Abstract
Life-long regular endurance exercise is known to counteract the deterioration of cardiovascular and metabolic function and overall mortality. Yet it remains unknown if life-long regular endurance exercise can influence the connective tissue accumulation of advanced glycation endproducts (AGEs) that is associated with aging and lifestyle-related diseases. We therefore examined two groups of healthy elderly men: 15 master athletes (64 ± 4 years) who had been engaged in life-long endurance running and 12 old untrained (66 ± 4 years) together with two groups of healthy young men; ten young athletes matched for running distance (26 ± 4 years), and 12 young untrained (24 ± 3 years). AGE cross-links (pentosidine) of the patellar tendon were measured biochemically, and in the skin, it was assessed by a fluorometric method. In addition, we determined mechanical properties and microstructure of the patellar tendon. Life-long regular endurance runners (master athletes) had a 21 % lower AGE cross-link density compared to old untrained. Furthermore, both master athletes and young athletes displayed a thicker patellar tendon. These cross-sectional data suggest that life-long regular endurance running can partly counteract the aging process in connective tissue by reducing age-related accumulation of AGEs. This may not only benefit skin and tendon but also other long-lived protein tissues in the body. Furthermore, it appears that endurance running yields tendon tissue hypertrophy that may serve to lower the stress on the tendon and thereby reduce the risk of injury.
Keywords: Master athletes, Life-long, Endurance training, AGE, Aging
Introduction
The extracellular matrix in collagen-rich connective tissues such as skin, cornea, cartilage, lung, arteries and tendon undergoes progressive, irreversible, and detrimental changes as part of the normal aging process (Monnier et al. 2005). The connective tissue of tendon has traditionally been considered relatively inert, but several reports demonstrate that young human tendons respond to physical activity by increased metabolic activity (Bojsen-Moller et al. 2006; Kalliokoski et al. 2005), and collagen synthesis (Langberg et al. 1999, 2001), increased size and altered mechanical properties (Kongsgaard et al. 2007; Seynnes et al. 2009; Arampatzis et al. 2007). In fact, human studies have shown that endurance runners have larger patellar tendon and Achilles tendon cross-sectional area (CSA; hypertrophy), which lowers the tendon stress (Westh et al. 2007; Magnusson et al. 2007; Rosager et al. 2002). Collectively, these data demonstrate that tendons readily respond and adapt to loading. However, it has been shown that fibroblast cell number decreases with age and it is therefore uncertain if tendons maintain this adaptive capacity throughout life (Nagy et al. 1969).
The collagen fibril is the fundamental tensile bearing structure of tendon connective tissue and it is made up of collagen molecules linked together by cross-links. These bonds provide structural integrity to the fibrils and ensure appropriate force transmission (Avery and Bailey 2005; Eyre et al. 1984). There are basically two different types of cross-links: one enzymatically controlled by lysyl-oxidase during maturation (Eyre et al. 1984; Fujimoto et al. 1978), and one formed via nonenzymatic glycation (Monnier et al. 2005). The latter results in the formation of irreversible covalent advanced glycation endproducts (AGE), i.e., Maillard reaction, which accompanies the aging process (Monnier and Cerami 1981; Maillard 1912).
AGE cross-links accumulate with age in long-lived proteins, such as collagen, resulting in markedly higher concentrations in older than in younger individuals (Chen et al. 2000; DeGroot et al. 2001; Haus et al. 2007; Bank et al. 1999; Suzuki et al. 2008). The accumulation appears to be accelerated with age-related chronic diseases such as diabetes (Dyer et al. 1993; Vogt et al. 1982) and is believed to influence the function of several tissues. This aging process may be the reason for the higher incidence of connective tissue injuries by age (Murrell and Walton 2001; Englund et al. 2012). In line with this, we recently demonstrated that both enzymatic hydroxylysyl pyridinoline (HP) and lysyl pyridinoline (LP) cross-links and pentosidine, which is a marker of AGE, were more abundant in the patellar tendon of old men compared with that of young men (Couppe et al. 2009). It is believed that greater AGE cross-link density increases tendon stiffness (Reddy et al. 2002; Galeski et al. 1977; Andreassen et al. 1988; Bai et al. 1992), however, mechanical properties of both animal and human tendon has been reported both to increase (Nielsen et al. 1998; Shadwick 1990; Kubo et al. 2007a; Wood et al. 2011), decrease (Stenroth et al. 2012; Karamanidis and Arampatzis 2006; Dressler et al. 2002; Onambele et al. 2006; Mian et al. 2007) or remain unaltered with aging (Couppe et al. 2009; Haut et al. 1992; Hubbard and Soutas-Little 1984; Johnson et al. 1994; Carroll et al. 2008). Thus, to what extent aging influence mechanical properties of tendon tissue remains largely unknown.
A modest reduction in AGE cross-link density in tendon has been shown after 12 weeks of physical resistance training (Kongsgaard et al. 2009), and 12 weeks of moderate walking exercise appears to lower serum levels of AGE (Yoshikawa et al. 2009). However, if long-term physical activity can modify the accumulation of enzymatic and AGE cross-links in human tendon with aging has never been investigated. It appears that endurance training in aging animals yield a stronger and more compliant tendon (Gosselin et al. 1998; Viidik et al. 1996; Simonsen et al. 1995; LaCroix et al. 2013; Nielsen et al. 1998), but this remains to be confirmed in a human model. Furthermore, at the microstructural level, the size and/or density of tendon collagen fibrils seems to be reduced with aging (Curwin et al. 1994; Nakagawa et al. 1994; Partington and Wood 1963; Patterson-Kane et al. 1997), but if these age-related adaptations are influenced by physical activity remains unknown (Edwards et al. 2005).
Collectively, it remains unknown if life-long regular endurance running in humans influence 1) accumulation of AGE cross-links in tendon connective tissue 2) tendon mechanical properties, and 3) tendon fibril morphology. We therefore sought to investigate the effect of aging and physical activity on collagen cross-linking and mechanical properties of the human patellar tendon, in vivo. We hypothesized that life-long regular endurance runners display lower AGE cross-link density and mechanical properties/fibril structure of their patellar tendon that are more similar to that observed in young individuals compared to age-matched sedentary controls.
Methods
Subjects
A total of 49 men were recruited for this study, 15 endurance runners [master athletes; running distance of 49 ± 3 km week−1 (4–6 running days week−1) over the last 28 ± 2 years (mean ± SE)], 12 old untrained weight-matched healthy controls (old untrained), and ten young men matched for current running distance to the old trained (young athletes; 43 ± 5 km week−1 (4–6 running days week−1), endurance runners during the last 6 ± 1 years), and 12 young untrained weight-matched controls (young untrained; Table 1). The trained subjects were moderately to well-trained runners (Saltin and Grimby 1968). The untrained subjects had been sedentary for at least 5 years and, currently, did not perform any type of regular physical activity. The trained individuals were all endurance runners. Moreover, neither the trained nor the untrained had any physically demanding occupational jobs. Thus, their physical activity level was primarily determined by their leisure time activities. All subjects were healthy normotensive (<140/90 mmHg) and nonobese (BMI < 28), did not take any prescription medication, had no overt signs or symptoms of diabetes, prediabetes, or atherosclerosis, and had no known joint, muscle, or tendon pathology. The groups had similar diet as assessed by a brief questionnaire. The questionnaire represents diet habits of typical food intake for the last 3 months. The questionnaire has been used in an investigation of the Danish population food habits (n = 1007) by the Danish Nutrition Council (http://sundhedsstyrelsen.dk/publ/mer/2002/Befolkningens_foedevareforbrug_1996_2_udg_2002.pdf). Before the test days, subjects abstained from caffeine intake and fasted overnight to be studied in the postabsorptive state. Subjects were studied at least 24 h after their last running session to avoid the immediate acute effects of exercise, but they were still considered in their regular training state. The Ethics Committee of the Capital Region of Denmark approved this study (journal number 25543), and all procedures conformed to the Declaration of Helsinki. Written, informed consent was obtained from all subjects before the study.
Table 1.
Subject characteristics and physical activity
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Subject characteristics | ||||||
| Number of subjects | 10 | 12 | 15 | 12 | ||
| Age (years) | 26 ± 4* | 24 ± 3§ | 64 ± 4 | 66 ± 4 | p < 0.001, K–W | |
| Height (cm) | 179 ± 4 | 178 ± 6 | 176 ± 5 | 175 ± 4 | ||
| Weight (kg) | 73 ± 6 | 70 ± 8 | 71 ± 6 | 75 ± 4 | ||
| BMI (kg m−12) | 23 ± 2 | 22 ± 2§ | 23 ± 2 | 25 ± 2 | p < 0.05 | |
| Waist circumference (cm) | 79 ± 5* | 80 ± 5§ | 83 ± 5 | 90 ± 4* | p < 0.001 | |
| Physical activity | ||||||
| Running distance per week (km) | 43 ± 5 | 49 ± 3 | ||||
| Running distance last week (km) | 48 ± 7 | 43 ± 5 | ||||
| Running years | 6 ± 1 | 28 ± 2 | ||||
| IPAQ (MET min week−1) | 3,724 ± 579 | 919 ± 199# | 8,881 ± 1,791 | 1,277 ± 575* | p < 0.001, K–W | |
| VO2 peak (ml O2 min−1 kg−1) | 59 ± 1 | 45 ± 1 | 48 ± 2 | 30 ± 2 | −13, 17 | A < 0.001, T < 0.001 |
| Knee extensor moment (Nm) | 202 ± 11, (56, YU), (50, MA) | 147 ± 10# | 152 ± 9# | 145 ± 10 | A < 0.05, T < 0.01, A × T < 0.05 | |
Height, weight, BMI and waist circumference are given as mean ± SD. Other data are given as mean ± SE. Single p values are shown for one-way ANOVA, and p values for effects are shown for two-way ANOVA (A, T, A × T). For further details, see text. Main effect sizes are given as old–young and athlete–untrained. Effect size is shown in parenthesis under the relevant group for significant interactions
K–W Kruskal–Wallis test, A age, T training, A × T interaction
*Different from master athletes (p < 0.05)
#Different from young athletes (p < 0.05)
§Different from old untrained (p < 0.05)
Study design
On the first test day, subjects arrived fasted in the morning and blood samples were obtained. An oral glucose tolerance test (OGTT) was performed, anthropometrics were measured, and questionnaires were filled up. Finally magnetic resonance image (MRI) scanning of the thigh was performed to determine the cross-sectional area (CSA) of the quadriceps muscle and patellar tendon, respectively. On the second test day, mechanical properties of the patellar tendon and muscle strength (knee extensor moment) was measured. An hour after consuming a light sandwich, fruit, and drinks, maximal oxygen consumption was measured. Test days 1 and 2 were performed on consecutive days. The anthropometric measures, blood samples, and physical activity data shown below are already reported elsewhere (Mikkelsen et al. 2013).
Anthropometric measures
Waist circumference was measured as the smallest circumference between anterior superior iliac spine and the lower ribs to the nearest millimeter. Height was measured to the nearest millimeter and weight to the nearest 100 g, wearing light clothes and without shoes. The latter was used to calculate body mass index, BMI (kg m−2).
Blood samples
Fasted plasma samples were drawn from an antecubital vein. For analyses of insulin, plasma was drawn into EDTA tubes and cooled on ice for 10 min, serum was allowed to clot at room temperature for 30 min followed by centrifugation of both plasma and serum (10 min at 3,060 × g at 4 °C), and the plasma or serum phase was stored at −80 °C until analysis.
A glucose tolerance test was performed by oral administration of 75 g glucose dissolved in water and glucose and insulin analysis of blood sampled before and after 2 h. In addition, blood lipid profile was determined by measuring triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL), low-density-lipoprotein cholesterol (LDL), and leucocytes. Blood samples were analyzed at the Clinical Biochemistry Department, Bispebjerg Hospital, Copenhagen. The blood samples were transported to the laboratory and analyzed no more than 2 h from the time of collection according to the laboratory standard operating procedures (Nordin et al. 2004). Authorized lab technicians handled the analyses. Cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride, and blood glucose concentrations were measured by a colorimetrical slide test in a Vitros 5.1 FS (Ortho Clinical Diagnostics, Raritan, NJ, USA; Sennels et al. 2011, 2012). Leucocyte concentrations were measured by optical spread light in a Sysmex XE (Sysmex, Kobe, Japan; Sennels et al. 2011). HbA1c was measured using an ion-exchange high-performance liquid chromatography (HPLC) method (Osoh G8 - Tosoh Bioscience, Yamaguchi, Japan; Dunn et al. 1979; Mosca et al. 1986).
Plasma insulin concentration was measured using a standard insulin ELISA kit, K6219 (Dako Denmark A/S, Glostrup, Denmark) at the Institute of Sports Medicine, Department of Orthopaedic Surgery M, Bispebjerg Hospital and the Center for Healthy Aging, Faculty of Health Sciences, University of Copenhagen, Denmark. The homeostatic model assessment (HOMA) used to quantify insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin as follows: HOMA-IR = (glucose in mmol/l × insulin in mU/l)/22.5. The quantitative insulin sensitivity check index (QUICKI) was derived from fasting insulin and fasting glucose as follows: QUICKI = 1 / [log (insulin μU ml−1) + log (glucose mg dl−1)].
Physical activity level
To quantify the physical activity level of the subjects, the International Physical Activity Questionnaire (IPAQ) was used (Craig et al. 2003). The IPAQ questionnaire quantifies the physical activity level of the subjects as the average weekly metabolic equivalent of task (MET) score, MET min week−1. One MET = 1 kcal · kg−1 · h−1 is equivalent to the resting metabolic rate (RMR) obtained during quiet sitting (Ainsworth et al. 2011). The World Health Organization (WHO) has defined a weekly MET score below 600 as low physical activity level and above 3,000 as a high physical activity level.
Peak oxygen consumption was measured on a Monark electronically braked ergometer bike (Monark 839 Ergomedic, Proterapi, Brøndby, Denmark) using an incremental protocol with a 25 W increase in load every minute starting at 50 W (old untrained) or 75 W (all other groups) until exhaustion which was reached within 4–12 min in all subjects. Subjects were instructed to aim at a pedalling frequency around 80–90 rounds min−1 (RPM) and were verbally encouraged to perform their max. Oxygen consumption (VO2), CO2 excretion, respiratory exchange ratio (RER), heart rate (HR), minute ventilation (VE), and cadence (RPM) were continuously recorded (breath-by-breath) using Masterscreen CPX equipment (Care Fusion, San Diego, CA, USA). All subjects reached RER values above 1.09 (range 1.09–1.34), indicating that high exertion levels were obtained. Peak oxygen uptake (VO2 peak) was determined as the highest mean VO2 value reached during a 15-s period (ignoring the highest and lowest value within that interval), and given as fitness level (ml O2 kg−1 min−1).
Muscle and tendon dimensions
The protocol for measuring anatomical cross-sectional area of the quadriceps femoris muscle (Q-CSA) and patellar tendon CSA/length has been described elsewhere (Couppe et al. 2008; Mikkelsen et al. 2013). In brief, Q-CSA of the mid-thigh level and tendon CSA/length was measured using magnetic resonance imaging (MRI; General Electric, Sigma Horizon LX 1.5 T, T1-weighted SE, Millwaukee, WI, USA). For the Q-CSA, T1-weighted (T1w) turbo spin echo (TSE) images were performed using a lower extremity coil and parameters as previously described. The lean muscle mass of the Q-CSA was measured (subcutaneous and intermuscular noncontractile tissue were excluded). The mean value of three measurements of the same image was used for further analysis. The typical error percentage was 2 %.
Patellar tendon CSA and length was determined by T1w TSE para axial and sagital plane MRI using a knee coil and parameters as described previously (Couppe et al. 2008). A plastic tube containing 1.0 % CuSO4 was placed in the FOV for normalization of tendon signal intensity. The axial scans were performed perpendicular to the patellar tendon. As described in detail elsewhere, the tendon CSA was measured region-specific (proximal, mid, and distal; Couppe et al. 2008). Average tendon CSA was calculated from the three sites and used for further analysis. Tendon length was obtained as previously described. The color range of each image was adjusted to set the minimum (black) at zero signal and the maximum (white) at the peak signal of the 1 % CuSO4 solution. Tendon CSA and length were measured using the grayscale image display. The typical error percent of repeated measures of site-specific tendon CSA was 3.1–5 %. Moreover, from the axial scans, tendon signal intensity [mean gray value (MGV)] were determined after measuring tendon CSA by using MATLAB (Carroll et al. 2008). The value of all slices from proximal to distal was averaged to determine mean tendon signal intensity. Both Q-CSA and patellar tendon CSA/length were manually outlined using the DICOM file viewer and associated measurement software Osirix 2.7.5 (OsiriX medical imaging software, Geneva, Switzerland). The MRI investigator (JFG) was blinded with regard to the subjects.
Mechanical properties of tendon
Details of the measurement, including the reliability of the method in our laboratory, has been reported previously (Hansen et al. 2006). The subjects were asked to refrain from strenuous exercise 48 h prior to the experiments. The subjects had 5 min warm-up on a stationary bike before the test, and all measurements were performed on the nondominant leg. In brief, subjects were seated in a rigid chair with 90 ° knee and hip angle. Knee extension ramps over 10 s were performed with a strain gauge attached to the lower leg measuring force production, while synchronized B-mode ultrasound of the patellar tendon was recorded. Displacement of the patella and tibial tuberosity was used to determine tendon deformation. Tendon force was calculated from the measured extensor moment, using the distance from the lateral epicondyle of the knee to the attachment point of the strain gauge as the external moment arm and estimating the tendon moment arm from the femur length (Visser et al. 1990). Each force–deformation curve was fitted to a second-order or third-order polynomial (r2 > 0.97), which has previously been shown to describe the data curves well. Force and deformation was normalized to stress and strain using the CSA and resting length determined by MRI. Stiffness and elastic modulus was determined in the final 20 % of the curve. To improve the comparability between subjects achieving different maximal tendon loads, the data was also analyzed at the greatest common force level across all subjects. One master athlete subject did not contribute to mechanical data due to technical error during mechanical testing.
Tendon biopsies
Tendon biopsies were obtained from the nondominant extremity (Couppe et al. 2008) according to procedures described in detail elsewhere (Kongsgaard et al. 2009). In brief, after sterilization of the insertion site, the skin was injected with local anesthetic (1 % lidocaine), and a 3- to 5-mm-long incision was made distally to the patella apex. Tendon biopsies (~8 mg) were obtained by using a 16 G Monopty biopsy instrument (Bard Inc, Covington, GA, USA) with a disposable core biopsy needle (14 gauge). The biopsy was taken at an angle of ~60° relative to the patella. Biopsies were cleared of external adipose tissue and blood. The piece used for analysis of collagen cross-links was snap-frozen in liquid nitrogen, and stored at −80 °C for subsequent analysis. Part of the tendon biopsy was used for transmission electron microscopy (TEM). The biopsy specimens for TEM were fixed in a 2 % glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.2) and stored at 4 °C until subsequent analyses. The procedure for TEM and the following measurements of collagen fibril diameter have previously been described in details (Magnusson et al. 2002).
Biochemical analysis of collagen cross-links
Freeze-dried tendon samples were hydrolyzed in 6° M HCl (+108 °C, 24 h), evaporated into dryness and dissolved in H2O. Hydroxyproline, the collagen-specific amino acid, was measured spectrophometrically to quantify collagen content assuming 13.7 % hydroxyproline by mass (Creemers et al. 1997). HP, LP, and pentosidine cross-link concentrations were determined in a single reversed-phase high-performance liquid chromatography (HPLC) run and detected on the basis of their natural fluorescence (Bank et al. 1997). At 0–16 min, the wavelength for HP and LP fluorescence was 400 nm for emission and 295 nm for excitation. The wavelengths were changed at 16–60 min to 378/328 nm to measure pentosidine. For the elution of the cross-links, a gradient was built up to contain 17 % eluent B (75 % acetonitrile with 0.13 % heptafluorobutyric anhydride) at 0 min and 25 % eluent B at 30 min. Eluent A was 0.13 % heptafluorobutyric anhydride. Flow rate was 1 ml min−1. HP was eluted at 12 min, LP at 13.5 min, and pentosidine at 23 min. The HPLC system used included Quaternary Gradient Pump unit, PU-2089 Plus, Intelligent AutosamplerAS-2057 Plus, and Intelligent Fluorescence Detector FP-2020 by Jasco. Data processing software was Jasco Chrompass. The LiChroCART 125-4 column was from Merck Hitachi. The results for HP, LP, and pentosidine are given compared with the standards injected at four different concentrations in each HPLC run. The intra-assay CV % based on duplicates within a run was 2.6, 3.7, and 3.9 % for HP, LP, and pentosidine, respectively. The detection limit for HP and LP is 0.4 and 0.05 pmol for pentosidine. Two subjects were not included in the final analysis due to an error in the original sample.
Stereology
A random sample of ten digitized electron microscopy images were obtained from each biopsy cross-section. The stereological analyses of collagen fibrils were carried out on a computer monitor onto which the digitized electron microscopy image was merged with a graphic representation of the stereological test systems (C.A.S.T.-grid software, The International Stereology Center at Olympus). Each TEM image was examined with 16 uniformly positioned points and 16 uniformly positioned unbiased counting frames (Gundersen et al. 1988), each of area 0.0426 μm2, in a fixed position relative to the image. The counting frames covered 3 % of the area of the TEM images. On average, 430 ± 66 fibrils (range 334–569) were analyzed per biopsy cross-section in the master athletes specimens, and 394 ± 88 from each of the old untrained specimens (range 276–584). Furthermore, 490 ± 186 fibrils (range 280–857) were analyzed per biopsy cross-section in the young athletes specimens, and 452 ± 104 from each of the young untrained specimens (range 320–678). All measurements were performed in a blinded fashion.
AGE-reader measurements
AGE levels on the volar side in the forarm skin were measured with the AGE reader, a noninvasive, portable device, which has been comprehensively validated with AGEs measured in skin biopsies (Meerwaldt et al. 2004), DiagnOptics BV, Groningen, the Netherlands. The AGE reader measurements were made after the main study due to the availability of the AGE reader equipment. All measurements were always performed by the same observer (RBS). The AGE reader illuminates a skin surface of 1 cm2, with an excitation light source using wavelengths between 300 and 420 nm. Only light from the skin is measured with a spectrometer in the 300–600 nm range, using 200 μm glass fiber. Autofluorescence (AF) was calculated as follows: ratio between average light intensity, measured in the range 420–600 nm and the average light intensity in the range 300–420 nm (emission/excitation), and this ratio is expressed as arbitrary units. Three consecutive measurements, from each patient, were obtained, of which the mean was calculated. Within-day test–retest reproducibility was assessed in eight subjects before and after 1 h in our lab. Typical error% was 4.8 %. No systematic difference was found between the first and second session. The coefficient of determination for multiple with-in day assessments averaged r2 = 0.81.
Statistical analysis
Effects of age (A), training (T), and age × training (A × T) were tested using two-way analysis of variance (ANOVA), with Holm–Sidak multiple comparison post hoc testing, if nothing else is stated. When a significant interaction was observed, the post hoc test determined effects of training within the old (master athletes vs. old untrained), training within the young (young athletes vs. young untrained), age within the trained (master athletes vs. young athletes), and age within the untrained (old untrained vs. young untrained). Nonparametric one-way ANOVA (Kruskal–Wallis) test, with Dunn’s multiple comparison post hoc test was used for Age and IPAQ (Table 1). Height, weight, BMI, and waist circumference were analysed using an ordinary one-way ANOVA with Sidak multiple comparison post hoc test to compare selected groups, as listed above. Data are given as mean (SD) or mean (SE), as stated in the “Results” section. SigmaPlot version 11 (Systat Software Inc) and Graph Pad Prism version 4 (GraphPad Software Inc., La Jolla, CA, USA) were used for statistical analyses.
Results
Subject characteristics
Subject characteristics are shown in Table 1. Old participants tended to be shorter than young participants (p = 0.052), and they had slightly higher BMI (p = 0.012). Waist circumference was higher in old than young groups (p < 0.001) and in the untrained than trained groups (p = 0.04).
Physical activity data
Data are shown in Table 1. Regular running distance per week was similar in old and young runners (master athletes 49 ± 3; young athletes 43 ± 5 km week−1). Likewise, running distance during the last week prior to the study was similar between these groups (master athletes 43 ± 5; young athletes 48 ± 7 km week−1). The master athletes had been running for 28 ± 2 years, and the young athletes for 6 ± 1 years. Physical activity level assessed by IPAQ was significantly higher in master athletes compared to that in old untrained (p < 0.001) and in young athletes than in young untrained (p < 0.05), but similar between master athletes vs. young athletes and old untrained vs. young untrained.
Muscle performance
In the following A, T, and A × T refer to effects of aging, training, and an interaction between aging and training, respectively. Data on muscle performance are shown in Table 1. Quadriceps muscle strength was greater in young than in old men (A, p < 0.05) and in athletes vs. untrained (T, p < 0.01). A training by age interaction was observed (A × T, p < 0.05) with young athletes being stronger than young untrained and master athletes. Likewise, VO2 peak (ml O2 min−1 kg−1) was higher in young than old men (A, p < 0.001) and in athletes vs. untrained individuals (T, p < 0.001), while no interaction between age and training was observed.
Blood lipid profile
Data are shown in Table 2. Athletes demonstrated lower triglyceride, total cholesterol, and LDL cholesterol levels (T, p < 0.01). Old men had higher total, LDL, and HDL cholesterol than their young counterparts (A, p < 0.01). The difference in HDL was largely explained by a higher HDL level in master athletes. No interaction of age by training was observed for the blood lipids.
Table 2.
Blood lipid profile
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 15 | N = 12 | ||
| Triglycerides (mmol/l) | 0.73 ± 0.06 | 1.21 ± 0.20 | 0.70 ± 0.04 | 1.24 ± 0.15 | −0.52 | T < 0.01 |
| Total cholesterol (mmol/l) | 4.44 ± 0.19 | 5.18 ± 0.42 | 5.76 ± 0.24 | 6.67 ± 0.27 | 1.40, −0.83 | A < 0.001, T < 0.01 |
| HDL cholesterol (mmol/l) | 1.49 ± 0.08 | 1.44 ± 0.07 | 1.93 ± 0.09 | 1.60 ± 0.11 | 0.30, 0.19 | A < 0.01 (T = 0.051) |
| LDL cholesterol (mmol/l) | 2.61 ± 0.15 | 3.20 ± 0.39 | 3.51 ± 0.18 | 4.51 ± 0.22 | 1.11, −0.79 | A < 0.001, T < 0.01 |
Data are given as mean ± SE. p Values for significant main effects of age (A) and training (T) are given. Main effect sizes are given as old–young and athlete–untrained
A age, T training, A × T interaction
Glucose homeostasis
Data are shown in Table 3. Fasting insulin was lower in the athletes than in the untrained (T, p < 0.001), but was unaffected by age. Fasting glucose was lower in young vs. old men (A, p < 0.001). A training by age interaction was observed for fasting glucose (A × T, p < 0.05), where old untrained had higher values than master athletes and young untrained. Likewise, blood glucose 2 h after glucose ingestion was lower in young vs. old men (A, p < 0.01). Glycosylated hemoglobin (HbA1c), which identifies the average plasma glucose concentration over prolonged periods of time, was also reduced in young compared to old men (A, p < 0.001) and was unaffected by training. Insulin resistance assessed by HOMA-IR was lower in athletes vs. untrained (T, p < 0.001) and was not affected by aging. Insulin sensitivity assessed by QUICKI was higher in athletes vs. untrained participants (T, p < 0.001) and was not affected by age.
Table 3.
Glucose homeostasis
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 15 | N = 12 | ||
| Insulin (fasted, pmol/l) | 14.9 ± 1.9 | 22.8 ± 2.8 | 13.9 ± 1.1 | 26.0 ± 2.4 | −10 | T < 0.001 |
| Glucose (fasted, mmol/l) | 4.66 ± 0.11 | 4.58 ± 0.10§ | 4.91 ± 0.09 | 5.26 ± 0.10*, (0.35, MA), (0.68, YU) | A < 0.001, A × T < 0.05 | |
| Glucose (2 h, mmol/l) | 4.49 ± 0.34 | 4.70 ± 0.15 | 5.05 ± 0.27 | 5.53 ± 0.20 | 0.69 | A < 0.01 |
| HbA1c (mean mmol/l) | 5.58 ± 0.16 | 5.51 ± 0.15 | 6.03 ± 0.16 | 6.45 ± 0.11 | 0.70 | A < 0.001 |
| HOMA-IR | 0.53 ± 0.09 | 0.79 ± 0.11 | 0.51 ± 0.04 | 1.02 ± 0.10 | −0.39 | T < 0.001 |
| QUICKI | 0.44 ± 0.01 | 0.41 ± 0.01 | 0.44 ± 0.01 | 0.39 ± 0.01 | 0.04 | T < 0.001 |
Data are given as mean ± SE. Main effect sizes are given as old–young) and athlete–untrained. Effect size is shown in parenthesis under the relevant group for significant interactions
A age, T training, A × T interaction***
*Different from master athletes (p < 0.05)
§Different from old untrained (p < 0.05)
Collagen concentration and cross-link density
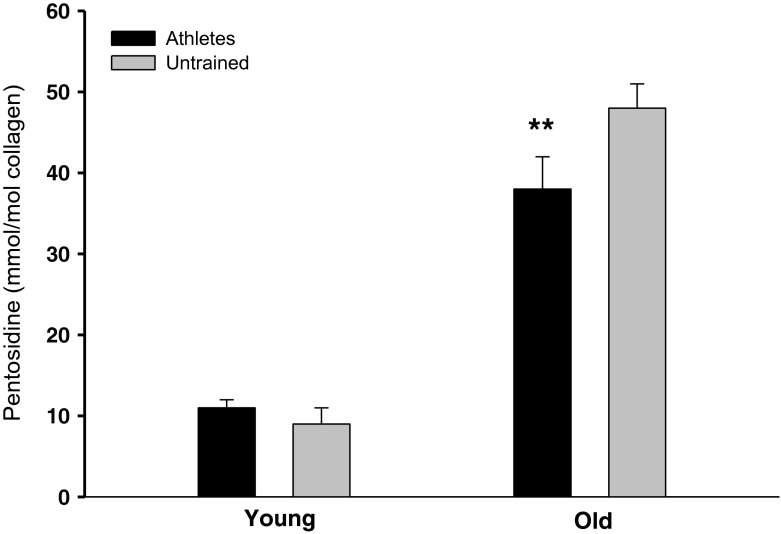
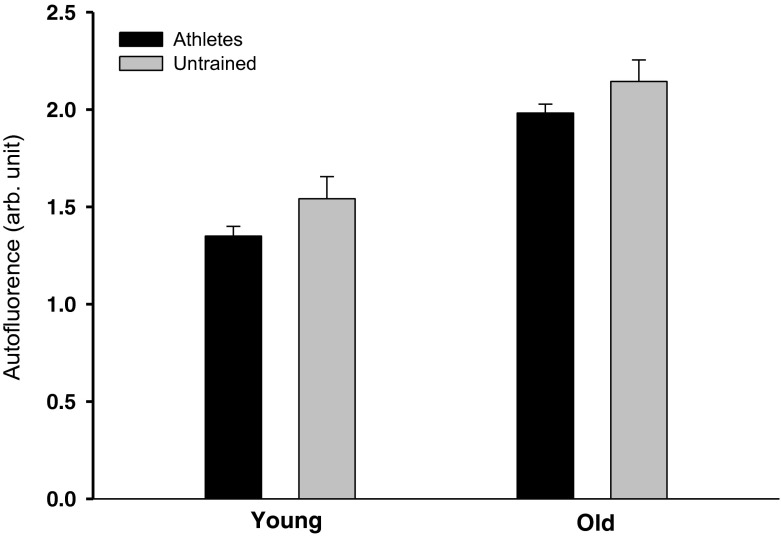
Data are shown in Table 4. Pentosidine density was higher with age (A, p < 0.001). There was an interaction between age and training (A × T, p < 0.05) with master athletes showing lower tendon pentosidine density than old untrained (p < 0.01 (Fig. 1). There was significant interaction for HP (A × T, p < 0.01), where HP was elevated in young athletes compared to young untrained (p < 0.05) and master athletes (p < 0.05). Effects of age (A, p < 001) and training (T, p < 0.05) were observed on skin AGE levels (autofluorescence; Fig. 2).
Table 4.
Tendon collagen cross-links
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 13 | N = 12 | ||
| Collagen (mg/mg dry wt) | 0.84 ± 0.04 | 0.85 ± 0.03 | 0.80 ± 0.03 | 0.79 ± 0.03 | ||
| Hydroxylysyl pyridinoline (HP, mmol/mol collagen) | 657 ± 48*, (142, YU), (197, MA) | 515 ± 44§ | 460 ± 42 | 563 ± 44 | A × T < 0.01 | |
| Lysyl pyridinoline (LP, mmol/mol collagen) | 31 ± 4 | 24 ± 2 | 32 ± 3 | 31 ± 3 | ||
| Pentosidine, (mmol/mol collagen) | 11 ± 2 | 9 ± 1 | 38 ± 4, (27, YA) | 48 ± 3**, (10, MA), (39, YU) | A < 0.001, A × T < 0.05 |
Data are given as mean ± SE. Effect size is shown in parenthesis under the relevant group for significant interactions
A age, T training, A × T interaction
*Different from master athletes (p < 0.05)
**Different from master athletes (p < 0.01)
§Different from young athletes (p < 0.05)
Fig. 1.
Pentosidine cross-link density (mmol/mol collagen). Pentosidine cross-link density was higher with age (p < 0.001). There was an interaction between age and training (p < 0.05), with **master athletes having a lower tendon pentosidine cross-link density than old untrained (p < 0.01). Data are given as mean ± SE
Fig. 2.
Skin autofluorescence (AGE levels). Data are given as mean ± SE. A significant effect of age (p < 001) and training (p < 0.05) was observed on skin autofluorescence. Data are given as mean ± SE
Muscle and tendon dimensions
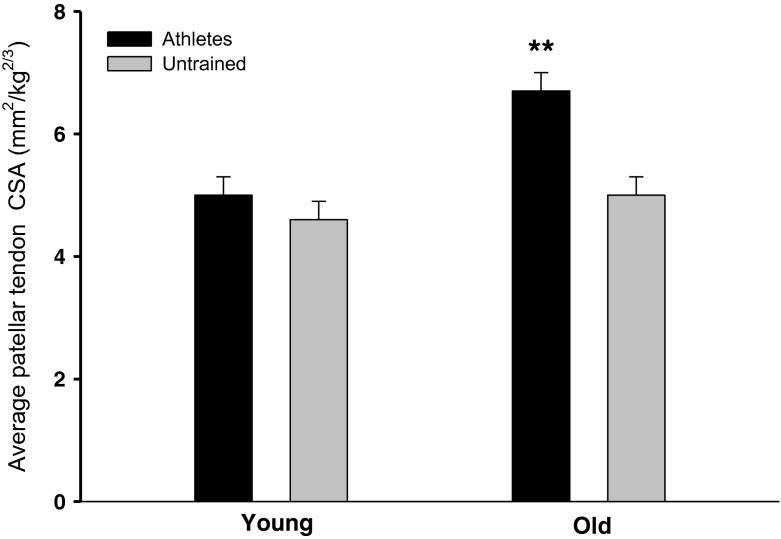
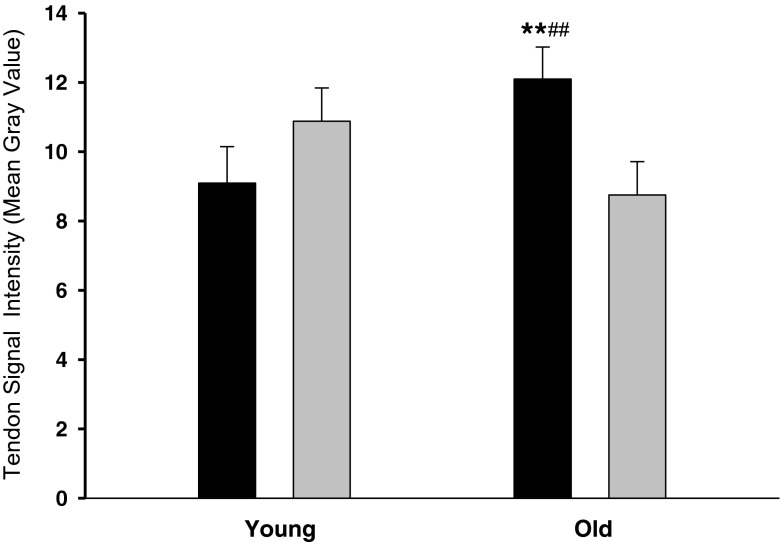
Data are shown in Table 5. Quadriceps muscle cross-sectional area (Q-CSA) as well as Q-CSA normalized with respect to body weight were larger in young than old men (A, p < 0.001) and in athletes vs. untrained (T, p < 0.001). In addition, an interaction between age and training was observed for Q-CSA (A × T, p < 0.05), where master athletes had a greater Q-CSA than old untrained (p < 0.001). Patellar tendon CSA demonstrated an interaction between age and training (A × T, p < 0.05), master athletes had larger tendons than any of the other groups (p < 0.01). Moreover, after normalizing patellar tendon CSA to body weight, a higher CSA was found with age (A, p < 0.0001) and training (T, p < 0.0001). Again, an interaction between age and training was demonstrated (A × T, p < 0.05), master athletes had larger tendons than any of the other individuals (p < 0.001; Fig. 3). Furthermore, young athletes tended to have greater patellar tendon CSA than young untrained (p = 0.103). Finally, an interaction between age and training (A × T, p < 0.01) was observed for tendon MRI signal intensity; master athletes had higher MRI signal intensity than old untrained men and young athletes (p < 0.05; Fig. 4).
Table 5.
Tendon and muscle dimensions
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 15 | N = 12 | ||
| Tendon length (mm) | 41 ± 2 | 42 ± 2 | 44 ± 2 | 42 ± 2 | ||
| Tendon CSA (mm2) | 84 ± 5** | 76 ± 5 | 110 ± 4, (26, YA), (24, OU) | 86 ± 5** | A < 0.001, T < 0.001, A × T < 0.05 | |
| Tendon CSA (mm2 kg−2/3) | 5.0 ± 0.3** | 4.6 ± 0.3 | 6.7 ± 0.3, (1.7, YA), (1.7, OU) | 5.0 ± 0.3** | A < 0.001, T < 0.001, A × T < 0.05 | |
| Quadriceps CSA (mm2) | 7,859 ± 201 | 6,792 ± 201 | 6,481 ± 200, (977, OU) | 5,504 ± 210* | A < 0.001, T < 0.001, A × T < 0.05 | |
| Quadriceps CSA (mm2 kg−2/3) | 45.1 ± 1.1 | 39.9 ± 1.0 | 38.3 ± 0.7, (7.2, OU) | 31.1 ± 1.3* | A < 0.001, T < 0.001, A × T < 0.05 |
Data are given as mean ± SE. Effect size is shown in parenthesis under the relevant group for significant interactions
A age, T training, A × T interaction
*Different from master athletes (p < 0.05)
**Different from master athletes (p < 0.01)
Fig. 3.
Average patellar tendon CSA normalized to body weight (mm2 kg−2/3) A greater effect of age (p < 0.0001) and training (p < 0.0001) was observed on weight-normalized tendon CSA. There was a significant interaction between age and training (p < 0.05), namely, the **master athletes had a greater tendon CSA than any of the other groups (p < 0.01). Data are given as mean ± SE
Fig. 4.
Tendon MRI signal intensity—mean gray value. A significant interaction between age and training (p < 0.01) was found for tendon MRI signal intensity—mean gray value. Master athletes had a significantly higher MRI signal intensity than **old untrained and ##young athletes (p < 0.01). Data are given as mean ± SE
Mechanical tendon properties
Mechanical properties of the tendon obtained at maximum force are shown in Table 6. Maximum patellar tendon force was greater in athletes vs. untrained men (T, p < 0.01). Furthermore, an interaction was found between age and training (A × T, p < 0.05), where young athletes had higher maximum force than master athletes (p < 0.01) and young untrained (p < 0.001). Tendon stress was significantly lower with age (A, p < 0.001). Likewise, there was a significant interaction between age and training for tendon stress (A × T, p < 0.05), with young athletes showing a greater stress than young untrained. However, no other effects were observed with respect to tendon deformation, stiffness, strain or Young’s modulus determined at maximal force. Mechanical tendon properties at a common force are shown in Table 7. For patellar tendon stress a main effect of both aging (A, p < 0.01) and training (T, p < 0.01) was detected, so that patellar tendon stress at common force levels was lower with age and training.
Table 6.
Tendon mechanical properties based on maximum force
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 14 | N = 12 | ||
| Max force (kN) | 6.1 ± 0.4**, (1.2, MA) | 4.4 ± 0.3§ | 4.9 ± 0.3 | 4.6 ± 0.3 | T < 0.001, A × T < 0.05 | |
| Deformation (mm) | 2.9 ± 0.2 | 2.8 ± 0.2 | 2.7 ± 0.2 | 2.5 ± 0.2 | ||
| Stiffness (kN mm−1) | 5.5 ± 0.5 | 4.2 ± 0.5 | 4.2 ± 0.4 | 4.1 ± 0.5 | ||
| Stress (MPa) | 74 ± 5**, (27, MA) | 61 ± 5 | 47 ± 4 | 55 ± 5 | A < 0.001, A × T < 0.05 | |
| Strain (%) | 7.4 ± 0.6 | 6.4 ± 0.5 | 6.3 ± 0.5 | 6.1 ± 0.5 | ||
| Modulus (GPa) | 2.7 ± 0.3 | 2.4 ± 0.3 | 2.0 ± 0.3 | 1.8 ± 0.2 | −0.7 | A < 0.01 |
Data are given as mean ± SE. Main effect sizes are given as old–young and athlete–untrained. Effect size is shown in parenthesis under the relevant group for significant interactions
A age, T training, A × T interaction
*Different from master athletes (p < 0.05)
**Different from master athletes (p < 0.01)
§Different from young athletes (p < 0.001)
Table 7.
Tendon mechanical properties determined based on common force
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 14 | N = 12 | ||
| Deformation (mm) | 2.1 ± 0.2 | 2.3 ± 0.2 | 2.1 ± 0.1 | 2.0 ± 0.2 | ||
| Stiffness (kN mm−1) | 3.2 ± 0.3 | 3.0 ± 0.3 | 3.2 ± 0.3 | 3.4 ± 0.3 | ||
| Stress (MPa) | 33 ± 2 | 38 ± 2 | 27 ± 2 | 33 ± 2 | 6, 6 | A < 0.01, T < 0.01 |
| Strain (%) | 5.2 ± 0.5 | 5.0 ± 0.4 | 4.8 ± 0.4 | 5.0 ± 0.4 | ||
| Modulus (GPa) | 1.6 ± 0.2 | 1.8 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.2 |
Data are given as mean ± SE. Main effect sizes are given as old–young and athlete–untrained
A age, T training, A × T interaction
Collagen fibril characteristics
Data are shown in Table 8. Tendon fibril density tended to be reduced with age (A, p = 0.084). An interaction between age and training was observed for fibril volume fraction (A × T, p < 0.05). Fibril volume fraction in old untrained strongly tended to be lower than in young untrained (p = 0.053). No other effects were observed.
Table 8.
Tendon fibril characteristics
| Variable | Young athletes (YA) | Young untrained (YU) | Master athletes (MA) | Old untrained (OU) | Main effect size | p Value |
|---|---|---|---|---|---|---|
| Number of subjects | N = 10 | N = 12 | N = 11 | N = 12 | ||
| Volume fraction (%) | 53 ± 2 | 57 ± 2§, (6, OU) | 56 ± 2 | 51 ± 2 | A × T < 0.05 | |
| Density (fibril μm2) | 92.8 ± 7.1 | 85.6 ± 6.2 | 81.1 ± 7.8 | 72.6 ± 6.2 | 12 | (A = 0.084) |
| Mean fibril diameter (nm) | 78 ± 2 | 83 ± 3 | 81 ± 4 | 83 ± 3 | ||
| Mean fibril area (nm2) | 6172 ± 624 | 6572 ± 541 | 6433 ± 678 | 6542 ± 541 |
Data are given as mean ± SE. Main effect sizes are given as old–young and athlete–untrained. Effect size is shown in parenthesis under the relevant group for significant interactions
A age, T training, A × T interaction
§Different from old untrained (p = 0.053)
Discussion
The benefits of life-long vigorous regular exercise on cardiovascular, metabolic parameters, and overall mortality are well documented (Chakravarty et al. 2008; Yataco et al. 1997; Tanaka and Seals 2008). These previous findings were confirmed by several of the variables obtained in the present study, where the master athletes had similar cardiovascular and metabolic profiles to that of the young sedentary controls. In both our old and young nonobese healthy men, regular long-term endurance running was associated with improved insulin sensitivity and lower triglyceride levels. Further, age-related changes in waist circumference, VO2 peak, cholesterol, LDL, and leg muscle size were counteracted by the effects of chronic physical activity.
To the best of our knowledge, this is the first study that has examined if aging and life-long endurance exercise in humans influence the accumulation of nonenzymatic (AGE) and enzymatic cross-links in connective tissue and fibril structure morphology. The main findings were that the master athletes had a 21 % lower AGE cross-link density (pentosidine) in their patellar tendons compared to old untrained men, and skin AGE levels (autofluorescence) were 11 % lower in athletes. Furthermore, both master athletes and young athletes had a higher cross-sectional area of the patellar tendon, which lowered the tendon stress for any given mechanical load. Master athletes also had a 30 % greater MRI signal intensity of their patellar tendon, in vivo. The present data suggest that life-long regular endurance exercise, such as running, can counteract the aging process in connective tissue of tendon and skin by reducing the age-related rise in AGE cross-link density, which may also affect other long-lived protein tissues. Furthermore, it appears that endurance running yields patellar tendon hypertrophy that reduces the stress exerted on the tendon and thereby lowers the risk of overuse injury.
Collagen cross-linking
AGE cross-links
AGE cross-links, including pentosidine, are formed when amino acid residues in the long-lived protein, such as the collagen triple helix, come into contact with glucose and thereby modify the structure of the collagen connective tissue (Monnier and Cerami 1981). These cross-links accumulate with aging (Monnier and Cerami 1981; Avery and Bailey 2005) and in chronic diseases such as diabetes (Brownlee 1995; Monnier et al. 2005), and this process may be one of the underlying reasons for the age-related higher incidence of connective tissue injury (Murrell and Walton 2001; Englund et al. 2012). Life-long endurance exercise has been shown to have several beneficial effects that can retard the normal age-related decline in physical, cardiovascular, and metabolic parameters (Tanaka and Seals 2008; Yataco et al. 1997; Chakravarty et al. 2008). However, to what extent life-long endurance exercise can decelerate this age-related accumulation of irreversible AGE cross-linking and the deterioration of tendon has, to our best knowledge, not been investigated. In the present study, we demonstrate that AGE cross-link density (pentosidine) in the patellar tendon was 21 % lower in master athletes compared to old untrained subjects and fourfold higher in old men compared to activity-matched young men.
We also determined the AGE accumulation in skin using a noninvasive method based on autofluorescence (AGE reader; Meerwaldt et al. 2004). It has been shown that the difference between young and older individuals in AGE cross-link density in collagen is twofold in skin (Verzijl et al. 2000) and skeletal muscle (Haus et al. 2007), fivefold in bone (Saito et al. 1997), sevenfold in tendon (Couppe et al. 2009), ninefold in ligaments (Chen et al. 2000), and 33-fold in cartilage (Verzijl et al. 2000) demonstrating marked age-related differences in tissue-specific turnover. The present data showed 45 % higher values in skin with aging, but also 11 % lower values by training, indicating that routine-running reduced the age-related AGE accumulation in skin. In contrast to AGE in the load-bearing tendon, the elevated AGE levels in the skin of the forearm of the athletes are likely the result of a systemic effect, which may also affect AGE accumulation in other tissues (Baynes 2000; Momma et al. 2012).
In the present study, master athletes demonstrated markedly lower fasting insulin and glucose levels, which is likely an effect of the life-long exposure to regular endurance running (Rogers et al. 1990; Seals et al. 1984). Elevated levels of blood glucose and reduced glucose tolerance could explain the higher AGE cross-link density in tendons of old untrained men in the present study. Likewise, improved glycemic control for many years may be the reason for lower AGE cross-link density in the tendons of the present master athletes (Lyons et al. 1991; Rogers et al. 1990). In individuals with normal glucose tolerance, it has been demonstrated that regular exercise can protect against the development of insulin resistance and the decline in glucose tolerance normally observed with sedentary aging. In addition, it has been shown that regular exercise can normalize glucose tolerance by means of short-term effects in individuals with abnormal glucose tolerance (Rogers et al. 1990). It has been proposed that glycation of collagen results in increased brittleness of connective tissue, which makes the connective tissue more prone to injury, while regular exercise may slow the glycation process (Avery and Bailey 2005). The mechanism of this process remains unclear but may relate to increased turnover of collagen with chronic exercise thereby attenuating AGE accumulation (Sell and Monnier 2012; Avery and Bailey 2005). In the present study, we assessed pentosidine, which only constitute a small fraction of the AGE cross-links, but there is strong evidence that it correlates with more prominent AGEs, such as glucosepane and carboxymethyl-lysine (CML; Dammann et al. 2012; Meerwaldt et al. 2004; Dyer et al. 1991). Altogether, the present data demonstrate that life-long regular physical activity influence the accumulation of nonenzymatic AGE cross-links tendon and skin and thereby partly counteract the aging process of the tissue.
The present study also raises questions as to the level of exercise that is needed in later life in order to reduce the magnitude of AGE cross-linking. There are prospective data indicating that AGE cross-link (pentosidine) density is reduced by 23 % with 12 weeks of heavy resistance training and that tendon mechanical properties can improve by short-term physical activity in elderly subjects, which indicates some kind of rejuvenation of tendon tissue (Kongsgaard et al. 2009). Future studies should explore whether short-term endurance running or other types of exercise of less or more vigorous intensity may reduce AGE cross-linking density in long-lived proteins and also whether AGE cross-linking in connective tissue of elderly can be reduced by short-term physical training.
Enzymatic cross-links
The density of mature lysyl oxidase-derived intermolecular covalent cross-links HP and LP gradually increase during tendon tissue maturation and are believed to be the chief contributors to the function and mechanical properties of tendons (Eyre et al. 1984; Fujimoto et al. 1978). Data on mature cross-link density of human tendons, and how these are influenced by aging, are sparse (Bank et al. 1999; Couppe et al. 2009). A small age-related increase of LP in the supraspinatus tendon has been reported in human cadavers (Bank et al. 1999), and we have previously shown that old men have greater HP (40 %) and LP (200 %) density in the patellar tendon compared to young men (Couppe et al. 2009). This is in contrast to the present findings where no age-related effect was observed for HP and LP cross-links. However, interestingly, the young athletes had a higher HP density compared to young untrained, suggesting a training effect. Up to a 37-fold increase (Heinemeier et al. 2007) in the expression of lysyl oxidase (LOX) mRNA in tendon tissue in response to acute training has been reported, which could indicate a loading-induced upregulation in enzymatic cross-linking (HP, LP) of collagen in tendon. This mechanism could explain the increased HP density in the young athletes. It is uncertain why this effect would be absent in aging master athletes, although it is known that age may blunt the LOX response possibly due to lack of tissue renewal associated to decline in tendon cell number and function with age (Nagy et al. 1969; Heinemeier et al. 2013; Thorpe et al. 2010).
Tendon collagen content
Collagen content of the patellar tendon (Haut et al. 1992) and tail-tendon (Vogel 1983) in animals has been shown to decrease or increase (Vailas et al. 1985) with age. In humans, we recently demonstrated that collagen concentration of the patellar tendon was 34 % lower in old men compared to that in young men (Couppe et al. 2009). In the present study, the reduction in collagen content with age was not significant, and there was no effect of training either. The reason for this difference is not clear, but may be related to a stricter matching between athletes and control subjects in the present study.
Structural and mechanical properties of tendon
Tendon dimensions
There is evidence that the size and mechanical properties of human tendons can be influenced by both the level of physical activity and the aging process. Recent reports demonstrate that young human tendons respond to physical activity by increasing tendon size, which is accompanied by altered functional mechanical properties (Kongsgaard et al. 2007; Seynnes et al. 2009). In animal models, aging is associated with an increase in tendon cross-sectional area (Nakagawa et al. 1996; Birch et al. 1999), and in humans, the Achilles tendon, but not the patellar tendon, appears to undergo hypertrophy with aging (Magnusson et al. 2003; Carroll et al. 2008). In the present study, we found a greater cross-sectional area of the patellar tendon in endurance athletes, which by definition resulted in a lower tendon stress for a given load. The master athletes had 30 % larger patellar tendon CSA than their sedentary healthy counterparts. Consequently, life-long regular endurance running appears to induce a hypertrophy response that lowers the stress on the tendon and potentially reduces the risk of injury. Moreover, the master athletes also had greater patellar tendon MRI signal intensity than old untrained and young athletes. It has been shown that MRI signal intensity correlate with histological findings such as tendon tissue hydration and collagen structure (Carroll et al. 2008; Harber et al. 2009). The present data collectively indicate that life-long endurance running increases tendon size and may change the internal milieu of tendon tissue.
Collagen fibril morphology
It has been previously shown that heavy slow resistance training is associated with changes in fibril morphology (Kongsgaard et al. 2010), and in addition, aging has been observed to reduce the size and/or density of collagen fibrils both in animals (Curwin et al. 1994; Nakagawa et al. 1994; Parry et al. 1978; Patterson-Kane et al. 1997) and humans (Sargon et al. 2005; Strocchi et al. 1991). In the present study, we did not find any significant effects on fibril morphology with age or training, although there was a tendency for lower collagen fibril density with age (p = 0.084).
Tendon mechanical properties
Stiffness of both animal and human tendons have been reported to increase (Shadwick 1990; Kubo et al. 2007a), decrease (Stenroth et al. 2012), or remain unchanged (Couppe et al. 2009) with aging. Vidiik et al. (Viidik et al. 1996) showed that life-long endurance training may have counteracting systemic effects on the aging process as reflected by less stiff tail tendon tissue. Simonsen et al. (1995) studied the effect of 38 weeks of strength training and swimming on Achilles tendon properties in rats and showed that ultimate failure decreased with aging, but that this age-related decline was counteracted only by swim training and not in rats subjected to strength training. However, this may be different in humans since improvement (change toward a young phenotype) of the mechanical properties of tendon has been observed with strength training in elderly persons (Reeves et al. 2003; Carroll et al. 2011; Seynnes et al. 2011). In the present study, the effect of chronic (life-long) endurance running appeared to yield a tendon hypertrophy response, which, however, was not accompanied by greater tendon modulus or stiffness at common force levels as may have been expected. The lack of a significant mechanical difference is likely due to different effects pulling in different directions, i.e., lower AGE cross-link density and greater tendon CSA.
Limitations
The present investigation is a cross-sectional case control study and, therefore, has inherent limitations. Natural selection cannot be excluded such that our runners have lower pentosidine levels and larger patellar tendon before loading their tendons. Furthermore, it must also be mentioned that there are considerable variations in tendon mechanics between subjects, and consequently, there is a risk that relevant differences were not found because a type 2 error was committed (Magnusson et al. 2001). Moreover, to avoid an underestimation of real PT force, the co-activation of the hamstrings should preferably be taken into account, and therefore we have previously measured the EMG (Electromyographic) activity from the biceps femoris during the ramped isometric extension (Hansen et al. 2006). Due to an unknown noise source during previous studies, which we were not able to filter, we chose to omit the biceps femoris EMG activity in the calculations. From our previous studies we calculated that this error would underestimate the tendon force and tendon stress by 5–20 %. However, studies have demonstrated that co-contraction does not seem to differ between master athletes and controls (Pearson et al. 2002) and, furthermore, does not change during maximal isometric contractions after 12 weeks of strength training (Kubo et al. 2007b; Reeves et al. 2003) or immobilization (Kubo et al. 2000, 2004). Therefore, it was assumed that this factor would remain constant.
Finally, the control subjects were carefully matched to our athletes and also had similar diets. Therefore, the sedentary old untrained males may not necessarily represent the average population of similar age with respect to BMI, waist circumference, and blood profile. This matching was necessary to ensure that these factors would not confound the age and training effects. Nevertheless, the master athlete model is likely a suitable model for the study of successful, optimal aging (Booth and Lees 2006) since changes that take place with time can be considered the results of primary (physiological) aging rather than secondary (lifestyle-related) aging (Tanaka and Seals 2008).
To our best knowledge, this study reports the first data to demonstrate that life-long regular running in male master athletes results in adaptations of tendon that occurs at different levels including lower AGE cross-link density, greater tendon MRI signal intensity, and increased tendon size. All of these changes can be considered beneficial to the mechanical integrity of the tendon, while, in particular, the increase in tendon size results in a reduced average tendon stress. As such, the present results suggest that life-long regular endurance exercise, such as running, can counteract the aging process in collagen-rich tissue (tendons, skin) and thereby possibly reduces the risk of tendon injury during running.
Acknowledgments
This study was supported by the Center for Healthy Aging, the University of Copenhagen, the Danish Physiotherapy Association, and the Danish Medical Research Council. We would like to thank the technical staff (Berith Sørensen and Janus Damm) at the Department of Radiology, Bispebjerg Hospital for helping obtaining the MRI images. Additionally, we thank Christina Eenberg Hansen, Simon Døssing, and Ann Christina R. Reimann for technical assistance. Kaisa-Leena Tulla (Department of Health Sciences, University of Jyväskylä) is acknowledged for expert technical assistance in collagen cross-link analyses. DiagnOptics BV, Groningen, the Netherlands enabled us to perform the AGE reader measurements free of charge.
References
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett Jr. DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS (2011) 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43(8):1575–1581 [DOI] [PubMed]
- Andreassen TT, Oxlund H, Danielsen CC. The influence of non-enzymatic glycosylation and formation of fluorescent reaction products on the mechanical properties of rat tail tendons. Connect Tissue Res. 1988;17(1):1–9. doi: 10.3109/03008208808992789. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol. 2007;210(Pt 15):2743–2753. doi: 10.1242/jeb.003814. [DOI] [PubMed] [Google Scholar]
- Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scand J Med Sci Sports. 2005;15(4):231–240. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- Bai P, Phua K, Hardt T, Cernadas M, Brodsky B. Glycation alters collagen fibril organization. Connect Tissue Res. 1992;28(1–2):1–12. doi: 10.3109/03008209209014224. [DOI] [PubMed] [Google Scholar]
- Bank RA, Beekman B, Verzijl N, de Roos JA, Sakkee AN, TeKoppele JM. Sensitive fluorimetric quantitation of pyridinium and pentosidine crosslinks in biological samples in a single high-performance liquid chromatographic run. J Chromatogr B Biomed Sci Appl. 1997;703(1–2):37–44. doi: 10.1016/S0378-4347(97)00391-5. [DOI] [PubMed] [Google Scholar]
- Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1999;58(1):35–41. doi: 10.1136/ard.58.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW. From life to death—the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology. 2000;1(3):235–246. doi: 10.1023/A:1010034213093. [DOI] [PubMed] [Google Scholar]
- Birch HL, McLaughlin L, Smith RK, Goodship AE. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Vet J Suppl. 1999;30:222–226. doi: 10.1111/j.2042-3306.1999.tb05222.x. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller J, Kalliokoski KK, Seppanen M, Kjaer M, Magnusson SP. Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J Appl Physiol. 2006;101(1):196–201. doi: 10.1152/japplphysiol.00004.2006. [DOI] [PubMed] [Google Scholar]
- Booth FW, Lees SJ. Physically active subjects should be the control group. Med Sci Sports Exerc. 2006;38(3):405–406. doi: 10.1249/01.mss.0000205117.11882.65. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Carroll CC, Dickinson JM, Haus JM, Lee GA, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of aging on the in vivo properties of human patellar tendon. J Appl Physiol. 2008;105(6):1907–1915. doi: 10.1152/japplphysiol.00059.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Weinheimer EM, Hollon CJ, Aagaard P, Magnusson SP, Trappe TA. Influence of acetaminophen and ibuprofen on in vivo patellar tendon adaptations to knee extensor resistance exercise in older adults. J Appl Physiol. 2011;111(2):508–515. doi: 10.1152/japplphysiol.01348.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168(15):1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Takahashi M, Kushida K, Suzuki M, Suzuki K, Horiuchi K, Nagano A. Direct detection of crosslinks of collagen and elastin in the hydrolysates of human yellow ligament using single-column high performance liquid chromatography. Anal Biochem. 2000;278(2):99–105. doi: 10.1006/abio.1999.4412. [DOI] [PubMed] [Google Scholar]
- Couppe C, Kongsgaard M, Aagaard P, Hansen P, Bojsen-Moller J, Kjaer M, Magnusson SP. Habitual loading results in tendon hypertrophy and increased stiffness of the human patellar tendon. J Appl Physiol. 2008;105(3):805–810. doi: 10.1152/japplphysiol.90361.2008. [DOI] [PubMed] [Google Scholar]
- Couppe C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009;107(3):880–886. doi: 10.1152/japplphysiol.00291.2009. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Creemers LB, Jansen DC, van Veen-Reurings A, van den BT, Everts V. Microassay for the assessment of low levels of hydroxyproline. Biotechniques. 1997;22(4):656–658. doi: 10.2144/97224bm19. [DOI] [PubMed] [Google Scholar]
- Curwin SL, Roy RR, Vailas AC. Regional and age variations in growing tendon. J Morphol. 1994;221(3):309–320. doi: 10.1002/jmor.1052210306. [DOI] [PubMed] [Google Scholar]
- Dammann P, Sell DR, Begall S, Strauch C, Monnier VM. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell’s mole-rat (Fukomys anselli) J Gerontol Ser A Biol Sci Med Sci. 2012;67(6):573–583. doi: 10.1093/gerona/glr208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGroot J, Verzijl N, Jacobs KM, Budde M, Bank RA, Bijlsma JW, TeKoppele JM, Lafeber FP. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthr Cartil. 2001;9(8):720–726. doi: 10.1053/joca.2001.0469. [DOI] [PubMed] [Google Scholar]
- Dressler MR, Butler DL, Wenstrup R, Awad HA, Smith F, Boivin GP. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res. 2002;20(6):1315–1322. doi: 10.1016/S0736-0266(02)00052-9. [DOI] [PubMed] [Google Scholar]
- Dunn PJ, Cole RA, Soeldner JS. Further development and automation of a high pressure liquid chromatography method for the determination of glycosylated hemoglobins. Metab Clin Exp. 1979;28(7):777–779. doi: 10.1016/0026-0495(79)90185-9. [DOI] [PubMed] [Google Scholar]
- Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266(18):11654–11660. [PubMed] [Google Scholar]
- Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91(6):2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Goodship AE, Birch HL, Patterson-Kane JC. Effect of exercise on age-related changes in collagen fibril diameter distributions in the common digital extensor tendons of young horses. Am J Vet Res. 2005;66(4):564–568. doi: 10.2460/ajvr.2005.66.564. [DOI] [PubMed] [Google Scholar]
- Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A. Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol. 2012;8(7):412–419. doi: 10.1038/nrrheum.2012.69. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Fujimoto D, Moriguchi T, Ishida T, Hayashi H. The structure of pyridinoline, a collagen crosslink. Biochem Biophys Res Commun. 1978;84(1):52–57. doi: 10.1016/0006-291X(78)90261-9. [DOI] [PubMed] [Google Scholar]
- Galeski A, Kastelic J, Baer E, Kohn RR. Mechanical and structural changes in rat tail tendon induced by alloxan diabetes and aging. J Biomech. 1977;10(11/12):775–782. doi: 10.1016/0021-9290(77)90091-4. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol. 1998;85(3):1011–1016. doi: 10.1152/jappl.1998.85.3.1011. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol Avon) 2006;21(1):54–58. doi: 10.1016/j.clinbiomech.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Douglass MD, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1452–R1459. doi: 10.1152/ajpregu.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J Appl Physiol. 2007;103(6):2068–2076. doi: 10.1152/japplphysiol.00670.2007. [DOI] [PubMed] [Google Scholar]
- Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech. 1992;25(2):163–173. doi: 10.1016/0021-9290(92)90273-4. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582(Pt 3):1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeier KM, Schjerling P, Heinemeier J, Magnusson SP, Kjaer M. Lack of tissue renewal in human adult Achilles tendon is revealed by nuclear bomb 14C. FASEB J. 2013 doi: 10.1096/fj.12-225599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RP, Soutas-Little RW. Mechanical properties of human tendon and their age dependence. J Biomech Eng. 1984;106(2):144–150. doi: 10.1115/1.3138471. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994;12(6):796–803. doi: 10.1002/jor.1100120607. [DOI] [PubMed] [Google Scholar]
- Kalliokoski KK, Langberg H, Ryberg AK, Scheede-Bergdahl C, Doessing S, Kjaer A, Boushel R, Kjaer M. The effect of dynamic knee-extension exercise on patellar tendon and quadriceps femoris muscle glucose uptake in humans studied by positron emission tomography. J Appl Physiol. 2005;99(3):1189–1192. doi: 10.1152/japplphysiol.00283.2005. [DOI] [PubMed] [Google Scholar]
- Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech. 2006;39(3):406–417. doi: 10.1016/j.jbiomech.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Reitelseder S, Pedersen TG, Holm L, Aagaard P, Kjaer M, Magnusson SP. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol (Oxford) 2007;191(2):111–121. doi: 10.1111/j.1748-1716.2007.01714.x. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Kovanen V, Aagaard P, Doessing S, Hansen P, Laursen AH, Kaldau NC, Kjaer M, Magnusson SP. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports. 2009;19(6):790–802. doi: 10.1111/j.1600-0838.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- Kongsgaard M, Qvortrup K, Larsen J, Aagaard P, Doessing S, Hansen P, Kjaer M, Magnusson SP. Fibril morphology and tendon mechanical properties in patellar tendinopathy: effects of heavy slow resistance training. Am J Sports Med. 2010;38(4):749–756. doi: 10.1177/0363546509350915. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol. 2000;83(6):463–468. doi: 10.1007/s004210000309. [DOI] [PubMed] [Google Scholar]
- Kubo K, Akima H, Ushiyama J, Tabata I, Fukuoka H, Kanehisa H, Fukunaga T. Effects of resistance training during bed rest on the viscoelastic properties of tendon structures in the lower limb. Scand J Med Sci Sports. 2004;14(5):296–302. doi: 10.1046/j.1600-0838.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- Kubo K, Ishida Y, Komuro T, Tsunoda N, Kanehisa H, Fukunaga T. Age-related differences in the force generation capabilities and tendon extensibilities of knee extensors and plantar flexors in men. J Gerontol Ser A Biol Sci Med Sci. 2007;62(11):1252–1258. doi: 10.1093/gerona/62.11.1252. [DOI] [PubMed] [Google Scholar]
- Kubo K, Morimoto M, Komuro T, Yata H, Tsunoda N, Kanehisa H, Fukunaga T. Effects of plyometric and weight training on muscle-tendon complex and jump performance. Med Sci Sports Exerc. 2007;39(10):1801–1810. doi: 10.1249/mss.0b013e31813e630a. [DOI] [PubMed] [Google Scholar]
- LaCroix AS, Duenwald-Kuehl SE, Brickson S, Akins TL, Diffee G, Aiken J, Vanderby R, Jr, Lakes RS. Effect of age and exercise on the viscoelastic properties of rat tail tendon. Ann Biomed Eng. 2013;41(6):1120–1128. doi: 10.1007/s10439-013-0796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521(Pt 1):299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534(Pt 1):297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Investig. 1991;87(6):1910–1915. doi: 10.1172/JCI115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol. 2001;531(Pt 1):277–288. doi: 10.1111/j.1469-7793.2001.0277j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Qvortrup K, Larsen JO, Rosager S, Hanson P, Aagaard P, Krogsgaard M, Kjaer M. Collagen fibril size and crimp morphology in ruptured and intact Achilles tendons. Matrix Biol. 2002;21(4):369–377. doi: 10.1016/S0945-053X(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci. 2003;58(2):123–127. doi: 10.1093/gerona/58.2.B123. [DOI] [PubMed] [Google Scholar]
- Magnusson SP, Hansen M, Langberg H, Miller B, Haraldsson B, Westh EK, Koskinen S, Aagaard P, Kjaer M. The adaptability of tendon to loading differs in men and women. Int J Exp Pathol. 2007;88(4):237–240. doi: 10.1111/j.1365-2613.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard LC. Action des acides aminés sur les sucres: formation des mélanoïdines par voie méthodique. C R Acad Sci. 1912;154:66–68. [Google Scholar]
- Meerwaldt R, Graaff R, Oomen PH, Links TP, Jager JJ, Alderson NL, Thorpe SR, Baynes JW, Gans RO, Smit AJ. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia. 2004;47(7):1324–1330. doi: 10.1007/s00125-004-1451-2. [DOI] [PubMed] [Google Scholar]
- Mian OS, Thom JM, Ardigo LP, Minetti AE, Narici MV. Gastrocnemius muscle-tendon behaviour during walking in young and older adults. Acta Physiol (Oxford) 2007;189(1):57–65. doi: 10.1111/j.1748-1716.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen UR, Couppé C, Karlsen A, Grosset JF, Schjerling P, Mackey AL, Klausen HH, Magnusson SP, Kjær M. Life-long endurance exercise in humans: circulating levels of inflammatorymarkers and leg muscle size. Mech Ageing Dev. 2013;134(11–12):531–540. doi: 10.1016/j.mad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Momma H, Niu K, Kobayashi Y, Guan L, Sato M, Guo H, Chujo M, Otomo A, Yufei C, Tadaura H, Saito T, Mori T, Miyata T, Nagatomi R (2012) Skin advanced glycation end-product accumulation is negatively associated with calcaneal osteo-sono assessment index among non-diabetic adult Japanese men. Osteoporos Int: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 23(6):1673–1681. doi:10.1007/s00198-011-1753-4 [DOI] [PMC free article] [PubMed]
- Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Monnier VM, Mustata GT, Biemel KL, Reihl O, Lederer MO, Zhenyu D, Sell DR. Cross-linking of the extracellular matrix by the maillard reaction in aging and diabetes: an update on “a puzzle nearing resolution”. Ann N Y Acad Sci. 2005;1043:533–544. doi: 10.1196/annals.1333.061. [DOI] [PubMed] [Google Scholar]
- Mosca A, Carpinelli A, Bonini P. Automated determination of glycated hemoglobins with a new high-performance liquid chromatography analyzer. Clin Chem. 1986;32(1 Pt 1):202–203. [PubMed] [Google Scholar]
- Murrell GA, Walton JR. Diagnosis of rotator cuff tears. Lancet. 2001;357(9258):769–770. doi: 10.1016/S0140-6736(00)04161-1. [DOI] [PubMed] [Google Scholar]
- Nagy IZ, Von Hahn HP, Verzar F. Age-related alterations in the cell nuclei and the DNA content of rat tail tendon. Gerontologia. 1969;15(4):258–264. doi: 10.1159/000211694. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T, Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand. 1994;152(3):307–313. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Hayashi K, Yamamoto N, Nagashima K. Age-related changes in biomechanical properties of the Achilles tendon in rabbits. Eur J Appl Physiol Occup Physiol. 1996;73(1–2):7–10. doi: 10.1007/BF00262803. [DOI] [PubMed] [Google Scholar]
- Nielsen HM, Skalicky M, Viidik A. Influence of physical exercise on aging rats: III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech Ageing Dev. 1998;100(3):243–260. doi: 10.1016/S0047-6374(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Nordin G, Martensson A, Swolin B, Sandberg S, Christensen NJ, Thorsteinsson V, Franzson L, Kairisto V, Savolainen ER. A multicentre study of reference intervals for haemoglobin, basic blood cell counts and erythrocyte indices in the adult population of the Nordic countries. Scand J Clin Lab Invest. 2004;64(4):385–398. doi: 10.1080/00365510410002797. [DOI] [PubMed] [Google Scholar]
- Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol. 2006;100(6):2048–2056. doi: 10.1152/japplphysiol.01442.2005. [DOI] [PubMed] [Google Scholar]
- Parry DA, Barnes GR, Craig AS. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978;203(1152):305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Partington FR, Wood GC. The role of non-collagen components in the mechanical behaviour of tendon fibres. Biochim Biophys Acta. 1963;69:485–495. doi: 10.1016/0006-3002(63)91298-8. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane JC, Parry DA, Birch HL, Goodship AE, Firth EC. An age-related study of morphology and cross-link composition of collagen fibrils in the digital flexor tendons of young thoroughbred horses. Connect Tissue Res. 1997;36(3):253–260. doi: 10.3109/03008209709160225. [DOI] [PubMed] [Google Scholar]
- Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, Harridge SD. Muscle function in elite master weightlifters. Med Sci Sports Exerc. 2002;34(7):1199–1206. doi: 10.1097/00005768-200207000-00023. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys. 2002;399(2):174–180. doi: 10.1006/abbi.2001.2747. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol Online. 2003;548(3):971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Effect of 10 days of physical inactivity on glucose tolerance in master athletes. J Appl Physiol. 1990;68(5):1833–1837. doi: 10.1152/jappl.1990.68.5.1833. [DOI] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sci Sports. 2002;12(2):90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem. 1997;253(1):26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- Saltin B, Grimby G. Physiological analysis of middle-aged and old former athletes. Comparison with still active athletes of the same ages. Circulation. 1968;38(6):1104–1115. doi: 10.1161/01.CIR.38.6.1104. [DOI] [PubMed] [Google Scholar]
- Sargon MF, Ozlu K, Oken F. Age-related changes in human tendo calcaneus collagen fibrils. Saudi Med J. 2005;26(3):425–428. [PubMed] [Google Scholar]
- Seals DR, Hagberg JM, Allen WK, Hurley BF, Dalsky GP, Ehsani AA, Holloszy JO. Glucose tolerance in young and older athletes and sedentary men. J Appl Physiol. 1984;56(6):1521–1525. doi: 10.1152/jappl.1984.56.6.1521. [DOI] [PubMed] [Google Scholar]
- Sell DR, Monnier VM. Molecular basis of arterial stiffening: role of glycation — a mini-review. Gerontology. 2012;58(3):227–237. doi: 10.1159/000334668. [DOI] [PubMed] [Google Scholar]
- Sennels HP, Jorgensen HL, Hansen AL, Goetze JP, Fahrenkrug J. Diurnal variation of hematology parameters in healthy young males: the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. 2011;71(7):532–541. doi: 10.3109/00365513.2011.602422. [DOI] [PubMed] [Google Scholar]
- Sennels HP, Jorgensen HL, Goetze JP, Fahrenkrug J. Rhythmic 24-hour variations of frequently used clinical biochemical parameters in healthy young males—the Bispebjerg study of diurnal variations. Scand J Clin Lab Invest. 2012;72(4):287–295. doi: 10.3109/00365513.2012.662281. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Erskine RM, Maganaris CN, Longo S, Simoneau EM, Grosset JF, Narici MV. Training-induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy, but not to strength gains. J Appl Physiol. 2009;107(2):523–530. doi: 10.1152/japplphysiol.00213.2009. [DOI] [PubMed] [Google Scholar]
- Seynnes OR, Koesters A, Gimpl M, Reifberger A, Niederseer D, Niebauer J, Pirich C, Muller E, Narici MV. Effect of alpine skiing training on tendon mechanical properties in older men and women. Scand J Med Sci Sports. 2011;21(Suppl 1):39–46. doi: 10.1111/j.1600-0838.2011.01340.x. [DOI] [PubMed] [Google Scholar]
- Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol. 1990;68(3):1033–1040. doi: 10.1063/1.346741. [DOI] [PubMed] [Google Scholar]
- Simonsen EB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and M. soleus of the rat. J Sports Sci. 1995;13(4):291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113(10):1537–1544. doi: 10.1152/japplphysiol.00782.2012. [DOI] [PubMed] [Google Scholar]
- Strocchi R, De Pasquale V, Guizzardi S, Govoni P, Facchini A, Raspanti M, Girolami M, Giannini S. Human Achilles tendon: morphological and morphometric variations as a function of age. Foot Ankle. 1991;12(2):100–104. doi: 10.1177/107110079101200207. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Takahashi M, Abe M, Nagano A. Biochemical study of collagen and its crosslinks in the anterior cruciate ligament and the tissues used as a graft for reconstruction of the anterior cruciate ligament. Connect Tissue Res. 2008;49(1):42–47. doi: 10.1080/03008200701820799. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Seals DR. Endurance exercise performance in Masters athletes: age-associated changes and underlying physiological mechanisms. J Physiol. 2008;586(1):55–63. doi: 10.1113/jphysiol.2007.141879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Streeter I, Pinchbeck GL, Goodship AE, Clegg PD, Birch HL. Aspartic acid racemization and collagen degradation markers reveal an accumulation of damage in tendon collagen that is enhanced with aging. J Biol Chem. 2010;285(21):15674–15681. doi: 10.1074/jbc.M109.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vailas AC, Pedrini VA, Pedrini-Mille A, Holloszy JO. Patellar tendon matrix changes associated with aging and voluntary exercise. J Appl Physiol. 1985;58(5):1572–1576. doi: 10.1152/jappl.1985.58.5.1572. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000;275(50):39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Viidik A, Nielsen HM, Skalicky M. Influence of physical exercise on aging rats: II. Life-long exercise delays aging of tail tendon collagen. Mech Ageing Dev. 1996;88(3):139–148. doi: 10.1016/0047-6374(96)01729-0. [DOI] [PubMed] [Google Scholar]
- Visser JJ, Hoogkamer JE, Bobbert MF, Huijing PA. Length and moment arm of human leg muscles as a function of knee and hip-joint angles. Eur J Appl Physiol Occup Physiol. 1990;61(5–6):453–460. doi: 10.1007/BF00236067. [DOI] [PubMed] [Google Scholar]
- Vogel HG. Age dependence of mechanical properties of rat tail tendons (hysteresis experiments) Aktuelle Gerontol. 1983;13(1):22–27. [PubMed] [Google Scholar]
- Vogt BW, Schleicher ED, Wieland OH. epsilon-Amino-lysine-bound glucose in human tissues obtained at autopsy. Increase in diabetes mellitus. Diabetes. 1982;31(12):1123–1127. doi: 10.2337/diacare.31.12.1123. [DOI] [PubMed] [Google Scholar]
- Westh E, Kongsgaard M, Bojsen-Moller J, Aagaard P, Hansen M, Kjaer M, Magnusson SP (2007) Effect of habitual exercise on the structural and mechanical properties of human tendon, in vivo, in men and women. Scand J Med Sci Sports [DOI] [PubMed]
- Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011;111(4):999–1006. doi: 10.1152/japplphysiol.00460.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yataco AR, Busby-Whitehead J, Drinkwater DT, Katzel LI. Relationship of body composition and cardiovascular fitness to lipoprotein lipid profiles in master athletes and sedentary men. Aging. 1997;9(1–2):88–94. doi: 10.1007/BF03340132. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Miyazaki A, Fujimoto S. Decrease in serum levels of advanced glycation end-products by short-term lifestyle modification in non-diabetic middle-aged females. Med Sci Monitor Int Med J Exp Clin Res. 2009;15(6):PH65–PH73. [PubMed] [Google Scholar]