Abstract
Age-related muscle loss, termed sarcopenia, has been linked to functional deficits and an increased risk of falling. Such risk is of alarming concern due to the high disability and mortality rates associated with falling in older adults. Our laboratory recently developed a prediction model for fat-free mass index (FFMI) and, subsequently, sarcopenia within a community-dwelling older adult population using functional measures that are easily accessible to clinicians. The purpose of this study was to (1) determine how our prediction model performed in an older and less mobile assisted-living population, and if performance of the model was poor; (2) to improve and modify our previous prediction model using data acquired from this unique population. Forty assisted-living older adults (10 males) aged 86.1 ± 6.2 years participated in the study. Each completed four questionnaires to examine their mental and physical health status and anxiety levels related to falling. Anthropometric, balance, strength, and gait tests were conducted. Fat-free mass values, determined by bioelectrical impedance analysis, were normalized by height to obtain FFMI. Using an algorithm proposed by the European Working Group on Sarcopenia in Older People, FFMI along with grip strength and gait speed were used to identify sarcopenic individuals. FFMI was significantly correlated with sex, body mass index (BMI), circumference measures, handgrip strength, gait velocity, and measures of gait variability. The percentage of the variable variation explained by our previous model was reduced for a population of assisted-living older adults (R2 of 0.6744 compared to the reported R2 of 0.9272 for community-dwelling older adults; McIntosh et al. Age (Dordrecht, Netherlands), 2013). The prediction equation that accounted for the greatest variability of FFMI for the assisted living group included the independent variables of forearm circumference, BMI, handgrip strength, and variability of the double support time during gait (adjusted R2 = 0.7950). This prediction model could be used by clinicians working in an assisted-living facility to identify individuals with reduced muscle mass and, once identified, aid with the planning and implementation of appropriate intervention strategies to attenuate the progression of additional muscle loss and improve quality of life.
Keywords: Sarcopenia, Assisted-living older adults, Fat-free mass index (FFMI), Mobility, Gait speed, Grip strength
Introduction
Sarcopenia is a universal phenomenon that is increasing in prominence due to the aging population. The term refers to a severe age-related loss of muscle mass and comes from the Greek word sarx meaning flesh and penia meaning loss (Rosenberg 2011). Although the natural aging process will inevitably result in wear and tear of the body, sarcopenia is a progressive condition that is believed to begin as early as the fourth decade of life (Waters et al. 2000) and unknowingly affects some individuals more than others. Sarcopenia has a complex, multifactorial etiology with underlying mechanisms that are not fully understood. Numerous factors are thought to perpetuate muscle loss, including motor neuron loss, muscle morphology, physical inactivity, and/or changes in the endocrine system (Roubenoff 2000; Janssen et al. 2002; Fielding et al. 2011). Sarcopenia can influence many aspects of life by causing a loss of strength, impairing the ability to perform activities of daily living, and increasing the risk for falls (Cruz-Jentoft et al. 2010). The increased risk for falls associated with sarcopenia is of significance as falls can lead to fractures, poorer quality of life, and even death (Roubenoff 2000). If it were possible to prevent or lessen the effects of sarcopenia, the number of incidences of falls and other negative consequences associated with muscle loss could be attenuated and the quality of life for many older adults could be improved.
Previous studies have identified a high prevalence of sarcopenia, ranging from 10–24 % in individuals between 65–70 years of age and 30–50 % in those over 80 years of age (Baumgartner et al. 1998; Iannuzzi-Sucich et al. 2002). More recent studies have reported prevalence rates of 25.3 % in hospitalized older adults (aged 82.8 ± 5.9 years; Smoliner et al. 2014) and 14.4–16.6 % in community-dwelling Brazilian older adults over 65 years of age (da Silva Alexandre et al. 2014). The range in prevalence can be attributed to ages of those studied as well as the lack of cohesion in how sarcopenia has been defined and diagnosed. Although the presence of sarcopenia has been documented in literature since (Rosenberg 1989), there is still no universally accepted definition, diagnostic criteria, or treatment for the syndrome. In 2009, the European Working Group on Sarcopenia in Older People (EWGSOP) was created to develop a more practical clinical definition and diagnostic criteria for sarcopenia (Cruz-Jentoft et al. 2010).
According to the EWGSOP, sarcopenia is a progressive process of age-related muscle loss, resulting in decreased strength and functionality. To incorporate the importance of muscle quantity and quality, the EWGSOP proposed an algorithm for detecting sarcopenia using the presence of both low muscle mass and low muscle function (Cruz-Jentoft et al. 2010). Using Janssen’s model (2002), the group considers reduced muscle mass to be two standard deviations (SD) below that of a young, healthy population and functional deficits to be decreased gait speed (<0.8 m/s) and decreased handgrip strength (<30 kg men, <20 kg women). The EWGSOP further suggests that individuals can be classified as pre-sarcopenic (reduced muscle mass without functional deficits), sarcopenic (reduced muscle mass with a deficit in gait speed or grip strength), or severely sarcopenic (reduced muscle mass, decreased gait speed, and decreased grip strength) (Cruz-Jentoft et al. 2010). To date, the EWGSOP has provided the most clear and in-depth definition of sarcopenia.
Given an increasingly larger aging baby-boomer population, a better understanding of the multifactorial nature of sarcopenia is timely and of critical importance to reduce related injuries and the strain imposed on the health care system. Previous work from our lab (McIntosh et al. 2013) used a combination of anthropometric, clinical, and biomechanical tests to successfully predict fat-free mass index (FFMI) and, subsequently, sarcopenia in community-dwelling older adults using the EWGSOP working definition of sarcopenia. Based on obtained results, we proposed a prediction equation for FFMI in community-dwelling older adults, which included sex, time outside of ellipse (TOE; a measure of balance), body mass index (BMI), and step time and obtained an adjusted R2 = 0.9272 for the prediction group and R2 = 0.8271 for the validation group. Our results suggested that functional changes (e.g., reductions in gait speed) might not be evident when using the EWGSOP definition of functional deficits related to sarcopenia. We argued that a more sensitive model is required to facilitate the detection of minor changes before major functional deficits occur; for example, we found that different components of gait speed (cadence and step length) were more closely related to FFMI than the overall measurement of gait speed. As such, we proposed that a more diverse and multifaceted working definition is required for clinicians working with this population, especially if early intervention is the ultimate goal.
Using a similar protocol, the aim of this work was to determine if a predication model based on data obtained from a community-dwelling population would maintain its high level of accuracy when tested on assisted-living older adults. While a single prediction model would be ideal, we must consider the fact that assisted-living older adults are generally older and have more complexities in terms of mobility and health than their community-dwelling counterparts. As such, the purpose of the current study was two-tiered: (1) to determine how our prediction model performed in an older and less mobile assisted-living population, and if performance of the model was poor; (2) to improve and modify this earlier prediction model using data acquired from this unique, assisted-living older adult population. A validated prediction model will enable clinicians to better detect sarcopenia before individuals are considered at risk of falling. Early detection would allow for interventions to attenuate the progression of sarcopenia in this cohort of older adults with the goal of decreasing falls and improving quality of life. It was hypothesized that smaller circumference measures, poorer balance and performance on clinical tests, and lower measures of strength and gait would be associated with reduced FFMI as suggested by our previous work (McIntosh et al. 2013).
Methods
Participants
Forty assisted-living older adults (10 males, 30 females) aged 86.1 ± 6.2 years participated in the current study. The study took place at four different facilities in the city of Guelph (pop. 117, 000), Ontario in order to attain a representative sample population of the community. Assisted-living facilities help to ensure the health and safety of older adults by providing assistance with activities of daily living, including but not limited to bathing, laundry, meal preparation, and drug administration. Residents at each facility signed up to participate in the study following a recruitment presentation; on average approximately 10 older adults volunteered from each of the four facilities (each housed ~ 100 residents). To be eligible for the study, individuals had to be 65 years of age or older, able to walk 12 m with or without a walking aid, and able to stand unaided for 1 min. Each participant was verbally asked a series of questions regarding their general physical and mental health in order to divulge past neurological and/or musculoskeletal medical conditions. Current medication use was reported due to an association between the numbers of drugs taken and falls risk (Huang et al. 2010). As per the guidelines established from the manufacturer of the bioelectrical impedance analysis device (BIA; Model 1500 from Bodystat, Isle of Man, British Isles) (Duren et al. 2008), those who had pre-existing heart conditions (i.e., congestive heart failure), kidney problems, or any implanted electronic devices (i.e., pacemaker) were excluded from participation in the study; the device induces an electrical current within the body which could pose a small health risk to these individuals.
Questionnaires
To begin the testing protocol, several surveys and questionnaires were conducted with each participant. The Mini Mental Status Examination (MMSE) was first performed to assess cognitive abilities (Folstein et al. 1975), with higher scores being indicative of better cognitive function. All participants achieved a score higher than 20 (out of 30), which was the minimum required to participate in the study (condition of the University Research Ethics Board and different assistive-living facilities for obtaining informed consent). All participants then completed a General Health and Information Questionnaire, which consisted of 18 questions to gather sociological data and to divulge past neurological, orthopedic, and musculoskeletal medical conditions. The questionnaire also required individuals to report the number of falls experienced over the past year; a fall was defined as an unexpected contact with the ground that did not occur due to fainting or illness. The Physical Activity Scale for the Elderly (PASE) survey was also completed to quantify each participant’s daily physical activity level. The survey provides weighted scores for participation in activities ranging from intense exercises (e.g., swimming; high values) to household chores (lower values) (Washburn et al. 1993). Finally, a short Falls Efficacy Scale (FES) was used to assess each participant’s concern of falling while performing seven nonhazardous activities of daily living (Tinetti et al. 1994). The test is scored out of 28, with a higher score indicating greater levels of concern about falling.
Anthropometric measurements
Participants removed their shoes prior to their height and weight being measured. All body weight measures were recorded using a standard scale and were validated with a portable force platform. Using a standard soft tape measure, circumferences were measured for the waist (WC), hip (HC), upper arm (AC), forearm (FC), calf (CC), and thigh (TC). All arm and leg measurements were taken from the participant’s dominant side. Both leg lengths were also measured from the greater trochanter to the floor with shoes on (participants wore their own footwear during walking trials) and used for subsequent gait analyses. All measurements were recorded to the nearest 0.1 kg or 0.1 cm.
Body composition
A dual-compartmental model of fat mass (FM) and fat-free mass (FFM) was measured using bioelectrical impedance analysis (BIA; model 1500, Bodystat Ltd., Douglas, Isle of Man). BIA uses a unique principle based upon conductance and impedance of certain tissues to determine body composition. Since the conductance of FM and FFM differ, an impedance value can be obtained and used to estimate these mass distributions (National Institutes of Health 1996). BIA was chosen to assess body composition for its simplicity, portability, and affordability. To ensure proper hydration status on the day of testing, each participant was instructed to drink 2–4 glasses of water within 2 h before the study. To further control for possible variation, participants were instructed not to consume alcohol 24 h beforehand, not to take part in any strenuous exercise within 12 h beforehand, and not to have any excessive caffeine or food intake within 4 h prior to testing. Participants voided their bladder and then lay on their bed in a supine position with limbs abducted for 5 min before analysis began. This method has been used often in the literature to provide valid, reliable estimates of skeletal muscle mass (Janssen et al. 2002; Castillo et al. 2003; Cruz-Jentoft et al. 2010; McIntosh et al. 2013).
Clinical tests
Two clinical tests were conducted to assess balance and mobility: the Timed Up and Go (TUG) and Dynamic Gait Index (DGI) tests. TUG assessed the time needed for each participant to stand up from a chair, walk forward 3 m, turn around, and return to seated position. Participants were encouraged not to use the chair arms as an aid for rising and lowering; however, it was deemed acceptable if needed. Three TUG trials were performed and the fastest time was recorded for data analysis. Individuals with a time greater than 14 s are typically considered to be at greater risk for falling (Shumway-Cook et al. 2000). The DGI required participants to perform a series of eight different functional walking tasks across a marked 6-m pathway. Tasks included fast/slow walking, horizontal and vertical head movements while walking, obstacle avoidance, and stair climbing. The test is designed to assess gait, balance, and risk for falls during perturbations and transitions (Herman et al. 2009) and yields a single score out of 24; a score of 19 or less is representative of increased falls risk (Shumway-Cook et al. 1997). For both clinical tasks, participants were allowed to use any walking aids that they normally require to perform their activities of daily living (e.g., rollator, cane, etc.).
Balance
Balance was measured using an AccuGait portable force platform (50 Hz; AMTI, MA, USA). Participants were asked to remove their footwear and stand quietly with their eyes fixed on a target in front of them and arms at their sides. Three, 1-min trials were conducted with the participant sitting between trials to prevent fatigue. Foot tracings were used to ensure similarity of foot placement between trials.
Strength measurements
Strength was measured using a Vernier digital hand dynamometer and collected using LoggerPro software (Vernier, OR, USA; 60 Hz). Subjects sat on a chair with their arms close to their thorax and elbows bent at a 90° angle while holding the dynamometer in a vertical position. Three isometric contractions were performed at a self-selected pace for both dominant and non-dominant hands. Visual feedback (via computer placed at eye level) and verbal encouragement were provided throughout each trial to motivate participants to improve their score and reach maximum strength. Peak forces (N) from the six trials were displayed and recorded. The highest peak force produced was recorded as maximum handgrip strength (MG), regardless of hand dominance.
Gait analysis
Gait was assessed using a 12-m GAITRite mat system (50 Hz; CIR Systems, Havertown, PA, USA). The mat measured spatial and temporal parameters of gait, including gait speed, cadence, stride length, etc. Participants walked 5 m before and after the GAITRite mat to ensure that steady state gait was captured. Subjects were asked to complete five trials at their preferred natural walking pace and were allowed the use of any walking aids if needed. Volunteers acted as spotters, walking alongside participants during each trial to ensure their safety and comfort.
Data analyses
Force plate data was filtered using a dual low-pass second-order Butterworth filter with a 10 Hz cutoff frequency. The first and last 5 s were removed from each trial to capture quiet stance in the remaining 50 s. Center of pressure (CoP) was then calculated (Reed-Jones et al. 2008) and normalized according to the mean CoP excursion in the medial-lateral and anterior-posterior directions. Means and standard deviations were computed for anterior-posterior and medial-lateral ranges, velocity, acceleration, cumulative path length (CPL), and the summation of time spent outside a 95 % confidence ellipse (TOE). TOE is a novel measure to characterize brief periods of instability and variability; these brief periods might otherwise be masked by calculations involving simple CoP averages (McIntosh et al. 2013). A longer duration outside of the 95 % confidence ellipse would be indicative of more periods of instability for a given participant.
Handgrip strength values that were collected in Newtons were converted to kilograms using the specifications listed on the Vernier website (Vernier 2012). Handgrip strength (kg) was then divided by forearm circumference (cm) to obtain an additional measure of handgrip strength that takes into account the anthropometrics (body dimensions) of each participant.
Using GAITRite software, any partial footfalls were removed as the participant stepped on/off the GAITRite mat. Velocity was normalized by each participant’s average leg length to allow for comparisons across individuals. Means and standard deviations were computed for cadence, velocity, normalized velocity, step length, stride length, step time, stride time, swing time, stance time, and single (SST) and double support time (DST). The mean standard deviation and standard deviation of the standard deviation were also calculated to provide insight into step-to-step gait variability.
Defining sarcopenia
The current study used the definition proposed by the EWGSOP to identify the presence of sarcopenia in a group of older adults residing in assisted-living facilities. FFMI was calculated to identify muscle loss, using the following formula:
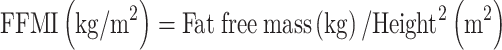 |
1 |
Participants with a FFMI below two standard deviations from a young adult reference population (Schutz et al. 2002, adapted from Janssen et al. 2002) were designated to have muscle loss (<15.5 kg/m2 males; <12.6 kg/m2 females). In keeping with the EWGSOP algorithm, participants were then classified as pre-sarcopenic, sarcopenic, or severely sarcopenic based on the presence of functional deficits. If individuals in the current study had a gait speed less than 0.8 m/s or a handgrip strength value less than 30 kg for men or 20 kg for women, they were classified as sarcopenic. If they did not present any functional deficits, they were classified as pre-sarcopenic. If an individual possessed muscle loss and both functional deficits, they were classified as severely sarcopenic (Cruz-Jentoft et al. 2010).
Statistical analyses
Two individuals with high BMI, one with a visual impairment, and one with edema were flagged as outliers, which resulted in the exclusion of their results from the study. Additionally, due to technical difficulties, data was not obtained for one individual’s balance data and another individual’s gait data. The remaining participants constituted the final sample size of 36 (but n = 35 for subsequent balance and gait data) available for further statistical analyses. Statistical analyses were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Tests of normality were assessed to identify individuals outside a 95 % confidence interval for all measures. Using the EWGSOP model, individuals were classified as sarcopenic or non-sarcopenic, and a one-way ANOVA was conducted to compare means between the populations for all measures. Pearson’s correlation coefficients were used to assess the relationship between FFMI and all measures obtained from the study.
As stated earlier, a goal of the current work was to validate our previously proposed prediction model generated using data acquired from community-dwelling older adults (McIntosh et al. 2013) to determine if such a tool is acceptable for use among an assisted-living older adult population. This previously proposed model suggested that sex, BMI, TOE, and step time were the most successful predictors of FFMI (Eq. 2).
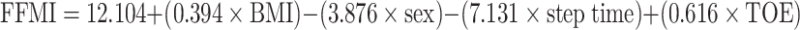 |
2 |
A secondary goal was to improve/modify our prediction model for the current assisted-living population if the adjusted R2 was markedly reduced from our previously proposed model (more than 10 % reduction in prediction capability). To this end, multiple linear regression analyses were performed to determine appropriate functional predictor variables for FFMI that could be easily measured by clinicians working with this unique older adult population. The goal was to predict the dependent variable, FFMI, from a combination of anthropometrics, clinical tests, and biomechanical measures. The highest correlated variables to FFMI from each methodological test were entered into the regression model based on Pearson’s correlation coefficients. Forward stepwise regressions (entry criteria α = 0.15) were performed on the prediction group (n = 36) by adding one variable at a time to the model, provided the F-statistic for each variable was significant. The forward stepwise option is generally used to test a large set of variables (Bowley 2008). Multicollinearity was assessed and the resulting prediction model was then internally validated on a subset of the data (n = 18). All errors were normally distributed, random, and independent as determined by a Shapiro-Wilk test and from visual inspection of residual plots.
Results
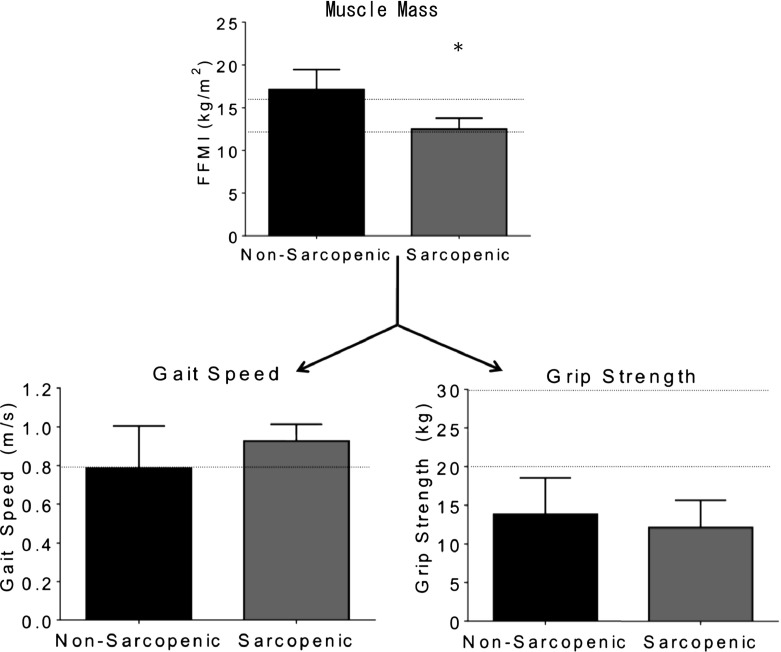
Tables 1 and 2 include the group characteristics and average results for anthropometric, clinical (MMSE, PASE, FES, DGI, and TUG), and biomechanical measures. Based on the EWGSOP diagnostic criterion (Cruz-Jentoft et al. 2010), all 36 (100 %) participants had grip strength deficits (<30 kg men, <20 kg women) and 15 (43 %) participants had gait impairments (<0.8 m/s), all of which were non-sarcopenic individuals. Eight (22 %) out of 36 individuals were classified as sarcopenic as their muscle mass was 2 SD lower than the reference population and they had a maximum grip strength lower than the EWGSOP proposed guidelines. No individuals within the current study showed muscle loss without the presence of functional deficits nor did they have muscle loss with the presence of both functional deficits (grip strength and gait speed). As such, no individuals were classified as pre-sarcopenic or severely sarcopenic, leaving 28 individuals who were classified as non-sarcopenic; see Fig. 1 for illustration of obtained results for muscle loss and functional deficits as they relate to the EWGSOP proposed guidelines.
Table 1.
General participant characteristics, including clinical test scores, anthropometrics, and body composition measures. Population data were split into two classifications for the sample population: sarcopenic and non-sarcopenic individuals. According to EWGSOP guidelines, there were no individuals in the current study classified as pre-sarcopenic or severely sarcopenic
| Characteristics | Total population (n = 36) | Sarcopenic (n = 8) | Non-sarcopenic (n = 28) |
|---|---|---|---|
| Mean (SD); range | Mean (SD); range | Mean (SD); range | |
| Age (years) | 86.7 (5.7); 70–96 | 88.8 (1.6); 86–91 | 86.1 (6.3); 70–96 |
| Mini Mental Status Exam (MMSE) | 27.2 (3.1); 20–30 | 28.0 (2.9); 22–30 | 27.0 (3.1); 20–30 |
| Physical Activity Scale for the Elderly (PASE) | 30.6 (20.2); 0–83.3 | 34.0 (20.9); 7.3–73.9 | 29.6 (20.3); 0–83.3 |
| Falls Efficacy Scale (FES) | 11.7 (5.1); 7–28 | 12.5 (5.5); 7–21 | 11.5 (5.0); 7–28 |
| Dynamic Gait Index (DGI) | 13.4 (5.3); 5–24 | 16.0 (4.6); 11–23 | 12.6 (5.3); 5-24 |
| Timed Up-and-Go (TUG) (s) | 15.0 (6.6); 7.7–43.8 | 13.6 (3.5); 9.1–19.4 | 15.4 (7.2); 7.7–43.8 |
| Height (cm) | 159.2 (8.8); 146–177 | 157.8 (9.4); 147.5–177 | 159.7 (8.7); 146–177 |
| Weight (kg)* | 68.9 (12.4); 50–97 | 59.9 (11.9); 50–85.2 | 71.5 (11.5); 53.6–97 |
| BMI (kg/m2)* | 27.0 (4.0); 20.4–35.8 | 24.5 (2.9); 20.4–28.6 | 27.7 (4.0); 20.5–35.8 |
| Waist circumference (cm)* | 100.1 (11.4); 75–127 | 92.9 (9.7); 75–107 | 102.1 (11.2); 84–127 |
| Hip circumference (cm) | 106.5 (8.4); 92–125.1 | 103.8 (5.8); 92–111 | 107.3 (8.9); 92.5–125.1 |
| Upper arm circumference (cm)* | 29.0 (3.7); 23–38.9 | 26.3 (1.9); 23–29 | 29.8 (3.7); 24.2–38.9 |
| Forearm circumference (cm)* | 21.9 (2.8); 16–27 | 18.7 (2.0); 16–22.5 | 22.9 (2.3); 18.3–27 |
| Calf circumference (cm) | 35.5 (3.4); 28.5–47 | 33.6 (2.7); 31–39 | 36.1 (3.4); 28.5–47 |
| Thigh circumference (cm) | 48.9 (4.7); 41–60 | 48.1 (5.6); 42–59 | 49.1 (4.5); 41–60 |
| Fat mass (%)* | 40.6 (8.4); 22.2–56.4 | 48.7 (4.9); 42.8–56.4 | 38.2 (7.7); 22.2–52.6 |
| Fat mass (kg) | 27.9 (7.0); 13–43.1 | 29.8 (5.6); 21.5–36.7 | 27.4 (7.3); 13–43.1 |
| Fat free mass (%)* | 59.4 (8.4); 43.6–77.8 | 51.3 (4.9); 43.6–57.2 | 61.8 (7.7); 47.4–77.8 |
| Fat free mass (kg)* | 41.2 (9.7); 27.1–61.2 | 31.4 (7.0); 27.1–48.5 | 44.0 (8.6); 29.3–61.2 |
| FFMI (kg/m2)* | 16.1 (2.9); 11.3–23.4 | 12.5 (1.3); 11.3–15.5 | 17.1 (2.3); 13.2–23.4 |
*Significant difference between sarcopenics and non-sarcopenics as determined using a one-way ANOVA
Table 2.
Gait and balance parameters for both sarcopenic and non-sarcopenic individuals
| Characteristics | Total population (n = 35) | Sarcopenic (n = 8) | Non-sarcopenic (n = 27) |
|---|---|---|---|
| Mean (SD); range | Mean (SD); range | Mean (SD); range | |
| Strength parametersa | |||
| Maximum grip strength (kg) | 13.4 (4.5); 5.7–23.4 | 12.1 (3.5); 8.2–19.3 | 13.8 (4.7); 5.7–23.4 |
| Normalized grip strength (kg/cm)b | 0.61 (0.19); 0.30–1.07 | 0.65 (0.17); 0.43–0.88 | 0.60 (0.20); 0.30–1.07 |
| Gait parameters | |||
| Cadence (steps/min) | 104.6 (14.6); 57.2–135.3 | 107.8 (7.5); 92.7–115.8 | 103.7 (16.1); 57.2–135.3 |
| Gait speed (m/s) | 0.82 (0.20); 0.47–1.21 | 0.93 (0.09); 0.82–1.04 | 0.79 (0.22); 0.47–1.21 |
| Normalized gait speed (s−1)c | 0.88 (0.22); 0.48–1.38 | 0.99 (0.09); 0.89–1.17 | 0.84 (0.23); 0.48–1.38 |
| Step length (cm) | 47.2 (7.8); 30.5–63.3 | 51.2 (3.9); 45.8–56.3 | 46.1 (8.3); 30.5–63.3 |
| Stride length (cm) | 93.0 (17.1); 61.4–126.9 | 102.9 (7.8); 92.0–112.7 | 90.1 (18.0); 61.4–126.9 |
| Step time (s) | 0.56 (0.11); 0.32–1.05 | 0.56 (0.04); 0.52–0.65 | 0.57 (0.13); 0.32–1.05 |
| Stride time (s) | 1.11 (0.24); 0.57–2.10 | 1.12 (0.08); 1.03–1.29 | 1.11 (0.27); 0.57–2.10 |
| Swing time (%GC) | 0.40 (0.08); 0.23–0.77 | 0.41 (0.03); 0.36–0.46 | 0.39 (0.09); 0.23–0.77 |
| Stance time (%GC) | 71.4 (16.0); 34.0–132.7 | 71.0 (6.0); 83.0–64.4 | 71.5 (18.0) 34.0–132.7 |
| Single support time (%GC) | 39.5 (8.4); 23.2–77.4 | 40.7 (3.0); 35.7–46.2 | 39.2 (9.4); 23.2–77.4 |
| Double support time (%GC) | 32.1 (9.7); 10.7–57.5 | 30.5 (4.3); 24.8–37.2 | 32.6 (10.8); 10.7–57.5 |
| Balance parameters | |||
| Time outside ellipse (s)* | 6.5 (0.6); 4.8–7.6 | 7.0 (0.5); 6.1–7.6 | 6.4 (0.5); 4.8–7.4 |
| Cumulative path length (cm) | 94.4 (60.0); 40.6–324.3 | 83.5 (25.8); 44.6–126 | 97.1 (65.9); 40.6–324.3 |
| Total sway velocity (cm/s) | 1.9 (1.2); 0.8–6.5 | 1.7 (0.5); 0.9–2.5 | 1.9 (1.3); 0.8–6.5 |
| Medial-lateral range (cm) | 2.6 (1.5); 0.9–8.9 | 2.6 (0.9); 1.5–3.6 | 2.5 (1.7); 0.9–8.9 |
| Anterior-posterior range (cm) | 3.1 (1.0); 1.4–5.5 | 3.1 (0.8); 2.0–4.4 | 3.1 (1.1); 1.4–5.5 |
| Frequency of excursions outside the 95 % confidence ellipse | 16 (7); 8–42 | 15 (5); 9–22 | 17 (7); 8–42 |
*p < 0.05, statistical significance found between sarcopenics and non-sarcopenics as determined using a one-way ANOVA
aStrength parameters include a sample size of n = 36 (non-sarcopenic, n = 28; sarcopenic, n = 8)
bHandgrip strength normalized to forearm circumference
cGait speed normalized to leg length
Fig. 1.
Results illustrating the differences in mean values between non-sarcopenic and sarcopenic populations obtained for muscle loss and functional deficits as they relate to the guidelines proposed by the EWGSOP (indicated by dotted horizontal lines). Asterisks (*) indicate a significant difference between non-sarcopenics and sarcopenics as determined using a one-way ANOVA
Tables 1 and 2 display results for the population as a whole, but also separate the results into sarcopenic and non-sarcopenic groupings. Biomechanical tests (Table 2) were separated into strength, gait, and balance measures for reporting purposes. Statistical differences between the two groups were noted for weight (p = 0.017; β = 0.680), BMI (p = 0.042; β = 0.537), WC (p = 0.043; β = 0.534), AC (p = 0.017; β = 0.680), FC (p = 0.0001; β = 0.995), FM (%; p = 0.001; β = 0.939), FFM (kg; p = 0.001; β = 0.956), FFM (%; p = 0.001; β = 0.939), FFMI (p = 0.0001; β = 0.999), TOE (p = 0.006; β = 0.808), and measures of variability, including swing time (p = 0.036; β = 0.563), SST (p = 0.036; β = 0.562), and DST (p = 0.024; β = 0.630). All anthropometric data, including height, weight, circumference measures, and BMI were lower in sarcopenic individuals than non-sarcopenics. Body composition measures showed that sarcopenics had higher FM, but lower FFM than non-sarcopenics. Balance measures showed that the CoP trajectories were outside of a 95 % confidence ellipse for a longer duration in sarcopenic individuals; recall that greater time spent outside of this ellipse may be indicative of more periods of instability for a given participant. Finally, the sarcopenic group showed less variability in their gait across five trials in terms of swing time, SST, and DST.
Correlation coefficients are listed in Table 3 for the full sample population as well as split between sarcopenic and non-sarcopenic groupings. As a whole population, FFMI was significantly correlated with sex, WC, HC, AC, FC, CC, BMI, handgrip strength, gait speed, step length, and stride length. The positive correlation between sex and FFMI indicates that males have significantly higher FFMI values than females. FFMI was also correlated with the variability, or SD, found within several gait parameters, including the following: step length, stride length, step time, stride time, swing time, stance time, SST, and DST. None of the mean balance measures examined were significantly correlated to FFMI for the whole population.
Table 3.
Results from Pearson correlation analyses; italicize values represent correlations between FFMI and measured variables for the population as well as for sarcopenic and non-sarcopenic classifications (p < 0.05)
| Characteristics correlation | FFMI population, (n = 36) | FFMI sarcopenic, (n = 8) | FFMI non-sarcopenic, (n = 28a) |
|---|---|---|---|
| Sex | 0.514 | 0.945 | 0.500 |
| Age | −0.290 | 0.107 | −0.232 |
| Number of medications | 0.096 | 0.615 | 0.039 |
| Number of falls in 1 year | 0.228 | 0.170 | 0.330 |
| Waist circumference | 0.605 | 0.519 | 0.552 |
| Hip circumference | 0.464 | 0.501 | 0.474 |
| Arm circumference | 0.605 | 0.247 | 0.520 |
| Forearm circumference | 0.807 | 0.748 | 0.664 |
| Calf circumference | 0.432 | 0.297 | 0.324 |
| Thigh circumference | 0.180 | 0.390 | 0.133 |
| BMI | 0.541 | 0.583 | 0.436 |
| TUG | 0.100 | 0.199 | 0.020 |
| DGI | −0.276 | −0.234 | −0.121 |
| CPL | 0.088 | 0.207 | 0.028 |
| Sway velocity | 0.088 | 0.206 | 0.040 |
| ML range | 0.187 | 0.589 | 0.228 |
| AP range | 0.262 | 0.866 | 0.275 |
| TOE | −0.207 | −0.022 | 0.158 |
| Maximum grip strength | 0.474 | 0.767 | 0.478 |
| Gait speed SD |
−0.426
0.296 |
0.240 0.889 |
−0.357 0.136 |
| Normalized Speed SD |
−0.495
0.087 |
−0.155 0.723 |
−0.437
−0.021 |
| Cadence SD |
−0.207 0.205 |
0.379 0.600 |
−0.210 0.095 |
| Step length SD |
−0.401
0.366 |
−0.104 0.621 |
−0.311 0.210 |
| Stride length SD |
−0.436
0.387 |
−0.111 0.652 |
−0.330 0.252 |
| Step time SD |
0.037 0.504 |
−0.335 0.709 |
0.043 0.423 |
| Stride time SD |
−0.035 0.513 |
−0.377 0.656 |
−0.013 0.424 |
| Swing time SD |
−0.103 0.515 |
−0.617 0.198 |
−0.042 0.414 |
| Stance time SD |
0.003 0.528 |
−0.225 0.677 |
0.004 0.441 |
| SST SD |
−0.103 0.515 |
−0.618 0.199 |
−0.043 0.413 |
| DST SD |
0.096 0.602 |
0.022 0.478 |
0.048 0.517 |
aFor the balance and gait parameters, non-sarcopenic group had a reduced sample size of n = 27
Our previously proposed prediction model (Eq. 2) was applied to our current data; however, the obtained adjusted R2 of 0.6744 was greatly reduced from the reported R2 of 0.9272 obtained from the previous sample population of community-dwelling older adults (McIntosh et al. 2013). Based on these findings, we then sought to improve the prediction results of our model based on acquired data for assisted-living older adults. To this end, the data were split into sarcopenic and non-sarcopenic categories, to identify common correlations with FFMI observed for both populations; these included sex, FC, handgrip strength, and variability in the amount of time spent in double support time during gait (SD of double support time). The highest correlated anthropometric, clinical, and biomechanical variables to FFMI were entered into a forward stepwise regression model. The resulting prediction equation that accounted for the greatest variability of FFMI in the current study included forearm circumference (FC), body mass index (BMI), maximum grip strength (MG), and standard deviation of the double support time (SDDST) as predictor variables (Eq. 3).
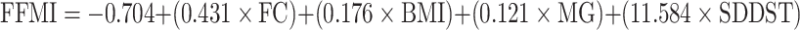 |
3 |
An adjusted R2 value of 0.7950 was obtained for the prediction group and a value of 0.7412 was obtained for the internal validation group. Beta values (Table 4) for the standardized coefficients were 0.38 for forearm circumference (p = 0.0551), 0.34 for body mass index (p = 0.0813), 0.24 for maximum grip strength (p = 0.1560), and 0.27 for standard deviation of the double support time (p = 0.0992).
Table 4.
Statistical results of the forward stepwise regression prediction model (n = 40; p < 0.05) conducted in SAS version 9.3 (SAS Institute Inc., Car, NC, USA)
| Variable | DF | Parameter estimate | Standard error | β value | Pr > |t| | Variance inflation factor |
|---|---|---|---|---|---|---|
| Intercept | 1 | −0.704 | 3.2146 | 0 | 0.8300 | 0 |
| Forearm circumference | 1 | 0.431 | 0.2044 | 0.38 | 0.0551 | 2.153 |
| Body mass index | 1 | 0.176 | 0.0929 | 0.34 | 0.0813 | 2.081 |
| Maximum grip strength | 1 | 0.121 | 0.0806 | 0.24 | 0.1560 | 1.620 |
| SDDST | 1 | 11.584 | 6.5234 | 0.27 | 0.0992 | 1.473 |
Discussion
Given that a major goal of this work was to compare performance of our prediction model for two cohorts of older adults, it is critical to explore characteristics of our older and less mobile assisted-living population and discuss the applicability of the European Working Group on Sarcopenia in Older People definition for this population. As expected, the prevalence of sarcopenia was 16 % higher in assisted-living older adults in comparison to their community-dwelling counterparts (5.9 % as reported by McIntosh et al. 2013). Within the current population, lower FFMI values were associated with females and reduced circumference, BMI, and grip strength measures (Table 3). Using the EWGSOP diagnostic criterion (Cruz-Jentoft et al. 2010), 22 % of our assisted-living population was classified as sarcopenic. Sarcopenia prevalence rates reported in the literature in persons over 80 years of age can range from approximately 30–50 % (Baumgartner et al. 1998) to 25.3 % in hospitalized older adults (aged 82.8 ± 5.9 years; Smoliner et al. 2014). The difference in prevalence rates could be attributed to the physical characteristics of the population studied (e.g., community-dwelling versus individuals with varying health problems and mobility challenges) as well as variability in how sarcopenia has been defined in the past. For example, the model used by Janssen et al. (2002) is more likely to include false positives in classifying sarcopenia due to the fact that anyone between 1–2 standard deviations below a reference population is classified as sarcopenic. It is also important to note that the current study included four test facilities with residents who are financially affluent. Research has suggested that individuals from more fiscally stable backgrounds generally live longer and healthier lives (Matthews et al. 2006).
Although significant differences between the sarcopenic and non-sarcopenic individuals were found for the majority of anthropometric measures (Table 1), it was also expected that differences would be found in more functional measures based on the EWGSOP model. To assess for functionality, we looked at a variety of clinical, balance, and biomechanical tests. It was expected that sarcopenic individuals would have poorer performances on the clinical measures of TUG and DGI, more specifically slower TUG times and lower DGI scores. It is also generally expected that the variability in balance and gait measures will increase while gait speed and strength will decrease in aging and/or pathological individuals (Parreira et al. 2012; Hegeman et al. 2007; Beauchet et al. 2009). In terms of biomechanical measures, TOE and measures of gait variability were the only variables that showed significant differences between the groups (Table 2). Perhaps the lack of significance found in our functional measures is an indication that other measures of physical function need to be assessed by clinicians. For example, the use of dynamic measures, such as a repeated sit-to-stand task, could be better indicators of functional ability due to the coordination and efforts required to successfully and safely complete them.
Although the EWGSOP has arguably created the most clear and concise method for identifying individuals with sarcopenia to date, there may be some shortfalls pertaining to the definition. As previously outlined, in our population of older adults residing in an assisted-living facility, we did not find any significant differences between sarcopenic and non-sarcopenic individuals for the functional measures proposed by the EWGSOP (i.e., grip strength and gait speed). Furthermore, all participants within the current study demonstrated grip strength deficits and interestingly, 42 % of the population that presented with gait speed deficits were non-sarcopenic (Fig. 1). Perhaps, there were other subclinical issues that had gone undetected such as neural deficits that would not be identified due to the current study’s protocol, which focused on physical factors (e.g., muscle strength, balance disturbances). However, the aforementioned points suggest that the guidelines proposed by the EWGSOP are set too high and are not sensitive enough for all older adult populations. Additionally, the proposed EWGSOP algorithm fails to break down key factors that may contribute to slower gait speed, such as cadence, step/stride length, and leg length. The way in which gait speed is altered in people with sarcopenia could be an important clinical measure that should be addressed by clinicians concerned with mobility. Finally, the current literature presents inconsistency in classifying gait impairments with cut-off values fluctuating between 0.8 and 1.0 m/s (see Table 5 in Cruz-Jentoft et al. 2010). The EWGSOP proposes that a gait speed of 0.8 m/s or less indicates a functional decline; however, fails to justify their use of 0.8 m/s as a measure for sarcopenia. Elble et al. (1991) observed that the mean walking speed for older adults (aged 74.7 ± 6.6) was 0.94 m/s, suggesting that 0.8 m/s is a considerably slow pace. While 0.8 m/s would identify older adults with marked mobility impairments, it may not be sensitive enough to predict the start of subtle functional declines.
The primary goal of the current work was to validate our previously proposed prediction model (which included sex, BMI, step time, and TOE; McIntosh et al. 2013); however when applied to the current population, the predictability of the variation of FFMI decreased by 25.3 %. This was likely due to the greater diversity and complexity of confounding health factors found within the older assisted-living population. The average age of our previously assessed community-dwelling population was 75.2 years (±5.7) and they were a considerably high functioning group (e.g., DGI score of 22 ± 4), whereas the current assisted-living population was 86.7 years (±5.7) of age and were arguably lower functioning (e.g., DGI score of 13 ± 5). Additionally, the current study had a small sample size of male participants (n = 10), which is common in this age demographic (Peebles and Norris 2003). Through comparison analysis, it became apparent that the two populations might need to be considered as separate entities. As such, our regression model evolved (purpose 2) to include a combination of predictor variables of FFMI that were best suited to an assisted-living population. Statistical analyses revealed that forearm circumference, BMI, grip strength, and variability (SD) within double support time during gait were the best predictors of FFMI. Although there are relatively few studies that validate the use of anthropometrics to estimate muscle mass and disability, we would argue that these are quick and simple measures that could easily be assessed by clinicians, in combination with other measures, to help predict sarcopenia. Grip strength is becoming a more frequently used tool and has recently been labeled as the best predictor for whole body strength and life longevity (Rantanen et al. 2012). Castillo et al. (2003) and Hausdorff et al. (2001) also reported that grip strength provides a better representation of muscle mass than tests involving knee strength. Furthermore, with the advancement of technology, we believed that the measurement of gait variability was reasonable to include in our prediction model geared towards clinicians. Simple gaming systems, such as the Microsoft Kinect, have been incorporated into motion capture studies and rehabilitation programs (Clark et al. 2013) and could easily be adapted by programmers as well as researchers and clinicians to measure variability within gait. The careful measurement and tracking of the predictor variables included in the current model should theoretically enable clinicians to predict and assess sarcopenia in an older adult population without the use of expensive body composition and biomechanical devices.
The largest limitation of this project was the small sample size. It is important to note that since a sample size calculation was not performed a priori as this study followed up on our previous work, which involved a population of 85 community-dwelling older adults. In the current work we hoped to recruit 10–12 participants from four different assisted-living facilities within our community (most house ~ 100 residents). We felt that this was possible/reasonable given our in/exclusion criterion and the nature of our goals for the project. Our small sample size does affect the direct applicably to predict FFMI and, subsequently, sarcopenia however, the results and implications of this analysis are important for future research on this topic. Future work will test this model in a larger more diverse population to reduce this limitation. Furthermore, the demographics of our current population may limit the predictability of the current equation to all ethnic groups and social classes of older adults. Although the current population was lower functioning than our previous community-dwelling population, participants could still be considered as high functioning simply as a result of our inclusion criteria. This is important to note, however, that differences between the populations were still observed. As previously mentioned, the lack of significance found in many of our functional measures may suggest that these tests were not sensitive enough to detect age-related changes in muscle mass. Future work will aim to use more dynamic measures to challenge this limitation.
Conclusion
The EWGSOP model started an important dialog between clinicians and researchers with a common interest in age-related muscle loss. Our work suggests that despite the strengths of this model, flaws may be present in detecting functional deficits among an assisted-living older adult population (i.e., gait speed). McIntosh et al. (2013) also found that subtle functional changes were not evident when using the definition in community-dwelling older adults. This suggests that the EWGSOP model is not quite sensitive enough for an older adult population who are undergoing numerous aging processes. We propose that a more diverse and multifaceted working definition is required for clinicians working with an older population. If early intervention is the ultimate goal, a more sensitive model is required to facilitate the detection of minor changes before major functional deficits occur. The reduced effectiveness of our previous prediction model is indicative of differences present between community-dwelling and assisted-living older adults, and suggests that the two populations need to be considered as separate entities in future studies. Although the current regression model predicts FFMI with a reduced degree of accuracy, it conveys the importance of using simple measurements that are readily available to clinicians to predict FFMI, and, subsequently, sarcopenia. Future work will aim to improve the accuracy of this model by involving a larger older adult test population and inclusion of more dynamic tasks that further challenge posture and balance. This ability to identify at-risk individuals prior to the onset of functional deficits will allow for early implementation of intervention strategies to attenuate, or possibly reverse, muscle loss and decrease falls risk.
Acknowledgments
We would like to thank our participants and staff from the Village of Riverside Glen (Kinesiologists Susan Brown, Jaimie Killbeck, Meghan McCutcheon, and Laura Cybulski), the Royal on Gordon (Kara Thomas), the Elliott Community (Paula Lannutti and Michelle Schefter), and Stone Lodge (Lambert Stillaway). We would also like to extend our appreciation to statistician Dr. Michelle Edwards, and to Dr. Lawrence Spriet for use of his BIA unit. Lastly, we would like to thank all of our volunteers for their help with data collection and entry and, in particular, Melissa Gurney for her help with data analysis. This work was financially supported by Ontario Neurotrauma Foundation Summer Internship (to T.M.C.; Grant No. 2011-PREV-INT-922).
Conflict of interest
The authors are unaware of any conflict of interest regarding this manuscript.
References
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Annweiler C, Bridenbaugh S, Assal F, Kressig RW, Herrmann FR. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55(6):702–706. doi: 10.1159/000235905. [DOI] [PubMed] [Google Scholar]
- Bioelectrical impedance analysis in body composition measurement: National institutes of health technology assessment conference statement. (1996). Am J Clin Nutr 64(3 Suppl): 524S- 532S [DOI] [PubMed]
- Bowley S. A hitchhiker's guide to statistics in plant biology. 2. Guelph: Ampersand Printing; 2008. [Google Scholar]
- Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25(3):226–231. doi: 10.1016/S0749-3797(03)00197-1. [DOI] [PubMed] [Google Scholar]
- Clark RA, Pua YH, Bryant AL, Hunt MA. Validity of the microsoft kinect for providing lateral trunk lean feedback during gait retraining. Gait Posture. 2013 doi: 10.1016/j.gaitpost.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Alexandre T, de Oliveira Duarte YA, Ferreira Santos JL, Wong R, Lebrao ML. Prevalence and associated factors of sarcopenia among elderly in Brazil: findings from the SABE study. J Nutr Health Aging. 2014;18(3):284–290. doi: 10.1007/s12603-013-0413-0. [DOI] [PubMed] [Google Scholar]
- Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, Cameron Chumlea W. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol. 2008;2(6):1139–1146. doi: 10.1177/193229680800200623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elble RJ, Thomas SS, Higgins C, Colliver J. Stride-dependent changes in gait of older people. J Neurol. 1991;238(1):1–5. doi: 10.1007/BF00319700. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- Hegeman J, Shapkova EY, Honegger F, Allum JH. Effect of age and height on trunk sway during stance and gait. J Vestib Res Equilib Orientat. 2007;17(2–3):75–87. [PubMed] [Google Scholar]
- Herman T, Inbar-Borovsky N, Brozgol M, Giladi N, Hausdorff JM. The dynamic gait index in healthy older adults: the role of stair climbing, fear of falling and gender. Gait Posture. 2009;29(2):237–241. doi: 10.1016/j.gaitpost.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ES, Karter AJ, Danielson KK, Warton EM, Ahmed AT. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med. 2010;25(2):141–146. doi: 10.1007/s11606-009-1179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannuzzi-Sucich M, Prestwood KM, Kenny AM. Prevalence of sarcopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol Ser A Biol Sci Med Sci. 2002;57(12):M772–M777. doi: 10.1093/gerona/57.12.M772. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Matthews RJ, Jagger C, Hancock RM. Does socio-economic advantage lead to a longer, healthier old age? Soc Sci Med (1982) 2006;62(10):2489–2499. doi: 10.1016/j.socscimed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- McIntosh EI, Smale KB, Vallis LA. Predicting fat-free mass index and sarcopenia: a pilot study in community-dwelling older adults. Age (Dordrecht Neth) 2013 doi: 10.1007/s11357-012-9505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parreira RB, Boer MC, Rabello L, Costa Vde S, de Oliveira E Jr, da Silva RA Jr (2013) Age-related differences in center of pressure measures during one-leg stance are time dependent. J Appl Biomech 29(3):312–6 [DOI] [PubMed]
- Peebles L, Norris B. Filling ‘gaps’ in strength data for design. Appl Ergon. 2003;34(1):73–88. doi: 10.1016/S0003-6870(02)00073-X. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordrecht Neth) 2012;34(3):563–570. doi: 10.1007/s11357-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Jones RJ, Vallis LA, Reed-Jones JG, Trick LM. The relationship between postural stability and virtual environment adaptation. Neurosci Lett. 2008;435(3):204–209. doi: 10.1016/j.neulet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Summary contents. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27(3):337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54(Suppl 3):S40–S47. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in caucasians aged 18-98 y. Int J Obes Relat Metab Disord J Int Assoc Study Obes. 2002;26(7):953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Gruber W, Baldwin M, Liao S. The effect of multidimensional exercises on balance, mobility, and fall risk in community-dwelling older adults. Phys Ther. 1997;77(1):46–57. doi: 10.1093/ptj/77.1.46. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- Smoliner C, Sieber CC, Wirth R. Prevalence of sarcopenia in geriatric hospitalized patients. J Am Med Dir Assoc. 2014;15(4):267–272. doi: 10.1016/j.jamda.2013.11.027. [DOI] [PubMed] [Google Scholar]
- Tinetti ME, Mendes de Leon CF, Doucette JT, Baker DI. Fear of falling and fall-related efficacy in relationship to functioning among community-living elders. J Gerontol. 1994;49(3):M140–M147. doi: 10.1093/geronj/49.3.M140. [DOI] [PubMed] [Google Scholar]
- Vernier (2012). Hand dynamometer. Retrieved August/10, 2012, from http://www.vernier.com/files/manuals/hd-bta.pdf
- Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- Waters DL, Baumgartner RN, Garry PJ. Sarcopenia: current perspectives. J Nutr Health Aging. 2000;4(3):133–139. [PubMed] [Google Scholar]