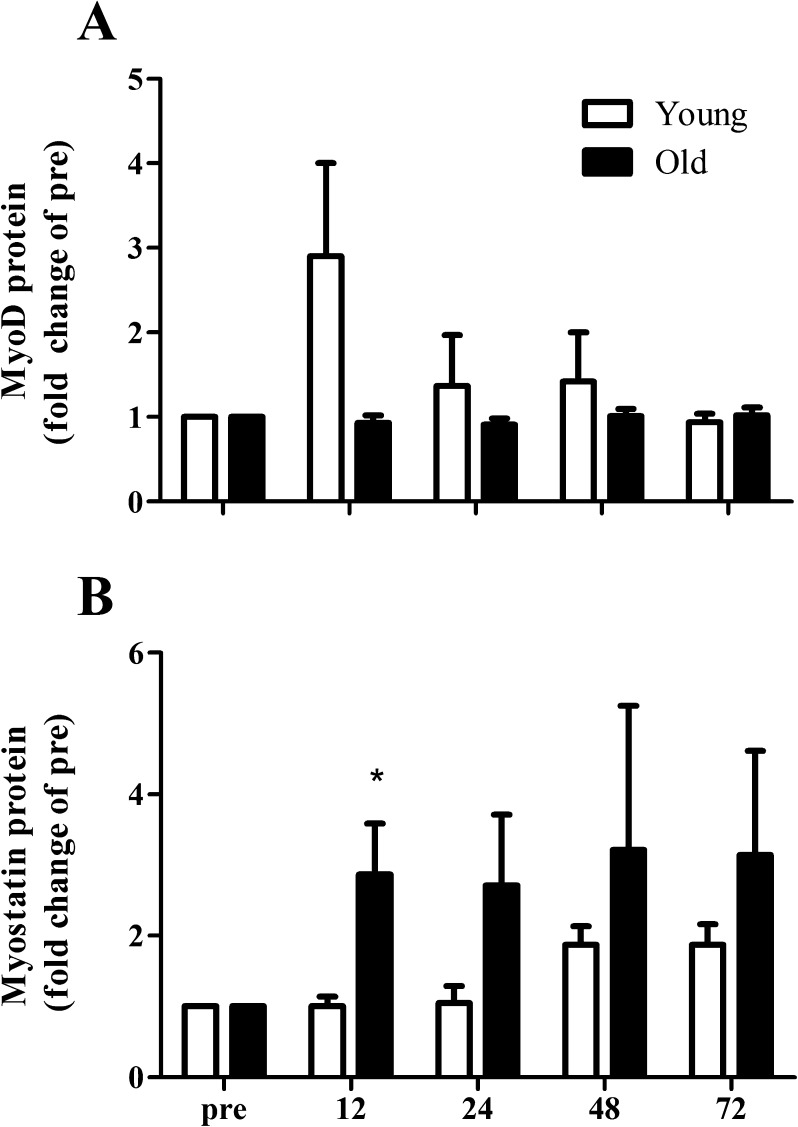
The increase in MyoD protein expression tended to be greater in the young compared to the elderly in response to the single bout of resistance-type exercise (Fig. 7b; time × group interaction; P = 0.092). In contrast, myostatin protein expression was significantly increased after 12 h of post-exercise recovery in the elderly men, whereas no changes were observed in the young men (Fig. 7c; time × group interaction; P = 0.021). In addition, myostatin protein expression tended to be increased in both groups at 72 h of post-exercise recovery in both the young and elderly men (Fig. 7c; main effect of time; P = 0.061)
Fig. 7.
Changes in MyoD (a) and myostatin (b) protein expression 12, 24, 48, and 72 h after a single bout of resistance-type exercise in young (n = 10) and old (n = 10) men. Protein expression was normalized to α-actin. Values represent means ± SEM. Asterisk indicates significant effect of time compared with pre-exercise value (P < 0.01)
Discussion
In the present study, we show that both type I and type II muscle fiber SC contents increase within 72 h of recovery from a single bout of resistance-type exercise in healthy young and elderly men. However, the post-exercise increase in type II muscle fiber SC content is delayed in the elderly when compared with that in the younger men. In addition, we observed an attenuated decline in the number of myostatin + SCs in the elderly when compared with the young men during recovery from exercise.
Exercise training has been established as an effective interventional strategy to stimulate skeletal muscle growth in both young and elderly people (Kosek et al. 2006; Petrella et al. 2006; Verdijk et al. 2009b; Fiatarone et al. 1994; Frontera et al. 1988; Leenders et al. 2013; Mackey et al. 2007; Tieland et al. 2012). The recruitment of SCs has been hypothesized to be imperative for sustained skeletal muscle hypertrophy to occur in response to anabolic stimuli (Adams et al. 2002; Rosenblatt and Parry 1992, 1993; Rosenblatt et al. 1994). Although some studies (McCarthy et al. 2011) have recently suggested that satellite cells are not necessary for muscle fiber growth in animal models, almost all human studies (Kadi and Thornell 2000; Petrella et al. 2008; Verdijk et al. 2009a; Verney et al. 2008) involving resistance-type exercise training show that muscle fiber hypertrophy is accompanied by myonuclear accretion, suggesting a contributing role of satellite cells. In line with previous studies (Dreyer et al. 2006; Cermak et al. 2013; McKay et al. 2009; McKay et al. 2012; O’Reilly et al. 2008; Snijders et al. 2012), we show that SC content increases by 49 ± 18 and 48 ± 15 % in the type I and type II muscle fibers during 72 h of post-exercise recovery in healthy young men (Fig. 3). Previous work has failed to show such measurable increases in type II muscle fiber SC content within 48 h of post-exercise recovery in elderly men (McKay et al. 2012). In agreement, we observed no changes in type II muscle fiber SC content during the initial 48 h of post-exercise recovery in our elderly subjects. However, we extend on previous work by showing a significant 34 ± 11 % increase in type II muscle fiber SC content following 72 h of post-exercise recovery in the older men (Fig. 3b). Interestingly, the increase in type II muscle fiber SC content assessed after 72 h of post-exercise recovery did not significantly differ between the healthy young and elderly men. This is the first study to show that the SC pool in the type II muscle fibers increases in response to a regular bout of resistance-type exercise in both young and elderly men, but this response is delayed in older compared with younger men.
To understand the mechanisms responsible for the delayed SC response to exercise in the older population, we determined the expression of MyoD and myogenin at the different time points. It is well established that MyoD acts to promote SC proliferation and transition of cells into differentiation (Megeney et al. 1996), while myogenin is known to drive terminal differentiation (Yablonka-Reuveni and Rivera 1994). In line with previous studies (McKay et al. 2008; Raue et al. 2006; Yang et al. 2005), we show that MyoD and myogenin mRNA expression are substantially upregulated in both young and elderly men during the first 12 to 48 h of post-exercise recovery. However, at 12 h post-exercise, the increase in myogenin mRNA expression was significantly greater in the young (∼9 ± 2-fold) compared with that in the elderly men (∼4 ± - fold). As mRNA expression is determined in mixed muscle tissue, changes in MyoD and myogenin mRNA expression do not necessarily reflect changes in SC function. Therefore, we also determined MyoD protein expression in the nuclear fraction of the muscle and assessed the number of MyoD + SCs in response to the single bout of exercise. In the present study, we show that MyoD expression tended to increase to a greater extent in the young (∼3-fold increase) compared to the elderly (no change) after 12 h of post-exercise recovery. In agreement, we observed a significant increase in the number of MyoD + SCs at 12 h of recovery in the young men, whereas no changes were observed in the elderly men (Fig. 4). The number of MyoD + SCs was significantly higher at 24, 48, and 72 h after exercise in both the young and older men when compared with baseline values, with a significantly greater increase observed after 48 h of post-exercise recovery in the young compared to the older men (Fig. 5). These results indicate that the SC regulatory pathways are activated to a lesser extent in the old compared with the healthy young during post-exercise recovery. This supports the idea that SC responsiveness is delayed and/or attenuated in healthy elderly men.
Myostatin has been shown to be a strong negative regulator of skeletal muscle growth. In animals, aging is accompanied by an increase in muscle myostatin concentrations (Kovacheva et al. 2010). In vitro studies have demonstrated that myostatin completely blocks myoblast proliferation and differentiation by the downregulation of MyoD (Taylor et al. 2001; Thomas et al. 2000; Langley et al. 2002). Likewise, the rate of SC proliferation in vivo has been reported to be significantly increased in myostatin knockout mice (McCroskery et al. 2003; McFarlane et al. 2011; Wagner et al. 2005). In contrast, pharmacological blockage of the myostatin/Activin A pathway appears to induce muscle fiber hypertrophy with little to no fusion of satellite cells to the myofiber (Lee et al. 2012). However, these in vitro and animal studies do not address the potential role of myostatin during muscle fiber growth in more physiological situations, like in response to a single bout of exercise or during adaptation to prolonged resistance-type exercise training in (aging) humans. In human skeletal muscle, myostatin protein expression has been reported to be ∼2-fold higher in healthy elderly men when compared with that in young controls (McKay et al. 2012). In addition, McKay et al. (2012) have recently shown that myostatin can be located within the SC in human skeletal muscle tissue and reported that the percentage of myostatin + SCs declines in skeletal muscle tissue during 48 h of post-exercise recovery. In line with these previous findings, the present study shows that the percentage of myostatin + SCs decreases substantially during recovery from exercise in both young and older men in response to a single bout of exercise (Fig. 4). However, after 12 and 48 h of post-exercise recovery, the decline in the number of myostatin + SCs was significantly smaller in the elderly when compared with the young. These results are in line with the blunted increase in the number of MyoD + SCs observed at the same time points. We speculate that during recovery from exercise, healthy elderly men are less able to downregulate myostatin in the SCs compared with their young controls. Such a reduced capacity to downregulate myostatin may be instrumental for the attenuated increase in SC number and, subsequently, a blunted myogenic response to prolonged exercise training.
Besides determining the localization of myostatin within the SC, we also assessed muscle myostatin mRNA and protein expression during post-exercise recovery. In the nuclear fraction of the muscle biopsy samples, myostatin protein expression was significantly upregulated within the first 12 h after exercise in the older men, whereas it had remained unchanged in the younger men. The upregulation of myostatin protein expression and/or translocation to the SC during post-exercise recovery provides further evidence that a blunted post-exercise myogenic response in the healthy elderly men may be mediated by myostatin. In contrast to myostatin protein expression, myostatin mRNA expression decreased significantly during post-exercise recovery, which is supported by many previous reports (Costa et al. 2007; Kim et al. 2005; McKay et al. 2012). Previous studies have shown that there is a Smad-7-dependent negative feedback loop through which increased myostatin protein expression is associated with decreased myostatin mRNA expression (Forbes et al. 2006; Kim et al. 2007). Such a relation may partly explain the observed discrepancy between myostatin mRNA and protein in the present study. Clearly, more research is warranted to elucidate the precise role of myostatin in skeletal muscle adaptation during post-exercise recovery, and the potential changes with aging herein.
Overall, the results from this study clearly indicate that the SC adaptive response during recovery from a single bout of resistance-type exercise is attenuated in the elderly subjects. This attenuated response may play an important role in the proposed reduced capacity of senescent muscle to increase muscle mass and/or fiber cross-sectional area after prolonged resistance-type exercise training (Kosek et al. 2006; Petrella et al. 2006). It could be speculated that after a single exercise session, elderly subjects require a more extensive recovery time between exercise bouts to optimize post-exercise reconditioning and, as such, maximize muscle hypertrophy. Studies that assess SC content and activation status at longer time intervals are required to elucidate the time it takes for SC content to peak in both young and elderly people during post-exercise recovery. Alternatively, elderly people may require subsequent bouts of exercise to increase SC content to the same level as healthy young individuals.
We conclude that type I and type II muscle fiber SC content increase during 72 h of recovery from a single bout of resistance-type exercise in both healthy young and elderly men. The increase in type II muscle fiber SC content during post-exercise recovery is delayed in the elderly and is accompanied by a blunted SC activation response.
References
- Adams GR, Caiozzo VJ, Haddad F, Baldwin KM. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J physiol Cell Physiol. 2002;283(4):C1182–1195. doi: 10.1152/ajpcell.00173.2002. [DOI] [PubMed] [Google Scholar]
- Bergström J (1975) Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35(7):609–916 [PubMed]
- Bish LT, Morine KJ, Sleeper MM, Sweeney HL. Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS One. 2010;5(4):e10230. doi: 10.1371/journal.pone.0010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ, Loon LJ. Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc. 2013;45(2):230–237. doi: 10.1249/MSS.0b013e318272cf47. [DOI] [PubMed] [Google Scholar]
- Costa A, Dalloul H, Hegyesi H, Apor P, Csende Z, Racz L, Vaczi M, Tihanyi J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol. 2007;101(4):427–436. doi: 10.1007/s00421-007-0510-z. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve. 2006;33(2):242–253. doi: 10.1002/mus.20461. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol. 2009;106(4):1403–1411. doi: 10.1152/japplphysiol.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol. 2006;206(1):264–272. doi: 10.1002/jcp.20477. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64(3):1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547(Pt 1):247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen JG, Nedergaard A, Andersen LL, Schjerling P, Andersen JL. Myostatin expression during human muscle hypertrophy and subsequent atrophy: increased myostatin with detraining. Scand J Med Sci Sports. 2011;21(2):215–223. doi: 10.1111/j.1600-0838.2009.01044.x. [DOI] [PubMed] [Google Scholar]
- Kadi F, Thornell LE. Concomitant increases in myonuclear and satellite cell content in female trapezius muscle following strength training. Histochem Cell Biol. 2000;113(2):99–103. doi: 10.1007/s004180050012. [DOI] [PubMed] [Google Scholar]
- Kadi F, Schjerling P, Andersen LL, Charifi N, Madsen JL, Christensen LR, Andersen JL. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558(Pt 3):1005–1012. doi: 10.1113/jphysiol.2004.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab. 2005;288(6):E1110–1119. doi: 10.1152/ajpendo.00464.2004. [DOI] [PubMed] [Google Scholar]
- Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated downregulation of myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol. 2007;103(5):1488–1495. doi: 10.1152/japplphysiol.01194.2006. [DOI] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101(2):531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151(2):628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277(51):49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- Lee S-J, Huynh T, Lee Y-S, Sebald S, Wilcox-Adelman S, Iwamori N, Lepper C, Matzuk M, Fan C-M. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A. 2012;109(35):60. doi: 10.1073/pnas.1211997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenders M, Verdijk LB, Hoeven LV, Kranenburg JV, Nilwik R, Wodzig WK, Senden JM, Keizer HA, Loon LJ. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542–552. doi: 10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed] [Google Scholar]
- Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Mackey AL, Esmarck B, Kadi F, Koskinen SO, Kongsgaard M, Sylvestersen A, Hansen JJ, Larsen G, Kjaer M. Enhanced satellite cell proliferation with resistance training in elderly men and women. Scand J Med Sci Sports. 2007;17(1):34–42. doi: 10.1111/j.1600-0838.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- Mackey A, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve. 2009;40(3):455–465. doi: 10.1002/mus.21369. [DOI] [PubMed] [Google Scholar]
- Mayhew JL, Prinster JL, Ware JS, Zimmer DL, Arabas JR, Bemben MG. Muscular endurance repetitions to predict bench press strength in men of different training levels. J Sports Med Phys Fitness. 1995;35(2):108–113. [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162(6):1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane C, Hui GZ, Amanda WZ, Lau HY, Lokireddy S, Xiaojia G, Mouly V, Butler-Browne G, Gluckman PD, Sharma M, Kambadur R. Human myostatin negatively regulates human myoblast growth and differentiation. Am J Physiol Cell Physiol. 2011;301(1):C195–C203. doi: 10.1152/ajpcell.00012.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle-lengthening contractions in humans. J Physiol. 2008;586(Pt 22):5549–5560. doi: 10.1113/jphysiol.2008.160176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, De Lisio M, Johnston AP, O’Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One. 2009;4(6):e6027. doi: 10.1371/journal.pone.0006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA, Parise G. Myostatin is associated with age-related human muscle stem cell dysfunction. FASEB J. 2012;26(6):2509–2521. doi: 10.1096/fj.11-198663. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94(23):12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev. 1996;10(10):1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve. 2008;38(5):1434–1442. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937–E946. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104(6):1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273(1 Pt 1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18-30 yr) and old (80-89 yr) women. J Appl Physiol. 2006;101(1):53–59. doi: 10.1152/japplphysiol.01616.2005. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Parry D. Gamma irradiation prevents compensatory hypertrophy of overloaded mouse extensor digitorum longus muscle. J Appl Physiol (Bethesda, Md : 1985) 1992;73(6):2538–2543. doi: 10.1152/jappl.1992.73.6.2538. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Parry D. Adaptation of rat extensor digitorum longus muscle to gamma irradiation and overload. Pflugers Arch - Eur J Physiol. 1993;423(3–4):255–264. doi: 10.1007/BF00374404. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Yong D, Parry D. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17(6):608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- Siriett V, Platt L, Salerno MS, Ling N, Kambadur R, Sharma M. Prolonged absence of myostatin reduces sarcopenia. J Cell Physiol. 2006;209(3):866–873. doi: 10.1002/jcp.20778. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, Beelen M, McKay BR, Parise G, Kadi F, van Loon LJ. A single bout of exercise activates skeletal muscle satellite cells during subsequent overnight recovery. Exp Physiol. 2012;97(6):762–773. doi: 10.1113/expphysiol.2011.063313. [DOI] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, McKay BR, Smeets JS, van Kranenburg J, Groen BB, Parise G, Greenhaff P, van Loon LJ. Acute dietary protein intake restriction is associated with changes in myostatin expression after a single bout of resistance exercise in healthy young men. J Nutr. 2014;144(2):137–145. doi: 10.3945/jn.113.183996. [DOI] [PubMed] [Google Scholar]
- Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, Kull FC, Jr, Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab. 2001;280(2):E221–228. doi: 10.1152/ajpendo.2001.280.2.E221. [DOI] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- Verney J, Kadi F, Charifi N, Feasson L, Saafi MA, Castells J, Piehl-Aulin K, Denis C. Effects of combined lower body endurance and upper body resistance training on the satellite cell pool in elderly subjects. Muscle Nerve. 2008;38(3):1147–1154. doi: 10.1002/mus.21054. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci U S A. 2005;102(7):2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witard OC, Tieland M, Beelen M, Tipton KD, van Loon LJ, Koopman R. Resistance exercise increases postprandial muscle protein synthesis in humans. Med Sci Sports Exerc. 2009;41(1):144–154. doi: 10.1249/MSS.0b013e3181844e79. [DOI] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164(2):588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol. 2005;98(5):1745–1752. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]
- Yin H, Price F, Rudnicki M. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]