Abstract
Cognitive deficiency and oxidative stress have been well documented in aging and in neurodegenerative disorders such as Alzheimer’s disease. In this study, we assessed the therapeutic effect of polyprenols on d-galactose-induced cognitive impairment in mice by testing on of behavioral and cognitive performance. In order to explore the possible role of polyprenols against d-galactose-induced oxidative damages, we assessed various biochemical indicators. Chronic administration of d-galactose (150 mg/kg·d, s.c.) for 7 weeks significantly impaired cognitive performance (both in step-through passive and active avoidance tests) and locomotor activity (in open-field test) and the ability of spatial learning and memory (in Morris water maze test) compared with the control group. The results revealed that polyprenols treatment for 2 weeks significantly ameliorated model mice’s cognitive performance and oxidative defense. All groups of polyprenols enhanced the learning and memory ability in step-through passive and active avoidance tests, locomotor activity in open-field test, and the ability of spatial learning and memory in Morris water maze test. Furthermore, high and middle level of polyprenols significantly increased total antioxidative capacity (T-AOC), glutathione peroxidase (GSH-Px), super oxide dismutase (SOD) activity, neprilysin (NEP), and β-site AβPP cleaving enzyme 1 (BACE1) expression, while nitric oxide (NO), nitric oxide synthase (NOS) activity, malondialdehyde (MDA) concentration, and the level of Aβ1-42 and presenilin 1 (PS1) were decreased. Polyprenols have a significant relieving effect on learning, memory, and spontaneous activities in a d-galactose-induced mouse model and ameliorates cognitive impairment and biochemical dysfunction in mice. In summary, we have demonstrated that polyprenols may ameliorate memory and cognitive impairment via enhancing oxidative defense and affecting generation and dissimilation of Aβ-related enzymes, suggesting that polyprenols represent a novel drug for treating Alzheimer’s disease.
Keywords: Alzheimer’s disease, Polyprenols, d-galactose, Oxidative stress, Amyloid-beta (Aβ)
Introduction
Alzheimer’s disease (AD) is the most common cause of senile dementia in an elderly population. This neurodegenerative disease is initially characterized by senile plaques consisting of extracellular accumulation of amyloid-beta (Aβ), intracellular neurofibrillary tangles composed of hyperphosphylated tau, and neuronal loss. At present, AD is rising distinctly and producing considerable social and financial problems (Kwon and Lee 2011; Marseille and Silverman 2006; Pamplona et al. 2005). Currently, various studies demonstrated different kinds of hypothesis related to AD such as low cholinergic function (Nunomura et al. 1999, 2001), gene mutation, beta amyloid (Soto et al. 1994), glycogen synthesis kinase (DaRocha-Souto et al. 2012), oxidative stress, excitotoxicity, and nerve cells apoptosis.
Polyprenols are isolated from the pine needles of Pinus massoniana and belong to the group of polyisoprenoid alcohols (Fig. 1). At present, polyprenols have been widely studied as multifunctional agents for the prevention and treatment of cancer and cardiovascular diseases. Polyprenols present intense protection on PC12 cells damage that is induced by Aβ1-42, which may be caused by inhibiting Aβ protein-induced oxygen radical. d-galactose serves as a feasible brain aging model for animals. Injection of d-galactose on long-term basis in mice could lead to a progressive deterioration in learning and memory ability and impairments in antioxidant capacity (Cui et al. 2006; Dumont and Beal 2011), mitochondrial function (Long et al. 2007), calcium homeostasis (Lu et al. 2006), inflammatory response (Lu et al. 2010), neuronal apoptosis (He et al. 2009), and cholinergic degeneration (Lei et al. 2008) in the brain. Accumulating pieces of evidence show that d-galactose eventually damages learning and memory function and induces higher levels of Aβ in cortex and hippocampus (Xian et al. 2011). The accumulation of Aβ in the brain is determined by the rate of production and degradation via several enzymes (Tian et al. 2009). Aβ is generated by the cleavage of amyloid precursor protein (APP) through β-secretase (BACE1) and γ-secretase. Presenilin 1(PS1) is the catalytic component of the γ-secretase complex, and its mutations are associated with early-onset AD (Wolfe 2012). Neprilysin (NEP) is the most important Aβ peptide-degrading enzyme in the brain. Selective inhibition of NEP resulted in Aβ1-42 deposition in rat hippocampus (Malito et al. 2008).
Fig. 1.
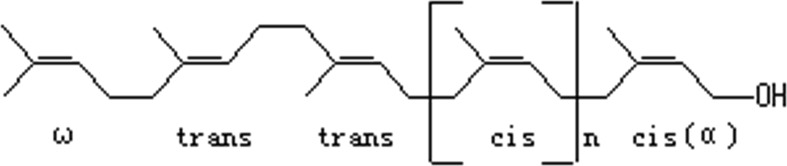
General polyprenol structure, where n = 11–19
Our previous studies have demonstrated that polyprenols could ameliorate cognitive impairment in a mouse model of Aβ(1–42)-induced amnesia, which improved passive avoidance ability and spatial memory using step-through passive avoidance test and Morris water maze. Polyprenols had the therapeutic potentials for AD-like donepezil (Choi et al. 2012); In vitro, Aβ(1–42)-induced apoptosis in PC12 cells was reversed by polyprenols, which significantly increased the PC12 cell viability and decreased lactic dehydrogenase activity (Zheng et al. 2011). These results indicate that polyprenols possess hopeful prospect to become an innovative drug for AD. In this study, we investigated a possible improvement of polyprenols on memory loss and learning impairment induced by d-galactose.
Materials and methods
Materials
Polyprenols were isolated from the pine needles of P. massoniana by the Institute of Chemical Industry of Forestry Products (Nanjing, China), which have the purity of 90 % (HPLC), prepared with plant oil and diluted with normal saline (NS) and Tween 80. Donepezil (batch number 091101) was purchased from Weicai Pharmaceutical Co. LTD (Shanghai, China), prepared with ethanol, and diluted with NS. d-galactose (batch number 0010828) was purchased from Shanghai Huixing Biochemicals Co. (Shanghai, China), prepared with NS and diluted with NS. Total antioxidant capacity assay kit, glutathione peroxidase (GSH-Px) assay kit, superoxide dismutase assay kit, nitric oxide (NO) assay kit, typing nitric oxide synthase (NOS) assay kit, and maleic dialdehyde assay kit were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Coomassie Brilliant Blue were purchased from Biosharp Co., Aβ enzyme-linked immunoassay kit, NEP enzyme-linked immunoassay kit, and BACE1 enzyme-linked immunoassay kit were purchased from Weiwo Biochemical Co. LTD (Nanjing, China). All other reagents and chemicals used in the study were of analytical grade or at the highest purity available.
Video analysis system of step-through active avoidance test and step-through passive avoidance test were purchased from Shanghai Mobiledatum Information Technology Co., LTD (Shanghai, China).
Animals and drug administration
Male ICR mice (18–22 g) were purchased from Animal Experimental Center of Yangzhou University (Yangzhou, China; with approval number SCXK (su)2007-0001). Pellet feed was purchased from the Animal Breeding Grounds of Nanjing Jiangning Qinglong Mountain (Nanjing, China). Animals were housed in a room that was automatically maintained at 21 ~ 25 °C and relative humidity (45 ~ 65 %) with a controlled light–dark cycle. The mice were under free access to food and water.
After 2 weeks of acclimatization, mice were randomly divided into six groups (each containing 12 mice): control (control), model (d-gal), positive (d-gal + donepezil), low-dosage, middle-dosage, and high-dosage polyprenol groups. Except for the control group, mice were subcutaneously injected with 3 % d-galactose at 150 mg/kg body weight once daily for 7 weeks while those of control group were treated with same volume NS. From the sixth week, polyprenol was administrated intragastrically with following three doses 20, 40, and 80 mg/kg body weight once daily for 2 weeks to low-dosage, middle-dosage, and high-dosage polyprenol groups. Donepezil was administrated intragastrically with a 3-mg/kg body weight dose once daily for 2 weeks to positive group. Same volume of NS was administrated to the control and model groups. The treatment was given 30 min prior to daily injection of d-galactose.
Behavioral tests
The behavioral tests were performed in a silent, isolated room at the temperature of 22 ± 2 °C. The experimenter and the devices for data acquisition and analysis were located in an adjacent room. A video-camera viewing the experimental area was positioned on the vertical form at the center of the arena and connected to a personal computer. Mice movements were tracked and analyzed with dedicated software (Any-maze™, Stoelting Co., Chicago, USA).
Step-through active avoidance test
The step-through active avoidance test was performed by using a two-compartment shuttle box (each 136 × 153 × 250 mm) adjacent to each other through an arch door (3 cm in diameter). The floor of the compartments was composed of 2-mm stainless-steel rods spaced 1 cm apart. In an acquisition trial, a mouse was initially placed in one compartment facing away from the door and allowed to acclimatize for 5 min. After the acclimatization, a buzzer was delivered in the compartments for 5 s. If the mouse was still present in this compartment, then it was shocked by an electric foot current (10–20 mA, 10–15 Hz) through the grid floor in this compartment until it went into the other compartment. The average intertrial interval was 60 s. Each test contained 20 buzzers and 20 electric foot shocks. Each mouse was trained for 20 trials/day for five consecutive days. At the last day, the final test results were recorded: (i) active avoidance response latency (AARL); (ii) escape response latency (ERL); (iii) active avoidance response times (AART), and (iv) escape-response times (ERT).
Step-through passive avoidance test
The step-through passive avoidance test was performed by using a two-compartment shuttle box that consisted of one illuminated and one dark compartment (each 136 × 153 × 250 mm) adjacent to each other through an arch door (3 cm in diameter). The floor of the dark compartment was composed of 2-mm stainless-steel rods spaced 1 cm apart. In an acquisition trial, a mouse was initially placed in the illuminated compartment facing away from the dark compartment and allowed to acclimatize for 5 min. After the acclimatization, an electric foot shock (36 V) was delivered through the grid floor in the compartments. If the mouse came into the dark compartment, it would be shocked and returned to the illuminated compartment immediately. Any mouse that did not go to the dark compartment within 5 min suggested that it was not sensitive to light and was ruled out of further experiments. After the 5-min training, the electric shock was stopped. The mouse was subjected to the same conditions for the retention trial 24 h later; as soon as it went into the dark compartment and was shocked, the test was terminated. The time that was taken for a mouse to enter the dark compartment the first time was defined as the latency for the trial. The latency was recorded for up to 300 s.
Open-field test
Open-field test is one of the most common tests to monitor general motor activity, exploratory behavior, and measures of anxiety. The open field is composed of a transparent arena (278 × 236 × 300 mm). Normal behavior of mice is to seek the protection of the periphery rather than the vulnerability of the center. Mice that are less anxious are expected to spend more time in the center. Mice that show signs of motor deficits are expected to show lower horizontal distances and vertical movements. Mice were individually placed in an open arena equipped with infrared photo beams to monitor mice behavior for 5 min without the experimenter being present in the room. The photo beams recorded the following activities in 5 min: (i) total time travelled in the central zone; (ii) total movement time in open field; (iii) total distance travelled in open field, and (iv) total number of line crossings in central square were monitored.
Morris water maze
Spatial learning and memory ability was tested by Morris water maze. The apparatus consisted of a black circular pool (180 cm in diameter and 80 cm in height) filled with water, which maintained at 25 ± 1 °C. The pool was divided into four quadrants of equal area: I, II, III, and IV. An escape platform (10 cm in diameter) was placed 1 cm below the water surface at the midpoint of the IV quadrant (the target quadrant). Several visual cues helping mice to learn the location of the hidden platform were arrayed surrounding the maze. The experiment included two phases—acquisition training session and the probe trail. In the acquisition training session, each mouse was submitted to four trials successively per day. In each trial, the time a mouse spent from being put into the water to finding and climbing onto the hidden platform was recorded as escape latency. Mice were given a maximum of 90 s for finding the platform. If a mouse failed to locate the platform within 90 s, it was placed on the platform and stayed there for 10 s, and its escape latency was recorded as 90 s. On the fifth day, the platform was removed from the pool for the probe trail. The mice were released into the water on the opposite side of the target quadrant and allowed to swim freely for 90 s. The total number of times each mouse crossed the position where the escape platform was once placed (crossing number) and time it spent in the IV quadrant (time in target quadrant%) were recorded.
Biochemical analyses
Tissue preparation for biochemical analyses
All the mice of each group were decapitated after behavioral tests, and the brains were removed and homogenized (1:10, w/v) in HEPES buffer (20 mM, pH 7.0). The tissue homogenates were centrifuged at 16.000 × g at 4 °C for 20 min, and the supernatants obtained were used for measurements of the biochemical analyses.
Determination of T-AOC, GSH-Px, SOD, NO, NOS, and MDA
For the determination of total antioxidative capacity (T-AOC), GSH-Px, super oxide dismutase (SOD), NO, NOS, and malondialdehyde (MDA), the operating manual was followed. T-AOC, GSH-Px, SOD, NO, and NOS levels were measured with the spectrophotometer, while MDA concentration was determined with thiobarbituric acid reaction (TBA).
Measurement of the level of Aβ1-42, NEP, BACE1, and PS1
The activities of Aβ1-42, NEP, BACE1, and PS1 were measured by ELISA according to the manufacturer’s instructions (Biovalue, Shanghai, China). Bradford protein assay was used to detect total protein concentrations in the supernatant. Data are represented as picograms (U) per milligram of protein.
Statistical analyses
All data were expressed as the mean ± SEM. The statistical significance in the behavioral tests was determined by one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05.
Results
Effects of polyprenols on a d-galactose-induced mouse model in step-through active avoidance test
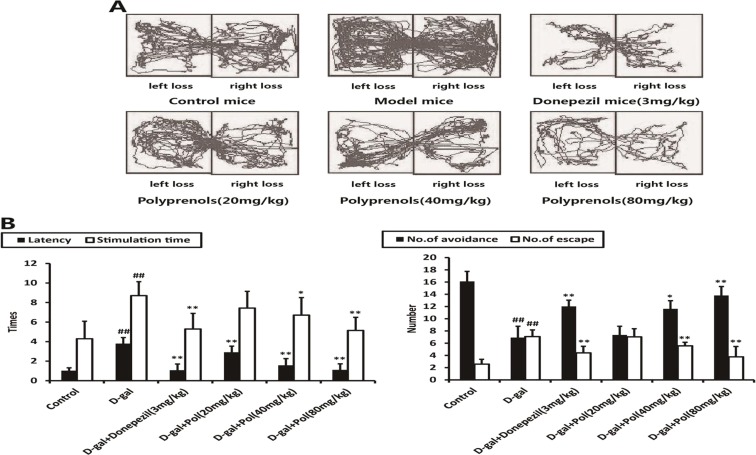
The mice with subcutaneous injections of d-galactose showed a significant increase in latency time, stimulation time, and number of escape and avoidance on the active avoidance test, as compared with the control group (p < 0.01). Conversely, all the groups of mice to which polyprenols were administrated intragastrically with a different concentrations of 20, 40, and 80 mg/kg showed varying degrees of reduced latency and stimulation time and number of escape and avoidance, especially significant differences in 40 and 80 mg/kg polyprenols (p < 0.01), as compared with the model group. A significant difference was observed in mice with 20 mg/kg polyprenols, including latency time (p < 0.01) and stimulation time (p < 0.05, Fig. 2).
Fig. 2.
Effects of polyprenols on a d-galactose-induced mouse model in step-through active avoidance test. Base on the tracks (a), the model mice showed an increase in latency time, stimulation time, and number of escape and avoidance, as compared with the control group. b Polyprenols (20, 40, and 80 mg/kg) groups showed varying degrees of reduced latency and stimulation time and number of escape and avoidance, especially in 40 and 80 mg/kg polyprenols, as compared with the model group. The data shown are mean ± SEM (n = 12). # p < 0.05; ## p < 0.01 vs control; *p < 0.05; **p < 0.01 vs model group
Effects of polyprenols on a d-galactose-induced mouse model in step-through passive avoidance test
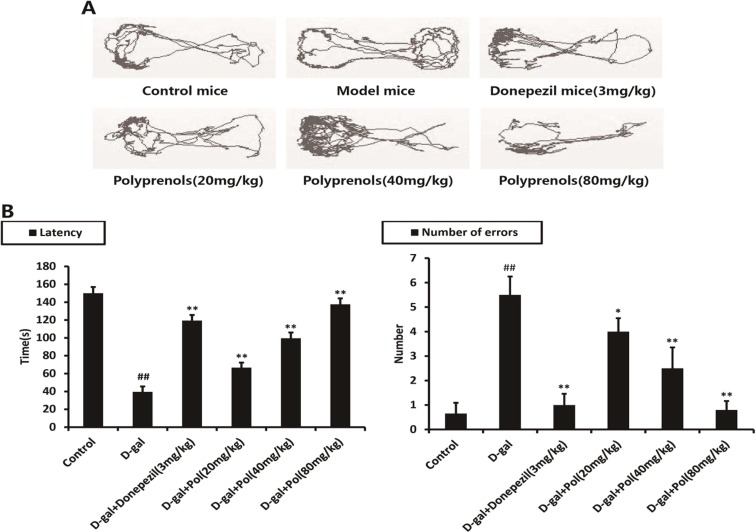
As it did in the passive avoidance test, injection of d-galactose effectively induced learning and memory deficiency on the passive avoidance test. All the polyprenols groups, however, reversed the d-galactose-induced learning and memory deficiency, as evidenced by improved latency time and reduced number of errors (p < 0.01, Fig. 3).
Fig. 3.
Effects of polyprenols on a d-galactose-induced mouse model in step-through passive avoidance test. Base on the tracks (a), the model mice showed decreased latency time and increased number of errors, as compared with the control group. (b) Polyprenols(20, 40, and 80 mg/kg) groups showed varying degrees of improved latency time and reduced number of errors, as compared with the model group. The data shown are mean ± SEM (n = 12). # p < 0.05; ## p < 0.01 vs control; *p < 0.05, **p < 0.01 vs model group
Effects of polyprenols on a d-galactose-induced mouse model in open field test
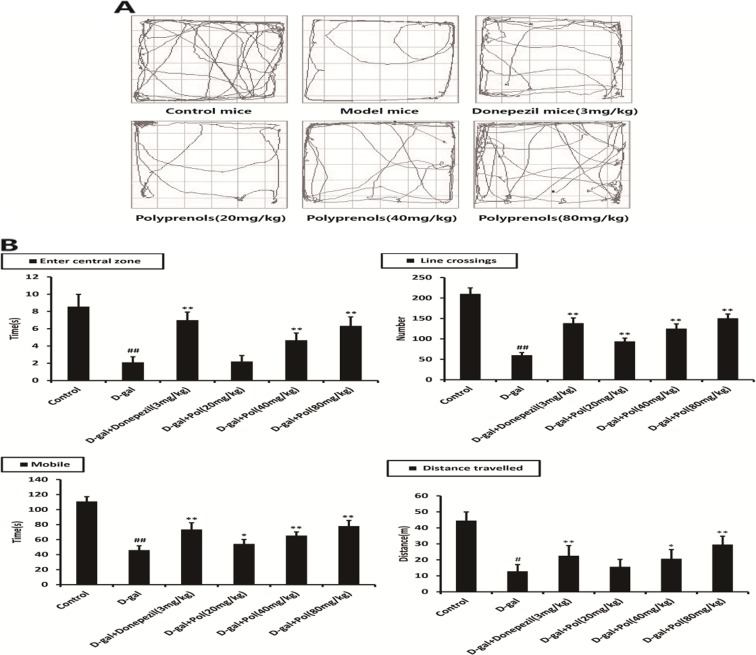
Compared with the values of the control group, the model group exerted a significant reduction of activities in central square (p < 0.01), a significant reduction of movement in open field (p < 0.01), a number of line crossings in central square arena of open field (p < 0.01), and a significant reduction of total distance travelled in open field (p < 0.01), which showed spontaneous activity defects in the model mice. All the polyprenol groups revealed significant differences in increased line crossing numbers and delayed mobile time (p < 0.01). The mice administrated intragastrically with 40 and 80 mg/kg polyprenols resulted in a profound increase in central square time and total distance travelled (p < 0.01, Fig. 4).
Fig. 4.
Effects of polyprenols on a d-galactose-induced mouse model in open-field test. Base on the tracks a, the model group exerted a reduction of activities in central square, movement in open field, number of line crossings in central square arena, and total distance travelled. b All the polyprenols group revealed increased line crossing numbers and delayed mobile time. The mice with 40 and 80 mg/kg polyprenols resulted in an increase in central square time and total distance travelled. The data shown are mean ± SEM (n = 12). # p < 0.05; ## p < 0.01 vs control; *p < 0.05; **p < 0.01 vs model group
Effects of polyprenols on a d-galactose-induced mouse model in Morris water maze test
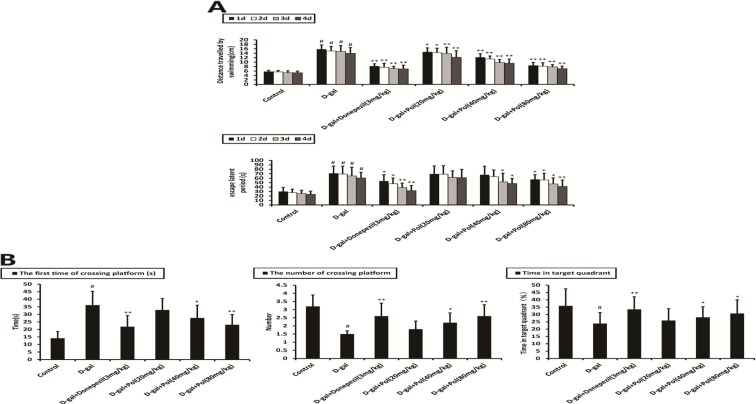
Morris water maze test was performed to detect the effects of polyprenols on spatial learning and memory ability. Figure 5a shows the escape latency during acquisition training. The model group mice exhibited obviously longer escape latency in training session than that of the mice in the control group (p < 0.01). Donepezil (3 mg/kg) and polyprenols (40 and 80 mg/kg) significantly shortened escape latency (p < 0.05).
Fig. 5.
a Effects of polyprenols on distance travelled by swimming and escape latency. The model group mice exhibited obviously longer escape latency in training session than that of mice in the control group (p < 0.01). Donepezil (3 mg/kg) and polyprenols (40 and 80 mg/kg) significantly shortened escape latency (p < 0.05). b The probe test was performed after the training test. The number of crossing platform and time spent in target quadrant were significantly lower in the model mice than the control mice (p < 0.05). Compared with the control group that showed a much increased presence in the target quadrant, a much more random swim trace in all four quadrants was observed in the model mice. Donepezil- and polyprenol-treated (40 and 80 mg/kg) mice spent more time in the target quadrant and cross the platform than model group mice (p < 0.01 and p < 0.05). The data shown are mean ± SEM (n = 12). # p < 0.05; ## p < 0.01 vs control; *p < 0.05; **p < 0.01 vs model group
The probe test was performed after the training test. The number of crossing platform and time spent in target quadrant were significantly lower in the model mice than the control mice (p < 0.05). Compared with the control group that showed a much increased presence in the target quadrant, a much more random swim trace in all four quadrants was observed in the model mice. The mice treated with donepezil (3 mg/kg) and polyprenols (40 and 80 mg/kg) spent more time in the target quadrant and cross the platform than the model group mice (p < 0.01 and p < 0.05). These results indicated that the ability of spatial learning and memory of mice was seriously impaired by d-galactose and polyprenols could improve this cognitive impairment (Fig. 5b).
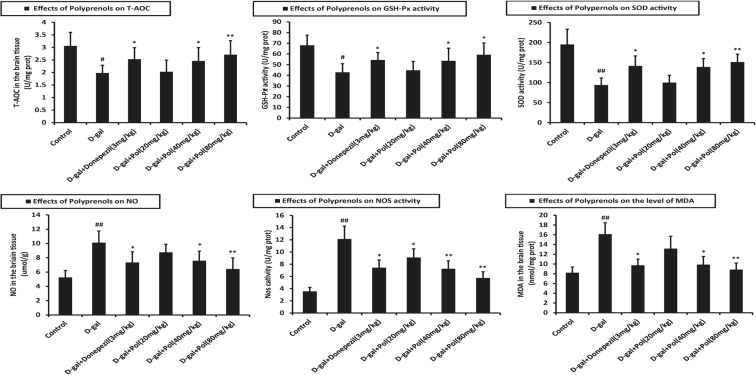
Effects of polyprenols on the level of T-AOC, GSH-Px, SOD, NO, NOS, and MDA
Once the behavior tests were completed, mice brain tissues were prepared for assessment of T-AOC, GSH-Px, SOD, NO, NOS, and MDA. The results of these biochemical analyses are shown in Fig. 6.
Fig. 6.
Effects of polyprenols on biochemical indicators in a d-galactose-induced mouse model. The group with d-galactose treatment exerted lower T-AOC, GSH-Px, SOD activity, higher NO, NOS activity, and MDA level than the control group(p < 0.01), respectively T-AOC, GSH-Px, and SOD activity improved significantly in the groups of 40 and 80 mg/kg, while NO, NOS activity, and MDA level fell significantly (p < 0.01). The data shown are mean ± SEM (n = 12). # p < 0.05; ## p < 0.01 vs control; *p < 0.05; **p < 0.01 vs model group
The group with d-galactose treatment exerted lower T-AOC, GSH-Px, and SOD activities and higher NO and NOS activities and MDA level than the control group (p < 0.01), whereas all the polyprenol groups exhibited statistically significant stimulative activity against mice with senile dementia. As shown in Fig. 6, injection of d-gatactose increased the degree of oxidative stress in the brain tissues. T-AOC, GSH-Px, and SOD activities improved significantly in the groups of 40 and 80 mg/kg, while NO and NOS activities and MDA level fell significantly (p < 0.01).
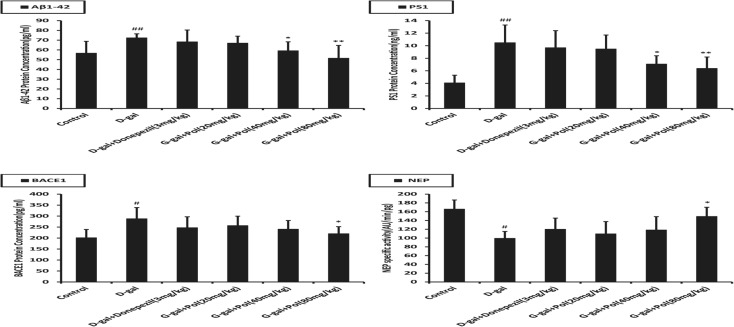
Effects of polyprenols on the level of Aβ1-42, BACE1, PS1, and NEP
As shown in Fig. 7, the concentration of Aβ1-42, BACE1, and PS1 in the brain of d-galactose-induced mice was increased while the NEP level was decreased significantly in comparison with the control group (p < 0.05). However, no significant difference was observed in the activity of Aβ1-42, BACE1, PS1, and NEP between the model and donepezil group (p > 0.05). Polyprenols (40 and 80 mg/kg) significantly lowered the level of Aβ1-42, PS1, and BACE1 while increasing NEP expression (p < 0.05). These suggested that polyprenols might be able to decrease deposition of Aβ by inhibiting its production and promoting its degradation.
Fig. 7.
Effects of .polyprenols on the expression level of Aβ1-42, PS1, BACE1, and NEP in a d-galactose-induced mouse model by ELISA (n = 20). The concentration of Aβ1-42, BACE1, and PS1 in the brain of d-galactose-induced mice was increased, whereas the NEP level was decreased significantly in comparison with control group (p < 0.05). Polyprenols (40 and 80 mg/kg) significantly lowered the level of Aβ1-42, PS1, and BACE1 while increased NEP expression (p < 0.05). # p < 0.05; ## p < 0.01 vs control; *p < 0.05; **p < 0.01 vs model group. The data shown are mean ± SEM (pg/mg protein)
Discussion
In the present study, we aimed to investigate the effects of polyprenols from pine needles of P. massoniana in a mouse model of AD. Consistent with previous reports, learning and memory dysfunction of a d-galactose-induced mouse model in the behavioral test was observed. Step-through active avoidance test is a quantitative determination of animal behavior changes. Shorter active avoidance time indicates quicker active avoidance reaction time and better learning and memory ability (Yang et al. 2004). Based on the skototaxis of mice, step-through passive avoidance test works by imposing electric shock on the dark room to compel mice to temporary tend to light, which reflects short memory ability of mice (Wang et al. 2013). Meanwhile, an open-field test is employed to evaluate animals’ autonomous behavior and exploratory behavior in an unfamiliar environment, which is based on phobotaxis. Activity in central square reflects spatial cognition and exploratory behavior, number of line crossing reflects excitability, while distance travelled reflects exploratory behavior (Marchei et al. 2009; Lei et al. 2013). Morris water maze test is one of the most frequently used method in behavioral study that relies on distal hint to navigate from origin around the perimeter of a pool of warm water (25 ± 1 °C) to locate the subaqueous escape platform. We could indicate that mice treated with polyprenols showed significant amelioration on cognitive impairment in a d-galactose-induced mouse model.
Long-term administration of d-galactose induces symptoms, which are similar to those in natural aging. In the present study, compared with the model, the groups treated with polyprenols significantly increased the activity and content of T-AOC, GSH-Px, and SOD and decreased activity of NO, NOS, and MDA. Increased GSH-Px eliminates harmful peroxide metabolites in the cells in order to block the lipid peroxidation chain reaction and prevents further hydrolysis to produce MDA (Valls et al. 2005; Dominique et al. 2004; Ozdemir et al. 2009; Briganti et al. 2008); SOD activity indirectly reflects the ability to remove oxygen-free radicals, while MDA indirectly reflects the severity of free radicals attack (Pan et al. 2013; Naghizadeh et al. 2013); NO is relatively stable, diffused through cell membrane, which inhibits platelet aggregation, vascular smooth muscle cells, and platelet activation factor induced neutrophil–endothelial cells interaction, adjusts vascular tension, stables circulation volume, mediated immune cells, and cytotoxic effect (Vanaja and Ekambaram 2004). NO level is highly related to learning and memory ability in the nervous system and moderately synthesized and released by neurons. If the release is excessive, nerve cells would be killed by toxicity and brain function would be declined (Gubandru et al. 2013). NOS is the key enzyme in the process of NO synthesis. Studies show that nNOS knockout mice have lower space learning ability, and large doses of nNOS-specific inhibitors could block long-term memory; neurons that contain NOS in the hippocampus play a significant role in learning and memory (Weitzdoerfer et al. 2004). Oxidative stress results in neuronal degeneration and impairs normal function of astrocytes, which has been implicated in the pathology of age-related neurodegenerative disorders such as AD, Parkinson’s disease, and cerebral stroke (Lei et al. 2013). Studies show that polyprenols are capable of accumulating endogenous antioxidants, degenerating free radicals, and inhibiting oxidative stress (Yu et al. 2012). These biochemical indicators in the brain tissues, as reported before, confirmed the notion that the increased oxidative stress may be a main pathogenic factor for the degeneration of astrocytes d-galactose-induced brain aging.
Generation and dissimilation of Aβ-related enzymes including Aβ1-42, BACE1, PS1, and NEP were detected. Polyprenols-treated model mice showed reduced level of Aβ1-42, PS1, and BACE1 and increased NEP expression, which suggested that cognitive improvement produced by polyprenols may be due, at least in part, to the reduction of brain Aβ. However, there was no significant difference between control group and donepezil group, which indicated that donepezil, a cholinesterase inhibitor, did not ameliorate cognitive impairment by affecting generation and dissimilation of Aβ-related enzymes.
In general, the results indicate that polyprenols has efficacy in the management of the experimental model of AD. It also has a profound effect on learning and memory dysfunction that are associated with AD. The application of polyprenols in treatment of AD is promising and worth investigating.
Acknowledgments
This study was supported by the National 12th Five-Year Plan “Major Scientific and Technological Special Project for Significant New Drugs Creation” project of “Novel G protein-coupled receptor targeted drug screening system and key technology research” (no. 2012ZX09504001-001); Program for New Century Excellent Talents in University (no. NCET-10-0817); Major Scientific and Technological Special Project of Guangdong Province (no. 2012A080201005); and the Fundamental Research Funds for the Central Universities (no. JKZ2009005 and no. JKY2011052).
Conflicts of interest
These authors have no conflict of interest to declare.
References
- Briganti S, Wlaschek M, Hinrichs C. Small molecular antioxidants effectively protect from PUVA-induced oxidative stress responses underlying fibroblast senescence and photoaging. Free Radic Biol Med. 2008;45:636–644. doi: 10.1016/j.freeradbiomed.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Choi D-Y, Lee Y-J, Hong JT, et al. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res Bull. 2012;87:144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Cui X, Zuo P, Zhang Q, Li X, Hu Y, Long J, et al. Chronic systemic d-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res. 2006;83:1584–1590. doi: 10.1002/jnr.20845. [DOI] [PubMed] [Google Scholar]
- DaRocha-Souto B, Coma M, Pérez-Nievas BG. synthase kinase-3 beta mediates β-amyloid induced neuritic damage in AD. Neurobiol Dis. 2012;45:425–437. doi: 10.1016/j.nbd.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominique BR, Christelle CA, Antonio T. Blood oxidative stress status in patients with macrophagic myofasciitis. Biomed Pharmacother. 2004;58(9):516–519. doi: 10.1016/j.biopha.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Dumont M, Beal MF. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic Biol Med. 2011;51:1014–1026. doi: 10.1016/j.freeradbiomed.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubandru M, Margina D, Tsitsimpikou C. Alzheimer’s disease treated patients showed different patterns for oxidative stress and inflammation markers. Food Chem Toxicol. 2013 doi: 10.1016/j.fct.2013.07.013. [DOI] [PubMed] [Google Scholar]
- He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Neuroprotective effects of (−)-epigallocatechin-3-gallate on aging mice induced by d-galactose. Biol Pharm Bull. 2009;32:55–60. doi: 10.1248/bpb.32.55. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Lee HK. Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta 25–35-induced learning and memory impairments in mice. Neurosci Lett. 2011;487:123–127. doi: 10.1016/j.neulet.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Lei M, Su Y, Hua X, Ding J, Han Q, Hu G, et al. Chronic systemic injection of D -galactose impairs the septohippocampal cholinergic system in rats. Neuroreport. 2008;19:1611–1615. doi: 10.1097/WNR.0b013e3283136a1f. [DOI] [PubMed] [Google Scholar]
- Lei M, Zhu Z, Wen Z, Ke S. Impairments of tight junctions are involved in d-galactose-induced brain aging. Neuroreport. 2013;24(12):671–676. doi: 10.1097/WNR.0b013e3283638f75. [DOI] [PubMed] [Google Scholar]
- Long J, Wang X, Gao H, Liu Z, Liu C, Miao M, et al. d-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology. 2007;8:373–381. doi: 10.1007/s10522-007-9081-y. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ. Quercetin reverses d-galactose induced neurotoxicity in mouse brain. Behav Brain Res. 2006;171:251–260. doi: 10.1016/j.bbr.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, et al. Ursolic acid attenuates d-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kB pathway activation. Cereb Cortex. 2010;20:2540–2548. doi: 10.1093/cercor/bhq002. [DOI] [PubMed] [Google Scholar]
- Malito E, Ralat LA, Manolopoulou M, et al. Molecular bases for the recognition of short peptide substrates and cysteine-directed modifications of human insulin-degrading enzyme. Biochemistry. 2008;47:12822–12834. doi: 10.1021/bi801192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchei P, Diverio S, Falocci NC. Breed differences in behavioural development in kittens. Physiol Behav. 2009;96(4):522–531. doi: 10.1016/j.physbeh.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Marseille DM, Silverman DHS. Recognition and treatment of Alzheimer’s disease: a case-based review. Am J Alzheimers Dis Other Dement. 2006;21:119–125. doi: 10.1177/153331750602100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghizadeh B, Mansouri MT, Ghorbanzadeh B. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–542. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J Neurosci. 1999;19(6):1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G. Oxidative damage is the earliest event in Alzheimer disease. Neuropathol Exp Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Ozdemir E, Cetinkaya S, Ersan S. Serum selenium and plasma malondialdehyde levels and antioxidant enzyme activities in patients with obsessive-compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:62–65. doi: 10.1016/j.pnpbp.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Dalfó E, Ayala V. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer's disease and identification of lipoxidation targets. J Biol Chem. 2005;280(22):21522–21530. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen Y, Li Q, Yu X, Wang J, Zheng J. The synthesis and evaluation of novel hydroxyl substituted chalcone analogs with in vitro anti-free radicals pharmacological activity and in vivo anti-oxidation activity in a free radical-injury Alzheimer’s model. Molecules. 2013;18:1693–1703. doi: 10.3390/molecules18021693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto C, Branes M, Alvarez J. Structural determinants of the Alzheimer’s amyloid beta-peptide. J Neurochem. 1994;63(4):1191–1198. doi: 10.1046/j.1471-4159.1994.63041191.x. [DOI] [PubMed] [Google Scholar]
- Tian J, Shi J, Yin J, et al. GEPT extract reduces Abeta deposition by regulating the balance between production and degradation of Abeta in APPV717I transgenic mice. Curr Alzheimers Res. 2009;6:118–131. doi: 10.2174/156720509787602942. [DOI] [PubMed] [Google Scholar]
- Valls V, Peiro C, Muniz P. Age-related changes in antioxidant status and oxidative damage to lipids and DNA in mitochondria of rat liver. Process Biochem. 2005;40(2):903–908. doi: 10.1016/j.procbio.2004.02.025. [DOI] [Google Scholar]
- Vanaja P, Ekambaram P. Demonstr ating the dose- and time-related effects of 7-nitroindazole on picrotoxin-induc ed convulsions, memory formation, brain nitric oxide synthase activity, and nitric oxide concentration in rats. Pharmacol Biochem Behav. 2004;77:1–8. doi: 10.1016/j.pbb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Wang CM, Liu MY, Wang F. Anti-amnesic effect of pseudoginsenoside-F11 in two mouse models of Alzheimer’s disease. Pharmacol Biochem Behav. 2013;106:57–67. doi: 10.1016/j.pbb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Weitzdoerfer R, Hoeger H, Engidawork E. Neuronal nitric oxide synthase knoekout mice show impaired cognitive performance. Nitric Oxide. 2004;10:130–140. doi: 10.1016/j.niox.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Processive proteolysis by γ-secretase and the mechanism of Alzheimer’s disease. Biol Chem. 2012;393:899–905. doi: 10.1515/hsz-2012-0140. [DOI] [PubMed] [Google Scholar]
- Xian YF, Lin ZX, Zhao M, Mao QQ, Ip SP, Che CT. Uncaria rhynchophylla ameliorates cognitive deficits induced by d-galactose in mice. Planta Med. 2011;77:1977–1983. doi: 10.1055/s-0031-1280125. [DOI] [PubMed] [Google Scholar]
- Yang J, He L, Wang J. Early administration of nicotinamide prevents learning and memory impairment in mice induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Pharmacol Biochem Behav. 2004;78:179–183. doi: 10.1016/j.pbb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Qian H, Zhao Y, Liu B, Fu C, et al. Polyprenols from Taxus chinensis var. mairei prevent the development of CCl4-induced liver fibrosis in rats. J Ethnopharmacol. 2012;142(1):151–160. doi: 10.1016/j.jep.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Zheng GY, HE L, BO CY, Wang MM. Protective effect of polyprenols from pine needles of Pinus massoniana on damaged PC12cells injury induced by β-amyloid protein, Chinese. Pharmacol Bull. 2011;27:581–582. [Google Scholar]