Abstract
In older persons, vitamin D insufficiency and a subclinical chronic inflammatory status frequently coexist. Vitamin D has immune-modulatory and in vitro anti-inflammatory properties. However, there is inconclusive evidence about the anti-inflammatory role of vitamin D in older subjects. Thus, we investigated the hypothesis of an inverse relationship between 25-hydroxyvitamin D (25(OH)D) and inflammatory markers in a population-based study of older individuals. After excluding participants with high-sensitivity C-reactive protein (hsCRP) ≥ 10 mg/dl and those who were on chronic anti-inflammatory treatment, we evaluated 867 older adults ≥65 years from the InCHIANTI Study. Participants had complete data on serum concentrations of 25(OH)D, hsCRP, tumor necrosis factor (TNF)-α, soluble TNF-α receptors 1 and 2, interleukin (IL)-1β, IL-1 receptor antagonist, IL-10, IL-18, IL-6, and soluble IL-6 receptors (sIL6r and sgp130). Two general linear models were fit (model 1—adjusted for age, sex, and parathyroid hormone (PTH); model 2—including covariates of model 1 plus dietary and smoking habits, physical activity, ADL disability, season, osteoporosis, depressive status, and comorbidities). The mean age was 75.1 ± 17.1 years ± SD. In model 1, log(25OH-D) was significantly and inversely associated with log(IL-6) (β ± SE = −0.11 ± 0.03, p = <0.0001) and log (hsCRP) (β ± SE = −0.04 ± 0.02, p = 0.04) and positively associated with log(sIL6r) (β ± SE = 0.11 ± 0.04, p = 0.003) but not with other inflammatory markers. In model 2, log (25OH-D) remained negatively associated with log (IL-6) (β ± SE = −0.10 ± 0.03, p = 0.0001) and positively associated with log(sIL6r) (β ± SE = 0.11 ± 0.03, p = 0.004) but not with log(hsCRP) (β ± SE = −0.01 ± 0.03, p = 0.07). 25(OH)D is independently and inversely associated with IL-6 and positively with sIL6r, suggesting a potential anti-inflammatory role for vitamin D in older individuals.
Keywords: Vitamin D, Inflammation, Inflammatory markers, Elderly subjects
Introduction
Vitamin D, calcitriol or 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), is a key endocrine regulator of calcium-phosphate homeostasis and bone metabolism. It is formed primarily, though not exclusively, in the kidney via 25-hydroxyvitamin D (25(OH)D) conversion, which represents the major circulating form of vitamin D and the most common marker of vitamin D status (Holick 2007). Studies have generally shown that concentration of 25(OH)D tends to decline with age (Hilger et al. 2014). Vitamin D insufficiency, as recommended by the Institute of Medicine, can be defined by 25(OH)D serum levels of less than 20 ng/mL (Ross et al. 2011). This phenomenon is an important potential public health issue by affecting more than one half of women and one third of middle-aged and older men living in the USA (Lips 2001). Several factors contribute to the age-related deterioration of vitamin D status, including insufficient exposure to sunlight, poor dietary intake of vitamin D, chronic diseases, pharmacological treatment, increase of fat mass, and disability (Holick 2007). Interestingly, in the elderly, low vitamin D levels frequently coexist with a subclinical proinflammatory status, characterized by a progressive increase of inflammatory cytokines and acute-phase reaction proteins (Ferrucci et al. 2005; Franceschi et al. 2000). Elevated concentrations of C-reactive protein (CRP), interleukin (IL)-6, and tumor necrosis factor (TNF)-α and suboptimal vitamin D levels have been associated with an increased risk of cardiovascular events, disorders of glucose metabolism, neurodegenerative diseases, and overall mortality (Pradhan et al. 2001; Il’yasova et al. 2005).
There is recent evidence that 25(OH)D is an immune modulator and that 1,25(OH)2D, formed in tissues outside of the kidney, can accumulate in the microenvironment of lymphoid organs, where it exerts specific autocrine and/or paracrine cell-specific activities (Hewison 2010; Lauretani et al. 2010). Studies of animal models and cell cultures showed both direct and indirect beneficial immune-modulating effects involving the T cells, B cells, and antigen-presenting cells (dendritic cells and macrophages) resulting in a switch from the Th1/Th17 response to the Th2/Treg profile (Helming et al. 2005). Interestingly, Th17 response plays a role in the pathogenesis of autoimmune diseases, and a suboptimal 25(OH)D status has been documented in a large number of autoimmune and inflammatory conditions including rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel diseases, and type 1 diabetes, but also infections, malignancies, transplant rejection, and cardiovascular disease (Querfeld 2013).
25(OH)D could play a role in signaling-related inflammation through a whole spectrum of underlying mechanisms including the downregulation of the proinflammatory transcription factor kappa B (NFκB) (Suzuki et al. 2009). On the contrary, the systemic inflammatory response, occurring after an inflammatory insult, could determine lower 25(OH)D concentration (Louw et al. 1992; Gray et al. 2005). Although a potential relationship between 25(OH)D and inflammation has been hypothesized, there is inconclusive evidence about the anti-inflammatory role of vitamin D, especially in the elderly. Moreover, the association between 25(OH)D serum concentration with multiple inflammatory markers has been addressed in few human studies and none of these has taken into account the complete role of different components of the IL-6, TNF-α, and IL-1 systems in older populations.
Using data from a large Italian older population-based study, we assessed whether serum 25(OH)D concentration is inversely associated with high-sensitivity C-reactive protein (hsCRP), TNF-α, soluble TNF-α receptors 1 and 2 (sTNFR1 and sTNFR2), IL-1 receptor antagonist (IL-1ra), IL-1β, IL-10, IL-18, IL-6, and IL-6 soluble receptors (sIL-6R and sgp130).
Subjects and methods
Subjects
InCHIANTI is an epidemiological study of risk factors for mobility disability in the elderly, designed by the Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence) and conducted on a representative sample of a population living in Greve in Chianti and Bagno a Ripoli, two small towns of the Chianti geographic area (Tuscany, Italy).
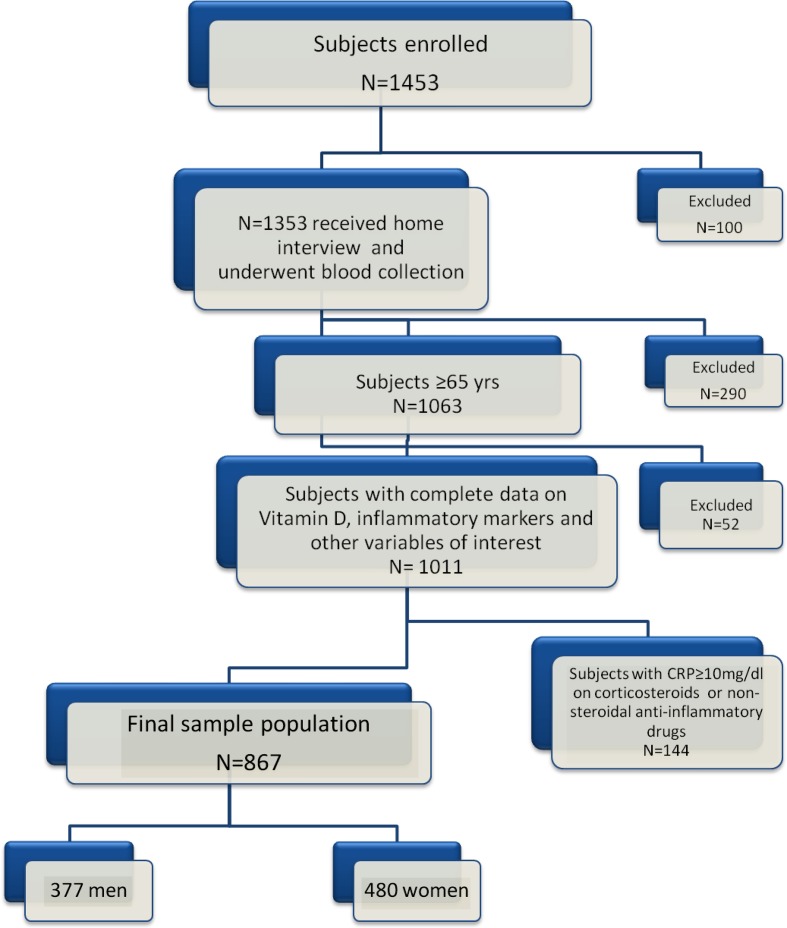
The population here evaluated was composed of 1,453 participants (aged 22–104) randomly selected from the residents of these two municipalities using a multistage stratified sampling method. Data collection started in September 1998 and was completed in March 2000. Detailed information on study design and data collection has been described elsewhere (Ferrucci et al. 2000). Of the whole study population, 1,353 donated a blood sample and 1,063 subjects were aged 65 years or older. One thousand eleven older subjects had complete data for the analysis presented here. Participants with hsCRP ≥10 mg/L (values potentially indicating an active inflammatory process) (Gabay and Kushner 1999), and those treated with corticosteroids and nonsteroidal anti-inflammatory drugs (N = 144) were excluded from the analysis. The final sample analysis was composed of 867 subjects (377 men and 490 women). The flow diagrams for the InCHIANTI and the post hoc data used in the current analysis are shown in Fig. 1.
Fig. 1.
Flow diagrams for the InCHIANTI study and the post hoc data used in the current analysis. Of the original 1,453 subjects, 1,353 subjects received home interview and underwent blood collection. One thousand sixty-three subjects were aged 65 years or older and 1,011 had complete data on serum concentrations of vitamin D, CRP, TNF-α, IL-6, sIL6r and sgp130, and other variables of interest. Since CRP values above 10 mg/L are considered to be consistent with an active inflammatory process, 144 participants with CRP ≥10 mg/L and those treated with corticosteroids and nonsteroidal anti-inflammatory drugs were excluded from our analysis. The final sample analysis was composed of 867 subjects (377 men and 490 women)
The study protocol was approved by local ethics committee. All participants received a detailed description of the purpose and design of the study and signed informed participation consent.
Laboratory measures
Blood samples were drawn in the morning after a 12-h overnight fast and resting period. All the routine blood tests were performed on fresh blood. 25(OH)D, parathyroid hormone (PTH), and IL-6 assays were performed on specimens previously stored at −80 °C. Serum 25(OH)D was measured by RIA (RIA kit; DiaSorin, Stillwater, MN, USA) after extraction of samples with acetonitrile. Intra- and inter-assay coefficient of variations (CVs) were 8.1 and 10.2 %, respectively. Serum IL-6, sIL-6r and TNF-α, sTNFR1 and R2, and IL-1ra and IL-1β were measured in duplicate high sensitivity enzyme-linked immunoabsorbent by assays (ELISA) using commercial kits (BioSource International Inc., Camarillo, CA, USA and human sIL-6R kit). The minimum detectable concentration (MDC) was 0.1 pg/ml for IL-6, 8.00 pg/ml for sIL-6r and 0.09 pg/ml for TNF-α, 8 pg/mL for sTNFR1 and sTNFR2, and 4.00 pg/mL for IL-1ra and 0.1 pg/mL for IL-1β. The intra-assay CV for sIL-6r was <6 %. The intra- and inter-assay CVs for TNF-α were 7 and <21 %, respectively. The interassay CV was 7 % for the other cytokines. Soluble glycoprotein 130 was assessed on plasma by ELISA (Quantikine Human Soluble gp130 Immunoassay, R&D Systems, Inc, Minneapolis, MN, USA). The MDC was approximately 25 ng/mL (at a 1:100 dilution). Inter-assay CV was approximately 10 %.
Serum IL-10 was detected by Human IL10 CytoSETS TM ELISA kits (BioSource International Inc.). The MDC was 1.00 pg/ml; the inter-assay CV was 8.6 %. Serum IL-18 level was detected in duplicate using highly sensitive quantitative sandwich assays (ELISA) (Quantikine HS, R&D Systems). The MDC was 0.7 pg/mL; the CV for IL-18 test was approximately 7 %.
C-reactive protein was measured with a high-sensitivity ELISA, a competitive immunoassay that uses purified protein and polyclonal anti-CRP antibodies. The inter-assay CV was 5 %. The MDC was 0.03 mg/l. Serum intact PTH was measured using a two-site immunoradiometric assay kit (N-tact PTHSP, DiaSorin Inc., Stillwater, MN, USA). The assay uses two affinity-purified polyclonal antibodies, one specific for the amino-terminal 1–34 portion of PTH molecule and the second specific for the 39–84 sequence of the hormone; the sensitivity assay was 1.2 ng/l. Intra- and interassay CVs were <3.0 and 5.5 %, respectively. Liver function was evaluated by aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Serum creatinine and urinary creatinine from the 24-h urine collection were measured using a modified Jaffe method. Serum creatinine and urinary creatinine from the 24-h urine collection were measured using a modified Jaffe method and used to calculate creatinine clearance as a measure of glomerular filtration rate.
Other measures
Obesity, physical activity, smoking habits, caloric and alcohol intake, disability, cognitive function, depressive status and chronic diseases, vitamin D supplement, number of medications, and season of blood collection were all considered as possible confounders of the association between vitamin D and inflammatory markers. Weight was measured to the nearest 0.1 kg using a high-precision mechanical scale with the participant wearing light clothes and without shoes. Standing height without shoes was measured to the nearest 0.1 cm. Body mass index was calculated as [weight (kg)]/[height (m)2], and obesity was defined as BMI > 30 kg/m2. The level of physical activity in the 12 months prior to the interview was assessed through an interviewer-administered questionnaire (Wareham et al. 2002) and was coded as sedentary (inactivity or light-intensity activity less than 1 h/week), light physical activity (light-intensity activity 2–4 h/week), and moderate-high physical activity (light-intensity activity ≥ 5 h/week or moderate activity ≥ 1–2 h/week). Smoking history was determined based on self-reports, and participants were categorized into never smokers, former smokers, and current smokers. To better describe the condition of current smokers, we introduced the variable pack-years, a measure of smoking exposure that combines intensity and duration, calculated as (packs smoked per day) × (years of smoking). Total years of cigarette smoking were also calculated to better address the condition of former smokers.
Disability was assessed using a self-administered scale (Katz Index of Independence in Activities of Daily Living, ADL) (Katz and Akpom 1976). Participants were classified as having ADL dependency when they reported need for help of another person in performing at least one ADL (≥1 ADLs) (getting out of a bed or chair, bathing or showering, or dressing). Daily caloric (kcal/day) and alcohol intake (g/day) were estimated by the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire (Kaaks and Riboli 1997). High alcohol intake was defined as more than 21 drinks/week for men and more than 14 drinks/week for women (≥30 g/day in men and ≥20 g/day in women) (Tenth Special Report to the U.S. Congress on Alcohol and Health from the Secretary of Health and Human Services. U.S. Department of Health and Human Services. Washington et al. 2000).
Global cognitive performance was assessed using the Mini-Mental State Examination (MMSE) performed by a trained geriatrician within a week of the blood draw. Depressive symptoms were evaluated with the Center for Epidemiologic Studies Depression Scale (CES-D) (Fava 1983). The CES-D is a 20-item self-report scale, ranging from 0 to 60. The CES-D has good psychometrics properties in assessing depressive symptoms and was validated both in older population-based studies and Italian population (Fava 1983). A score ≥16 was considered to indicate depressive symptoms. The prevalence of specific medical conditions was established using standardized criteria that combine information from self-reported history, medical records, and a clinical medical examination. Diagnostic algorithms were modified versions of those created for the Women’s Health and Aging Study (Kaaks and Riboli 1997). The following diseases were assessed: coronary heart disease (angina and acute myocardial infarction, CHD), peripheral arterial disease (PAD), stroke (and/or transient ischemic attack), hypertension, diabetes mellitus, congestive heart failure, chronic obstructive pulmonary disease (COPD), Parkinson’s disease, and cancer.
Because of the known seasonal variability in vitamin D concentration, season for blood collection was considered as categorical season variable with 3-month intervals. Season categories were identified as follows: winter (blood collections performed during the time period between December and February), spring (evaluations performed between March and May), summer (from June to August), and fall (from September to November).
Cortical volumetric bone mineral density (vBMDc) (mg/cm3), a selective measure of the apparent volumetric density of cortical bone and marker of bone material property, was measured at the 38 % site of the tibial length by peripheral quantitative computed tomography scans (pQCT) (XCT 2000 device, Stratec Medizintechnik, Pforzheim, Germany). The precision error of the XCT2000 was below 1 % for volumetric cortical density. We used vBMDc by pQCT because this parameter has been linked to osteoporosis and risk of osteoporotic fractures (Siris et al. 2001; Zebaze et al. 2010).
Statistical analysis
Variables normally distributed were reported as mean values ± SD, and categorical values as numbers and percentages. The 25(OH)D, PTH, IL-6, sIL-6R, sgp130, TNF-α, sTNFR1 and sTNFR2, IL-1ra, IL-1β, IL-10, IL-18, and hsCRP were described by reporting median values and interquartile ranges because of skewed distribution. To approximate normal distributions, log-transformed values for 25(OH)D, IL-6, sIL6r, sgp130, TNF-α, sTNFR1, and sTNFR2, IL-1β, IL-1ra, IL-10, IL-18, and hsCRP were used in the analysis and back-transformed for data presentation.
Vitamin D levels were also categorized into tertiles based on the distribution in the whole study population. Differences across tertile categories of 25(OH)D were determined by using Wilcoxon rank-sum test (Kruskal-Wallis test) adjusted for age and sex. Factors statistically correlated with 25(OH)D levels were identified by using an age-adjusted partial correlation coefficient and Spearman partial rank-order correlation coefficients as appropriate. Parsimonious models obtained by backward selection from initial fully adjusted models were used to identify independent factors of 25(OH)D levels. Model 1 was adjusted for age, sex, and PTH, while model 2 included the variables of model 1 plus physical activity, caloric and alcohol intake, season of blood collection, smoking, pack-years, years of cigarette smoking, vBMDc, ADL disability, CES-D, CHD, and creatinine.
Statistical significance was defined as p < 0.05. SAS 8.2 statistical package was used for all analyses (SAS Institute, Inc., Cary, NC, USA).
Results
Characteristics of the study population are presented in Table 1. The mean age was 75.1 ± 7.1 (years ± SD). According to a cutoff point of 50 nmol/L, 557 (64.2 %) were considered vitamin D-insufficient subjects.
Table 1.
Characteristics of study participants
| Parameter | All (N = 867) |
|---|---|
| Age (years) | 75.1 ± 7.1a |
| Sex, M (n, %) | 377 (43.5)b |
| BMI (kg/m2) | 27.3 ± 4.0 |
| Calcium (mg/dl) | 9.4 ± 0.44 |
| PTH (pmol/L) | 22.4 [16.1–32.8]c |
| Vitamin D (nmol/L) | 40.4 [26.7–64.6] |
| IL-6 (pg/ml) | 1.3 [0.8–2.0] |
| CRP (μg/mL) | 2.3 (1.2–4.3) |
| sIL6r (ng/mL) | 93.9 [68.8–125.5] |
| sgp130 (ng/mL) | 308.3 [273.3–345.9] |
| TNF-α (pg/mL) | 4.52 [3.24–5.94] |
| sTNFR1 (pg/mL) | 1,356.3 [1,101.8–1,746.8] |
| sTNFR2 (pg/mL) | 2,608.1 [2,272.2–3,131.1] |
| IL-1β (pg/mL) | 0.12 [0.08–0.18] |
| IL-10 (pg/mL) | 0 [0–5.2] |
| IL-18 (pg/mL) | 376.5 [293.8–471.9] |
| IL-1ra (pg/mL) | 126.9 [93.4–174.9] |
| Caloric intake (kcal/day) | 1,857.9 [1,513.3–2,239.3] |
| High alcohol intake (n, %)d | 172 (19) |
| Smoking (n, %)e | |
| Never smokersf | 522 (60.2) |
| Former smokers | 229 (26.4) |
| Current smokers | 116 (13.3) |
| Pack of cigarette smoking (years) | 0 [0–18.5] |
| Total years of cigarette smoking (years) | 0 [0–33] |
| vBMDc (mg/cm3) | 999.3 ± 72.47 |
| No of drugs | 2[1–3] |
| Vitamin D supplements (n, %) | 32 (3.7) |
| Physical activity (n, %)g | |
| Sedentaryf | 166 (19.1) |
| Light physical activity | 657 (75.8) |
| Moderate–high physical activity | 44 (5.1) |
| ADL dependency (n, %)h | |
| Disabledf | 62 (7.2) |
| Not disabled | 805 (92.8) |
| Season of blood collection (n, %)i | |
| 1f | 247 (28.4) |
| 2 | 123 (14.2) |
| 3 | 145 (16.7) |
| 4 | 352 (40.6) |
| MMSE (total score) | 24.7 ± 4.5 |
| CES-D (total score) | 10 [6–17] |
| PAD (n, %) | 119 (13.7) |
| Parkinson disease (n, %) | 16 (1.8) |
| CHD (n, %) | 211 (24.3) |
| AST (U/L) | 20 [17–23] |
| ALT (U/L) | 17 [14–22] |
| Creatinine (mg/dL) | 0.9 [0.80–1.0] |
| COPD (n, %) | 81 (9.7) |
| Cancer (n, %) | 55 (6.3) |
PTH parathyroid hormone, CRP C-reactive protein, sIL6r soluble IL-6 receptor, sgp130 signaling-trasducing 130-kDa component, sTNFR1 soluble TNF-α receptors 1, sTNFR2 soluble TNF-α receptors 2, IL-1Ra IL-1 receptor antagonist, vBMDc cortical volumetric bone mineral density, ADL activities of daily living, MMSE Mini-Mental State Examination, CES-D Center for Epidemiologic Studies Depression Scale, PAD peripheral arterial disease, CHD coronary heart disease, AST aspartate aminotransferase, ALT alanine aminotransferase, COPD chronic obstructive pulmonary disease
aMean ± SD (all such values)
bAll such values are number of cases (percentage)
cAll such values are medians (IQR)
dHigh alcohol intake was defined as more than 21 drink/week for men and more than 14 drinks/week for women
eBased on self-reports scored from never smokers to current smokers
fReference category
gDerived from the interviewer-administered questionnaire scored from sedentary (inactivity or light-intensity activity less than 1 h/week), to moderate–high physical activity (light-intensity activity ≥5 h/week or moderate activity ≥1–2 h/week)
hDerived from the Katz’s Index categorized as disabled or not-disabled (6/6 ADL independent and loss of at least one activity was considered dependent)
iSeasons was categorized in four periods: 1 = winter (December, January, and February), 2 = spring (March, April, and May), 3 = summer (June, July, and August), and 4 = autumn (September, October, and November)
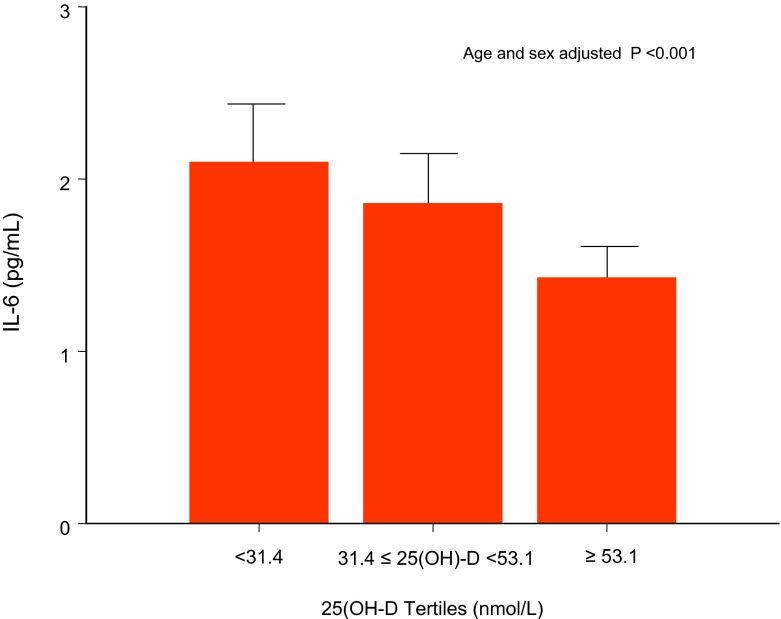
Levels of inflammatory markers across 25OH-D tertiles were reported as mean values ± SD (Table 2). There was a significant difference in IL-6 (Table 2 and Fig. 2), sIL-6r , hsCRP, sgp130, TNF-α, sTNFR1, and sTNFR2 across 25OHD tertiles (p < 0.05) with higher levels of these markers in the lowest 25OH-D (<31.4 nmol/L) tertile. There was no significant difference in IL1ra, IL-1ß, IL-10, and IL-18 across 25OH-D tertiles.
Table 2.
Differences in inflammatory markers across 25(OH)D tertiles
| Serum 25(OH)D tertiles | ||||
|---|---|---|---|---|
| Tertile 1 n = 289 (<31.4 nmol/L) |
Tertile 2 n = 287 (≤31.4 < 53.1 nmol/L) |
Tertile 3 n = 291 (≥53.1 nmol/L) |
P a | |
| IL-6 (pg/ml) | 2.04 ± 1.87 | 1.72 ± 1.63 | 1.41 ± 1.21 | <0.001 |
| sIL-6r (ng/mL) | 102.42 ± 49.4 | 98.17 ± 54.08 | 110.09 ± 54.10 | 0.01 |
| sgp130 (ng/mL) | 320.76 ± 60.52 | 315.52 ± 6.76 | 303.61 ± 55.64 | 0.001 |
| CRP (μg/mL) | 3.42 ± 2.56 | 2.98 ± 2.21 | 2.72 ± 2.16 | 0.004 |
| TNF-α (pg/mL) | 5.39 ± 4.31 | 5.00 ± 2.80 | 9.85 ± 87.19 | 0.02 |
| sTNFR1 (pg/mL) | 1,568.92 ± 810.61 | 1,497.77 ± 641.03 | 1,366.13 ± 478.39 | 0.005 |
| sTNFR2 (pg/mL) | 2,894.80 ± 847.46 | 2,773.12 ± 717.88 | 2,632.08 ± 663.02 | <0.001 |
| IL-1ß (pg/mL) | 0.38 ± 2.22 | 0.21 ± 0.73 | 0.40 ± 1.92 | 0.41 |
| IL1ra (pg/mL) | 139.75 ± 67.83 | 145.98 ± 86.30 | 142.46 ± 84.94 | 0.85 |
| IL-10 (pg/mL) | 96.05 ± 381.91 | 66.11 ± 263.97 | 73.48 ± 312.67 | 0.15 |
| IL-18 (pg/mL) | 395.24 ± 159.53 | 405.03 ± 145.45 | 405.77 ± 146.56 | 0.31 |
aThe analysis (age and sex adjusted) by Wilcoxon-Rank-sum test (Kruskal-Wallis test)
Fig. 2.
IL-6 median values across 25(OH)D tertiles (nmol/L), based on the distribution in the whole study population. The 25(OH)D tertiles were as follows: tertile 1 (25OH-D <31.4 nmol/L), tertile 2 (31.4 ≤ 25OH-D <53.12 nmol/L), tertile 3 (25OH-D ≥53.1 nmol/L). Differences in inflammatory markers across 25(OH)D tertiles were compared by Wilcoxon rank-sum test (Kruskal-Wallis test) adjusted for age and sex. There was a significant difference in IL-6 levels across 25OHD tertiles with higher levels of this marker in the lowest 25OH-D tertile (p < 0.001)
Log (25OH-D) levels were inversely related to log (IL-6) (r = −0.10; p = 0.003), log (hsCRP) (r = −0.08; p = 0.02), and log (sgp130) (r = −0.08; p = 0.02) and positively correlated to log (sIL6r) (r = 0.08; p = 0.02). Log (25OH-D) was not significantly correlated with other inflammatory markers (Table 3). To further explore factors independently associated with vitamin D levels, we fit parsimonious models, including only independent variables significantly associated with 25(OH)D (Table 4). Table 4 shows covariate-adjusted associations between 25(OH)D concentrations and inflammatory markers. In model 1 (adjusted for age, sex, and PTH), log (25OH-D) was significantly and inversely associated with log (IL-6) (β ± SE = −0.11 ± 0.03, p = <0.0001) and log (hsCRP) (β ± SE = −0.04 ± 0.02, p = 0.04) and positively associated with log (sIL6r) (β ± SE = 0.11 ± 0.04, p = 0.003). In the fully adjusted model (model 2), log (25OH-D) was still negatively associated with log (IL-6) (β ± SE = −0.10 ± 0.03, p = 0.0001) and positively associated with log (sIL6r) (β ± SE = 0.11 ± 0.03, p = 0.004). Log (25OH-D) concentration was also significantly associated with log (sgp130) (β ± SE = −0.0008 ± 0.0003, p = 0.01). The association between log (25OH-D) and log (hsCRP) approached the statistical significance (β ± SE = −0.01 ± 0.03, p = 0.07).
Table 3.
Age-adjusted partial correlation coefficients evaluating the relationship of 25OHD and inflammatory markers and other potential confounding variables
| Log (25OHD), nmol/L | ||
|---|---|---|
| r | P a | |
| Log (IL-6), pg/mL | −0.10 | 0.003 |
| Log (sIL6r), ng/mL | 0.08 | 0.02 |
| Log (sgp130), ng/mL | −0.08 | 0.02 |
| Log (CRP), μg/mL | −0.08 | 0.02 |
| Log (TNF-α), pg/mL | 0.004 | 0.92 |
| Log (IL1ra), pg/mL | 0.05 | 0.29 |
| Log (IL-1β), pg/mL | 0.003 | 0.95 |
| Log (IL-10), pg/mL | −0.009 | 0.86 |
| Log (sTNFR1), pg/mL | 0.04 | 0.16 |
| Log (sTNFR2) , pg/mL | 0.01 | 0.71 |
| Log (IL-18), pg/mL | 0.11 | 0.03 |
| BMI, kg/m2 | −0.04 | 0.71 |
| ALT, U/L | −0.029 | 0.44 |
| AST, U/L | −0.027 | 0.42 |
| Creatinine, mg/dL | 0.09 | 0.008 |
| Log (PTH), pmol/L | −0.24 | <0.0001 |
| Calcium, mg/dl | −0.05 | 0.13 |
| ADL disability, 2 categories | −0.08 | 0.02 |
| Physical activity, 3 levels | 0.15 | <0.0001 |
| High Alcohol intake, g/day | 0.12 | 0.0006 |
| Season, 4 categories | 0.18 | <0.0001 |
| vBMDc, mg/cm3 | 0.14 | <0.0001 |
| Vitamin D supplements, n | −0.003 | 0.92 |
| MMSE, n | 0.05 | 0.13 |
| Smoking status, 3 categories | 0.11 | 0.001 |
| Total years of cigarette smoking (years) | 0.15 | <0.0001 |
| Pack of cigarette smoking (years) | 0.12 | 0.0007 |
| Caloric intake (kcal/day) | 0.14 | <0.0001 |
| CES-D, n | −0.13 | 0.0003 |
| CHD, n | −0.10 | 0.005 |
| Cancer, n | −0.06 | 0.13 |
| Parkinson, n | −0.004 | 0.90 |
| COPD, n | −0.002 | 0.53 |
| PAD, n | 0.02 | 0.56 |
sIL-6r soluble IL-6 receptor, sgp130 signaling-transducing 130-kDa component, CRP C-reactive protein, TNF-α tumor necrosis factor-α, IL-1Ra IL-1 receptor antagonist, sTNFR1 soluble TNF-α receptor 1, sTNFR2 soluble TNF-α receptor 2, AST aspartate aminotransferase, ALT alanine aminotransferase, PTH parathyroid hormone, ADL activities of daily living, vBMDc cortical volumetric bone mineral density, MMSE Mini-Mental State Examination, CES-D Center for Epidemiologic Studies Depression Scale, CHD coronary heart disease, COPD chronic obstructive pulmonary disease, PAD peripheral arterial disease
aAge-adjusted
Table 4.
Relationship between vitamin D and inflammatory markers
| Model 1a | Model 2b | |||
|---|---|---|---|---|
| β ± SE | P | β ± SE | P | |
| Log (IL-6) | −0.11 ± 0.03 | <0.0001 | −0.10 ± 0.03 | 0.0001 |
| Log (sIL6r) | 0.11 ± 0.04 | 0.003 | 0.11 ± 0.03 | 0.004 |
| Log (sgp130) | −0.0006 ± 0.0003 | 0.07 | −0.0008 ± 0.0003 | 0.01 |
| Log (CRP) | −0.04 ± 0.02 | 0.04 | −0.01 ± 0.03 | 0.07 |
| Log (IL-18) | −0.035 ± 0.06 | 0.53 | −0.01 ± 0.06 | 0.83 |
aParsimonious models obtained by backward selection method from initial models including all covariates reported in the table and age, sex and PTH. PTH parathyroid hormone, CRP C-reactive protein, sIL6r soluble IL-6 receptor, sgp130 signaling-transducing 130-kDa component
bParsimonious models also adjusted for physical activity, caloric and alcohol intake, season of blood collection, smoking, pack-years, years of cigarette smoking, cortical bone mineral density, ADL disability, CES-D, CHD, creatinine; ADL, Katz’s Index; CES-D Center for Epidemiologic Studies Depression Scale; CHD coronary heart disease
Discussion
In our study, 25(OH)D serum concentration was independently and inversely associated with the proinflammatory cytokine IL-6 and its soluble receptor sgp130 and positively associated with sIL6r.
To the best of our knowledge, this is the first study assessing the relationship between vitamin D levels and inflammatory cytokines IL-6, IL-10, IL-18, TNF-α, IL-1β, and hsCRP also focusing on the role of the main proinflammatory cytokine receptors including sIL6r, sgp130, sTNFR1, sTNFR2, and IL-1ra in a well-represented cohort of older subjects. Our findings are of importance because little it is known about the relationship between 25(OH)D levels and immune markers of inflammation, especially in the elderly.
The few published data coming from observational studies (Amer and Qayyum 2012; Reyman et al. 2013; Laird et al. 2014; Ngo et al. 2010; Peterson and Heffernan 2008) support a primary anti-inflammatory role of vitamin D. In a very recent observational investigation conducted in 957 older Irish adults (>60 years of age), Laird et al. (Laird et al. 2014) showed a significant association between low vitamin D status (25OH-D <25 nmol/L) and markers of inflammation including IL-6, TNF-α, IL-10, hsCRP, and the ratio of IL-6 to IL-10.
Ngo et al. (Ngo et al. 2010), in a cohort of 253 middle-aged and older subjects (mean, 63.4 ± 6 years), have found that low 25(OH)D levels were inversely associated with hsCRP levels. Consistently, an inverse relationship between serum 25(OH)D status and TNF-α concentrations was documented by Peterson et al. (Peterson and Heffernan 2008) in a study population of 25(OH)D-deficient women (age range, 25–82 years).
On the contrary, several investigations failed to prove any significant association between 25(OH)D and the main proinflammatory cytokines (Gannagé-Yared et al. 2003; Vilarrasa et al. 2010; Shea et al. 2008; Clendenen et al. 2011). Interestingly, scarce attention has been devoted to the relationship between 25(OH)D and IL-6. This is surprising because IL-6 is the primary biomarker of phenotypes of aging, underlying not detected conditions (Li et al. 2014) and adverse outcomes, including disability and death (Maggio et al. 2006).
Studies in nasal polyposis cultures documented a potential inhibitory effect of vitamin D on proinflammatory cytokines including IL-6 (Rostkowska-Nadolska et al. 2010). In humans, the link between vitamin D, IL-6, and systemic inflammation is especially evident during chronic inflammatory conditions and metabolic disorders. Dobnig et al. (Dobnig et al. 2008), in a prospective cohort study of more than 3,000 consecutive patients (mean [SD] age, 62 (Lauretani et al. 2010) years) undergoing coronary angiography, showed that low 25(OH)D concentrations were significantly correlated with IL-6 and CRP levels. Interestingly, Thorand et al. observed that the significant inverse association between serum 25(OH)D and incident type 2 diabetes was attenuated after adjustment for IL-6 and other inflammatory markers (Thorand et al. 2011). Interestingly, studies in CKD children also showed a significant relationship between 25(OH)D and IL-6 independent of the severity of kidney disease (Suzuki et al. 2009). Finally, in a cohort from the Baltimore Hip Studies, women aged ≥65 years, 25(OH)D deficient at the time of hip fracture, had higher serum IL-6 levels in the year after fracture (Miller et al. 2007).
The molecular mechanisms underlying the relationship between vitamin D and IL-6 could be found in the inhibitory action of vitamin D on both cellular expression and activation of the proinflammatory transcription factor kappa B (NFκB). In this way, vitamin D might regulate the release of the inflammatory cytokine IL-6, which represents a downstream target of NFκB activation (Suzuki et al. 2009; Jablonski et al. 2011). The anti-inflammatory properties of vitamin D could be mediated by vitamin D receptor (VDR) (expressed by many types of immune cells) which is part of the inactivating complex with the nuclear p65 subunit of NFκB (Equils et al. 2006; Provvedini et al. 1983). Studies conducted in VDR-null mice have shown that VDR deletion abolished VDR/P65 binding and results into upregulation of NFκB transcriptional activity and increased IL-6 circulating levels (Wu et al. 2010).
Our findings also provide novel insight on the relationship between vitamin D and IL-6, focusing on the role of different components of IL-6 system. In fact, we showed for the first time in the specific setting of elderly subjects, not selected for specific diseases or conditions, a significant inverse relationship between vitamin D and IL-6 and sgp130 and a positive association between 25(OH)D and sIL-6r. These data are of importance because soluble IL-6 receptors, fluctuating remarkably much less than IL-6, give a more precise information about the activation of IL-6 pathway (Maggio et al. 2006). Interestingly, our data would suggest that vitamin D might also influence IL-6 system by modulating IL-6 “trans signaling”. Trans signaling mechanism, by the formation of circulating IL-6/sIL-6r complex, causes activation of the IL-6 pathway in cells without IL-6 membrane receptor and accounts for most of IL-6 biological activity (Maggio et al. 2006). Although there are limited and equivocal data about the role played by sIL-6r in the inflammatory pathway, several lines of evidence support the hypothesis of an anti-inflammatory role for sIL-6r by the upregulation of cellular gp130 expression, which is capable to impair IL-6 biological activity on membrane receptors (Ferrucci et al. 2005). Our findings are consistent to those published by Ferrucci et al. (Ferrucci et al. 2005) showing an impairment of sIL-6r serum concentrations in inflammatory conditions that stimulate IL-6 production.
By analyzing thoroughly the relationship between vitamin D and IL-6 system, we also observed an independent inverse relationship between vitamin D and sgp130. Soluble gp130 is considered the natural inhibitor of the IL-6/sIL-6r complex (Maggio et al. 2006). Consistently to what has been suggested by Zuliani et al. (Zuliani et al. 2010), documenting both higher IL-6 and sgp130 levels in clinical conditions associated with “low-grade” systemic inflammation, we can speculate that the negative association we have found between vitamin D and sgp130 is related to the activation of inflammatory processes involving the IL-6 and might be considered as a strategy to avoid IL-6 trans-signaling. Interestingly, we also found an inverse relationship between vitamin D and hsCRP in the crude model and in the linear multivariate regression analysis including age, sex, and PTH. Moreover, hsCRP levels were found higher in the lowest tertiles of vitamin D (<31.4 nmol/L). However, the relationship between vitamin D and hsCRP only approached the statistical significance in the fully adjusted model including chronic inflammatory diseases. These data could suggest that the global health status expressed as the combination of physical function and activity as well as some chronic conditions influence hsCRP concentration more than vitamin D alone.
We failed to detect any association between vitamin D and TNF-α. These results are only partially surprising. The inter-assay and intra-assay variability of TNF-α is quite high and most of the previous investigations conducted in the InCHIANTI study have not shown any significant association between hormones other than 25(OH)D and TNF-α (Melzer et al. 2008).
To overcome this specific issue, we also tested the relationship between vitamin D and soluble TNF-α receptors, sTNFR1 and sTNFR2, that have been shown to be more sensitive inflammatory markers than TNF-α in the assessment of different inflammatory diseases (Owczarek et al. 2012). However, we did not find any association between vitamin D and soluble TNF-alpha receptors suggesting that vitamin D might not affect the TNF-α system.
Finally, because IL-1ra bioactivity seems to be regulated by proinflammatory cytokines including IL-6, as an acute-phase protein, and patients with a variety of infectious and immune conditions or low muscle strength showed both higher IL-1ra and IL-6 levels (Stenholm et al. 2010; Gabay et al. 1997), we also investigated the relationship between 25(OH)D and IL-1ra. However, we did not appreciate any significant relationship between vitamin D and IL-1ra.
The cross-sectional nature of this study does not allow us to determine the direction of the association between vitamin D and inflammatory markers. We cannot ignore that low 25(OH)D could represent a marker of ill health resulting from inflammatory processes involved in disease occurrence and clinical course. This hypothesis has been raised from a very recent systematic review documenting a discrepancy between the existing observational and intervention studies on the role of 25(OH)D concentrations in nonskeletal health outcomes (Autier et al. 2014). The lack of evidence that raising of 25(OH)D concentration can modify the occurrence or clinical course of diseases is not causal and may explain why low vitamin D status is reported in a wide range of disorders. In fact, a fluctuation in vitamin D levels has been described after an inflammatory insult, such as surgical stress (Louw et al. 1992; Gray et al. 2005). In patients who underwent cardiac surgery, a transitory decline in 1-25-hydroxyvitamin D is observed together with a significant increase in inflammatory markers including IL-6. This phenomenon seems to be remarkably stronger in older than younger individuals (Börgermann et al. 2012). However, 25(OH)D concentrations remain low 3 months after surgery (Reid et al. 2011) despite the normalization of CRP status, suggesting that factors other than inflammation may be responsible for the postsurgical decline in vitamin D.
Study limitation and strengths
Some limitations of the study must be recognized. First, because of the cross-sectional nature of the study, we cannot define cause–effect relationships between vitamin D and inflammatory markers. Second, we did not measure the plasma levels of the IL-6/sIL-6r complex, and this would add important information about the real regulation of the IL-6 “trans-signaling” pathway in vitamin D insufficiency. We are also aware that other methodologies such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) are considered the gold standard in the assessment of 25(OH)D levels.
Despite these limitations, our study has multiple important strengths. This is the first attempt to provide novel insight of the relationship between 25(OH)D and inflammatory markers in a cohort of community-dwelling older adults, focusing on the role of different components of the IL-6, TNF-α, and IL-1 systems. IL-1 receptor antagonist, sTNFR1 and sTNFR2, and sgp130 and sIL-6r were used in addition to IL-1β and TNF-α and IL-6 soluble receptors because they are more sensitive than those respective cytokines and/or provide additional information on cytokine biological activities [35; 46-48]. Moreover, previous studies have been conducted in young adults with chronic inflammatory conditions. The population here evaluated had also detailed and complete data on serum concentrations of hsCRP, IL-1β, IL-10, and IL-18 as well as factors known to influence both 25(OH)D and inflammatory status, that are not easily found in large population studies, especially of older adults. All analyses were accurately assessed by ultrasensitive method (ELISA). Season of the blood collection was also included in the analysis to account for seasonal vitamin D variability. Finally, to avoid the influence of acute inflammatory status and anti-inflammatory medications on the hypothesis tested, we excluded participants with hsCRP ≥10 mg/dl and those who were on chronic treatment with corticosteroids and nonsteroidal inflammatory drugs.
In conclusion, we found that in older individuals 25(OH)D concentration is independently and inversely associated with IL-6 and positively with sIL6r, suggesting a potential anti-inflammatory role for vitamin D in older individuals. These results, if confirmed by longitudinal studies, may better delineate the direction of this association, explaining the mechanisms by which the reduced levels of 25(OH)D facilitate the development of proinflammatory conditions in the elderly. Randomized controlled studies, examining the effects of vitamin D administration on inflammatory markers and clinical outcomes are clearly needed.
Acknowledgments
We acknowledge Maurizio Conca, Pasquale Rosanova, and Maria Teresa Zanelli for their technical support.
The InCHIANTI Study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336), and by the Intramural Research Program of the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here. None of the authors had a conflict of interest.
References
- Amer M, Qayyum R. Relation between serum 25-hydroxyvitamin D and C-reactive protein in asymptomatic adults (from the continuous National Health and Nutrition Examination Survey 2001 to 2006) Am J Cardiol. 2012;109:226–230. doi: 10.1016/j.amjcard.2011.08.032. [DOI] [PubMed] [Google Scholar]
- Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- Börgermann J, Lazouski K, Kuhn J, Dreier J, Schmidt M, Gilis-Januszewski T, Knabbe C, Gummert JF, Zittermann A. 1,25-Dihydroxyvitamin D fluctuations in cardiac surgery are related to age and clinical outcome. Crit Care Med. 2012;40:2073–2081. doi: 10.1097/CCM.0b013e31824e8c42. [DOI] [PubMed] [Google Scholar]
- Clendenen TV, Koenig KL, Arslan AA, et al. Factors associated with inflammation markers, a cross-sectional analysis. Cytokine. 2011;56:769–778. doi: 10.1016/j.cyto.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin and 1,25-dihydroxyvitamin D levels with all cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- Equils O, Naiki Y, Shapiro AM, Michelsen K, Lu D, Adams J, Jordan S. 1,25-dihydroxyvitamin D inhibits lipopolysaccharide-induced immune activation in human endothelial cells. Clin Exp Immunol. 2006;143:58–64. doi: 10.1111/j.1365-2249.2005.02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol. 1983;39:249–251. doi: 10.1002/1097-4679(198303)39:2<249::AID-JCLP2270390218>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1 Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannagé-Yared MH, Azoury M, Mansour I, Baddoura R, Halaby G, Naaman R. Effects of a short-term calcium and vitamin D treatment on serum cytokines, bone markers, insulin and lipid concentrations in healthy post-menopausal women. J Endocrinol Invest. 2003;26:748–753. doi: 10.1007/BF03347358. [DOI] [PubMed] [Google Scholar]
- Gray A, McMillan DC, Wilson C, Williamson C, O’Reilly DS, Talwar D. The relationship between the acute changes in the systemic inflammatory response, lipid soluble antioxidant vitamins and lipid peroxidation following elective knee arthroplasty. Clin Nutr. 2005;24:746–750. doi: 10.1016/j.clnu.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Helming L, Böse J, Ehrchen J, Schiebe S, Frahm T, Geffers R, Probst-Kepper M, Balling R, Lengeling A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Colbert LH, Harris TB, Newman AB, Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26:S15–S25. doi: 10.1093/ije/26.suppl_1.S15. [DOI] [PubMed] [Google Scholar]
- Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv. 1976;6:493–508. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- Laird E, McNulty H, Ward M, Hoey L, McSorley E, Wallace JM, Carson E, Molloy AM, Healy M, Casey MC, Cunningham C, Strain JJ. Vitamin D deficiency is associated with inflammation in Older irish adults. J Clin Endocrinol Metab. 2014;25:c20133507. doi: 10.1210/jc.2013-3507. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Maggio M, Valenti G, Dall’Aglio E, Ceda GP. Vitamin D in older population: new roles for this ‘classic actor’? Aging Male. 2010;13:215–232. doi: 10.3109/13685538.2010.487551. [DOI] [PubMed] [Google Scholar]
- Li H, Weng P, Najarro K, Xue QX, Semba RD, Margolick JB, Leng SX. Chronic CMV infection in older women: longitudinal comparisons of CMV DNA in peripheral monocytes, anti-CMV IgG titers, serum IL-6 levels, and CMV pp 65 (NLV)-specific CD8(+) T-cell frequencies with twelve year follow-up. Exp Gerontol. 2014;54:84–89. doi: 10.1016/j.exger.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- Louw JA, Werbeck A, Louw ME, Kotze TJ, Cooper R, Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit Care Med. 1992;20:934–941. doi: 10.1097/00003246-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–784. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Perry JR, Hernandez D, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs) PLoS Genet. 2008;4:e1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RR, Hicks GE, Shardell MD, Cappola AR, Hawkes WG, Yu-Yahiro JA, Keegan A, Magaziner J. Association of serum vitamin D levels with inflammatory response following hip fracture: the Baltimore Hip Studies. J Gerontol A Biol Sci Med Sci. 2007;62:1402–1406. doi: 10.1093/gerona/62.12.1402. [DOI] [PubMed] [Google Scholar]
- Ngo DT, Sverdlov AL, McNeil JJ, Horowitz JD. Does Vitamin D modulate asymmetricdimethylarginine and C-reactive protein concentrations? Am J Med. 2010;123:335–341. doi: 10.1016/j.amjmed.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Owczarek D, Cibor D, Głowacki MK, Cieśla A, Mach P. TNF-α and soluble forms of TNF receptors 1 and 2 in the serum of patients with Crohn’s disease and ulcerative colitis. Pol Arch Med Wewn. 2012;122:616–623. doi: 10.20452/pamw.1537. [DOI] [PubMed] [Google Scholar]
- Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Querfeld U. Vitamin D and inflammation. Pediatr Nephrol. 2013;28:605–610. doi: 10.1007/s00467-012-2377-4. [DOI] [PubMed] [Google Scholar]
- Reid D, Toole BJ, Knox S, Talwar D, Harten J, O’Reilly DS, Blackwell S, Kinsella J, McMillan DC, Wallace AM. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–1011. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]
- Reyman M, Verrijn Stuart AA, van Summeren M, Rakhshandehroo M, Nuboer R, de Boer FK, van den Ham HJ, Kalkhoven E, Prakken B, Schipper HS. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int J Obes (Lond) 2013;38:46–52. doi: 10.1038/ijo.2013.75. [DOI] [PubMed] [Google Scholar]
- Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostkowska-Nadolska B, Sliupkas-Dyrda E, Potyka J, Kusmierz D, Fraczek M, Krecicki T, Kubik P, Zatonski M, Latocha M. Vitamin D derivatives: calcitriol and tacalcitol inhibits interleukin-6 and interleukin-8 expression in human nasal polyp fibroblast cultures. Adv Med Sci. 2010;55:86–92. doi: 10.2478/v10039-010-0012-9. [DOI] [PubMed] [Google Scholar]
- Shea MK, Booth SL, Massaro JM, et al. Vitamin K and vitamin D status: associations with inflammatory markers in the Framingham Offspring Study. Am J Epidemiol. 2008;167:313–320. doi: 10.1093/aje/kwm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM (2001) Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA 2815-2822 [DOI] [PubMed]
- Stenholm S, Maggio M, Lauretani F, Bandinelli S, Ceda GP, Di Iorio A, Giallauria F, Guralnik JM, Ferrucci L. Anabolic and catabolic biomarkers as predictors of muscle strength decline: the InCHIANTI study. Rejuvenation Res. 2010;13:3–11. doi: 10.1089/rej.2009.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: Implication for the treatment of kawasaki disease. J Steroid Biochem Mol Biol. 2009;113:134–138. doi: 10.1016/j.jsbmb.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Tenth Special Report to the U.S. Congress on Alcohol and Health from the Secretary of Health and Human Services. U.S. Department of Health and Human Services. Washington, DC: National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism (NIAAA); June 2000. NIH publication no. 00-1583
- Thorand B, Zierer A, Huth C, Linseisen J, Meisinger C, Roden M, Peters A, Koenig W, Herder C. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care. 2011;34:2320–2322. doi: 10.2337/dc11-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarrasa N, Vendrell J, Maravall J, Elío I, Solano E, San José P, García I, Virgili N, Soler J, Gómez JM. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010;38:235–242. doi: 10.1007/s12020-010-9379-4. [DOI] [PubMed] [Google Scholar]
- Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, Sun J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Galvani M, Maggio M, Volpato S, Bandinelli S, Corsi AM, Lauretani F, Cherubini A, Guralnik JM, Fellin R, Ferrucci L. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010;213:319–324. doi: 10.1016/j.atherosclerosis.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]