Abstract
The activation of the Pi3k-Akt1-FOXO pathway seems to be involved in the extended longevity observed in growth hormone receptor/growth hormone binding protein knockout (GHRKO) mice and is related to the growth of primordial ovarian follicles. The aim of this work was to measure the expression of genes in the ovaries of GHRKO and normal (N) mice treated with phorbol 12-myristate 13-acetate (PMA), an inhibitor of GH and IRS1 signaling. For this study, a group of N (n = 10) and GHRKO (n = 10) mice, N mice treated (n = 10) or not (n = 10) with PMA, and GHRKO mice treated (n = 10) or not (n = 10) with PMA were used. All were 6-month-old female mice. After the last PMA injection, the ovaries were collected for gene expression analysis. Expression of Amh, Gdf9, and Bmp15 was higher in GHRKO than N mice (P < 0.05), but was not different between PMA-treated N mice (P > 0.10). Expression of Amh and Gdf9 was higher (P < 0.05) for GHRKO PMA-treated mice. In addition, we observed a higher expression of Socs3 (P < 0.001) in GHRKO than N mice and a tendency for increased expression of Foxo3a (P = 0.07). For GHRKO PMA-treated mice, Foxo3a mRNA expression was higher (P = 0.02) and a tendency for higher expression of Mtor (P = 0.06) and Socs3 (P = 0.10) in GHRKO PMA-treated mice was observed. To summarize, the present data further confirm the previous histological observations that GHRKO mice have an ovarian phenotype characteristic of younger mice indicated by higher expression of Amh, Gdf9, and Bmp15 mRNA. In addition, we have shown a higher expression of Socs3 in GHRKO mice and higher Foxo3a expression in PMA-treated GHRKO mice, suggesting a role for these mediators in the process of ovarian aging.
Keywords: GHRKO, FOXO3a, AMH, Aging, Ovary
Introduction
Mice with a disruption of the growth hormone receptor (GHR)/GH binding protein gene (GHRKO) are characterized by a reduction of 90 % in hepatic production of insulin-like growth factor (IGF-I) and a reduced adult body size (Zhou et al. 1997). This phenotype is accompanied by an increased lifespan of GHRKO mice when compared to littermate controls (List et al. 2011). GHRKO mice are also hypoinsulinemic and have increased insulin sensitivity (Bonkowski et al. 2009). Similarly to GHRKO mice, Ames dwarf (df/df) mice have a mutation that decreases pituitary GH secretion and consequently reduces serum concentrations of IGF-I (Chandrashekar and Bartke 1993). These mice are also characterized by a smaller body size and increased lifespan (Brown-Borg et al. 1996). On the other hand, mice that express a GH transgene are characterized by significant reduction of their lifespan when compared to their normal (N) controls (Bartke et al. 2002). These lines of evidence suggest that the GH/IGF-I axis plays a major role in the process of aging (Barbieri et al. 2003). Therefore, the study of the intracellular mediators of tissue-specific responses is important to determine how the process of aging is coordinated in the body, including the female reproductive tissues.
A functional GH/IGF-I axis is important for maximum reproductive efficiency (Chandrashekar et al. 2004). GHRKO mice are fertile, although they have reduced ovulation rate and smaller litter size than N females (Danilovich et al. 1999), and IGFKO mice are infertile (Baker et al. 1996), while mice with liver-specific IGF-I gene disruption have N fertility (Liu et al. 2000). This evidence suggests that GH is important for maximal reproductive efficiency; however, systemic IGF-I is essential for N follicle development, and females are not able to reproduce in its absence. Additionally, GHRKO females have an increased number of primordial follicles and a reduced number of healthy antral follicles (Zaczek et al. 2002; Slot et al. 2006), which can explain the reduced ovulation rate. However, treating GHRKO females with IGF-I decreases the number of primordial follicles and increases the number of healthy antral follicles (Slot et al. 2006), suggesting that IGF-I-induced signaling promotes primordial follicle activation and antral follicle survival. In addition, previous evidence indicates that GHRKO female mice have a prolonged reproductive lifespan, as indicated by the presence of ovarian activity at a later age (older than 20 months) when N mice have already depleted the ovarian follicular reserves (Sluczanowska-Glabowska et al. 2012).
The activation of the phosphoinositide 3-kinase (Pi3k)/protein kinase B (Akt1) pathway and the downstream transcription factor Forkhead Box O3a (FOXO3a) is essential for growth initiation of the ovarian primordial follicle reserve (Castrillon et al. 2003; Reddy et al. 2008; Brown et al. 2010). Hyperphosphorylation of FOXO3a results in its nuclear export, culminating with the global activation of primordial follicles and premature ovarian failure (Kalich-Philosoph et al. 2013). In addition, the mammalian target of rapamycin (mTOR) activation accelerates primordial follicle activation in a synergistic way with the Pi3k/Akt1 pathway (Adhikari et al. 2010; Caron et al. 2010). The activation of the Pi3k-Akt1-FOXO pathway is promoted by both insulin and IGF-I and reduced signaling via this pathway seems to be involved in the extended longevity observed in GHRKO mice (Bartke 2008). Therefore, it is possible that the extended reproductive lifespan and slower primordial follicle activation observed in GHRKO mice can be also related to altered activation of this pathway.
Phorbol 12-myristate 13-acetate (PMA) is a compound that can induce the proteolysis of cell surface GHR, therefore reducing GH signaling (Alele et al. 1998). As a consequence, its administration can reduce IGF-I mRNA production in some tissues (Li et al. 1999). In addition, PMA also functions as a protein kinase C (PKC) activator, decreasing insulin-induced tyrosine phosphorylation of IRS-1 and its ability to bind and activate Pi3k (Zheng et al. 2000). Since IRS-1 is an essential cellular mediator of IGF-I signaling, treatment of cells with PMA can significantly reduce Pi3k/Akt1 activation induced by IGF-I (Zheng et al. 2000). Therefore, we tested the short-term effects of reduced IGF-I signaling on the genes involved in the activation of primordial follicles and compared to the long-term effects of reduced IGF-I signaling in GHRKO mice.
Based on this evidence, the aim of this work was to measure the expression of genes related to the ovarian pathway for activation of primordial follicles in GHRKO and N mice and in N and GHRKO mice treated acutely with PMA.
Methods
Normal and GHRKO mice (all 6-month-old females) were bred and maintained under temperature- and light-controlled conditions (22 ± 2 °C, 12 h light/12 h dark cycle) (Masternak et al. 2004). Normal mice (n = 10, N PMA) and GHRKO mice (n = 10, GHRKO PMA) were treated with three intraperitoneal injections every 48 h of PMA dissolved in DMSO (0.1 mg/kg; Sigma-Aldrich Corp., St. Louis, MO, USA) (Erroi et al. 1993). The control group consisted of N mice (n = 10, N DMSO) and GHRKO mice (n = 10, GHRKO DMSO) treated with DMSO. Another group of 20 mice consisted of non-treated GHRKO mice (n = 10, GHRKO NT) and non-treated N mice (n = 10, N NT). Twenty-four hours after the last PMA injection, all animals were anesthetized and euthanized after overnight fasting, and the ovaries were collected and stored at −80 °C.
The ovaries were removed from the −80 °C and homogenized with 400 μL of Qiazol (Qiagen, Valencia, CA, USA) using 0.5-mm zirconium oxide beads in the Bullet Blender 24 (Next Advance, Averill Park, NY, USA) for 4 min at speed 10. After that, 300 μL of Qiazol was added to the samples and incubated for 5 min at room temperature. Next, 170 μL of chloroform was added, and the samples were homogenized and incubated for 3 min at room temperature. Samples were then centrifuged at 4 °C at 12,000×g for 15 min and the clear upper phase was transferred to a new tube with 525 μL of cold ethanol. The solution was transferred to columns (miRNeasy Mini Kit, Qiagen), and RNA was isolated according to kit instructions. On-column DNase treatment (RNase-free DNase Set, Qiagen) following manufacturer's instructions was performed. The quantity of RNA was determined using a spectrophotometer (Epoch Microplate Spectrophotometer, Biotek, Winooski, VT, USA) and diluted to 200 ng/μL. Reverse transcription reactions were performed with 1 μg of RNA (5 μL) using iScript Synthesis Kit (Biorad, Hercules, CA, USA) in a 20-μL volume incubated for 5 min at 25 °C, 30 min at 42 °C, and 5 min at 85 °C (MJ Mini Personal Thermal Cycler, Biorad). The final cDNA solution was diluted to 10 ng/μL before use.
Real-time PCR using SYBR Green dye was used to evaluate gene expression. β2 microglobulin expression was used as an internal control. The primer sequences are listed in Table 1. The PCR reactions were performed in duplicate in a 20-μL volume using 5 μL of Fast SYBR Green Mastermix (Applied Biosystems, Foster City, CA, USA), 0.4 μL of each primer (10 μM stock), and 2 μL of cDNA. Fluorescence was quantified with the ABI Prism 7500 Fast Real-Time PCR System (Applied Biosystems). For each assay, 45 PCR cycles were run (95 °C for 3 s and 60 °C for 30 s), and a dissociation curve was included at the end of the reaction to verify the amplification of a single PCR product. Analyses of amplification plots were performed with the 7500 Software (Applied Biosystems). Each assay plate included a negative control. The coefficient of variation was below 5 % for all the primer pairs used. Relative expression was calculated from the equation 2A−B/2C−D (where A is the cycle threshold [Ct] number for the gene of interest in the first control sample, B is the Ct number for the gene of interest in the analyzed sample, C is the Ct number for β2 microglobulin in the first control sample, and D is the Ct number for β2 microglobulin in the analyzed sample). The first control sample was expressed as 1.00 by this equation, and all other samples were calculated in relation to this value. Afterward, the results in the control groups (N mice non-treated, N mice treated with DMSO, and GHRKO mice treated with DMSO) were averaged, and all other outputs were divided by the mean value of the relative expression in the control group to yield the fold change of the genes of interest expression compared to the control group (Masternak et al. 2005).
Table 1.
Primer pairs used in the experiment
| Gene | Primer sequence |
|---|---|
| β2M |
For AAGTAT ACT CAC GCC ACC CA Rev CAG GCGTAT GTATCA GTC TC |
| Ghr |
For AGGTCTCAGGTATGGATCTTTGTCA GCCAAGAGTAGCTGGTGTAGCCT |
| Igf1 |
For CTGAGCTGGTGGATGCTCTT Rev CACTCATCCACAATGCCT |
| Socs2 |
For CTG CGC GAG CTC AGT CAA A Rev ATC CGC AGGTTA GTC GGT CC |
| Socs3 |
For TGT CGG AAG ACT GTC AAC GG Rev GAA GAA GCC AAT CTG CCC CT |
| Akt1 |
For CCG GTT CTT TGC CAA CAT CG Rev ACA CACTCC ATG CTG TCA TCT T |
| Pi3k |
For TAGCTGCATTGGAGCTCCTT Rev TACGAACTGTGGGAGCAGAT |
| Mtor |
For CGG CAA CTT GAC CAT CCT CT Rev TGCTGG AAG GCGTCA ATC TT |
| Irs1 |
For CGG GGA AGA CGA GAT GCT TT Rev TAC TGG AGC CTT GCG GCA C |
| Foxo3a |
For TCCCAGATCTACGAGTGGATGG Rev CCTTCATTCTGAACGCGCAT |
| Bmp15 |
For GAGCGAAAATGGTGAGGCTG Rev GGCGAAGAACACTCCGTCC |
| Gdf9 |
For GCTCTATAAGACGTATGCTACC Rev CAGAGTGTATAGCAAGACCGAT |
| Amh |
For TCCTACATCTGGCTGAAGTGATATG Rev CAGGTGGAGGCTCTTGGAACT |
β2M β-2 microglobulin, Ghr growth hormone receptor, Igf1 insulin-like growth factor I, Socs2 suppressor of cytokine signaling 2, Socs3 suppressor of cytokine signaling 3, Akt1 protein kinase B, Pi3k phosphoinositide 3-kinase, Mtor mammalian target of rapamycin, Irs1 insulin receptor substrate 1, Foxo3a Forkhead Box O3a, Bmp15 bone morphogenetic factor 15, Gdf9 growth and differentiation factor 9, Amh anti-Müllerian hormone
The results are presented as mean ± standard error of the mean (SEM). All statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). A t test was performed for comparison between groups. A P value lower than 0.05 was considered statistically significant and as a tendency between 0.05 and 0.10.
Results
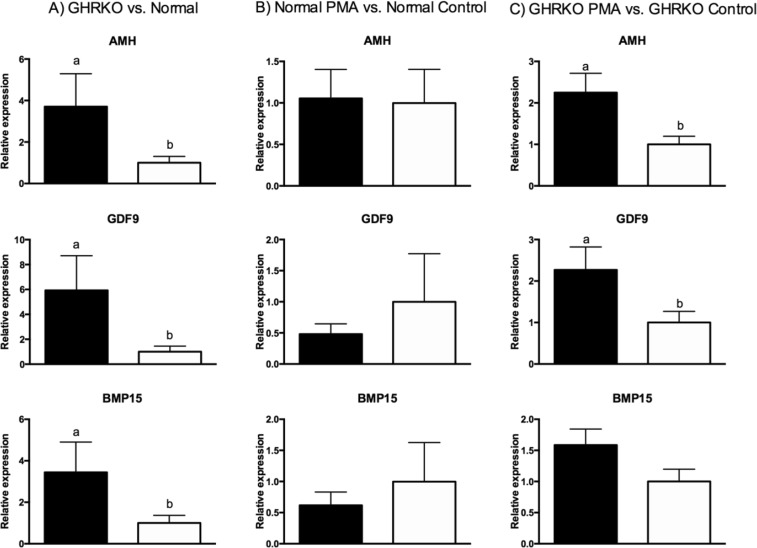
Expression of Amh, Gdf9, and Bmp15 was higher in GHRKO than N non-treated mice (P < 0.05; Fig. 1a). Despite that, expression of Amh, Gdf9, and Bmp15 was not different between N mice treated with PMA or DMSO for a week (P > 0.10; Fig. 1b). Expression of Amh and Gdf9 was higher (P < 0.05) for GHRKO PMA-treated than control GHRKO mice, although Bmp15 only tended to be higher in GHRKO PMA-treated mice (P = 0.07; Fig. 1c).
Fig. 1.
Expression of Amh, Bmp15, and Gdf9 mRNA in a GHRKO (black bar) and normal mice (white bar), b normal mice treated with PMA (black bar) or DMSO (white bar), and c GHRKO mice treated with PMA (black bar) and DMSO (white bar)
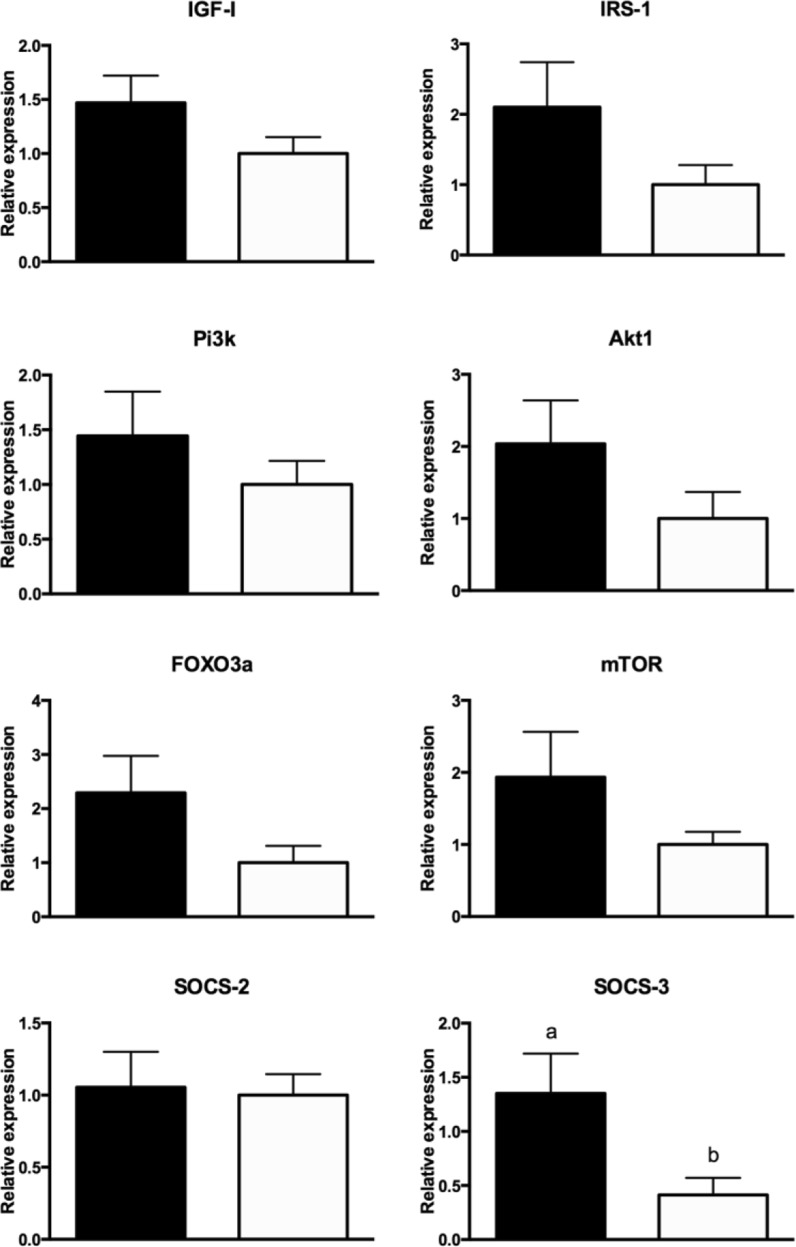
Regarding the expression of the genes in the pathway of primordial follicle activation (summarized in Fig. 2), we observed a higher expression of Socs3 (P < 0.001) in GHRKO than N non-treated mice and a tendency for increased expression of Foxo3a (P = 0.07) in GHRKO than in N non-treated mice. No difference in the expression of Pi3k, Socs2, Mtor, and Igf1 between N and GHRKO non-treated mice was observed (P > 0.10).
Fig. 2.
Expression of Igf1, Irs1, Pi3k, Akt1, Foxo3a, Mtor, Socs2, and Socs3 mRNA in GHRKO (black bar) and normal mice (white bar)
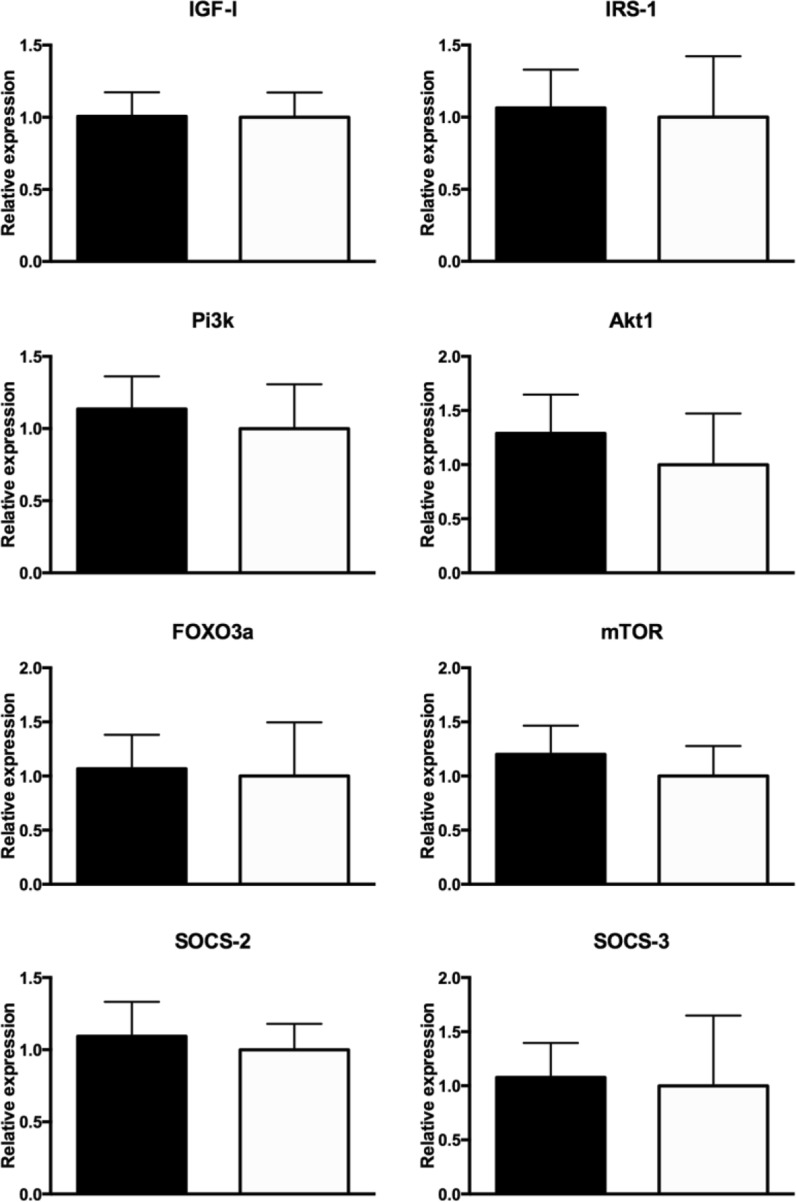
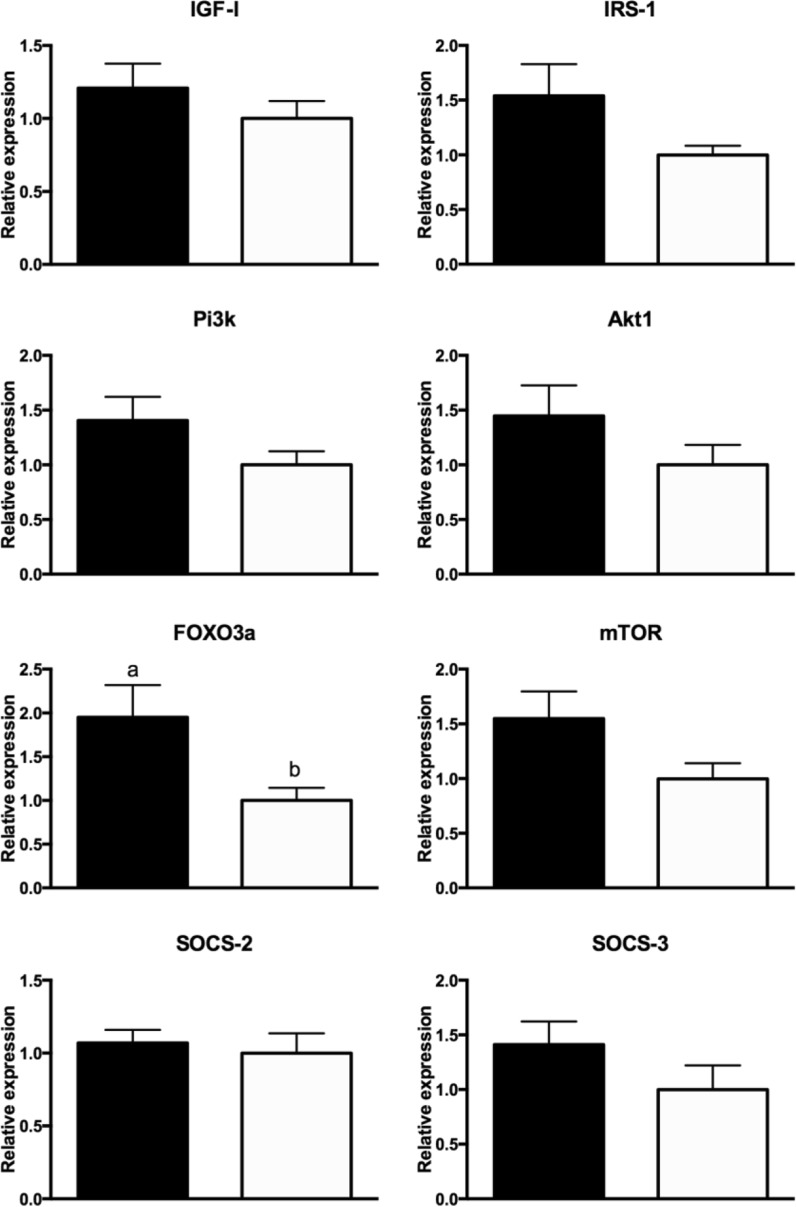
No differences in the expression of Igf1, Irs1, Pi3k, Akt1, Foxo3a, Mtor, Socs2, and Socs3 were observed between N mice treated with PMA and DMSO for a week (P > 0.10; Fig. 3). Foxo3a mRNA expression was higher (P = 0.02) and a tendency for higher expression of Mtor (P = 0.06) and Socs3 (P = 0.10) in PMA-treated vs DMSO-treated GHRKO mice was observed (Fig. 4). No difference was observed in the expression of Igf1, Irs1, Pi3k, Akt1, and Socs2 when comparing PMA with DMSO-treated GHRKO mice (P > 0.10; Fig. 4).
Fig. 3.
Expression of Igf1, Irs1, Pi3k, Akt1, Foxo3a, Mtor, Socs2, and Socs3 mRNA in normal mice treated with PMA (black bar) or DMSO (white bar)
Fig. 4.
Expression of Igf1, Irs1, Pi3k, Akt1, Foxo3a, Mtor, Socs2, and Socs3 mRNA in GHRKO mice treated with PMA (black bar) or DMSO (white bar)
Discussion
In the present study, we observed that expression of Amh, Gdf9, and Bmp15 was higher in GHRKO than in N mice of the same age. AMH is exclusively produced by developing preantral and small antral follicles (Jeppesen et al. 2013), and it is used to estimate the size of the ovarian reserve (Visser et al. 2006). GDF9 and BMP15 are also produced exclusively by growing oocytes in the mouse ovary (McGrath et al. 1995; Dube et al. 1998) and are involved in follicle maturation and oocyte quality (Dong et al. 1996; Yan et al. 2001). Therefore, the increased expression of Amh, Gdf9, and Bmp15 in GHRKO mice suggests that these mice have a larger ovarian reserve than N mice of the same age. This observation confirms previous histological findings that 2-year-old GHRKO mice still have visible ovarian structures (antral follicles and corpora lutea) while N mice of the same age have already completely depleted their ovarian reserve (Sluczanowska-Glabowska et al. 2012). Despite that, the expression of Amh, Gdf9, and Bmp15 was not altered by short-term PMA treatment in N mice, indicating that changes observed in the ovarian reserve of GHRKO female mice can be due to chronic suppression of IGF-I signaling. On the other hand, GHRKO mice treated with PMA expressed higher levels of Amh and Gdf9, indicating a possible interaction between the gene disruption and PMA treatment, which would further preserve the follicular reserve. Although the difference between PMA-treated and control GHRKO mice was more subtle (two times higher) than that observed between GHRKO and N mice (almost four times higher), this effect was not previously observed and can indicate that PMA treatment in GHRKO mice could further disrupt the progression of follicles to the primary stage, increasing the size of the ovarian reserve and possibly further reducing the ovulation rate. It is also possible that the divergent impact of PMA treatment on ovarian gene expression in GHRKO as compared to N mice was related to different regulation of IGF-1 signaling in the ovaries of these animals. In GHRKO mice, both direct and indirect actions of GH on the ovary have been eliminated by deletion of the GH receptors with concomitant reduction of IGF-1 from the systemic circulation. However, it was shown that despite elimination of GH signal and reduction of serum IGF-1, local IGF-1 expression persists in ovaries from GHRKO mice (Zaczek et al. 2002). Since PMA disrupts IGF-1 signaling, the presence of IGF-1 mRNA in GHRKO’s ovaries can provide possible explanation for the interaction between genotype and treatment.
The most significant difference observed between GHRKO and N mice was the increased expression of Socs3 mRNA. It has been previously demonstrated that SOCS-1 and SOCS-3 act as negative regulators of insulin signaling and that Socs3 overexpression reduces IRS1 phosphorylation (Ueki et al. 2004). Therefore, the higher expression of Socs3 mRNA observed in GHRKO mice could be blocking insulin signaling and therefore preventing the activation of the Pi3k/Akt1 pathway and follicle progression from primordial to primary stage, reducing the rate of the ovarian reserve depletion in these mice. Other genes were not differently expressed between GHRKO and N mice and therefore were probably not related directly at the transcriptional level to the process of ovarian aging. What is also interesting and likely biologically significant is the tendency for increased expression of Foxo3a in the ovaries of GHRKO compared to N mice. Moreover, this difference reached significance in GHRKO mice treated with PMA in comparison to control GHRKO mice. FOXO3a is crucial, while not phosphorylated, for the maintenance of follicles in the primordial quiescent stage (Castrillon et al. 2003). Therefore, the higher expression of Foxo3a indicates that follicles could be trapped in the primordial stage. This hypothesis agrees with histological observations in long-living GH-resistant GHRKO mice, where a higher number of follicles in the primordial stage are observed concomitantly with fewer follicles in the primary, secondary, and tertiary stages (Slot et al. 2006). A recent study has demonstrated that oocyte expression of a constitutively active form of Foxo3 in a transgenic mouse model is associated with the preservation of the ovarian reserve (Pelosi et al. 2013) and, therefore, can be related to an ovarian phenotype characteristic of younger mice as observed in GHRKO mice in the current and previous studies (Sluczanowska-Glabowska et al. 2012). Studies in other tissues generally indicate that higher FOXO3 mRNA expression predicts also higher level of nuclear FOXO3 (Essaghir et al. 2009; Turrel-Davin et al. 2010). Despite that, it is known that cellular localization (nuclear vs. cytoplasmic) of FOXO3 rather than the level of expression truly indicates the activation of this protein. It is well established that FOXO3 is moved to the cytoplasm once activated by Akt; therefore, we could speculate less cytoplasmic FOXO3 in GHRKO mice due to the reduced IGF-1/insulin signaling. However, further studies will need to be completed to confirm the activity of FOXO3 in GHRKO mice and the specific role of PMA on localization of FOXO3 in N and GHRKO animals.
As stated before, the importance of the endocrine versus the paracrine/autocrine IGF-I is controversial. However, in the present study, we clearly demonstrate that there is ovarian expression of Igf1 mRNA and that it is independent of the presence of a functional GH receptor since both GHRKO mice and N mice treated with PMA expressed similar levels of Igf1 mRNA to their respective controls. Therefore, these results indicate that a local functional GHR is not needed for ovarian IGF-I production, which must be regulated mainly by other factors. Ovarian IGF-I production seems to be stimulated by estradiol and inhibited by GH in rats, although the combined effect of estradiol and GH is stimulatory (Hernandez et al. 1989). It is also interesting to notice that neither of the PMA-treated groups had any changes in the expression of Igf1 mRNA, which should disrupt insulin/IGF-I signaling. This indicates that the level of circulating IGF-I is not playing a role in the local production of IGF-I by the Pi3k/Akt1 pathway.
Conclusion
To summarize, the present data further confirm the previous histological observations that GHRKO mice have ovarian phenotype characteristic of younger mice indicated by higher expression of ovarian markers of the size of the ovarian reserve (Amh, Gdf9, and Bmp15 mRNA). Also, we have shown that short-term treatment with PMA did not alter the profile of these ovarian markers in N mice, but increased them in GHRKO mice. In addition, we have shown a higher expression of Socs3 in GHRKO mice and higher expression of Foxo3a in the ovaries of GHRKO mice treated with PMA, which suggests a role for these mediators in the process of ovarian aging. However, we are aware that the genes measured relate to total protein expression and do not represent any post-translational modifications (e.g., phosphorylation status), which makes it important to continue with future studies to determine the action of these intracellular signaling intermediates and the relation with ovarian physiology of aging.
Acknowledgments
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under award numbers R01AG032290 and P01AG031736. A. Schneider was supported by the Federal University of Pelotas during the period of the research.
References
- Adhikari D, Zheng W, Shen Y, Gorre N, Hamalainen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397–410. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology. 1998;139:1927–1935. doi: 10.1210/endo.139.4.5906. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafe M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am J Physiol Endocrinol Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Bartke A. Impact of reduced insulin-like growth factor-1/insulin signaling on aging in mammals: novel findings. Aging Cell. 2008;7:285–290. doi: 10.1111/j.1474-9726.2008.00387.x. [DOI] [PubMed] [Google Scholar]
- Bartke A, Chandrashekar V, Bailey B, Zaczek D, Turyn D. Consequences of growth hormone (GH) overexpression and GH resistance. Neuropeptides. 2002;36:201–208. doi: 10.1054/npep.2002.0889. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brown C, LaRocca J, Pietruska J, Ota M, Anderson L, Smith SD, Weston P, Rasoulpour T, Hixon ML. Subfertility caused by altered follicular development and oocyte growth in female mice lacking PKB alpha/Akt1. Biol Reprod. 2010;82:246–256. doi: 10.1095/biolreprod.109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, Perreault C, Roux PP, Kitano H. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Bartke A. Induction of endogenous insulin-like growth factor-I secretion alters the hypothalamic-pituitary-testicular function in growth hormone-deficient adult dwarf mice. Biol Reprod. 1993;48:544–551. doi: 10.1095/biolreprod48.3.544. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V, Zaczek D, Bartke A. The consequences of altered somatotropic system on reproduction. Biol Reprod. 2004;71:17–27. doi: 10.1095/biolreprod.103.027060. [DOI] [PubMed] [Google Scholar]
- Danilovich N, Wernsing D, Coschigano KT, Kopchick JJ, Bartke A. Deficits in female reproductive function in GH-R-KO mice; role of IGF-I. Endocrinology. 1999;140:2637–2640. doi: 10.1210/endo.140.6.6992. [DOI] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- Erroi A, Fantuzzi G, Mengozzi M, Sironi M, Orencole SF, Clark BD, Dinarello CA, Isetta A, Gnocchi P, Giovarelli M, et al. Differential regulation of cytokine production in lipopolysaccharide tolerance in mice. Infect Immun. 1993;61:4356–4359. doi: 10.1128/iai.61.10.4356-4359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essaghir A, Dif N, Marbehant CY, Coffer PJ, Demoulin JB. The transcription of FOXO genes is stimulated by FOXO3 and repressed by growth factors. J Biol Chem. 2009;284:10334–10342. doi: 10.1074/jbc.M808848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ER, Roberts CT, Jr, LeRoith D, Adashi EY. Rat ovarian insulin-like growth factor I (IGF-I) gene expression is granulosa cell-selective: 5′-untranslated mRNA variant representation and hormonal regulation. Endocrinology. 1989;125:572–574. doi: 10.1210/endo-125-1-572. [DOI] [PubMed] [Google Scholar]
- Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, Raine-Fenning N, Campbell BK, Yding Andersen C. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–527. doi: 10.1093/molehr/gat024. [DOI] [PubMed] [Google Scholar]
- Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, Wolf I, Kanety H, Sredni B, Meirow D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5:185ra162. doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- Li F, Cao W, Steinberg RH, LaVail MM. Basic FGF-induced down-regulation of IGF-I mRNA in cultured rat Muller cells. Exp Eye Res. 1999;68:19–27. doi: 10.1006/exer.1998.0572. [DOI] [PubMed] [Google Scholar]
- List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Yakar S, LeRoith D. Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc Soc Exp Biol Med. 2000;223:344–351. doi: 10.1046/j.1525-1373.2000.22349.x. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey K, Bonkowski MS, Panici J, Sun L, Wang J, Przybylski GK, Bartke A. Divergent effects of caloric restriction on gene expression in normal and long-lived mice. J Gerontol A Biol Sci Med Sci. 2004;59:784–788. doi: 10.1093/gerona/59.8.B784. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Bonkowski MS, Panici JA, Przybylski GK, Bartke A. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, Schlessinger D, Ottolenghi C. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun. 2013;4:1843. doi: 10.1038/ncomms2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Slot KA, Kastelijn J, Bachelot A, Kelly PA, Binart N, Teerds KJ. Reduced recruitment and survival of primordial and growing follicles in GH receptor-deficient mice. Reproduction. 2006;131:525–532. doi: 10.1530/rep.1.00946. [DOI] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Glabowski W, Kopchick JJ, Bartke A, Kucia M, Ratajczak MZ. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res. 2012;5:18. doi: 10.1186/1757-2215-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrel-Davin F, Tournadre A, Pachot A, Arnaud B, Cazalis MA, Mougin B, Miossec P. FoxO3a involved in neutrophil and T cell survival is overexpressed in rheumatoid blood and synovial tissue. Ann Rheum Dis. 2010;69:755–760. doi: 10.1136/ard.2009.109991. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Mullerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Zaczek D, Hammond J, Suen L, Wandji S, Service D, Bartke A, Chandrashekar V, Coschigano K, Kopchick J. Impact of growth hormone resistance on female reproductive function: new insights from growth hormone receptor knockout mice. Biol Reprod. 2002;67:1115–1124. doi: 10.1095/biolreprod67.4.1115. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Quirion R. Stimulation of protein kinase C modulates insulin-like growth factor-1-induced akt activation in PC12 cells. J Biol Chem. 2000;275:13377–13385. doi: 10.1074/jbc.275.18.13377. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]