Abstract
The acute effects of stretching on peak force (Fpeak), percent voluntary activation (%VA), electromyographic (EMG) amplitude, maximum range of motion (MROM), peak passive torque, the passive resistance to stretch, and the percentage of ROM at EMG onset (%EMGonset) were examined in 18 young and 19 old men. Participants performed a MROM assessment and a maximal voluntary contraction of the plantarflexors before and immediately after 20 min of passive stretching. Fpeak (−11 %), %VA (−6 %), and MG EMG amplitude (−9 %) decreased after stretching in the young, but not the old (P > 0.05). Changes in Fpeak were related to reductions in all muscle activation variables (r = 0.56–0.75), but unrelated to changes in the passive resistance to stretch (P ≥ 0.24). Both groups experienced increases in MROM and peak passive torque and decreases in the passive resistance to stretch. However, the old men experienced greater changes in MROM (P < 0.001) and passive resistance (P = 0.02–0.06). Changes in MROM were correlated to increases in peak passive torque (r = 0.717), and the old men also experienced a nonsignificant greater (P = 0.08) increase in peak passive torque. %EMGonset did not change from pre- to post-stretching for both groups (P = 0.213), but occurred earlier in the old (P = 0.06). The stretching-induced impairments in strength and activation in the young but not the old men may suggest that the neural impairments following stretching are gamma-loop-mediated. In addition, the augmented changes in MROM and passive torque and the lack of change in %EMGonset for the old men may be a result of age-related changes in muscle-tendon behavior.
Keywords: Stretching-induced force deficit, Muscle activation, Range of motion, Stretch tolerance, Passive torque
Introduction
Since the original work of Kokkonen et al. (1998) and Fowles et al. (2000), many authors have examined the acute effects of stretching on various measures of muscular performance. Recent reviews (Kay and Blazevich 2012; McHugh and Cosgrave 2010) have suggested that prolonged passive stretching may temporarily compromise the muscles’ ability to produce maximal force, a phenomenon that has been termed the “stretching-induced force deficit.” As a result of these findings, the American College of Sports Medicine (ACSM 2010) recently revised their stretching guidelines and suggested that “…for sport activities where muscular strength, power, and endurance are important for performance, it is recommended that stretching be performed following activity rather than during the warm-up period” (p. 173).
There have been two general hypotheses to explain the stretching-induced force deficit: (a) neural factors or a reduction in muscle activation and (b) mechanical factors, whereby a reduction in the passive resistance to stretch results in a subsequent alteration of the angle-torque relationship. For example, previous authors have reported stretching-induced reductions in surface electromyographic (EMG) amplitude, percent voluntary activation (%VA), and V-wave amplitude (Behm et al. 2001; Cramer et al. 2005; Fowles et al. 2000; Herda et al. 2008, 2009; Trajano et al. 2013).
Although the precise mechanism responsible for the reduction in central drive is unclear, previous authors (Herda et al. 2009) have suggested that the reduction in muscle activation is due to an altered gamma loop. This was suggested by Herda et al. (2009) who demonstrated that 20 min of passive stretching and Achilles tendon vibration resulted in similar decreases in isometric peak toque, %VA, and EMG amplitude. The authors (Herda et al. 2009) proposed that passive stretching like vibration may suppress Ia-afferent activity and diminish gamma loop function. Interestingly, Richardson et al. (2006) further investigated gamma loop function by examining the influence of aging and anterior cruciate ligament (ACL) reconstruction on Ia-afferent activity and reported that the young healthy group experienced decreases in maximal strength and EMG amplitude following 20 min of infrapatellar vibration, whereas the ACL reconstructed and elderly group experienced no changes in either strength or muscle activation following vibration. The authors (Richardson et al. 2006) suggested that the elderly and ACL reconstructed group had an existing impaired gamma loop, which may be due to decreased muscle spindle sensitivity causing the vibration to have no influence on strength or muscle activation. Thus, based on these findings, if an impaired gamma loop is the neural mechanism responsible for the stretching-induced force deficit, it is possible that older adults would not experience any decrements in %VA or EMG amplitude following passive stretching due to an existing impaired gamma loop.
Other studies (Fowles et al. 2000; Herda et al. 2008; McHugh and Nesse 2008; Weir et al. 2005) have indicated that mechanical factors may also explain the stretching-induced force deficit. In theory, decreases in the passive resistance to stretch may result in a rightward shift in the angle-torque relationship, where isometric strength decreases at the shortest muscle lengths (Herda et al. 2008; McHugh and Nesse 2008). In addition, the stretching-induced increase in muscle-tendon unit compliance has also been reported to reduce evoked peak twitch force or torque (Fowles et al. 2000; Ryan et al. 2008b; Trajano et al. 2013) and increase the electromechanical delay (Costa et al. 2010). Therefore, the purpose of the present study was to examine the influence of an acute prolonged bout of intermittent passive stretching of the plantarflexors on maximal strength, muscle activation, maximum joint range of motion (MROM), stretch tolerance (i.e., peak passive torque), the passive resistance to stretch, and the percentage of ROM at EMG onset (%EMGonset) in both young and old adults. We hypothesized that passive stretching would elicit similar mechanical changes in both the young and old men; however, the old but not the young men would not experience stretching-induced reductions in muscle activation following the passive stretching due to an existing impaired gamma loop.
Methods
Subjects
Eighteen young (mean ± SD age = 22.6 ± 1.9 years; mass = 81.6 ± 13.7 kg; stature = 176.8 ± 5.9 cm) and 19 old (69.2 ± 4.9 years; 82.6 ± 12.1 kg; 176.1 ± 7.4 cm) men volunteered for this investigation. None of the participants reported any current or ongoing neuromuscular diseases or musculoskeletal injuries specific to the ankle, knee, or hip joints. The young men self-reported participating in 2.1 ± 1.4 h of aerobic activities (i.e., walking, jogging), 3.7 ± 2.3 h of resistance training, and 1.9 ± 2.1 h of recreational sports per week. The older men self-reported participating in 4.5 ± 4.1, 1.6 ± 1.9, and 1.0 ± 2.4 h of aerobic, resistance training, and recreational sports per week, respectively. This study was approved by the University Institutional Review Board for Human Subjects Research.
Experimental design
A between-subjects design with repeated measures [time (pre- vs. post-stretching) × group (young vs. old)] was used to examine the acute effects of repeated passive stretching on isometric peak force (Fpeak), %VA, EMG amplitude of the soleus (SOL) and medial gastrocnemius (MG) muscles, MROM, peak passive torque, the passive resistance to stretch, and %EMGonset of the plantarflexor muscles. The participants visited the laboratory on two occasions separated by 2–7 days. The first visit was a familiarization trial, and the subsequent visit was the experimental session. During the experimental session, each participant underwent a MROM and isometric strength assessment prior to and immediately following nine stretches held for 135 s at their maximum tolerated torque threshold (described below) with 20 s of rest between stretches (approximately 20 min of time under stretch) (Herda et al. 2009). A 5-min rest period was allowed between the pre-stretching assessments and the stretching protocol. All post-testing strength and ROM assessments were performed 9.1 ± 1.2 min following the end of the stretching protocol.
Familiarization trial
Prior to the experimental session, each participant signed a written informed consent form, completed a health status questionnaire, and practiced the twitch interpolation procedure and the MROM assessments to ensure that they were comfortable with the procedures and to minimize any potential learning effects. The stretch intensity or maximum tolerable torque threshold was determined during a series of passive stretches with the dynamometer programmed in passive mode as described previously (Ryan et al. 2008a). The torque threshold was progressively increased to the point of discomfort, but not pain, as verbally acknowledged by the participant. This predetermined torque threshold was used during the experimental session for the repeated passive stretching protocol.
Isometric strength assessment
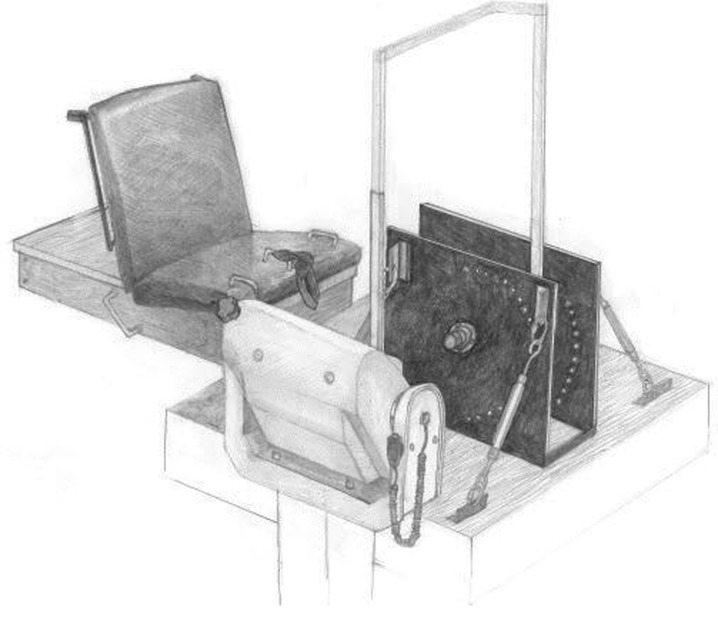
To determine Fpeak and %VA, each participant performed two 3–5-s isometric maximal voluntary contractions (MVCs) before and after the repeated stretching protocol at an ankle joint angle of approximately 1° of dorsiflexion (DF) between the foot and leg (0° = neutral) with a knee flexion angle of 0° below the horizontal plane (full extension). Each MVC was performed on a custom-built load cell (Omegadyne, model LC402, range 0–5,000 lbs; Stamford, CT, USA) apparatus (Fig. 1) attached to a calibrated Biodex System 3 dynamometer (Biodex Medical Systems, Inc. Shirley, NY, USA). The load cell was bolted between 0.38 in. (9.5 mm) steel plates, with the foot strapped to the top plate that was bolted to the compression side of the load cell. The metatarsals (ball of the foot) were positioned over the center of the load cell. The participants were seated with restraining straps over the pelvis and thigh with the lateral malleolus of the fibula aligned with the input axis of the dynamometer. In addition, the foot was secured in a heel cup attached to a footplate with the toe and ankle straps over the metatarsals and malleoli. Two minutes of rest was allowed between each MVC and each participant was instructed to give a maximal voluntary effort for each trial while strong verbal encouragement was provided by the investigators.
Fig. 1.
A picture of the custom-built load cell apparatus attached to a calibrated isokinetic dynamometer
Percent voluntary activation
The twitch interpolation technique was used to determine %VA. Transcutaneous electrical stimuli were delivered to the tibial nerve using a high-voltage (maximal voltage = 400 V) constant-current stimulator (Digitimer DS7AH, Herthfordshire, UK). The cathode was a metal probe (8 mm diameter) with the tip covered in a saline-soaked sponge, which was pressed over the tibial nerve in the popliteal fossa. The anode was a 9.5-cm rectangular self-adhesive electrode (Durastick Supreme, Chattanooga Group, Hicton, TN, USA) that was positioned between the patella and the tibial tuberosity. Single square wave stimuli (1 ms in duration) were used to determine the optimal probe location (40 mA) as well as the maximal compound muscle action potential (M-wave) with incremental amperage increases. Once a plateau in the peak-to-peak M-wave was determined, despite amperage increases, 20 % was added to the amperage that yielded the highest peak-to-peak M-wave to assure a supramaximal stimulus. Doublets (two single stimuli delivered successively at 100 Hz) were administered approximately 500 ms into the MVC plateau (superimposed twitch) and then again 3 s after the MVC trial at rest (potentiated twitch) to increase the signal-to-noise ratio and to minimize the series of elastic effects on evoked torque production (Desbrosses et al. 2006). Percent voluntary activation was calculated using the following equation described by Allen et al. (1995):
 |
Surface electromyography
Preamplified bipolar, active surface electrodes (EL254S Biopac Systems, Santa Barbara, CA, USA; gain = 350 and interelectrode distance of 20 mm) were placed over the MG and SOL muscles (Hermens et al. 1999). A single, pre-gelled, disposable reference electrode (Ag-Ag Cl Quinton Quick Prep; Quinton Instruments Co, Bothell, WA) was placed over the spinous process of the seventh cervical vertebra (Hermens et al. 1999). The skin was shaved, lightly abraded, and cleaned with isopropyl alcohol prior to the placement of the electrodes.
Maximum range of motion
MROM was determined for each participant using a Biodex isokinetic dynamometer programmed in passive mode. Each participant completed two assessments (at both pre- and post-stretching) where the dynamometer passively dorsiflexed the ankle at 5° s−1 starting at 25° of plantar flexion (PF) to the participants’ maximally tolerated range of motion as described previously by Magnusson et al. (1996). The dynamometer was manually stopped by the investigators when the participant acknowledged the momentary onset of pain and was then immediately returned to the starting position. All participants were instructed to remain as quiet and relaxed as possible. The assessment with the greatest MROM value was used for all subsequent analyses at both pre- and post-stretching. In addition, all MROM values are reported as the total ROM achieved from 25° of PF to maximum DF (e.g., 25°PF + 20°DF = 45° of ROM).
Signal processing
The torque (Nm), position (°), and EMG (μV) signals were sampled simultaneously at 1 kHz for the MROM assessments, and the force (N) and EMG (μV) signals were sampled at 2 kHz for the twitch interpolation procedure with a Biopac data acquisition system (MP150WSW; Biopac Systems). All signals were stored on a personal computer (Dell Inspiron 8200; Dell, Inc., Round Rock, TX) to be processed offline using custom written software (LabVIEW 8.5; National Instruments). All EMG signals were filtered with a pass band of 10–500 Hz using a zero-phase shift fourth-order Butterworth filter. The torque and position signals from the dynamometer and the force signal from the load cell were smoothed with a zero-phase shift 100-point moving averager. During the twitch interpolation procedure, the amplitude of the EMG signal was calculated using a root mean squared (RMS) function and normalized to the pre-stretching MVC values. Isometric MVC Fpeak was calculated as the average force value during a 250-ms epoch taken immediately before the superimposed twitch. The same concurrent 250-ms epochs were selected for the EMG signals of the MG and SOL muscles. During the MROM assessment, peak and joint angle-specific torque values were calculated at 10°PF, 5°PF, neutral, 5°DF, and 10°DF, following a gravity correction for the weight of the footplate. In addition, EMG onset was defined as the joint angle at which EMG activity was three standard deviations above baseline for 100 ms for either the MG or SOL muscles (Blazevich et al. 2012).
Statistical analyses
Physical characteristics of the participants were examined using independent samples t tests. Six separate two-way mixed factorial ANOVAs [time × group] were used to analyze Fpeak, %VA, normalized EMG amplitude for the SOL and MG, MROM, peak passive torque, and %EMGonset. A three-way mixed factorial ANOVA [time × group × angle] was used to analyze joint angle-specific passive torque values. When a significant interaction was found, follow-up analyses were performed using lower ordered ANOVAs with Bonferroni corrections and t tests. In addition, to examine the differences between groups at post-stretching, all non-normalized post-stretching values were examined using an ANCOVA (Vickers 2001) with the pre-stretching values serving as the covariate following verification of the homogeneity of regression assumption. Non-normally distributed variables were log-transformed before analysis. Pearson product moment correlation coefficients were computed to examine the relationships between changes in Fpeak and changes in muscle activation (%VA, normalized EMG amplitude of the SOL and MG) and changes in the passive resistance to stretch. The relationship between changes in MROM and peak passive torque was also examined. Statistical analyses were performed using SPSS version 19.0 (IBM SPSS Data Collection, Chicago, IL, USA). An alpha of P ≤ 0.05 was used to determine statistical significance.
Results
Table 1 contains the raw pre- and post-stretching values (mean ± SE) for all the dependent variables. There was a significant difference in age (P < 0.001), but not body mass (P = 0.81) or stature (P = 0.73) between the young and old.
Table 1.
Pre- and post-stretching values (mean ± SE) for all dependent variables
| Age group | Pre-stretching | Post-stretching | |
|---|---|---|---|
| Isometric peak force (N) | Y | 493.5 ± 20.3** | 438.5 ± 22.3*,** |
| O | 379.2 ± 24.0 | 370.5 ± 24.9*,*** | |
| Voluntary activation (%) | Y | 93.1 ± 1.2 | 87.6 ± 2.9* |
| O | 92.1 ± 1.4 | 91.9 ± 1.3 | |
| SOL EMG amplitude (%MVC) | Y | 100 | 100.5 ± 5.0 |
| O | 100 | 107.5 ± 2.6 | |
| MG EMG amplitude (%MVC) | Y | 100 | 90.5 ± 4.7 |
| O | 100 | 104.0 ± 3.7** | |
| Maximum range of motion (°) | Y | 45.0 ± 2.1 | 50.8 ± 2.2* |
| O | 39.7 ± 1.8 | 48.7 ± 2.0*,*** | |
| Peak passive torque (Nm) | Y | 44.4 ± 5.1 | 49.2 ± 5.6* |
| O | 37.5 ± 3.2 | 47.6 ± 4.3* | |
| Passive torque 10°PF (Nm) | Y | 6.0 ± 0.5 | 4.6 ± 0.5* |
| O | 6.7 ± 1.0 | 3.9 ± 0.8*,*** | |
| Passive torque 5°PF (Nm) | Y | 8.5 ± 0.5 | 6.6 ± 0.5* |
| O | 9.8 ± 1.5 | 6.1 ± 1.0*,*** | |
| Passive torque neutral (Nm) | Y | 11.7 ± 0.7 | 9.2 ± 0.7* |
| O | 14.2 ± 2.1 | 8.9 ± 1.3*,*** | |
| Passive torque 5°DF (Nm) | Y | 16.0 ± 1.0 | 12.8 ± 0.9* |
| O | 18.5 ± 2.5 | 13.0 ± 1.7*,*** | |
| Passive torque 10°DF (Nm) | Y | 22.3 ± 1.5 | 17.5 ± 1.3* |
| O | 23.8 ± 2.9 | 18.6 ± 2.3* | |
| % Range of motion at EMG onset (%)a | Y | 82.1 ± 4.2 | 85.1 ± 4.4 |
| O | 66.5 ± 6.0 | 69.4 ± 4.2 |
Y young, O old, SOL soleus, MG medial gastrocnemius, EMG electromyography
*P ≤ 0.05, significant difference from pre-stretching; **P ≤ 0.05, significant difference between young and old; ***P ≤ 0.05, significant difference between young and old via an ANCOVA
aEMG onset data includes only the participants that displayed EMG activity; see text for more details
For MVC Fpeak (Fig. 2), there was a two-way interaction (P < 0.01). MVC Fpeak was greater in the young compared to the old at all time points (P ≤ 0.05); however, Fpeak decreased from pre- to post-stretching (P < 0.001) for the young (−11.36 ± 10.90 %), but did not change for the old (−2.49 ± 7.32 %; P = 0.18). The ANCOVA results demonstrated that the adjusted post-stretching Fpeak values were greater (P = 0.01) in the old compared to the young. In addition, there were significant correlations between changes in Fpeak and changes in %VA (r = 0.754; P < 0.001), EMG SOL (r = 0.559; P < 0.001), and EMG MG (r = 0.668; P < 0.001); however, there were no significant correlations (P ≥ 0.24) between changes in Fpeak and changes in the passive resistance to stretch (10°PF–10°DF).
Fig. 2.
The percent change values from pre- to post-stretching for isometric peak force, percent voluntary activation (%VA), and normalized electromyographic (EMG) amplitude for the soleus (SOL) and medial gastrocnemius (MG) muscles in young (shaded bars) and old (open bars) men.*P ≤ 0.05 indicates a significant difference between young and old men. ANCOVA results are presented for isometric peak force and %VA values. Values represent the means and the error bars represent the SE
For %VA (Fig. 2), there was a two-way interaction (P = 0.04). %VA decreased from pre- to post-stretching for the young (−5.89 ± 11.88 %; P = 0.04), but did not change for the old (−0.02 ± 4.65 %; P = 0.91). In addition, there was no difference in %VA between groups at baseline (P = 0.57). The ANCOVA results demonstrated that the adjusted post-stretching %VA values were greater (P = 0.05) for the old.
For normalized SOL EMG amplitude (Fig. 2), there was no two-way interaction (P = 0.21) and no main effect for time (P = 0.16) or group (P = 0.21). Normalized SOL EMG amplitude was not different between the young and old at any time point and did not change from pre- to post-stretching for both the young and old. For normalized MG EMG amplitude (Fig. 2), there was a two-way interaction (P = 0.03). Normalized MG EMG amplitude was greater for the old (104.04 ± 16.05 %) compared to the young (90.50 ± 19.87 %) post-stretching (P = 0.03).
For MROM (Fig. 3), there was a two-way interaction (P = 0.01). MROM was greater for the young and approached statistical significance at pre- (P = 0.06) but was similar at post-stretching (P = 0.48) between the young and old. MROM also increased from pre- to post-stretching for both groups (young = 13.54 ± 7.65 %; old = 23.37 ± 9.68 %; P < 0.001). The ANCOVA results demonstrated that the adjusted post-stretching MROM values were greater for the old compared to the young (P = 0.01). In addition, there was a significant correlation (r = 0.717; P < 0.001) between changes in MROM and changes in peak passive torque.
Fig. 3.
The percent change values from pre- to post-stretching for maximum range of motion (MROM) and peak passive torque for the young (shaded bars) and old (open bars) men. *P < 0.05 indicates a significant difference between young and old men via an ANCOVA. There was also a significant (P < 0.05) relationship between changes in MROM and peak passive torque. Values represent the means and the error bars represent the SE
For peak passive torque (Fig. 3), there was a two-way interaction (P = 0.05). However, the post hoc comparisons demonstrated that there were no differences in peak passive torque between groups at both time points (P ≥ 0.34), although both groups increased (P ≤ 0.03) from pre- to post-stretching (young = 12.05 ± 20.61 %; old = 30.41 ± 29.11 %). The ANCOVA results demonstrated that the adjusted post-stretching values were greater for the old when compared to the young but did not reach statistical significance (P = 0.08).
For the passive resistance to stretch (Fig. 4), there was no three-way (P = 0.29) or two-way interactions (P ≥ 0.06), or a main effect for group (P = 0.48); however, there was a main effect for time and angle (P < 0.001). Passive torque increased across all joint angles (10°PF − 10°DF) and decreased from pre- to post-stretching at all joint angles (P < 0.001) for both the young and old. The ANCOVA results demonstrated that adjusted post-stretching passive torque values were greater for the young at all joint angles (P ≤ 0.05) except at 10°DF were statistical significance (P = 0.06) was almost obtained.
Fig. 4.

The pre- (shaded squares) and post-stretching (open squares) passive torque values for the a young and b old men at each joint angle (10°PF, 5°PF, neutral, 5°DF, and 10°DF). *P < 0.05 indicates a progressive increase in passive torque at each joint angle at pre- and post-stretching. **P < 0.05 indicates a significant difference between pre- and post-stretching at all joint angles. ***P < 0.05 indicates post-stretching values were significantly greater for the young men at all joint angles except 10°DF (P = 0.06) via an ANCOVA. Values represent the means and the error bars represent the SE
For the %EMGonset, there was no two-way interaction (P = 0.62), no main effect for time (P = 0.21) or group (P = 0.06). The %EMGonset did not change from pre- to post-stretching for either the young or old, but occurred at a nonsignificantly (P = 0.06) greater percentage of MROM for the young (83.56 ± 17.99 %) compared to the old (67.92 ± 22.30 %). The ANCOVA results demonstrated that the adjusted post-stretching %EMGonset values were similar between the young and old (P = 0.34). In addition, similar to the findings recently reported by Blazevich et al. (2012), EMG onset was not detected in seven participants (six young and one old) at pre- or post-testing, and these participants were not included in these analyses.
Discussion
The present study examined the influence of an acute bout of prolonged (~20 min) passive stretching on plantar flexion strength and MROM in both young and old men. The primary findings were that (1) the young but not the old men experienced stretching-induced decreases in Fpeak which were strongly related to changes in muscle activation; (2) the older men experienced a greater increase in MROM which was strongly related to changes in peak passive torque and a greater decrease in the passive resistance to stretch; and (3) the %EMGonset did not change from pre- to post-stretching for both groups.
Stretching-induced force deficit
Maximal isometric strength decreased by 11.4 % for the young men but did not change for the older men. These findings are consistent with previous studies that have reported decreases in isometric plantar flexion strength following intermittent passive stretching protocols lasting 5–30 min (Fowles et al. 2000; Herda et al. 2009; Trajano et al. 2013; Weir et al. 2005) in young men. We are aware of only two recent studies (Gurjao et al. 2009; Goncalves et al. 2013) that have examined the stretching-induced force deficit in older adults. These authors (Gurjao et al. 2009; Goncalves et al. 2013) reported contrasting findings following the same stretching protocol (three 30-s static stretches) of the quadriceps muscles. Our results are in agreement with the findings of Goncalves et al. (2013) who reported that an acute bout of passive stretching did not influence Fpeak in older women (65 ± 4 years).
There are two general hypotheses to explain the stretching-induced force deficit: (a) neural factors that involve decreases in muscle activation (Trajano et al. 2013; Herda et al. 2009; Fowles et al. 2000) and (b) mechanical factors that involve alterations of the length-tension relationship (McHugh and Nesse 2008; Herda et al. 2008, 2011). The current study reported decreases in %VA and normalized EMG amplitude of the MG muscles for the young men only (Fig. 2). These findings are consistent with previous studies which have utilized the twitch interpolation technique in the plantarflexor muscles (Trajano et al. 2013; Herda et al. 2009; Fowles et al. 2000) in young men. It is possible that the conflicting findings for EMG amplitude of the SOL muscles in our study and those reported by Trajano et al. (2013) may be due to the normalization procedures employed. Our post-stretching EMG amplitude values were normalized to pre-stretching values, whereas Trajano et al. (2013) normalized EMG amplitude values to the maximal M-wave. In addition, similar to the findings by Trajano et al. (2013), changes in Fpeak were strongly correlated to changes in all muscle activation variables (Fig. 5) which may suggest that a reduction in central drive is a major contributor to the stretching-induced force deficit. Previous authors have also suggested that reductions in muscle activation may be a result of a persistent Golgi tendon organ reflex, fatigue-related mechanisms, neural tension, and/or a reduced motor cortical drive from the mild pain response experienced during prolonged stretching (Trajano et al. 2013; Fowles et al. 2000; McHugh et al. 2013). However, an interesting study by Herda et al. (2009) reported similar decreases in muscle activation following both prolonged passive stretching and vibration and suggested that passive stretching of sufficient duration (Ryan et al. 2008b) causes a temporary inhibition of the gamma loop. Specifically, the authors hypothesized that passive stretching like vibration may result in a suppression of spindle-mediated Ia-afferent activity and subsequent inhibition of the gamma loop feedback mechanisms required for type II motor unit activation (Kouzaki et al. 2000), thereby resulting in a reduction in maximal voluntary strength. The lack of change in muscle activation and Fpeak among the older men in this study may provide further support of this hypothesis. For example, Richardson et al. (2006) and Konishi et al. (2007) demonstrated that prolonged vibration resulted in decreases in maximal strength and muscle activation (EMG amplitude) in young adults, but no change in the older group. These authors suggested that older adults may have an impaired gamma loop as a result of decreased muscle spindle sensitivity causing the vibration to have no influence on strength and activation. Collectively, these findings and the results from our current study may provide a tentative support that the decreases in voluntary strength and muscle activation seen following prolonged passive stretching are gamma-loop-mediated.
Fig. 5.
The relationship between changes in isometric peak force and a percent voluntary activation (%VA) and normalized electromyographic (EMG) amplitude of the b medial gastrocnemius (MG) and c soleus (SOL) muscles for the young (black circles) and old (gray circles) men. All relationships were statistically significant (P < 0.001)
The stretching-induced force deficit may also be related to mechanical alterations whereby the passive resistance to stretch (i.e., passive torque) is reduced resulting in a rightward shift of the angle-torque curve (Herda et al. 2008; McHugh and Nesse 2008; McHugh et al. 2013). The results of the current study demonstrated that both the young and old men experienced decreases in the resistance to stretch at all joint angles (10°PF–10°DF; Fig. 4). These reductions, however, were not correlated with the changes in Fpeak. Previous studies have reported stretching-induced reductions in evoked twitch properties (Behm et al. 2001; Ryan et al. 2008b; Trajano et al. 2013), which may reflect the muscles’ reduced capacity to generate force as a result of an increase in muscle-tendon compliance. However, Behm et al. (2001)) and Trajano et al. (2013) demonstrated that the increase in skeletal muscle compliance is overcome by high-frequency tetanic forces. It is likely that the longer duration and greater force production generated during an MVC is also sufficient to overcome the increase in tissue compliance. Thus, these findings may suggest that the stretching-induced decreases in voluntary force production do not appear to be significantly influenced by decreases in the passive resistance to stretch. However, it is possible that the mechanical contributions to the stretching-induced force deficit may be more noticeable when tested at shorter muscle lengths as seen in previous studies examining isometric strength at both short and long muscle lengths (Herda et al. 2008; McHugh and Nesse 2008; McHugh et al. 2013). Furthermore, the current study did not examine changes in resting twitch properties to determine if the stretching-induced force deficit was due to changes in peripheral function. However, previous authors have indicated that changes in resting twitch properties were not related to the stretching-induced force deficit (Trajano et al. 2013), and the lack of change in M-wave amplitude (Costa et al. 2010) and conduction velocity (Ce et al. 2008) may suggest that passive stretching does not influence the excitation-contraction coupling.
Maximum range of motion changes
Our findings indicated that both groups experienced increases in MROM, decreases in the passive resistance to stretch, and were able to tolerate a greater passive load at stretch termination (i.e., stretch tolerance) following an acute bout of stretching. These findings are similar to previous authors that have reported acute stretching-induced increases in MROM (Herda et al. 2011; Morse et al. 2008; Ryan et al. 2008b; Mizuno et al. 2013) and peak passive torque (Magnusson et al. 1998; Mizuno et al. 2013) and decreases in the passive resistance to stretch (Herda et al. 2011; Morse et al. 2008; Mizuno et al. 2013). However, an unexpected finding was that the older men experienced greater changes in MROM and the passive resistance to stretch (Figs. 3 and 4). In addition, there was a strong positive relationship between changes in MROM and peak passive torque (r = 0.717; P < 0.001) with the older men experiencing a nonsignificant (P = 0.08) greater change in peak passive torque (young = 12 %; old = 30 %). These findings are in agreement with the results reported by Morse et al. (2008) who indicated that the stretching-induced reduction in passive resistance and increase in MROM were primarily a result of changes within the muscle (i.e., altered perimysium) as there was no change in tendon properties. However, in a recent comparison between flexible and inflexible young men, Blazevich et al. (2012) demonstrated that although the rate of change of muscle and tendon length was not different between groups, the flexible participants experienced significantly greater tendon lengthening near MROM. Furthermore, Kato et al. (2005) reported that females were more flexible and exhibited less passive resistance than their age-matched male counterparts as a result of a more compliant tendon. Thus, it is possible that the augmented changes seen among the older men may be a result of the age-related increases in tendon compliance (Mian et al. 2007; Narici et al. 2008; Stenroth et al. 2012), whereby the older adults experience similar changes in muscle compliance as seen with younger participants (Morse et al. 2008), and perhaps an additional increase in tendon compliance following an acute bout of prolonged passive stretching. Age-related changes in muscle-tendon behavior were demonstrated previously by Mian et al. (2007) who reported that young and old adults have similar changes in the lengthening of the muscle-tendon complex during the stance phase of walking; however, the older adults exhibited greater changes in tendon length which was attributed to an age-related increase in tendon compliance. Although we did not directly measure tendon compliance in the current study, our findings may support the hypothesis that tendon compliance is greater in older adults when compared to younger adults which has been demonstrated recently by Stenroth et al. (2012).
A recent investigation by Blazevich et al. (2012) demonstrated a moderate relationship (r = 0.60) between the angle at which EMG onset occurred and MROM. These authors (Blazevich et al. 2012) have suggested that EMG onset may be related to an “…appendicular tonic stretch reflex feedback mechanism” that may prevent excessive muscle stretching (p. 1453). Our findings indicated that %EMGonset did not change from pre- to post-stretching in the young or old groups. Thus, although both groups experienced different increases in MROM (young = 14 %; old = 23 %) post-stretching, the onset of muscle activity occurred at the same percentage of MROM. These findings may suggest that the augmented acute stretching-related changes in the older men may be unrelated to alterations in stretch reflex feedback mechanisms. It also important to note that the onset of EMG activity occurred earlier during the MROM assessment in the older men (young = 84 %; old = 68 %; P = 0.059) at both pre- and post-stretching. Although the specific mechanisms cannot be determined from the current study, we may speculate that %EMGonset may occur earlier in older adults due to an altered muscle-tendon behavior, whereby a passively stiffer muscle will lengthen less and a more compliant tendon will lengthen more during the MROM assessment when compared to younger adults. It is clear that more work in this area is needed and future studies using ultrasonography assessments may help determine the mechanisms underlying an earlier onset of muscle activation during passive stretching in older adults.
Conclusion
In summary, an acute and prolonged intermittent bout of passive stretching of the plantarflexors resulted in a significant decrease in Fpeak for the young men only, which was strongly related to changes in muscle activation and unrelated to changes in the passive resistance to stretch. The lack of changes in Fpeak and muscle activation for the older men provides further support that the stretching-induced force deficit may be gamma-loop-mediated. Furthermore, the older men experienced greater increases in MROM which were strongly related to changes in stretch tolerance (i.e., peak passive torque) and greater decreases in the passive resistance to stretch. These findings in conjunction with the lack of change in %EMGonset may be a result of the age-related increase in tendon compliance. Lastly, it is important to note that these changes were a result of a 20-min stretching protocol. Future studies using ultrasonography assessments and more practical stretching durations should be employed using the same controlled conditions of the current study.
Acknowledgments
The authors would like to thank the National Strength and Conditioning Association Foundation for helping to fund this project. We would also like to thank Matthew LaFleur for the drawing of Fig. 1.
Conflict of interest
Dr. Cramer is the principal investigator or co-investigator of current research or service agreements at the University of Nebraska-Lincoln with Rock Creek Pharmaceuticals, Abbott Nutrition, General Nutrition Center, and Stepan Lipid Nutrition.
References
- ACSM’s Guidelines for exercise testing and prescription. Philadelphia (PA): Lippincott, Williams & Wilkins; 2010. [Google Scholar]
- Allen GM, Gandevia SC, McKenzie DK. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve. 1995;18(6):593–600. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- Behm DG, Button DC, Butt JC. Factors affecting force loss with prolonged stretching. Can J Appl Physiol. 2001;26(3):261–272. doi: 10.1139/h01-017. [DOI] [PubMed] [Google Scholar]
- Blazevich AJ, Cannavan D, Waugh CM, Fath F, Miller SC, Kay AD. Neuromuscular factors influencing the maximum stretch limit of the human plantarflexors. J Appl Physiol. 2012;113(9):1446–1455. doi: 10.1152/japplphysiol.00882.2012. [DOI] [PubMed] [Google Scholar]
- Ce E, Paracchino E, Esposito F. Electrical and mechanical response of skeletal muscle to electrical stimulation after acute passive stretching in humans: a combined electromyographic and mechanomyographic approach. J Sports Sci. 2008;26(14):1567–1577. doi: 10.1080/02640410802277429. [DOI] [PubMed] [Google Scholar]
- Costa PB, Ryan ED, Herda TJ, Walter AA, Hoge KM, Cramer JT. Acute effects of passive stretching on the electromechanical delay and evoked twitch properties. Eur J Appl Physiol. 2010;108(2):301–310. doi: 10.1007/s00421-009-1214-3. [DOI] [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Weir JP, Johnson GO, Coburn JW, Beck TW. The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol. 2005;93(5–6):530–539. doi: 10.1007/s00421-004-1199-x. [DOI] [PubMed] [Google Scholar]
- Desbrosses K, Babault N, Scaglioni G, Meyer JP, Pousson M. Neural activation after maximal isometric contractions at different muscle lengths. Med Sci Sports Exerc. 2006;38(5):937–944. doi: 10.1249/01.mss.0000218136.58899.46. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Sale DG, MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89(3):1179–1188. doi: 10.1152/jappl.2000.89.3.1179. [DOI] [PubMed] [Google Scholar]
- Goncalves R, Gurjao AL, Jambassi Filho JC, Farinatti Pde T, Gobbi LT, Gobbi S. The acute effects of static stretching on peak force, peak rate of force development and muscle activity during single- and multiple-joint actions in older women. J Sport Sci. 2013;31(7):690–698. doi: 10.1080/02640414.2012.746727. [DOI] [PubMed] [Google Scholar]
- Gurjao AL, Goncalves R, de Moura RF, Gobbi S. Acute effect of static stretching on rate of force development and maximal voluntary contraction in older women. J Strength Cond Res. 2009;23(7):2149–2154. doi: 10.1519/JSC.0b013e3181b8682d. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Cramer JT, Ryan ED, McHugh MP, Stout JR. Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J Strength Cond Res. 2008;22(3):809–817. doi: 10.1519/JSC.0b013e31816a82ec. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Ryan ED, Smith AE, Walter AA, Bemben MG, Stout JR, Cramer JT. Acute effects of passive stretching vs vibration on the neuromuscular function of the plantarflexors. Scand J Med Sci Sports. 2009;19(5):703–713. doi: 10.1111/j.1600-0838.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Costa PB, Walter AA, Ryan ED, Hoge KM, Kerksick CM, Stout JR, Cramer JT. Effects of two modes of static stretching on muscle strength and stiffness. Med Sci Sports Exerc. 2011;43(9):1777–1784. doi: 10.1249/MSS.0b013e318215cda9. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G (1999) European recommendations for surface electromyography: results of the SENIAM project. Roessingh Research and Development
- Kato E, Oda T, Chino K, Kurihara T, Nagayoshi T, Fukunaga T, Kawakami Y. Musculotendinous factors influencing difference in ankle joint flexibility between women and men. Int J Sport Health Sci. 2005;3:218–225. doi: 10.5432/ijshs.3.218. [DOI] [Google Scholar]
- Kay AD, Blazevich AJ. Effect of acute static stretch on maximal muscle performance: a systematic review. Med Sci Sports Exerc. 2012;44(1):154–164. doi: 10.1249/MSS.0b013e318225cb27. [DOI] [PubMed] [Google Scholar]
- Kokkonen J, Nelson AG, Cornwell A. Acute muscle stretching inhibits maximal strength performance. Res Q Exerc Sport. 1998;69(4):411–415. doi: 10.1080/02701367.1998.10607716. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Kasukawa T, Tobita H, Nishino A, Konishi M. Gamma loop dysfunction of the quadriceps femoris of elderly patients hospitalized after fall injury. J Geriatr Phys Ther. 2007;30(2):54–59. doi: 10.1519/00139143-200708000-00004. [DOI] [PubMed] [Google Scholar]
- Kouzaki M, Shinohara M, Fukunaga T. Decrease in maximal voluntary contraction by tonic vibration applied to a single synergist muscle in humans. J Appl Physiol. 2000;89(4):1420–1424. doi: 10.1152/jappl.2000.89.4.1420. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Simonsen EB, Aagaard P, Sorensen H, Kjaer M. A mechanism for altered flexibility in human skeletal muscle. J Physiol. 1996;497(Pt 1):291–298. doi: 10.1113/jphysiol.1996.sp021768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Aagard P, Simonsen E, Bojsen-Moller F. A biomechanical evaluation of cyclic and static stretch in human skeletal muscle. Int J Sports Med. 1998;19(5):310–316. doi: 10.1055/s-2007-971923. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Cosgrave CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports. 2010;20(2):169–181. doi: 10.1111/j.1600-0838.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Nesse M. Effect of stretching on strength loss and pain after eccentric exercise. Med Sci Sports Exerc. 2008;40(3):566–573. doi: 10.1249/MSS.0b013e31815d2f8c. [DOI] [PubMed] [Google Scholar]
- McHugh MP, Tallent J, Johnson CD. The role of neural tension in stretch-induced strength loss. J Strength Cond Res. 2013;27(5):1327–1332. doi: 10.1519/JSC.0b013e31828a1e73. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Minetti AE, Narici MV. Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol. 2007;189(1):57–65. doi: 10.1111/j.1748-1716.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Matsumoto M, Umemura Y. Decrements in stiffness are restored within 10 min. Int J Sports Med. 2013;34(6):484–490. doi: 10.1055/s-0032-1327655. [DOI] [PubMed] [Google Scholar]
- Morse CI, Degens H, Seynnes OR, Maganaris CN, Jones DA. The acute effect of stretching on the passive stiffness of the human gastrocnemius muscle tendon unit. J Physiol. 2008;586(1):97–106. doi: 10.1113/jphysiol.2007.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil. 2008;30(20–22):1548–1554. doi: 10.1080/09638280701831058. [DOI] [PubMed] [Google Scholar]
- Richardson MS, Cramer JT, Bemben DA, Shehab RL, Glover J, Bemben MG. Effects of age and ACL reconstruction on quadriceps gamma loop function. J Geriatr Phys Ther. 2006;29(1):28–34. doi: 10.1519/00139143-200604000-00006. [DOI] [PubMed] [Google Scholar]
- Ryan ED, Beck TW, Herda TJ, Hull HR, Hartman MJ, Costa PB, Defreitas JM, Stout JR, Cramer JT. The time course of musculotendinous stiffness responses following different durations of passive stretching. J Orthop Sports Phys Ther. 2008;38(10):632–639. doi: 10.2519/jospt.2008.2843. [DOI] [PubMed] [Google Scholar]
- Ryan ED, Beck TW, Herda TJ, Hull HR, Hartman MJ, Stout JR, Cramer JT. Do practical durations of stretching alter muscle strength? A dose-response study. Med Sci Sports Exerc. 2008;40(8):1529–1537. doi: 10.1249/MSS.0b013e31817242eb. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S Finni T (2012) Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113(10):1537–1544. doi:10.1152/japplphysiol.00782.2012 [DOI] [PubMed]
- Trajano GS, Seitz L, Nosaka K, Blazevich AJ. Contribution of central vs. peripheral factors to the force loss induced by passive stretch of the human plantarflexors. J Appl Physiol. 2013;115(2):212–218. doi: 10.1152/japplphysiol.00333.2013. [DOI] [PubMed] [Google Scholar]
- Vickers A. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1(6):1–4. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir DE, Tingley J, Elder GC. Acute passive stretching alters the mechanical properties of human plantarflexors and the optimal angle for maximal voluntary contraction. Eur J Appl Physiol. 2005;93(5–6):614–623. doi: 10.1007/s00421-004-1265-4. [DOI] [PubMed] [Google Scholar]