Abstract
Offspring of parents with exceptional longevity (OPEL), who are more likely to carry longevity-associated genotypes, may age more successfully than offspring of parents with usual survival (OPUS). Maintenance of physical function is a key attribute of successful aging. While many genetic and non-genetic factors interact to determine physical phenotype in aging, examination of the contribution of exceptional parental longevity to physical function in aging is limited. The LonGenity study recruited a relatively genetically homogenous cohort of Ashkenazi Jewish (AJ) adults age 65 and older, who were defined as either OPEL (having at least one parent who lived to age 95 or older) or OPUS (neither parent survived to age 95). Subjective and objective measures of physical function were compared between the two groups, accounting for potential confounders. Of the 893 LonGenity subjects, 365 were OPEL and 528 were OPUS. OPEL had better objective and subjective measures of physical function than OPUS, especially on unipedal stance (p = 0.009) and gait speed (p = 0.002). Results support the protective role of exceptional parental longevity in preventing decline in physical function, possibly via genetic mechanisms that should be further explored.
Keywords: Aging, Genetics, Longevity, Physical Function
Introduction
Maintenance of physical function is a key component of most definitions of successful aging (Peel et al. 2005; Rowe and Kahn 1998). Good physical function is rated highly among attributes of successful aging in surveys of the general elderly population (Bowling and Dieppe 2005; Rowe and Kahn 1998). Emerging evidence from human and animal studies shows that genetics partially determine exceptional longevity, and associated successful aging phenotypes (Adams et al. 2008; Barzilai et al. 2006; Barzilai et al. 2003; Murabito et al. 2012; Newman et al. 2011). Offspring of parents with exceptional longevity (OPEL) who are more likely to carry longevity-associated genotypes may age more successfully than offspring of parents with usual survival (OPUS) (Barzilai et al. 2006; Barzilai et al. 2003). While many genetic and non-genetic factors (e.g., environment and disease) interact to determine the final physical phenotype in aging, there has been limited examination of the contribution of exceptional parental longevity to maintenance of physical function in aging (Frederiksen et al. 2002; Newman et al. 2011).
The LonGenity study, established in 2007, recruited a cohort of Ashkenazi Jewish (AJ) adults age 65 and older, who were defined as either OPEL (having at least one parent who lived to age 95 or older) or OPUS (neither parent survived to age 95). Relative genetic homogeneity of the AJ population (Seldin et al. 2006) provides a unique sample for comparisons within the cohort (Barzilai et al. 2006). Our group has identified several biomarkers and candidate mechanisms associated with longevity in a separate cohort of AJ adults not included in this study (Atzmon et al. 2009; Atzmon et al. 2008; Atzmon et al. 2005; Barzilai et al. 2006; Barzilai et al. 2003). In addition, we reported that OPEL have a healthier phenotype than OPUS with lower prevalence of chronic illnesses such as hypertension, diabetes mellitus, heart attacks, and strokes (Atzmon et al. 2004). This is consistent with results from studies of longevity in other cohorts indicating that offspring of centenarians are less susceptible to age-related diseases than those without parents with exceptional longevity (Adams et al. 2008; Terry et al. 2004). Since these risk factors are also implicated in the maintenance of physical function, we hypothesized that OPEL would have better physical function compared to OPUS.
Methods
The goal of the LonGenity study is to identify genotypes associated with longevity and their association with successful aging in AJ seniors. Majority of AJ participants in the LonGenity study were systematically recruited using public records such as voter registration lists. A smaller AJ sample was identified through contacts at synagogues, community organizations, and advertisements in Jewish newspapers (Verghese et al. 2006). Potential participants were contacted by mail and then by telephone to assess interest and eligibility. AJ adults age 65 and above were invited to our research center for participation. Exclusion criterion included diagnosis of dementia, severe visual or hearing impairments as well as having a sibling already enrolled in the study. The eligible sample included 365 OPEL and 528 OPUS participants.
Frailty diagnosis was operationalized using the Cardiovascular Health Study criteria defined as meeting three of more of the following criteria: unintentional weight loss, self-reported exhaustion, weak grip strength (handgrip dynamometer), slow gait speed, and low physical activity (Fried et al. 2001). Presence of depression, diabetes, heart failure, hypertension, myocardial infarction, strokes, Parkinson’s disease, chronic obstructive lung disease, and arthritis was used to calculate a summary illness index as previously described (Holtzer et al. 2006; Verghese et al. 2012).
Physical function—subjective:
Research assistants interviewed participants at the Aging Research Center at Albert Einstein College of Medicine using validated mobility assessment questionnaires (Verghese et al. 2004). The four physical function related questions examined for this study were: (1) “How far can you walk without a break on level ground?” (abnormal response: ¼ mile or less); (2) “Do you have difficulty walking?” (abnormal response: yes); (3) “Have you ever used a cane or walker?” (abnormal response: yes); and (4) “Do you have difficulty climbing up or down stairs?” (abnormal response: yes) (Verghese et al. 2008). Abnormal responses to the questions were determined based on sampling distributions in our studies, literature review, and clinical experience (Verghese et al. 2004). These questions were reported to be highly reliable, and have been validated against physical performance measures and functional status in older adults (Verghese et al. 2004; Verghese et al. 2008).
Physical function—objective:
We selected four established objective clinical markers of physical function focusing on lower extremity to complement our subjective measures. Gait speed is considered a geriatric vital sign, and predicts multiple adverse outcomes in older adults (Studenski et al. 2011; Verghese et al. 2009; Verghese et al. 2007b). As previously described (Verghese et al. 2007b), research assistants measured steady state gait using an 8.5-m long computerized walkway (180 × 35.5 × 0.25 in.) with embedded pressure sensors (GAITRite; CIR Systems, PA). Participants were asked to walk at their normal pace in a quiet well-lit hallway wearing comfortable footwear and without any attached monitoring devices. Start and stop points were marked by white lines on the floor, and included four feet from the walkway edge for initial acceleration and terminal deceleration. Based on footfalls recorded on the walkway, the software automatically computes gait parameters such as gait speed (cm/s). The GAITRite system is widely used in clinical and research settings, and excellent reliability has been reported in our and other centers (Devos et al. 2007; Verghese et al. 2007a; Verghese et al. 2007b). Research assistants also measured time in seconds to climb up three steps, a quick and valid clinical measure for assessing risk of functional decline (Oh-Park et al. 2012). Another physical assessment included unipedal stance, which measures the ability to stand on one foot (maximum 30 s), and is a clinical test of balance that is also a good predictor of falls (Hurvitz et al. 2001; Hurvitz et al. 2000). Lastly, time to get up five times from a chair unassisted was evaluated as a measure of lower extremity strength and balance (Guralnik et al. 2000).
Statistical analysis:
Subject characteristics were summarized with descriptive statistics. We used multivariate logistic (categorical) and linear (continuous) regression analysis to test cross-sectional associations of OPEL versus OPUS status with the subjective and objective physical function measures, adjusted for age, gender, years of education, and body mass index (BMI). Results are reported as adjusted mean differences in OPUS and OPEL samples. Odds ratios (OR) (logistic) or beta coefficients (linear) with 95 % confidence intervals (CI) are also reported using OPUS as the reference group. All analyses were performed using SPSS, version 20.
Results
Study population:
Table 1 presents subject characteristics. The 365 (40.9 %) OPEL were younger than the 528 (59.1 %) OPUS (75.1 vs. 77.1 years, p < 0.001), and included a higher proportion of women (59.2 vs. 52.1 %, p = 0.036). Education was statistically higher in OPEL, though the absolute difference was only 0.5 years. OPEL reported fewer illnesses than OPUS (1.19 vs. 1.45, p = 0.001). Among individual illnesses, OPUS had significantly higher prevalence of hypertension and strokes. Prevalence of frailty was higher in OPUS than OPEL (12.5 vs. 11.4 %), though the difference was not significant.
Table 1.
Subject characteristics of OPUS and OPEL
| Description | OPEL | OPUS | p value |
|---|---|---|---|
| (n = 365) | (n = 528) | ||
| Age, mean ± SD | 75.04 ± 6.07 | 77.14 ± 7.04 | <0.001 |
| Sex (% female), mean ± SD | 59.18 ± 0.49 | 52.20 ± 0.50 | 0.036 |
| Education (years), mean ± SD | 17.50 ± 2.97 | 17.00 ± 2.95 | 0.013 |
| Grip strength (kg/m2), mean ± SD | 12.64 ± 11.21 | 12.45 ± 11.58 | 0.810 |
| BMI (kg/m2), mean ± SD | 27.72 ± 6.03 | 27.60 ± 4.55 | 0.753 |
| Summary illness index, mean ± SD | 1.19 ± 1.05 | 1.45 ± 1.09 | 0.001 |
| Depression, n (%) | 68 (20) | 96 (21) | 0.709 |
| Diabetes, n (%) | 26 (8) | 52 (11) | 0.079 |
| Heart failure, n (%) | 3 (1) | 8 (2) | 0.296 |
| Hypertension, n (%) | 132 (43) | 224 (52) | 0.015 |
| Myocardial infarction, n (%) | 15 (4) | 34 (7) | 0.075 |
| Stroke, n (%) | 5 (1) | 26 (6) | 0.002 |
| Parkinson’s disease, n (%) | 3 (1) | 8 (2) | 0.298 |
| Chronic obstructive lung disease, n (%) | 13 (4) | 17 (4) | 0.943 |
| Arthritis, n (%) | 141 (43) | 191 (45) | 0.696 |
| aFrailty (%) | 11.43 | 12.50 | 0.793 |
aMeets Cardiovascular Health Study criteria for frailty (3 or more out of 5 features) (Fried et al. 2001)
Physical function:
Table 2 indicates that OPEL reported fewer problems on all subjective physical function questions compared to OPUS, though the differences were only significant for use of an assistive device (OR 0.51, 95 % CI 0.28–0.93) when adjusted for age, sex, education, and BMI.
Table 2.
Subjective and objective physical function measures in OPEL and OPUS adjusted for age, sex, and education years
| Subjective physical function measuresa | OPEL | OPUS | Adjusted odds ratio (95 % CI) | p value |
|---|---|---|---|---|
| Walk ≤ ¼ mile without break, % (SE) | 9.4 (1.7) | 13.5 (1.5) | 0.58 (0.33; 1.02) | 0.059 |
| Has difficulty walking, % (SE) | 14.5 (2.0) | 16.8 (1.7) | 0.84 (0.53; 1.32) | 0.442 |
| Used a cane or walker, % (SE) | 7.5 (1.7) | 12.6 (1.4) | 0.51 (0.28; 0.93) | 0.027 |
| Difficulty climbing up or down stairs, % (SE) | 2.98 (2.5) | 36.6 (2.1) | 0.70 (0.50; 0.99) | 0.045 |
| Objective physical function measuresb | Beta coefficient (95 % CI) | |||
| Gait speed (cm/s), mean (SE) | 111.16 (1.18) | 106.67 (1.01) | 4.49 (1.41; 7.58) | 0.004 |
| Stair climb up (s), mean (SE) | 1.96 (0.06) | 2.08 (0.05) | −0.12 (−0.27; 0.03) | 0.110 |
| Unipedal stance (s), mean (SE) | 16.94 (0.53) | 14.94 (0.44) | 2.00 (0.63; 3.36) | 0.004 |
| Chair rise (s), mean (SE) | 10.70 (0.22) | 11.21 (0.19) | −0.51 (−1.07; 0.05) | 0.075 |
aBinary logistic regression adjusted for age, sex, education, and BMI
bLinear regression adjusted for age, sex, education, and BMI
OPEL also performed better on all objective physical function measures compared to OPUS. Differences were significant for gait velocity (p = 0.004) and unipedal stance (p = 0.004), after adjustments for age, sex, education, and BMI. Inclusion of quadratic term in the model to account for possible non-linear age trends did not materially change the results (data not shown). All continuous traits were examined visually and statistically, and met normality assumptions except for unipedal stance. The result remained significant when comparing log transformed unipedal stance values between OPEL and OPUS after adjustments for age, sex, education (0.07, 95 % CI 0.02–0.13, p = 0.007). OPUS took longer to climb up stairs and perform the chair rise assessment, though differences were not significant in adjusted models.
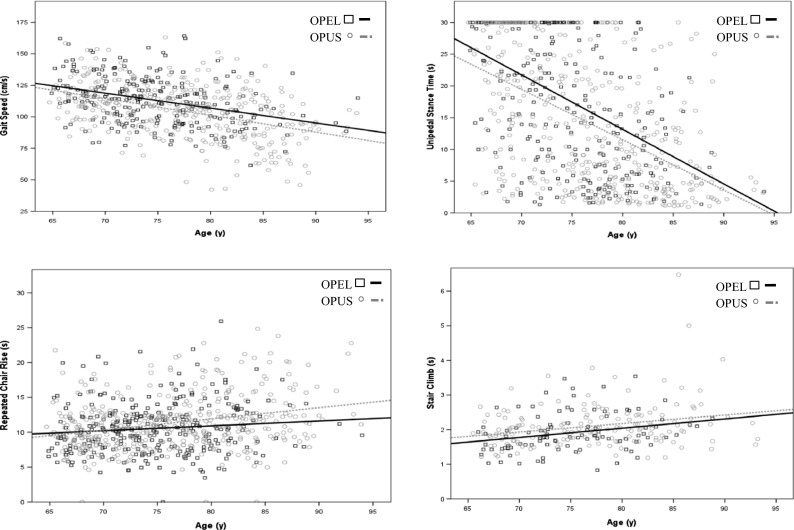
Figure 1 graphically presents the association of age with the physical function measures by parental longevity. The figures show that at any given age, OPEL have better physical function than OPUS.
Fig. 1.
Scatter Plots of quantitative physical function measures for OPEL and OPUS vs. age
Discussion
Overall, the OPEL in the LonGenity cohort had better performance on the four selected subjective and four objective physical function measures compared to OPUS, though differences were significant on few measures. These results are consistent with our hypothesis that persons with long-lived parents may enjoy not only a longer life but one relatively spared from physical functioning declines. Our results are supported by a previous analysis of three Danish population-based studies that reported parental longevity was associated with better physical and cognitive function measures in the adult offspring; almost all the effects were seen solely in the cohort of 70 + year-olds (similar to the mean age of the LonGenity cohort), but not among middle-aged or nonagenarian subjects (Frederiksen et al. 2002). While lifestyle factors are important in maintaining physical function in aging, a previous study showed that physical activity, smoking, alcohol consumption, and dietary habits in OPEL did not significantly differ from the general population (Rajpathak et al. 2011). An analysis in the population-based Danish twin registry showed that the effect of genetic factors on functional abilities increases with age and accounts for one third to one half of the variation among women aged 80 years and older (Christensen et al. 2000).
The LonGenity cohort includes a non-disabled, ambulatory, community-dwelling sample, which might have minimized subjective reports of physical limitations overall and between our study groups. However, the differences on all subjective measures between OPUS and OPEL were in the expected direction, with worse performance reported in the OPUS group. A stronger trend was seen with the objective physical function measures, which may be more sensitive to early physical function decline in high functioning adults (Verghese et al. 2012). These results are consistent with conclusions described by Newman and colleagues indicating that continuous measures of physical function, like gait speed, may be sensitive for detecting “rate of aging” and early signs of future disability (Newman et al. 2011). Closer examination of the objective measures showed that age adjusted mean performance for both groups was in the normal range reported in our and other studies indicating the overall high functional status of our cohort (Guralnik et al. 2000; Oh-Park et al. 2010; Oh-Park et al. 2011; Springer et al. 2007). Hence, the group differences seen in this study might be identifying early and mild signs of age-related physical function decline. In particular, the small variance in frailty prevalence along with a strong group difference in gait speed, one of the key criteria of frailty (Fried et al. 2001), indicate support of early signs of decline in physical function in the OPUS group.
The large sample of a relatively genetically homogeneous population and use of validated subjective and objective measures of physical function are strengths of this study; however, limitations are noted. The cross-sectional design does not permit causal inferences. Although previous studies from our group, conducted in a separate cohort of AJ adults, suggest a genetic explanation for phenotypic differences (Atzmon et al. 2005; Barzilai et al. 2006), further mechanistic studies are required. Physical function measures such as gait speed may be linked with specific genotypes via the effect of gene(s) on both brain and peripheral (muscle, nerve, and vasculature) processes. Our studies in other aging cohorts have linked functional polymorphisms in COMT and APOE genotypes to gait velocity in older adults (Holtzer et al. 2010; Holtzer et al. 2013). While stronger associations with physical function measures might be seen in individuals with both parents with exceptional longevity, we only had 12 such individuals, which was not sufficient to test this hypothesis. The influence of health behaviors such as physical activity, which play an important healthy aging, on longitudinal changes in physical function in OPUS and OPEL should be further studied. We mainly focused on lower extremity subjective and objective measures in this analysis but other physical measures might shed additional light on functional correlates of exceptional parental longevity. The physical function comparisons were adjusted for age, sex, education, and BMI; however, given the higher mean age of OPUS, group differences in disease prevalence should be cautiously interpreted.
Results of this study and others (Atzmon et al. 2005; Frederiksen et al. 2002; Holtzer et al. 2010) provide evidence that variation in late-life physical function and frailty is attributable to both environmental and genetic factors, and that genetic factors may become increasingly important with aging. Longitudinal studies of physical function and the effect of parental longevity are necessary to validate results of this study and provide important clues of the genetic factors that mediate aging and susceptibility to frailty and functional decline.
Acknowledgments
This work was supported by grants from NIH P01AG021654 (NB, JC), R01 AG 046949 (Nir Barzilai, Sofiya Milman, Jill P. Crandall) the Nathan Shock Center of Excellence for the Biology of Aging P30AG038072 (NB), Glenn Center for the Biology of Human Aging Paul Glenn Foundation Grant (Nir Barzilai). Sofiya Milman is a recipient of the Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Research in Aging Grant. Jill P. Crandall is a recipient of The Paul Glenn Foundation Award for Research in the Biological Mechanisms of Aging. The project described was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Adams ER, Nolan VG, Andersen SL, Perls TT, Terry DF. Centenarian offspring: start healthier and stay healthier. J Am Geriatr Soc. 2008;56:2089–2092. doi: 10.1111/j.1532-5415.2008.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Schechter C, Greiner W, Davidson D, Rennert G, Barzilai N. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- Atzmon G, Rincon M, Rabizadeh P, Barzilai N. Biological evidence for inheritance of exceptional longevity. Mech Ageing Dev. 2005;126:341–345. doi: 10.1016/j.mad.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Atzmon G, et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci. 2008;63:447–453. doi: 10.1093/gerona/63.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzmon G, Barzilai N, Surks MI, Gabriely I. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab. 2009;94:4768–4775. doi: 10.1210/jc.2009-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. Jama. 2003;290:2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Derby CA, Bauman JM, Lipton RB. A genotype of exceptional longevity is associated with preservation of cognitive function. Neurology. 2006;67:2170–2175. doi: 10.1212/01.wnl.0000249116.50854.65. [DOI] [PubMed] [Google Scholar]
- Bowling A, Dieppe P. What is successful ageing and who should define it? BMJ. 2005;331:1548–1551. doi: 10.1136/bmj.331.7531.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, McGue M, Yashin A, Iachine I, Holm NV, Vaupel JW. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2000;55:M446–452. doi: 10.1093/gerona/55.8.M446. [DOI] [PubMed] [Google Scholar]
- Devos D, et al. Improvement of gait by chronic, high doses of methylphenidate in patients with advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:470–475. doi: 10.1136/jnnp.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen H, et al. Do children of long-lived parents age more successfully? Epidemiology. 2002;13:334–339. doi: 10.1097/00001648-200205000-00015. [DOI] [PubMed] [Google Scholar]
- Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.M221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Ozelius L, Xue X, Wang T, Lipton RB, Verghese J. Differential effects of COMT on gait and executive control in aging. Neurobiol Aging. 2010;31:523–531. doi: 10.1016/j.neurobiolaging.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, Wang C, Verghese J. Performance variance on walking while talking tasks: theory, findings, and clinical implications, age. Netherlands: Dordrecht; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurvitz EA, Richardson JK, Werner RA, Ruhl AM, Dixon MR. Unipedal stance testing as an indicator of fall risk among older outpatients. Arch Phys Med Rehabil. 2000;81:587–591. doi: 10.1016/S0003-9993(00)90039-X. [DOI] [PubMed] [Google Scholar]
- Hurvitz EA, Richardson JK, Werner RA. Unipedal stance testing in the assessment of peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:198–204. doi: 10.1053/apmr.2001.17830. [DOI] [PubMed] [Google Scholar]
- Murabito JM, Yuan R, Lunetta KL. The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J Gerontol A Biol Sci Med Sci. 2012;67:470–479. doi: 10.1093/gerona/gls089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, et al. Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging. 2011;3:63–76. doi: 10.18632/aging.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Park M, Holtzer R, Xue X, Verghese J. Conventional and robust quantitative gait norms in community-dwelling older adults. J Am Geriatr Soc. 2010;58:1512–1518. doi: 10.1111/j.1532-5415.2010.02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Park M, Wang C, Verghese J. Stair negotiation time in community-dwelling older adults: normative values and association with functional decline. Arch Phys Med Rehabil. 2011;92:2006–2011. doi: 10.1016/j.apmr.2011.07.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-Park M, Perera S, Verghese J. Clinically meaningful change in stair negotiation performance in older adults. Gait Posture. 2012;36:532–536. doi: 10.1016/j.gaitpost.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel NM, McClure RJ, Bartlett HP. Behavioral determinants of healthy aging. Am J Prev Med. 2005;28:298–304. doi: 10.1016/j.amepre.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Rajpathak SN, Liu Y, Ben-David O, Reddy S, Atzmon G, Crandall J, Barzilai N. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Kahn R. Successful aging. New York: Random House (Pantheon); 1998. [Google Scholar]
- Seldin MF, et al. European population substructure: clustering of northern and southern populations. PLoS Genet. 2006;2:e143. doi: 10.1371/journal.pgen.0020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer BA, Marin R, Cyhan T, Roberts H, Gill NW. Normative values for the unipedal stance test with eyes open and closed. J Geriatr Phys Ther. 2007;30:8–15. doi: 10.1519/00139143-200704000-00003. [DOI] [PubMed] [Google Scholar]
- Studenski S, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry DF, Wilcox MA, McCormick MA, Pennington JY, Schoenhofen EA, Andersen SL, Perls TT. Lower all-cause, cardiovascular, and cancer mortality in centenarians’ offspring. J Am Geriatr Soc. 2004;52:2074–2076. doi: 10.1111/j.1532-5415.2004.52561.x. [DOI] [PubMed] [Google Scholar]
- Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall CB, Lipton RB. Reliability and validity of a telephone-based mobility assessment questionnaire. Age Ageing. 2004;33:628–632. doi: 10.1093/ageing/afh210. [DOI] [PubMed] [Google Scholar]
- Verghese J, LeValley A, Hall CB, Katz MJ, Ambrose AF, Lipton RB. Epidemiology of gait disorders in community-residing older adults. J Am Geriatr Soc. 2006;54:255–261. doi: 10.1111/j.1532-5415.2005.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X, Buschke H, Pahor M. Walking while talking: effect of task prioritization in the elderly. Arch Phys Med Rehabil. 2007;88:50–53. doi: 10.1016/j.apmr.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Xue X, Holtzer R. Self-reported difficulty in climbing up or down stairs in nondisabled elderly. Arch Phys Med Rehabil. 2008;89:100–104. doi: 10.1016/j.apmr.2007.08.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60:1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]