Abstract
Changes in the activities of FoxOs caused by phosphorylation, acetylation, or ubiquitination induce expressional changes in the genes involved in the modulation of oxidative stress by modifying histones and chromatins and can substantially alter cellular functions during aging and age-related diseases. However, the precise role that FoxO6, a novel member of the FoxO class of transcription factors, plays in the aging kidney has not been determined. The purpose of this study was to determine the role played by FoxO6 in the maintenance of redox homeostasis in HEK293T cells and aged kidney tissues isolated from ad libitum (AL)-fed and 40 % calorie restriction (CR) rats. The results obtained from AL-fed rats showed that diminished FoxO6 activity during aging was caused by FoxO6 phosphorylation, which disabled its transcriptional activity. In contrast, CR rats were found to have significantly higher FoxO6 activities and maintained redox balance. To determine the molecular mechanism responsible for FoxO6 modification by age-related oxidative stress, we examined H2O2-treated HEK293T cells in which FoxO6 was inactivated by phosphorylation and found that H2O2-induced oxidative stress promoted FoxO6 phosphorylation via PI3K/Akt signaling. The results of this study show that the protective role of FoxO6 in the aging process may in part be related to its ability to attenuate oxidative stress by upregulating catalase expression, as shown in CR. This delineation of the role of FoxO6 expands understanding of the pathological and physiological mechanisms of aging.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9679-3) contains supplementary material, which is available to authorized users.
Keywords: FoxO6, Phosphorylation, PI3K/Akt pathway, Aging, Caloric restriction, Oxidative stress
Introduction
Many recent investigations have demonstrated that FoxO transcription factors play key roles in the inductions of various downstream target genes, which include regulators of metabolism, cell cycle, cell death, and the oxidative stress response (Accili and Arden 2004). Furthermore, it has been established that in mammals, the FoxO (Forkhead transcription factor) family consists of the evolutionally highly conserved forkhead transcription factors, FoxO1, FoxO3a, FoxO4, and FoxO6 (Van der Heide et al. 2004).
FoxO family members also play important roles in the aging process (Chung et al. 2011), particular, by suppressing the generation of reactive oxygen species (ROS), although it should be noted that FoxO4 plays a detrimental role by upregulating the expressions of MAFbx and MURF1 during muscle aging (Clavel et al. 2006). The crucial roles played by the FoxO family in downstream of PI3K/Akt pathways that might affect insulin levels and be involved in oxidative stress-related diseases and the aging process are also well appreciated (Karger et al. 2009).
The FoxO is a key epigenetic mechanism in aging (Ni et al. 2012; Ribarič 2012). In response to insulin or oxidative stress, FoxO proteins are phosphorylated by protein kinase B (PKB, also known as Akt), a downstream kinase of phosphatidylinositol 3-kinase (PI3K), and this phosphorylation leads to the translocation of these proteins from the nucleus into the cytoplasm (Brunet et al. 1999). However, another epigenetic mechanism involves the posttranslational acetylations of FoxO3 and FoxO4 (Brunet et al. 1999) by cAMP-response element-binding protein (CREB)-binding protein (CBP). Furthermore, CBP triggers the transactivation functions of FoxO1 and FoxO4, whereas their acetylation by CBP attenuates their transcriptional activities (Brunet et al. 1999). In addition, FoxO4 has been shown to reduce cellular oxidative stress by directly increasing manganese superoxide dismutase (MnSOD) and catalase levels (Fukuoka et al. 2003).
Oxidative stress damages all cellular components, including protein, lipid, and DNA, and thereby, promotes cellular senescence (de Magalhães and Church 2006), compromises cell function, and threatens cell survival (Zanichelli et al. 2012). Thus, oxidative stress can disrupt the normal mechanisms of cellular signaling. Interestingly, intricate interactions between Akt and FoxO have been recently reported in the context of the mechanisms of cellular regulation. For example, in yeast, a mutation of Sch 9, which is homologous to Akt, extended lifespan (Fabrizio et al. 2001), and an insulin receptor mutation that decreased the activity of the insulin/IGF-1-like pathway has been reported to increase longevity in fruit flies (Tatar et al. 2001), mice (Bluher et al. 2003), and human (de Magalhães et al. 2012).
It is also interesting that these lifespan-extending mutations are associated with increased resistance to oxidative stress, which is partly mediated by the increased expression of antioxidant genes (Honda and Honda 1999). Other investigators have shown that PKB-regulated FoxO can reduce levels of cellular oxidative stress by directly increasing the messenger RNA (mRNA) and protein levels of MnSOD and catalase (Burgering and Medema 2003). However, little information is available on the status of FoxO6 during aging or of its modulation by various signaling factors and calorie restriction (CR), the gold standard of aging intervention.
Previous studies have shown that FoxO6 plays an important role in the regulation of hepatic glucose homeostasis in mice (Kim et al. 2011) and in the development of gastric carcinoma. Furthermore, Chung et al. (2013) reported that FoxO6 and PGC-1a form a regulatory loop that sets the level of oxidative metabolism in skeletal muscle. However, the role played by FoxO6 in aging has not been well defined.
CR has been shown to delay age-related biologic changes and to suppress a number of age-associated pathologic abnormalities, regardless of sex, in mammalian and nonmammalian species (Yu 2005). Furthermore, CR is known to suppress oxidative-related alterations and oxidative-induced age-related diseases and to extend lifespan (Stepanyan et al. 2006).
In the present study, to obtain a better understanding of the mechanism underlying the oxidative-dependent aging process, we investigated the effect of FoxO6 on oxidative stress in aged kidney. In addition, we explored FoxO6 phosphorylation and the process whereby PI3K/Akt signaling modulates FoxO6 activities during aging by studying HEK293T cells and aged kidney tissue isolated from ad libitum-fed (AL) and 40 % CR rats.
Materials and methods
Animals
Specific pathogen-free male Fischer 344 rats (6 or 24 months old) were obtained from Samtako (Osan, Republic of Korea) and fed a diet of the following composition: 21 % soybean protein, 15 % sucrose, 43.65 % dextrin, 10 % corn oil, 0.15 % α-methionine, 0.2 % choline chloride, 5 % salt mix, 2 % vitamin mix, and 3 % Solka-Floc fiber. The ad libitum (AL)-fed group had free access to both food and water, but animals in the CR were fed 60 % of the food intake of their AL-fed littermates, beginning at 6 weeks of age.
At prescribed times, rats were fed-conditioned and sacrificed by decapitation, and kidneys were quickly removed, rinsed in iced-cold buffer [100 mM Tris, 1 mM EDTA, 0.2 mM phenylmethyl-sulfonylfluoride (PMSF), 1 μM pepstatin, 2 μM sodium orthovanadate (pH 7.4)], immediately frozen in liquid nitrogen, and stored at −80 °C. The kidney is metabolically active and sensitive to many age-related changes including redox responsive molecular events, which make the kidney suitable for the study. In addition, our laboratory has substantial experience and data on age-related renal changes. The animal protocol used in this study was reviewed and approved by the Pusan National University-Institutional Animal Care and use Committee (PNU-IACUC).
Cell culture system
Human embryo kidney 293 T cells (HEK293T) cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) (Nissui Co., Tokyo) supplemented with 10 % heat-inactivated (56 °C for 30 min) fetal bovine serum (Gibco, Grand Island, NY), 233.6 mg/ml glutamine, 100 mg/ml penicillin streptomycin, 0.25 μg/ml amphotericin B, and 10 % heat-inactivated fetal bovine serum. Cells were maintained at 37 °C in a 5 % CO2 humidified atmosphere.
Materials
All chemical reagents were obtained from Sigma (St. Louis, MO, USA), except where noted. 2′,7′-Dichlorodihydrofluorescein was from Molecular Probes, Inc. (Eugene, OR, USA). Western blotting detection reagents were obtained from Amersham (Bucks, UK). RNAzolTM B was obtained from TEL-TEST, Inc. (Friendwood, TX, USA). Antibodies against FoxO1, p-FoxO1 (Thr24), catalase, MnSOD, β-actin, histone H1, p-Akt, and total-Akt were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against p-FoxO1 (Ser256) were obtained from Cell Signaling (New England BioLabs, Hertsfordshire, UK). Antibodies against FoxO6, p-FoxO6 (Ser184) and FoxO6-wt, FoxO6-siRNA, and CA-Akt virus were obtained from Dr. H.H. Dong (University of Pittsburgh, PA). Anti-rabbit IgG-horseradish peroxidase-conjugated antibody and anti-mouse IgG-horseradish peroxidase-conjugated antibody were from Amersham (Bucks, UK). Horseradish peroxidase-conjugated donkey anti-sheep/goat IgG was purchased from Serotec (Oxford, UK). Polyvinylidene difluoride (PVDF) membranes were obtained from Millipore Corporation (Bedford, MA, USA).
Nuclear extract preparation
Frozen rat kidney tissues (0.2–0.4 μg) were rinsed in PBS buffer and then transferred to a Dounce tissue grinder (Wheaton Manufacturers, NJ). Solution A (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol (DTT), 0.5 mM PMSF) was added at 2.5 ml/g tissue. Five strokes of pestle were used to homogenize tissue to a liquid mass. After the addition of NP-40 (0.5 %), five additional strokes of homogenization were performed. The homogenates were transferred to Eppendorf tubes and centrifuged in a microcentrifuge (Beckman) for 1 min.
The supernatant contained predominantly cytoplasmic constituents. To obtain a nuclear pellet, 400 μl of solution C (20 mM HEPES pH 7.9, 0.4 M NaCl, 1 mM of each of EDTA, EGTA, DTT, and PMSF) was added. Tubes were mixed thoroughly and placed on a small rotatory shaker for 15 min. Finally, the mixture was centrifuged at 12,000 rpm for 3 min in a microcentrifuge. Supernatants, which contained nuclear proteins, were then removed, transferred carefully to a fresh tube, and stored at −80 °C until required for Western blotting. Protein contents were determined using the bicinchoninic acid protein assay (Sigma, ST. Louis, MO, USA).
Western blotting
Western blotting was carried out as described previously (Kim et al. 2008). Homogenized samples were boiled for 5 min with a gel-loading buffer (125 mM Tris-HCl, 4 % sodium dodecyl sulfate (SDS), 10 % 2-mercaptoethanol, pH 6.8, 0.2 % bromphenol blue) at a ratio of 1:1. Total protein-equivalents for each sample were separated by SDS-polyacrylamide gel electrophoresis (PAGE) using acrylamide gels as described by Laemmli (1970) and transferred to a PVDF membrane at 15 V for 1 h in a semidry transfer system. The membrane was immediately placed into a blocking buffer (1 % nonfat milk) in 10 mM Tris, pH 7.5, 100 mM NaCl, and 0.1 % Tween-20. The blot was allowed to block at room temperature for 1 h. The membrane was incubated with specific primary antibody at 25 °C for 1 h, followed by a horseradish peroxidase-conjugated secondary antibody at 25 °C for 1 h. Antibody labeling was detected using enhanced chemiluminescence per the manufacturer’s instructions. Prestained protein markers were used for molecular weight determinations.
Transfection and luciferase reporter assay
Catalase activities were estimated using catalase-Luc vector (Dr. Dong, University of Pittsburgh, PA, USA) that contained a specific binding sequence for FoxO. Transfection was carried out using Lipofectamine 2000 (Invitrogen). Briefly, 1 × 104 cells per well were seeded in 48-well plates. When cultured cells reached about 40 % confluence, they were treated with 1 μg DNA/0.5 μl Lipofectamine 2000 complexes in 500 μl normal media (10 % serum contained) for 24 h and then treated with the virus of FoxO6 (100 multiplicity of infection (MOI)) and FoxO6-siRNA (100 MOI) 24 h after transfection. Subsequently, 100 μM of H2O2 was treated for 2 h, and the cells were washed with PBS and subjected to the Steady-Glo Luciferase Assay System (Promega, Madison, WI, USA). Luciferase activity was measured by a luminometer (GENious, TECAN, Salzburg, Austria).
Assay for insulin
Insulin levels were measured using a rat insulin ELISA kit (Shibayagi Co, Japan). Briefly, 100 μl of biotin-conjugated anti-insulin solution was added to 10 μl of sample per well. HRP-conjugated avidin solution and chromogenic substrate solution were then added. After incubation for 30 min at room temperature, 100 μl of reaction stopper was added. Optical densities were measured at 450 nm using a microplate reader (TECAN, Salzburg, Austria).
Reactive species (RS) scavenging activity
RS generation was measured as previously described (Kim et al. 2008) in tissue. One hundred and twenty-five micromolars of 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) was added to the homogenate with buffer for a final volume of 250 μl.
For the determination of intracellular RS generation activity, HEK293T cells were seeded in a 96-well plate. After 1 day, the medium was changed to a fresh, serum-free medium. The cells were treated with or without FoxO6-wt, FoxO6-siRNA, or CA-Akt virus and were pre-incubated for 1 day. After treatment with H2O2 (100 μM) for 2 h, the medium was replaced with a fresh, serum-free medium, and DCFDA (2.5 μM) was added. The fluorescence intensity of DCF was measured every 5 min for 1 h using the microplate fluorescence reader TECAN (Salzburg, Austria) with excitation and emission wavelengths of 485 and 535 nm, respectively.
Immunoprecipitation (IP) of nuclear extracts
Nuclear extracts were immunoprecipitated in a buffer containing 40 mM Tris-HCl (pH 7.6), 120 mM NaCl, 20 mM β-glycerophosphate, 20 mM NaF, 2 mM sodium orthovanadate, 5 mM EDTA, 1 mM PMSF, 0.1 % NP-40 containing leupeptin (2 μg/ml), aprotinin (1 μg/ml), and pepstatin A (1 μg/ml) (Kim et al. 2008). Aliquots of nuclear extracts were then precleared using a 50 % protein A agarose for 30 min at 4 °C. The nuclear extracts were centrifuged at 12,000 × g at 4 °C for 15 min, incubated overnight at 4 °C with the required antibody, and then incubated overnight at 4 °C with 50 % protein A agarose slurry. After washing the immunoprecipitates three times with the IP buffer, the immunoprecipitated proteins were analyzed by SDS-PAGE, and Western blotting analysis was performed as described above.
Immunostaining
HEK293T cells were seeded at 1 × 104 cells per well in a 12-well plate, incubated for 24 h, fixed in 4 % paraformaldehyde solution (15 min at room temperature), washed with PBS buffer, blocked with 3 % normal goat serum (Gibco, Grand Island, USA), and immunostained using rabbit anti-FoxO6 antibody (1:1,000 dilution, Santa Cruz, CA) at 4 °C overnight. Cells were then washed with TBS and incubated for 3 h in the presence of anti-rabbit IgG labeled with Alexa Fluor 488 (1:200; Invitrogen, CA, USA). Cell nuclei were visualized by immunostaining with Hoechst 33342 (1:1,000; Invitrogen), and FoxO6 was determined by confocal laser scanning microscopy (TCS SP2, Leica, Wetzler, Germany).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was used to study the interaction between FoxO6 and catalase promoter in HEK293T cells. Cells (1 × 105) were cultured in a medium supplemented with H2O2 with or without 100 MOI FoxO6 viruses. After incubation for 24 h, cells were cross-linked with 1 % formaldehyde and ultrasonicated (VCX-600 ultrasonicator; Sonics & Materials Inc., Danbury, CT) at 30 % of maximum power five times for 20 s. After centrifugation at 18,000 × g for 10 min, the supernatant was incubated with 5-μg polyclonal rabbit anti-FoxO6 antibody that was generated (Kim et al. 2011) and immunoprecipitated using a ChIP assay kit (Upstate Biotechnology, Lake Placid, NY). Immunoprecipitates were analyzed by PCR using catalase promoter-specific primers (GenBank accession number AY545477, 372 bp; forward 5′-GAGCTGAGAAAGCATAGCTATG-3′, reverse 5′-CAGCCAATCAGCACCACCCCC-3′).
Statistical analysis
ANOVA was conducted to analyze significant differences among all groups. Fisher’s protected LSD post hoc test was used to determine the significance between group means. Statistical significance was accepted at p value of <0.05.
Results
FoxO6 activity was downregulated by oxidative stress and aging
It has been reported that in mice, FoxO6 mRNA can be detected in the kidney, lung, and muscle in the fed condition, and that low levels are detectable in the liver (Kim et al. 2011). In the present study, we investigated FoxO6 function in aged kidney tissues.
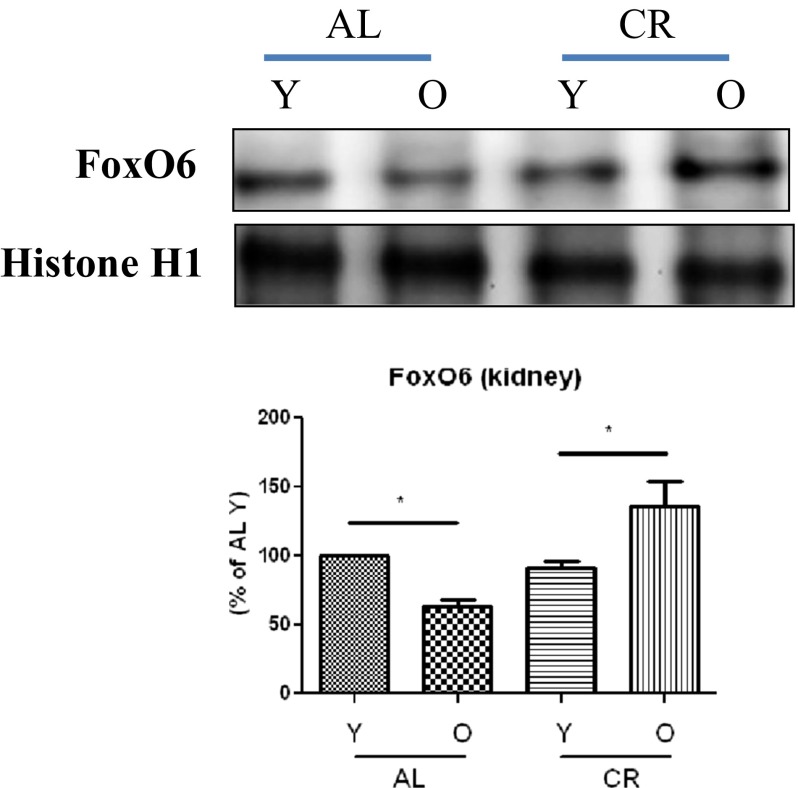
We found FoxO6 levels were lower in the kidney tissues of old (24 months) rats than in those of young (6 months) rats and that CR animals had higher FoxO6 levels than AL animals of the same age (Fig. 1).
Fig. 1.
Effects of FoxO6 on oxidative stress-induced aging and CR. Levels of FoxO6 in young and old, AL and CR rat kidney tissues as assessed by Western blotting (n = 6 in each group). One representative result for each protein is shown of three experiments that yielded similar results. Results of one-factor ANOVA: * p < 0.05 vs. young AL or CR rats. Y young (6-month-old), O old (24-month-old), AL ad libitum, CR calorie restriction
To identify the molecular events underlying H2O2-induced FoxO6 activation in HEK293T cells, Western blot analysis was used to examine the cytosolic translocation of FoxO6. When HEK293T cells were treated with 100 μM H2O2 in serum-free media for 0.5 to 8 h (Fig. 2a), nuclear FoxO6 protein levels were found to noticeably decrease and cytoplasmic FoxO6 levels were markedly increased 1 h after treatment. Furthermore, when HEK293T cells were treated with H2O2 at concentration from 50 to 1,000 μM for 1 h, FoxO6 protein showed a remarkable shift from the nucleus to the cytoplasm at concentrations above 50 μM (Fig. 2b). Moreover, RS levels were found to be upregulated in an H2O2 concentration-dependent manner (Fig. 2c). These results suggest that oxidative stress regulates FoxO6. In addition, immunostaining showed that treatment with 100 μM H2O2 induced the translocation of FoxO6 from the nucleus to the cytoplasm.
Fig. 2.
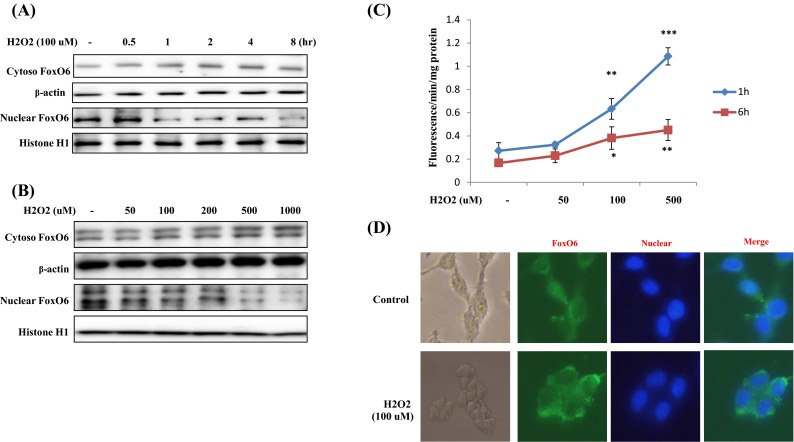
Translocation of FoxO6 by oxidative stress. a H2O2 (100 μM) time-dependently inhibited the activation of FoxO6. Western blot was used to detect FoxO6 in the cytoplasmic and nuclear extracts (20 μg protein) of HEK293T cells. b H2O2 dose-dependently inhibited the activation of FoxO6. Western blot was used to detect FoxO6 cytoplasmic and nuclear extracts (20 μg protein) of HEK293T cells. c DCFDA fluorescence intensities were measured after treating HEK293T cells with vehicle or H2O2 for 1 or 6 h. Results of one-factor ANOVA: *** p < 0.001; ** p < 0.01; * p < 0.05; vs. H2O2 untreated group, respectively. d Indirect immunohistochemistry findings showing the effect of FoxO6 on H2O2-induced subcellular localization in HEK293T cells
Effects of age and CR on FoxO6 phosphorylation
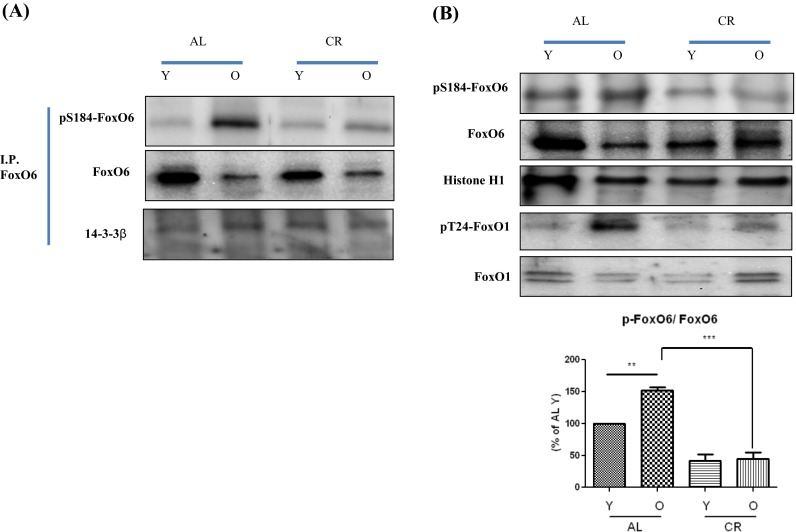
Because FoxO transcription factors play a central role in the regulation of stress response (Kim et al. 2005), we investigated their modifications during aging. As shown in Fig. 3a, FoxO6 phosphorylation increased as FoxO6 activity decreased during aging, but this imbalance was effectively counterbalanced by CR-induced dephosphorylation.
Fig. 3.
Increased FoxO6 phosphorylation and activation during aging and their inhibition by CR. a Nuclear extracts were prepared from young and old rat kidneys. Immunoprecipitation showed FoxO6 was physically associated with p-FoxO6 and 14-3-3β. b Western blot analyses for renal nuclear p-FoxO6 and FoxO6 were performed on nuclear proteins from AL and CR rats. The results shown are representative of three experiments. Results of one-factor ANOVA: ** p < 0.01 vs. young age AL rats; *** p < 0.001 vs. old age AL rats
Furthermore, FoxO6 phosphorylation levels were higher in old rats than in young rats, whereas CR animals had lower FoxO6 levels than AL animals of the same age (Fig. 3b). FoxO1 (Thr24) phosphorylation levels were also higher in old rats, and CR animals showed lower FoxO1 levels than the same aged AL animals (Fig. 3b). These results suggest similar roles for FoxO1 and FoxO6 during aging.
To identify the mechanism responsible for the inability of FoxO6 to undergo subcellular redistribution, we examined the association between FoxO6 and 14-3-3β, a scaffold protein known to bind FoxO and modify its transcriptional activity (Nielsen et al. 2008). We found increased association between FoxO6 and 14-3-3β during aging in the control animals and a decreased interaction between FoxO6 and 14-3-3β in the CR animals (Fig. 3a). These data indicate the incapability of FoxO6 to undergo aging-dependent interaction with 14-3-3β.
Changes in expression of FoxO6 target genes during aging and CR
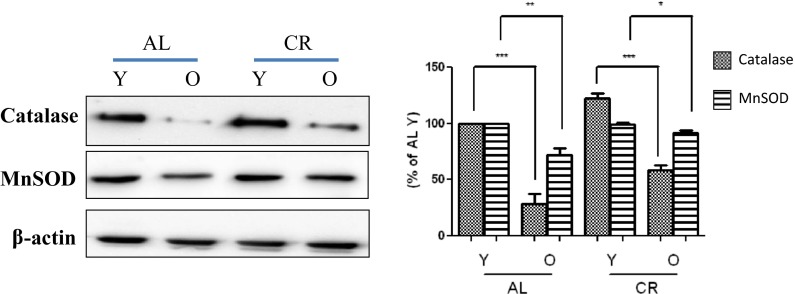
Other investigators have shown that Akt-regulated FoxO can reduce the level of cellular oxidative stress by directly increasing protein levels of MnSOD and catalase (Burgering and Medema 2003), which are the two major antioxidant enzymes responsible for protecting organisms against oxidative stress by reducing ROS. In the present study, the levels of antioxidant enzymes during aging and CR were analyzed by Western blotting. As shown in Fig. 4, although MnSOD and catalase levels decreased during aging, MnSOD levels were similar in the AL and CR groups. On the other hand, catalase protein levels were greater in the CR group. These observations indicate the importance of the role played by FoxO6 regarding antioxidant enzyme expression during aging.
Fig. 4.
Effects of age and CR on gene expressions of MnSOD, catalase, and FoxO6-dependent genes. Effects of age and CR on the expression of catalase and MnSOD. Western blotting was used to determine catalase and MnSOD protein levels in the kidney tissues of young (6-month-old) and old (24-month-old) AL and CR rats. The results shown are representative of three experiments. Results of one-factor ANOVA: *** p < 0.001 vs. Y-AL control; *** p < 0.001 vs. Y-CR control; ** p < 0.01 vs. age-matched AL rats; * p < 0.05 vs. age-matched CR rats. Y young, O old, AL ad libitum, CR calorie restriction
Modulation of insulin level, RS, and the PI3K/Akt signaling pathway by CR during aging
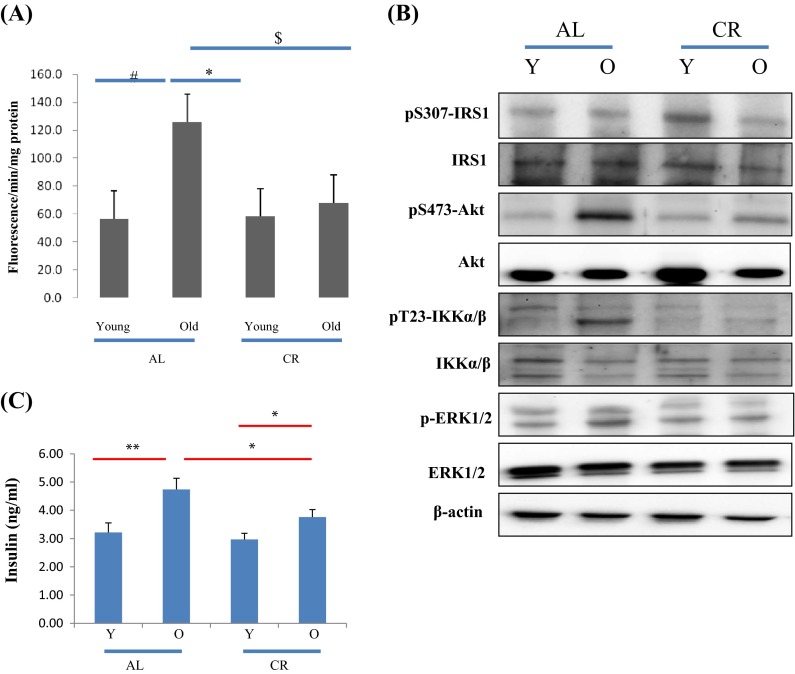
To assess overall oxidative status, total RS levels were measured in kidney tissue using a DCFDA probe, as evidence indicates that mammalian FoxO protects human cells from oxidative damage (Kops et al. 2002). In the present study, RS levels were found to be upregulated in rats and CR reduced this upregulation (Fig. 5a), indicating that CR acts to redress redox imbalance during aging.
Fig. 5.
Effects of age and CR on insulin signaling molecules. a RS generation in aged rats and the effects of aging and CR were investigated using DCFDA and kidney homogenates. Values are the mean ± SEs of five rats. Results of one-factor ANOVA: # p < 0.05 vs. young age AL rats; * p < 0.05 vs. old age AL rats. $ p < 0.05 vs. Old AL rats. b CR prevented age-related activation of the PI3K/Akt, IKK, and ERK pathways. Western blotting was used to determine total and phosphorylated Akt (Ser 473) levels in cytoplasmic extracts (20 μg protein) from AL or CR rats. c Levels of insulin in the serum of young and old AL or CR rat were measured using a chemical reagent kit (n = 6 per group). The results shown are representative of three experiments. Results of one-factor ANOVA: ** p < 0.01 vs. young AL rats; * p < 0.05 vs. old AL rats
In addition, we examined the signaling molecules that lead to Akt activation (via PI3K activation) by oxidative stress. To determine whether FoxO phosphorylation is induced by activation of the PI3K/Akt pathway, we examined phosphorylated Akt (the active form of Akt) levels. Although total Akt levels did not change, aging was found to increase Akt phosphorylation at Ser473 (Fig. 5), and CR suppressed this effect (Fig. 5b). These findings suggest that PI3K/Akt signaling, which is upregulated by increased oxidative stress, is associated with FoxO6 phosphorylation during aging and that CR inhibits these effects.
We also examined the expressions of key signals of Akt activation and insulin upregulation. Insulin levels were observed to increase during aging (Fig. 5c), and CR markedly reduced these increases (Fig. 5c). Furthermore, it is well established that insulin activates PI3K and its downstream target, Akt. These results suggest that PI3K/Akt signaling, which is upregulated by increased insulin levels and oxidative stress, is associated with FoxO6 phosphorylation during aging and that CR inhibits these effects.
Verification of enhanced FoxO6 phosphorylation in H2O2-treated HEK293T cells
We examined FoxO6 expression in HEK293T cells exposed to oxidative stress, by treating FoxO6-virus-treated cells with or without different concentrations (50 to 500 μM) of H2O2. As shown in Fig. 6a, treatment with 100 μM H2O2 induced a remarkable shift of FoxO6 from the nucleus to the cytoplasm. Furthermore, levels of catalase were increased by enhanced FoxO6 expression but reduced by adding H2O2 (Fig. 6b). In addition, we found that RS levels were significantly increased by H2O2 in the presence of empty vector and decreased by FoxO6 virus (Fig. 6c).
Fig. 6.
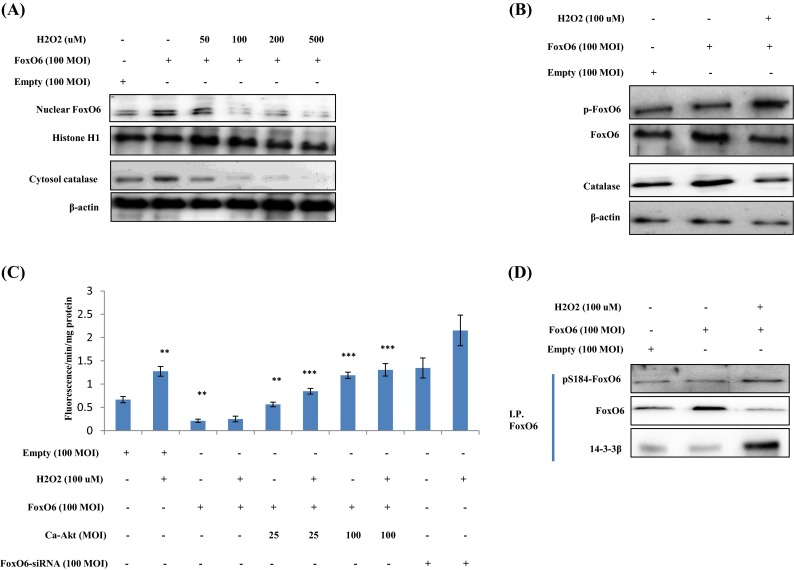
Enhancement of FoxO phosphorylation in H2O2-treated HEK293T cells. HEK293T cells were treated with H2O2 at various concentrations, and FoxO6 levels were determined by Western blotting. Samples loaded on sample gels were probed with β-actin and histone H1. a Levels of nuclear FoxO6 and cytoplasmic catalase were noticeably diminished after treatment with H2O2 at 50 to 500 μM. b Nuclear phosphorylated FoxO6 and catalase levels were noticeably decreased by 100 μM H2O2 in FoxO6-transfected (100 MOI) cells. c Quantitative analysis was performed by measuring DCFDA fluorescence after treating cells with vehicle or 100 μM H2O2 in the absence or presence of FoxO6 (100 MOI), CA-Akt (25, 100 MOI), or FoxO6-siRNA (100 MOI) for 1 day. Results of one-factor ANOVA: ** p < 0.01 vs. H2O2 untreated cells; *** p < 0.001 vs. H2O2-treated cells. d Immunoprecipitated FoxO6 was found to be physically associated with 14-3-3β by Western blotting
We also examined the effect of oxidative stress on FoxO phosphorylation. As shown in Fig. 6d, H2O2 enhanced FoxO6 phosphorylation at Ser184 and reduced unphosphorylated FoxO6 levels in H2O2-treated HEK293T cells. Phosphorylated FoxO6 is known to associate with 14-3-3β (a scaffold protein), which binds with FoxO6 and modifies its transcriptional activity (Kim et al. 2011), and thus, we generated an adenoviral vector expressing a constitutively active allele of FoxO6-CA by converting the conserved Akt phosphorylation site at Ser184 to Ala184 (Kim et al. 2011). However, it was found that FoxO6-CA did not change FoxO6 phosphorylation (Supp. 1) or H2O2 levels.
Regulation of FoxO6 activity by oxidative stress in HEK293T cells
The transcriptional activities of the FoxO family proteins have been reported to increase when insulin levels are reduced (Barthel et al. 2005). To examine the hypothesis that FoxO6 targets the catalase gene for transactivation, we examined the ability of FoxO6 to stimulate catalase expression in HEK293T cells. FoxO6 was shown to be associated with binding and activity to conserve H2O2 in the catalase promoter, as determined by luciferase (Fig. 7a) and the chromatin immunoprecipitation assay (Fig. 7b) in the empty vector and the FoxO6 virus-transduced HEK293T cells. FoxO6 associated with the catalase promoter DNA by FoxO6 virus cells, compared with the empty vector in HEK293T cells, enhanced the stimulatory effect of FoxO6 on catalase expression. This action was abolished in response to H2O2 in kidney cells (Fig. 7b). On the other hand, catalase promoter activity was reduced in FoxO6-siRNA-treated cells (Fig. 7a). It also reduced catalase levels in FoxO6-siRNA vector-transduced cells (Supp. 2). These observations support the idea that FoxO6 targets the catalase gene for transactivation and, thus, contributes to the regulation of oxidative stress.
Fig. 7.
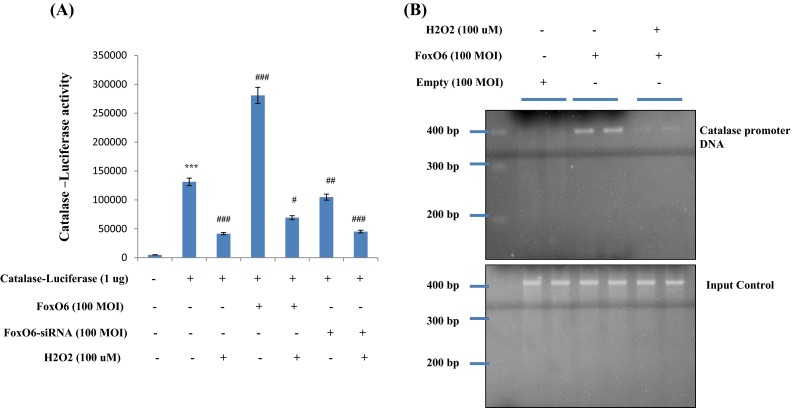
Activation of FoxO6 by oxidative stress in kidney cells. a HEK293T cells were transiently transfected with a catalase-containing plasmid linked to the luciferase gene, preincubated with FoxO6 (100 MOI) or FoxO6-siRNA (100 MOI) for 8 h, and then treated with H2O2 for 2 h. Results are presented in relative luminescence units (RLU). Results of one-factor ANOVA: *** p<0.001 vs. untreated control group, # p<0.05; ## p<0.01;### p<0.001 vs. reporter-only-transfected group. b FoxO6 bound to catalase promoter in HEK293T cells. Cells were transfected with catalase-Luc in the presence of FoxO6 vector at 100 MOI. After incubation for 24 h, cells were subjected to chromatin immunoprecipitation (ChIP) assay using rabbit pre-immune IgG (lanes 1 and 2), anti-FoxO6 antibody (lanes 3 and 4), and 100 μM H2O2 (lanes 5 and 6). Immunoprecipitates were subjected to PCR using the catalase promoter
Effect of oxidative stress on FoxO6 phosphorylation and the PI3K/Akt pathway
Several authors have suggested that members of the FoxO family are regulated by the PI3K/Akt pathway. More specifically, Akt (a key downstream effector of PI3K) is believed to phosphorylate FoxOs directly or to promote their phosphorylation by other kinases (Brunet et al. 1999). We examined the PI3K/Akt pathway and its effect on FoxO6 phosphorylation utilizing constitutively active Akt (CA-Akt) and measured FoxO6 phosphorylation by Akt. We found that FoxO6 reduction due to its phosphorylation increased when the concentration of Akt was increased (Fig. 8a).
Fig. 8.
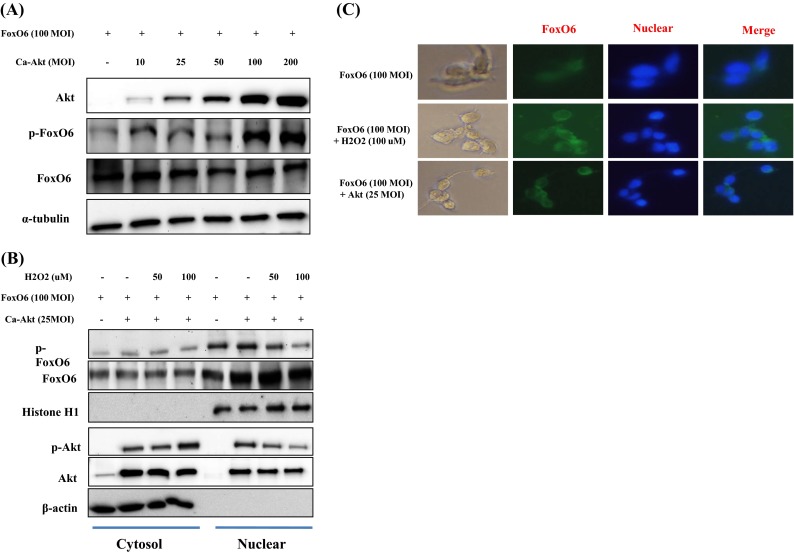
Activation of FoxO6 phosphorylation through the PI3K/Akt pathway by H2O2. HEK293T cells were grown to 80 % confluence in 100 mm dishes in DMEM medium, pretreated (1 day) with or without FoxO6 (100 MOI) plus CA-Akt (25 MOI), and then stimulated with 100 μM H2O2. a Cells were pretransduced with 100 MOI of FoxO6 vector in the absence or presence of Ca-Akt vector (10–200 MOI) and analyzed by Western blotting using antibody. b After stimulation with H2O2 (50 or 100 μM) in the absence or presence of FoxO6 plus CA-Akt (25 MOI), levels of phosphorylated FoxO6 were determined in the cell extract. c HEK293T cells were pretransduced with 100 MOI of FoxO6 vector with or without Ca-Akt vector (25 MOI) for 24 h and then treated with or without H2O2 (100 μM) for 1 h. Cells were immunostained using a rabbit anti-FoxO6 antibody, followed by IgG conjugated with fluorescein isothiocyanate (green). Bar = 100 μm
The nuclear and cytoplasmic levels of p-Akt have been reported in 97 and 100 %, respectively, of astrocytomas (El-Habr et al. 2010), and Chang et al. (2011) reported that Akt activity was suppressed by the dephosphorylation of Akt. Accordingly, we treated HEK293T cells with FoxO6 or CA-Akt (25 MOI) for 1 day, incubated them with 100 μM H2O2 for 1 h, and then assessed p-FoxO6 and p-Akt levels. As shown in Fig. 8b, in HEK293T cells, FoxO6 remained predominantly in the nucleus regardless of CA-Akt. These results indicate that the phosphorylation of FoxO6 in kidney cells is associated with the phosphorylation and nuclear accumulation of Akt.
Furthermore, immunostaining showed that FoxO6, although phosphorylated in response to H2O2, was localized in the cytoplasm in H2O2-treated HEK293T cells, as determined by immunostaining (Fig. 8c); otherwise, FoxO6 did not undergo a low (25 MOI) Akt-dependent nuclear exclusion. These findings indicate that H2O2 phosphorylates FoxO6 in an Akt-dependent manner and that this results in its cytoplasmic translocation.
Discussion
In the present study, we provide new evidence that the FoxO6 phosphorylation that occurs during aging can be prevented by CR. Our study also shows that serum insulin levels and ROS increase with age and that CR suppresses these increases. Changes in insulin level and its modulation are deemed significant because increased FoxO6 phosphorylation during aging is blunted by activated PI3K/Akt, the level of which is insulin dependent. As was expected, the insulin-suppressive action of CR prevented age-associated FoxO6 decrease by inhibiting the PI3K/Akt pathway (Fig. 5). Zemva et al. (2012) reported that FoxO6 mRNA levels are upregulated in aged mouse brain, which implies that the insulin/FoxO6 signaling pathway is involved in aging. However, clinical studies have failed to reveal any association between FoxO6 and life expectancy in human (Kleindorp et al. 2011). The most significant finding of this study is that decreased FoxO binding activity during aging is blunted by PI3K/Akt activation during aging under states of increased insulin and oxidative stress. As one might expect, our data on CR shown in Fig. 1 suggest that the oxidative stress-suppressive effect of CR prevents FoxO6 reduction by inhibiting the PI3K/Akt pathway.
The protective role of FoxO during aging has been suggested by several authors; for example, in one study, the loss of FoxO3 activity in explanted vascular smooth muscle of aged animals appeared to limit antioxidant properties in tissues by downregulating MnSOD and enhancing cell injury (Li et al. 2006). Yamaza et al. (2010) reported that FoxO1 also regulates some genes involved in cell cycle arrest, DNA repair, apoptosis, and stress reactions in response to oxidative stress in the liver. Nuclear FoxO1 activates the transcriptions of genes involved in stress response, including MnSOD, to counteract the effect of enhanced mitochondrial ROS production (Senapedis et al. 2011). Furthermore, the FoxO1-mediated transcriptions of antioxidant genes, such as MnSOD, are facilitated by the activation of FoxO1 via its deacetylation by Sirt1 (Jian et al. 2011). However, no previous study has investigated the role of FoxO6 during aging.
Initial clues that PI3K controls FoxO activity resulted from studies on the nematode, Caenorhabditis elegans. Genetic studies showed that PI3K suppresses the function of DAF-16 (a Forkhead transcription factor) (Lin et al. 1997), and it was subsequently found that PI3K is critical for the proper control of metabolism and cell survival (Birkenkamp and Coffer 2003). However, age-related interactions between PI3K and FoxO6 activities have not been fully explored (Zemva et al. 2012).
The aging process and ad libitum feeding of animals are well known to increase insulin and IGF-1 plasma levels (Coschigano et al. 2003). In response to insulin, FoxO proteins are phosphorylated by PKB, a downstream kinase of PI3K, and this phosphorylation leads to the translocation of these proteins from the nucleus to the cytoplasm (Brunet et al. 1999). The inhibition of FoxO and other related proteins through phosphorylation by Akt also has been reported (Tang et al. 1999). Moreover, Lin et al. (2004) reported that FoxO3a may have similar immunoregulatory functions in vivo. Protein kinase B (PKB/c-Akt) mediates many of the anti-apoptotic effects of PI3K signaling. However, although a large number of PKB substrates have been implicated in the regulation of cellular survival, little is known as to how PI3K/Akt signaling regulates cellular ROS levels. Earlier investigations have shown that Akt-regulated FoxO can reduce cellular oxidative stress by directly increasing mRNA and protein levels of MnSOD and catalase (Burgering and Medema 2003). In the present study, a similar situation was observed whereby Akt activation decreased catalase levels and, thus, probably increased cellular ROS.
Our results characterize FoxO6 as a novel transcription factor that plays an important function in the aged kidney, as was demonstrated by the modulation of FoxO6 by insulin signaling in cultured HEK293T cells. Furthermore, we found that the PI3K/Akt pathway was activated, FoxO6 was phosphorylated (Ser184), and target gene expressions were downregulated when these cells were exposed to H2O2 (Fig. 6). It is known that FoxO1 is regulated by cyclin-dependent kinases (CDK1/2) (Yuan et al. 2008); FoxO3 is phosphorylated by Ste20-like kinase (MST1) (Lehtinen et al. 2006); and FoxO4 is phosphorylated by stress-activated protein kinase (JNK), and that all of these processes result in relocalization from the nucleus to the cytoplasm (Oh et al. 2005). Kim et al. (2011) found that in liver cells, FoxO6 is mainly located in the nucleus in serum-containing media and that the nuclear localization of FoxO6 implies transcriptional activity under these conditions. On the other hand, FoxO6 was found not to be regulated by nucleocytoplasmic shuttling (Jacobs et al. 2003). Our observation that the cytosolic level of FoxO6 was slightly increased by H2O2 indicates the ability of FoxO6 to translocate; however, we also found that the FoxO6 protein was predominantly localized in the nucleus (Fig. 8c).
The present study provides additional information on FoxO6 transcriptional activity and reveals the phosphorylation of PI3K/Akt during aging. Furthermore, it suggests that these changes are closely related to age-related increases in insulin and oxidative stress levels. More importantly, in the context of aging, these age-related changes were found to be counterbalanced by the anti-aging effect of CR. Based on our in vivo and in vitro observations, we propose that the age-related phosphorylation of FoxO6 represses the expressions of catalase and MnSOD. Furthermore, we suggest that FoxO6 are regulated by PI3K/Akt activation induced by age-related oxidative stress, which in turn can be modulated by the anti-aging effect of CR.
Electronic supplementary material
Interactions of FoxO6 in HEK293T kidney cells. Nuclear extracts were prepared from young and old rat kidneys. Immunoprecipitated FoxO6 was found to be physically associated with p-FoxO6 and 14-3-3 β by Western blotting. (PPT 243 kb)
Catalase expression of FoxO6-dependent genes after FoxO6 knockdown. Western blot analysis was used to assess catalase protein levels in FoxO6-siRNA treated HEK293T cells. Results of one-factor ANOVA: ##p < 0.01, ###p < 0.001 vs. H2O2 nontreated cells. (PPT 352 kb)
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIP) (Grant no. 2009-0083538). We also take this opportunity to thank the Aging Tissue Bank (Busan, Republic of Korea) for supplying research materials.
Conflict of interest
The authors have no conflict of interest to disclose.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/S0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. doi: 10.1042/BST0310292. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Chang WH, Liu TC, Yang WK, Lee CC, Lin YH, Chen TY, Chang JG. Amiloride modulates alternative splicing in leukemic cells and resensitizes Bcr-AblT315l mutant cells to imatinib. Cancer Res. 2011;71:383–392. doi: 10.1158/0008-5472.CAN-10-1037. [DOI] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- Chung SY, Huang WC, Su CW, Lee KW, Chi HC, Lin CT, Chen ST, Huang KM, Tsai MS, Yu HP, Chen SL. FoxO6 and PGC-1a form a regulatory loop in myogenic cells. Biosci Rep. 2013;33:485–497. doi: 10.1042/BSR20130031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1, and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Aging Dev. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor 1 levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006;41:1–10. doi: 10.1016/j.exger.2005.09.002. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-environment interactions that modulate aging: powerful targets for drug discovery. Pharmacol Rev. 2012;64:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Habr EA, Tsiorva P, Theodorou M, Levidou G, Korkolopoulou P, Vretakos G, Petraki L, Michalopoulos NV, Patsouris E, Saetta AA. Analysis of PIK3CA and B-RAF gene mutations in human astrocytomas: association with activation of ERK and AKT. Clin Neuropathol. 2010;29:239–245. doi: 10.5414/NPP29239. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- Fukuoka M, Daitoku H, Hatta M, Matsuzaki H, Umemura S, Fukamizu A. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int J Mol Med. 2003;12:503–508. [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003;278:35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- Jian B, Yang S, Chen D, Chaudry I, Raju R. Influence of aging and hemorrhage injury on Sirt1 expression: possible role of myc-Sirt1 regulation in mitochondrial function. Biochim Biophys Acta. 2011;12:1446–1451. doi: 10.1016/j.bbadis.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karger S, Weidinger C, Krause K, Sheu SY, Aigner T, Gimm O, Schmid KW, Dralle H, Fuhrer D. FOXO3a: a novel player in thyroid carcinogenesis? Endocrinol Relat Cancer. 2009;16:189–199. doi: 10.1677/ERC-07-0283. [DOI] [PubMed] [Google Scholar]
- Kim HS, Skurk C, Maatz H, Shiojima I, Ivashchenko Y, Yoon SW, Park YB, Walsh K. Akt/FoxO3a signaling modulates the endothelial stress response through regulation of heat shock protein 70 expression. FASEB J. 2005;19:1042–1044. doi: 10.1096/fj.04-2841fje. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim JY, Yu BP, Chung HY. The activation of NF-kappaB through Akt-induced FOXO1 phosphorylation during aging and its modulation by calorie restriction. Biogerontology. 2008;9:33–47. doi: 10.1007/s10522-007-9114-6. [DOI] [PubMed] [Google Scholar]
- Kim DH, Perdomo G, Zhang T, Slusher S, Lee S, Phillips BE, Fan Y, Giannoukakis N, Gramignoli R, Strom S, Ringquist S, Dong HH. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774. doi: 10.2337/db11-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorp R, Flachsbart F, Puca AA, Malovini A, Schreiber S, Nebel A. Candidate gene study of FoxO1, FoxO4, and FoxO6 reveals no association with human longevity in Germans. Aging Cell. 2011;10:622–628. doi: 10.1111/j.1474-9726.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansn TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la lglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends lifespan. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Li M, Chiu JF, Mossman BT, Fukaqawa NK. Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J Biol Chem. 2006;281:40429–40439. doi: 10.1074/jbc.M606596200. [DOI] [PubMed] [Google Scholar]
- Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- Lin L, Hron JD, Peng SL. Regulation of NF-κB, Th activation, and autoinflammation by the Forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Ni Z, Ebata A, Alipanahiramandi E, Lee SS. Two SET domain containing genes link epigenetic changes and aging in Caenorhabditis elegans. Aging Cell. 2012;11:315–325. doi: 10.1111/j.1474-9726.2011.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 2008;7:688–699. doi: 10.1111/j.1474-9726.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribarič S. Diet and aging. Oxidative Med Cell Longev. 2012;2012:2012. doi: 10.1155/2012/741468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapedis WT, Kennedy CJ, Boyle PM, Silver PA. Whole genome siRNA cell-based screen links mitochondria to Akt signaling network through uncoupling of electron transport chain. Mol Biol Cell. 2011;22:1791–1805. doi: 10.1091/mbc.E10-10-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan Z, Hughes B, Cliché DO, Camp D, Hekimi S. Genetic and molecular characterization of CLK-1/mCLK1, a conserved determinant of the rate of aging. Exp Gerontol. 2006;41:940–951. doi: 10.1016/j.exger.2006.06.041. [DOI] [PubMed] [Google Scholar]
- Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999;274:16741–16746. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends lifespan and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Van der Heide LP, Hoekman MFM, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, Shimokawa I. FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell. 2010;9:372–382. doi: 10.1111/j.1474-9726.2010.00563.x. [DOI] [PubMed] [Google Scholar]
- Yu BP (2005) Calorie restriction as a potent anti-aging intervention: suppression of oxidative stress. In: Suresh Rattan (ed) Aging intervention and therapies, World Sci. Pub, p 193-217
- Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, Schaefer EM, Bonni A. Activation of FoxO1 by cdk1 in cycling cells and postmitotic neurons. Science. 2008;319:1665–1668. doi: 10.1126/science.1152337. [DOI] [PubMed] [Google Scholar]
- Zanichelli F, Capasso S, di Bernardo G, Cipollaro M, Pagnotta E, Carteni M, Casale F, Iori R, Giordano A, Galderisi U. Low concentrations of isothiocyanates protect mesenchymal stem cells from oxidative injuries, while high concentrations exacerbate DNA damage. Apoptosis. 2012;17:964–974. doi: 10.1007/s10495-012-0740-3. [DOI] [PubMed] [Google Scholar]
- Zemva J, Schilbach K, Stohr O, Moll L, Franko A, Krone W, Wiesner RJ, Schubert M. Central FoxO3a and FoxO6 expression is down-regulated in obesity induced diabetes but not in aging. Exp Clin Endocrinol Diabetes. 2012;120:340–350. doi: 10.1055/s-0032-1330107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Interactions of FoxO6 in HEK293T kidney cells. Nuclear extracts were prepared from young and old rat kidneys. Immunoprecipitated FoxO6 was found to be physically associated with p-FoxO6 and 14-3-3 β by Western blotting. (PPT 243 kb)
Catalase expression of FoxO6-dependent genes after FoxO6 knockdown. Western blot analysis was used to assess catalase protein levels in FoxO6-siRNA treated HEK293T cells. Results of one-factor ANOVA: ##p < 0.01, ###p < 0.001 vs. H2O2 nontreated cells. (PPT 352 kb)