Abstract
Aging is associated with decline in cardiovascular, autonomic function, and brain-derived neurotropic factor (BDNF). Reports are scanty regarding whether yoga can improve age-related degenerative changes in healthy active men. This study is designed to appraise the role of yoga in improving age-related degenerative changes in cardiometabolic risk profile, autonomic function, stress, and BDNF. Healthy active males of three age groups (20–29, 30–39, and 40–49 years) were randomly assigned to practice yoga daily 1 h for 3 months. Significantly higher values of heart rate (HR), blood pressure (BP), load in heart (DoP), myocardial oxygen consumption (RPP), and total cholesterol (TC) were noted in senior age group. HR, BP, DoP, RPP, and TC decreased significantly following yogic practice. High frequency (HF), total power (TP), all time domain variables of heart rate variability (HRV), and skin conductance (SC) were significantly decreased with advancement of age. HF, TP, and time domain parameters of HRV and SC increased significantly following yogic practice. Higher levels of catecholamines and low frequency (LF) power of HRV was noted with advancement of age. Levels of catecholamines and LF significantly decreased following yogic practice. Cortisol and adrenocorticotropic hormone (ACTH) level raised in senior age group. BDNF, serotonin, and dopamine were low in higher age group. Significant decrement of cortisol; ACTH; and increment in serotonin, dopamine, and BDNF was noted following yogic practice. This study revealed that yogic practices might help in the prevention of age-related degeneration by changing cardiometabolic risk factors, autonomic function, and BDNF in healthy male.
Keywords: Yoga, Aging, Cardiovascular system, Autonomic function, BDNF
Introduction
There are many theories to explain the aging phenomenon; among these most widely accepted biological theories are (1) wear and tear theory, (2) neuro-endocrine theory, (3) genetic control theory, (4) free radical theory, (5) mitochondrial theory, (6) waste accumulation theory, and (7) telomerase theory (Frisard and Ravussin 2006; Farley et al. 2006; Steen 2001; Burzynski 2005; Viña et al. 2007; Alexeyev et al. 2004; Terman 2006; Bekaert et al. 2005). The above-mentioned theories can explain physiological changes, which occurs with advancement of age. Cardiovascular aging in terms of decline in maximum heart rate, oxygen extraction, aerobic capacity, arterial stiffening, vasoconstriction, elevated systolic blood pressure, thickening of the left ventricle wall, reduced diastolic filling rate, impaired cardiac reserve, alterations in heart rate rhythm, and prolonged cardiac action potential were described earlier by different researcher (Landahl et al. 1986; Reardon and Malik 1996; Jensen-Ustad et al. 1997). Aging was found to be directly associated with structural and functional changes in autonomic nervous system (ANS). Changes were found in autonomic nerves and ganglia. Recording from sympathetic nerves of skeletal muscle also revealed significant changes. Researchers suggested that aging might enhance basal norepinephrine level and depress heart rate variability (Shimazu et al. 2005; Reardon and Malik 1996; Jensen-Ustad et al. 1997). Brain-derived neurotropic factor (BDNF), a potent marker of adult neurogenesis and cognitive performance, declines with aging (Erickson et al. 2010). Age-related changes in levels of cortisol, adrenocorticotropic hormone, dehydroepiandrosterone (DHEA) and of its sulfate (DHEAS), serotonin, and dopamine have been reported in literatures (Kuzina et al. 2010; Garau et al. 2006 and Yonezawa et al. 1989; Dreher et al. 2008). Electrical flux has beneficial role to prevent any wound- and age-related emotions and has negative correlation with age (Barontini et al. 1997). Early studies had implicated aging decrements in conditioning of skin conductance (SC) responses (Botwinick and Kornetsky 1960).
Physical training and/or regular moderate exercise has profound effect on nervous system, cardiovascular system, respiratory system, and metabolism (Leosco et al. 2013; Kirk-Sanchez and McGough 2014; Fahri 2010). Physical training is beneficial for physical and mental health improvement and it has rejuvenating effect on brain development in terms of moods, behavior, stress, anxiety, cognitive performance, levels of BDNF, and social interpersonal interactions (Leosco et al 2013; Kirk-Sanchez and McGough 2014; Tapp and Signorile 2014). Physical training or exercise is beneficial beyond doubt, but it needs more space and good health to perform exercise. Adverse environmental conditions like extreme cold, extreme heat, and rains limit outdoor exercise. Specific exercise can be performed with electronic devices at home or gym, which is costly. Furthermore, studies comparing the effects of yoga and exercise seem to indicate that, in both healthy and disease population, yoga may be as effective or better than exercise at improving a variety of health-related outcome measures such as heart rate variability (HRV; Bowman et al. 1997), cognitive performance (Gothe et al. 2013), blood glucose (Gordon et al. 2008; Singh et al. 2008), blood lipids (Singh et al. 2008), salivary cortisol (Smith et al. 2011), and oxidative stress (Sinha et al. 2007). Except all these, yoga is helpful for improving subjective measures of fatigue (Oken et al. 2004, 2006), pain, and sleep in healthy and ill population (Yurkuran et al. 2007). Yoga can improve cardiovascular system by decreasing heart rate and blood pressure (Selvamurthy et al. 1998). Yogic practice is helpful for improving heart rate variability components such as low frequency band (LF), high frequency band (HF), and LF/HF in healthy males and females (Huang et al. 2013; Papp et al. 2013). Hatha yoga practice may be associated with the promotion of neuroplasticity changes in brain systems such as increase gray matter volume (Froeliger et al. 2012) and levels of circulating BDNF in depressed patients (Naveen et al 2013). Yogic practice has advantageous role to modulate stress response systems by reducing perceived stress and anxiety (Smith et al. 2007).
Studies regarding the role of yoga on BDNF, catecholamines, and HRV on healthy active males has rarely been reported. In addition to that, the effect of yogic practice on age revealed degenerative changes in cardiometabolic risk factor, HRV, circulating BDNF, catecholamines, and stress hormones of physically active males has not been assessed earlier. We tested on healthy physically active population of different age groups a hypothesis that advancement of age has degenerative effects and yogic practice have beneficial role to improve age-related changes on cardiometabolic risk profile (i.e., body weight, body mass index, heart rate, blood pressure, load on the heart, myocardial oxygen consumption, and lipid profile), autonomic nervous system (i.e., HRV, epinephrine, and norepinephrine), circulating levels of BDNF, serotonin, dopamine, and stress hormones of physically active men.
Material and methods
Study volunteers
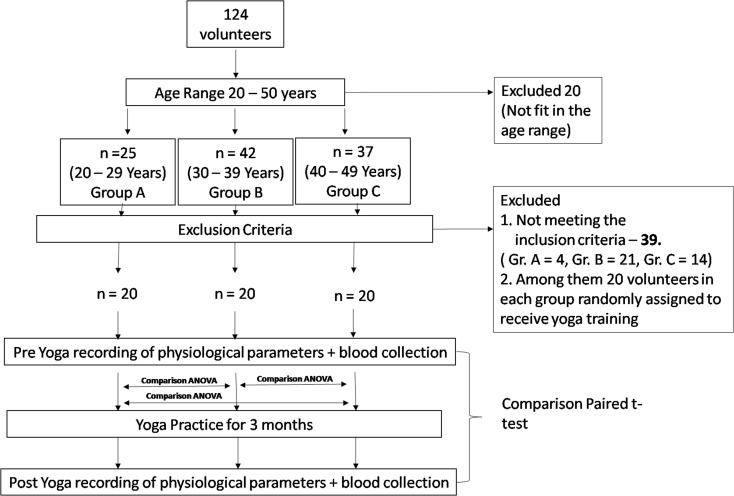
A total of 124 healthy physically active male volunteers of age ranged 20–50 years old were randomly selected and divided into three different age groups, viz. 20–29, 30–39, and 40–50 years old designated as groups A, B, and C, respectively. Twenty volunteers did not match with any of these age groups, so they were excluded from the study. Among 104 volunteers, groups A, B, and C were comprised of 25, 42, and 37 volunteers, respectively. Thirty-nine participants/volunteers did not match inclusion criteria. Among them, we randomly assign 20 volunteers in each group. Participant’s selection has been summarized in Fig. 1. Physical characteristics of the participants are summarized in Table 1. Participants were undergoing similar physical activity and food intake.
Fig. 1.
Schematic representation of volunteers distribution and parameters recording schedule
Table 1.
Physical characteristics of volunteers
| Parameters | 20–29 years (A) | 30–39 years (B) | 40–50 years (C) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Height (cm) | 171.5 ± 1.6 | 169.8 ± 1.0 | 170.1 ± 1.4 | |||
| Weight (kg) | 64.4 ± 1.4 | 65.6 ± 1.3 | 71.9 ± 1.5* | 71.3 ± 1.7 | 73.3 ± 1.6** | 72.5 ± 1.6 |
| BMI (kg/m2) | 22.0 ± 0.6 | 22.4 ± 0.6 | 24.9 ± 0.4*** | 24.7 ± 0.7 | 25.3 ± 0.5* | 25.1 ± 0.5 |
Data are expressed as mean±standard error of the mean (SEM)
BMI body mass index
*p < 0.01; **p < 0.001; ***p < 0.05; as compared to pre-exposure values of group A; one-way ANOVA followed was by Tukey–Kramer’s multiple comparison post hoc test were used to find out the difference between different age groups
Inclusion criteria included (1) normal healthy and physically active male; (2) absence of disease which could have contributed to obesity, hypertension, and neurological disorders; (3) not in medication; (4) no prior knowledge of yoga; and (5) smokers, alcoholics, and tobacco eaters were excluded from study.
Design of the study
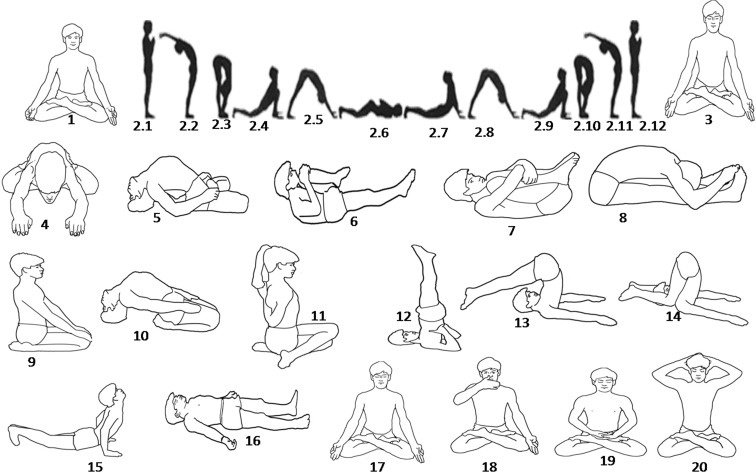
This is a longitudinal study of three different age groups. The study protocol has been summarized in Fig. 1. In addition to their routine activities, participants practiced yogasana, pranayama, and meditation daily for 1 h, 6 days a week, for a period of 3 months in the morning with ambient temperature under supervision of qualified yoga instructor. Yoga protocol has been summarized in Table 2 and Fig. 2. Anthropometric and physiological parameters of the participants were recorded in the field laboratory. Ambient room temperature and relative humidity varied from 22–24 °C and 40–50 %, respectively, during the period of anthropometric and physiological data collection and blood sample collection. Blood samples were collected in the morning after overnight fasting before and after 3 months yogic practice for biochemical estimation.
Table 2.
Details of yogic module
| Cleansing processes | Session (min) |
|---|---|
| Kapalbhati (Rapid shallow breathing) | 2 |
| Yogasanas (yogic postures) | |
| Suryanamaskar (sun salutation in 12 different postures, 1 round) | |
| Padmasana | 40 |
| Yogamudra | |
| Matsayasana | |
| Suptapavanmuktasana | |
| Pavanmuktasana | |
| Paschimottanasana | |
| Vajrasana | |
| Suptavajrasana | |
| Gomukhasana | |
| Sarvangasana | |
| Halasana | |
| Karnapedasana | |
| Bhujangasana | |
| Shavasana (relaxed supine posture) | |
| Pranayama (breathing exercises) | |
| Bhastrika (forceful expulsion of breathing) | 10 |
| Anulom-vilom (alternative nostril breathing) | |
| Bhramri (producing buzzing sound of bee with closed ear and lips) | |
| Meditation | |
| Omkar meditation (Om Chant) | 8 |
| Total session | 60 |
Fig. 2.
Various yoga postures as practiced by the volunteers. 1 Kapalbhati, 2 Suryanamaskar, 2.1 Pranamasana (pose 1), 2.2 Hasta Uttanasana (pose 2), 2.3 Pada hastasana (pose 3), 2.4 Asva Sancalanasana (pose 4), 2.5 Parvatasana (pose 5), 2.6 Astanga Namaskarasana (pose 6), 2.7 Bhujangasana (pose 7), 2.8 Parvatasana (pose 8), 2.9 Asva Sancalanasana (pose 9), 2.10 Pada hastasana (pose 10), 2.11 Hasta Uttanasana (pose 11), 2.12 Pranamasana (pose 12), 3 Padmasana, 4 Yogamudra, 5 Matsayasana, 6 Suptapavanmuktasana, 7 Pavanmuktasana, 8 Paschimottanasana, 9 Vajrasana, 10 Suptavajrasana, 11 Gomukhasana, 12 Sarvangasana, 13 Halasana, 14 Karnapedasana, 15 Bhujangasana, 16 Shavasana, 17 Bhastrika, 18 Anuloma-Viloma, 19 Meditation, 20 Bhramari
Anthropometric measurements
Anthropometric variables of the volunteers were measured with minimal clothing (only inner wears). Body weight (BW) in kilograms was recorded using an electronic weighing machine (Delmar, India) with least count of 0.1 kg. Standing height from the sole of the feet to the vertex in erect body position was measured in centimeters using anthropometric rod. Body mass index (BMI) was calculated as the ratio of weight to height in meter squared (kg/m2).
Resting physiological parameters
Resting heart rate and blood pressure
Resting heart rate (HR) and blood pressure (BP) was recorded using Omron automatic blood pressure monitor model HEM-7111 (Omron Healthcare Singapore PTE LTD. Singapore, Japan). Pulse pressure (PP) was calculated as difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP). Mean blood pressure (MBP) was calculated as DP + 1/3 PP. Double product (DoP) is an easily measurable index of load in the heart, and rate pressure product (RPP) is an easily measurable index of myocardial oxygen consumption, which were measured using heart rate and blood pressure (Gobel et al. 1978). RPP was calculated as SBP×HR. DoP was calculated as MBP×HR. The value of DoP was expressed as mmHg beats per minute (bpm) and value of RPP was expressed as mmHg bpm.
Resting heart rate variability
HRV was measured using Biograph Physiological Suite, from the raw electrocardiogram (ECG) recorded on Procomp® Infiniti Polygraph system (Thought Technology Limited, Canada) in fully resting and lying condition for 5 min after getting electrocardiographic baseline. R-R interval (time duration between two consecutive R waves of ECG) was used for HRV analysis. A careful manual editing was performed using Kubios HRV analysis 2.0 software (Biosignal Analysis and Medical Imaging Group, University of Eastern Finland, Finland) by visual inspection to mark the peaks. This was to remove artifacts as well as insert missing peaks or delete false peaks and artifacts. Analysis of the detected RR waveform was carried out in three domains—time domain, frequency domain, and nonlinear analysis. Kubios HRV calculates time domain matrices like mean of RR interval (mean RR), standard deviation of RR interval (SDNN), square root of mean of the sum of squares of different between adjustment RR interval (RMSSD), percent difference between adjacent RR intervals that are greater than 50 ms (pNN50), and frequency domain matrices like LF, HF, LF/HF, and total power (TP).
Skin conductance
SC was measured using biograph physiological monitoring suite, recorded on hardware Procomp® Infiniti Polygraph system (Thought Technology Limited) in fully resting and lying condition.
Biochemical estimation
Collection of samples
Blood samples of volunteers were collected from an antecubital vein in ethylene diamino tetra acetic acid (EDTA)-treated vial in morning in resting condition after overnight fasting. Volunteers were advised not to take alcohol or tobacco and smoke within 12 h prior to blood collection. The blood samples were centrifuged at 1,000 × g for 15 min to collect plasma. Samples were maintained at −20 °C at a field location and stored them at −80 °C until assayed in laboratory.
Lipid profile
Total cholesterol (TC), total triglyceride (TG), high-density lipoprotein-cholesterol (HDL-cholesterol) and low-density lipoprotein (LDL-cholesterol) were measured by using commercially available diagnostics kits (Giesse Diagnostics Snc, Italy). Intensity of color was measured using spectrophotometer (Model SmartSpec 3000, BIORAD, Hercules, CA, USA).
Epinephrine
Epinephrine (Epi) levels was measured from plasma using a Labor Diagnostika Nord kit (Cat No BA E-6200, Labor Diagnostika Nord Gmb & Co. KG, Nordhorn, Germany). Absorbance was read using microplate reader (Model Versamax, Molecular Device, Sunnyvale, CA, USA).
Norepinephrine
Norepinephrine (NE) levels were measured from plasma using a Labor Diagnostika Nord kit (Cat No BA E-6200, Labor Diagnostika Nord Gmb & Co. KG). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Dopamine
Dopamine was measured from plasma using Labor Diagnostika Nord kit (Cat No BA E-6200, Labor Diagnostika Nord Gmb & Co. KG). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Serotonin
Serotonin was measured from plasma using Labor Diagnostika Nord kit (Cat No BA E-6200, Labor Diagnostika Nord Gmb & Co. KG). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Cortisol
Cortisol was measured in plasma using the Cayman Kit (Cat No. 500360, Cayman Chemical Laboratories, USA). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Adrenocorticotropic hormone
Adrenocorticotropic hormone (ACTH) was estimated from plasma using DRG ELISA kit (cat No. EIA-3647, DRG International Inc, USA). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Brain-derived neurotropic factor
BDNF was measured from plasma using the Syd Lab ELISA kit (Cat No EK000004-EK0307, Syd Lab Inc, Boston, MA, USA). Absorbance was read using microplate reader (Model Versamax, Molecular Device).
Statistical analysis
Linear regression was carried out to evaluate the correlation between age and other variables. Significant level was set at p value of <0.05. All intergroup comparisons were done by one-way ANOVA. When significant differences were found, Tukey–Kramer’a multiple comparisons tests were done as post hoc analysis. Comparison of data of before and after yogic practice in each group was made using paired “t” test. All the statistics was performed using SPSS version 17.0 for windows (SPSS Software, IBM Corporation, USA). Data shown in the tables are mean±standard error of mean (SEM).
Results
Anthropometric parameters
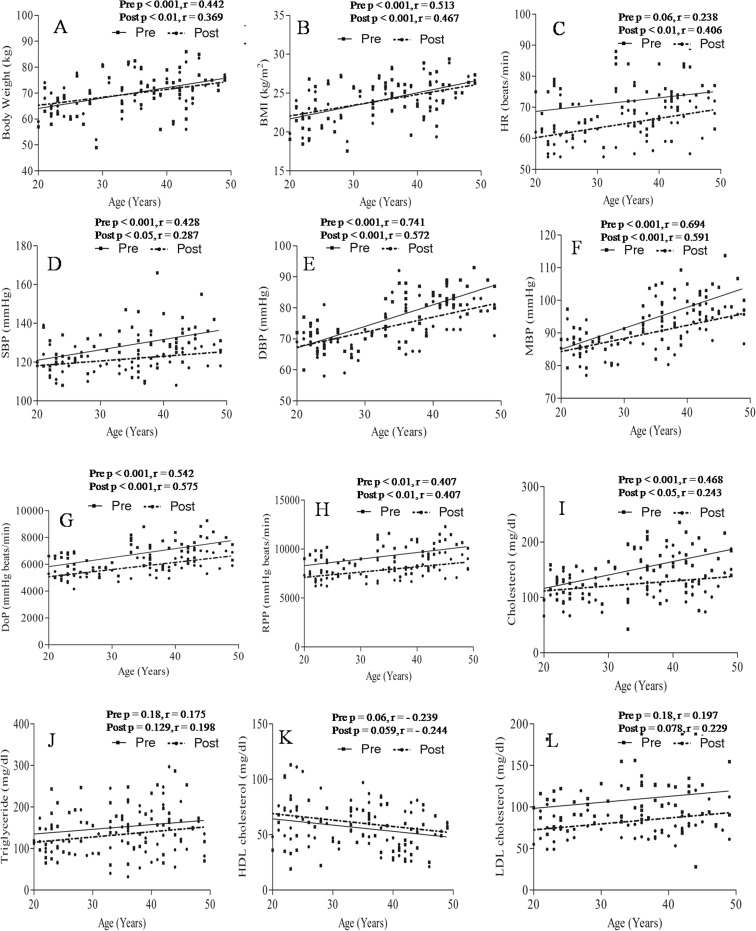
Significant positive correlation was found between BW and age before (p < 0.001, r = 0.442) and after (p < 0.01, r = 0.369) yogic practice (Fig. 3a). BW was significantly higher in groups B (p < 0.01) and C (p < 0.001) as compared to group A (Table 1). Significant positive correlation was found between BMI and age before (p < 0.001, r = 0.513) and after (p < 0.001, r = 0.467) yogic practice (Fig. 3b). BMI was significantly higher in groups B (p < 0.05) and C (p < 0.01) as compared to group A (Table 1). BW and BMI did not show any significant change following yogic practices in all groups (Table 1).
Fig. 3.
Correlation between age with anthropometric parameters and metabolic risk factors before and after yogic practice. a Correlation of age with body weight, b correlation of age with body mass index, c correlation of age with heart rate, d correlation of age with systolic blood pressure, e correlation of age with diastolic blood pressure, f correlation of age with mean blood pressure, g correlation of age with double product, h correlation of age with rate pressure product, i correlation of age with total cholesterol, j correlation with triglceride, k correlation of age with high-density lipoprotein cholesterol, and l correlation of age with low density lipoprotein cholesterol
Resting physiological variables and cardiometabolic risk factors
HR did not show any significant correlation with age before (p = 0.06, r = 0.238) yogic practice where as it was positively correlated with age after (p < 0.01, r = 0.406) yogic practice as shown in Fig. 3c. HR reduced significantly (p < 0.01) following yogic practice in each age group as depicted in Table 3. SBP showed a significant positive correlation with age before (p < 0.001, r = 0.428) and after (p < 0.05, r = 0.287) yogic practice (Fig. 3d). SBP was significantly (p < 0.01) higher in group C as compared to group A, and SBP reduced significantly (p < 0.05) in groups B and C following yogic practice (Table 3). Significant positive correlation was found between DBP and age before (p < 0.001, r = 0.741) and after (p < 0.001, r = 0.572) yogic practice (Fig. 3e). DBP was significantly (p < 0.001) higher in groups B and C as compared to group A, and it was significantly (p < 0.05) more in group C as compared to group B (Table 3). DBP decreased significantly (p < 0.01) following yogic practice in group C (see Table 3). MBP showed significantly positive correlation with age before (p < 0.001, r = 0.694) and after (p < 0.001, r = 0.591) yogic practice (Fig. 3f). MBP was significantly (p < 0.01) higher in group C as compared to group B (Table 3). Significant reduction of MBP was noted in groups B (p < 0.01) and C (p < 0.01) following yogic practice (Table 3). A significant positive correlation was noted between DoP and age before (p < 0.001, r = 0.542) and after (p < 0.001, r = 0.575) yogic practice (Fig. 3g). DoP was significantly higher in group C as compared to group A (p < 0.001) and as compared to group B (p < 0.05; see Table 3). DoP decreased significantly following yogic practice in groups A (p < 0.01), B (p < 0.001), and C (p < 0.001) as shown in Table 3. Significant and positive correlation was found between RPP and age before (p < 0.01, r = 0.407) and after (p < 0.01, r = 0.407) yogic practice (Fig. 3h). RPP was significantly (p < 0.001) higher in group C as compared to group A, and RPP was reduced significantly in groups A (p < 0.05), B (p < 0.001), and C (p < 0.001) following yogic practice (Table 3). Levels of TC were significantly higher in groups B (p < 0.01) and C (p < 0.001) as compared to group A (Table 3). Levels of TC decreased significantly following yogic practice in groups B (p < 0.05) and C (p < 0.01; see Table 3). Significant positive correlation was found between age and levels of TC before (p < 0.001, r = 0.468) and after (p < 0.05, r = 0.243) yogic practice (Fig. 3i). The present study did not establish any significant correlation between age with TG, HDL-cholesterol, and LDL-cholesterol before and after yogic practice (see Fig. 3j–l); insignificant change was found in the TG and HDL-cholesterol levels following yogic practice of the different age groups. Levels of LDL-cholesterol decreased significantly in groups A (p < 0.01), B (p < 0.01), and C (p < 0.05) following yogic practice (Table 3).
Table 3.
Effect of yogic practice on cardio metabolic risk factors of different age groups
| Parameters | 20–29 years (A) | 30–39 years (B) | 40–50 years (C) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| HR (beats/min) | 69.0 ± .46 | 61.6 ± 1.33** | 71.3 ± 1.97 | 64.6 ± 1.58 | 72.2 ± 1.61 | 67.9 ± 1.18* |
| SBP (mmHg) | 122.6 ± 1.60 | 119.5 ± 1.68 | 129.5 ± 2.95 | 120.8 ± 1.60** | 133.6 ± 1.8¥¥ | 124.6 ± 1.43*** |
| DBP (mmHg) | 69.3 ± 0.99 | 68.1 ± 1.15 | 77.6 ± 1.50¥¥¥ | 75.7 ± 1.57 | 84.4 ± 0.89¥¥¥ϕ ϕ | 79.1 ± 0.97** |
| MBP (mmHg) | 87.0 ± 0.95 | 85.2 ± 1.00 | 95.0 ± 1.66¥¥¥ | 90.7 ± 1.26** | 100.8 ± 1.03¥¥¥ϕ ϕ | 94.3 ± 0.73*** |
| DoP (mmHg bpm) | 6,005.6 ± 145.9 | 5,254.3 ± 139.5 | 6,783.4 ± 234.3 | 5,858.2 ± 170.5 | 7,572.8 ± 180.7¥¥¥ϕ | 6,395.4 ± 125.1*** |
| RPP (mmHg bpm) | 8,462.8 ± 223.2 | 7,365.3 ± 200.3 | 9,269.5 ± 344.8 | 7,791.9 ± 203.5 | 10,051.02 ± 271.6¥¥¥ | 8,455.7 ± 191.0 *** |
| TC (mg/dl) | 119.4 ± 5.14 | 112.0 ± 6.34 | 162.3 ± 10.00¥¥ | 128.9 ± 6.60* | 171.7 ± 9.88¥¥¥ | 132.6 ± 7.48** |
| TG (mg/dl) | 141.0 ± 12.42 | 119.3 ± 8.00 | 146.7 ± 11.23 | 131.0 ± 12.54 | 165.4 ± 13.4 | 148.9 ± 14.74 |
| HDL-C (mg/dl) | 61.7 ± 5.97 | 66.9 ± 4.49 | 60.8 ± 3.90 | 63.5 ± 4.24 | 46.0 ± 3.17 | 51.5 ± 4.35 |
| LDL-C (mg/dl) | 99.6 ± 5.64 | 75.0 ± 4.12** | 111.1 ± 5.01 | 84.3 ± 6.88** | 115.4 ± 9.29 | 88.6 ± 6.38* |
Values are expressed as mean±SEM
HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, MBP mean blood pressure, DoP double product, RPP rate pressure, product and bpm beats per minute, TC total cholesterol, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
*p < 0.05; **p < 0.01; and ***p < 0.001; comparisons were made between initial base line values and the values obtained after yogic practice of the respective group using paired “t” test. ¥¥ p < 0.01 and ¥¥¥ p < 0.001, as compared to pre-exposure values of group A; one-way ANOVA followed by Tukey–Kramer’s multiple comparison post hoc test was used to find out the difference between different age groups. ϕ p < 0.05; ϕϕ p < 0.01; as compared to pre-exposure values of group B; one-way ANOVA followed by Tukey–Kramer’s multiple comparison post hoc test were used to find out difference between different age groups
Autonomic function, heart rate variability, and skin conductance
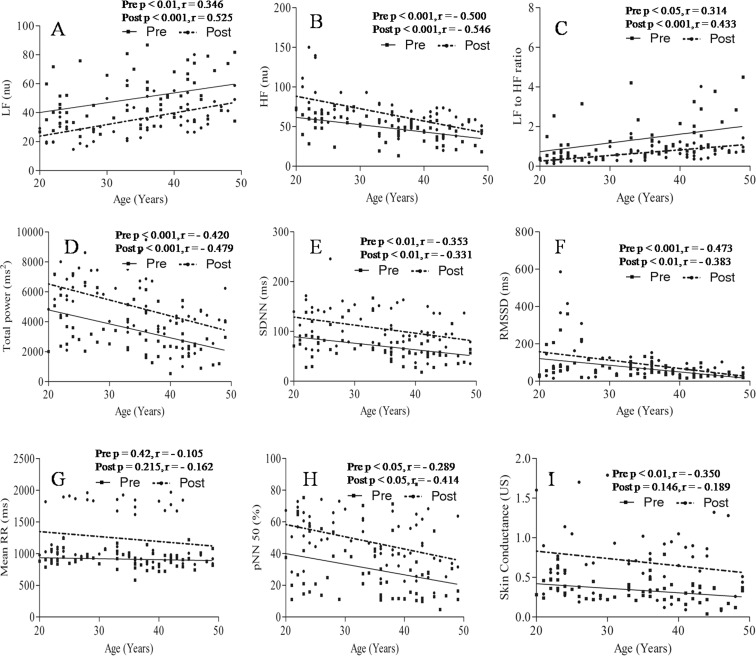
Significant positive correlation was found between age and low frequency component of HRV (LF) before (p < 0.01, r = 0.346) and after (p < 0.001, r = 0.525) yogic practice (Fig. 4a). LF was found significantly (p < 0.01) higher with advancement of age in group C as compared to group A (Table 4). It was decreased significantly in groups A (p < 0.001), B (p < 0.01), and C (p < 0.01) following yogic practice (Table 4). High-frequency component of HRV (HF) showed a significant negative correlation with age before (p < 0.001, r = −0.500) and after (p < 0.001, r = −0.546) yogic practice (Fig. 4b). HF was significantly low in the higher age group, group C (p < 0.001) while compared with group A. It was increased significantly in groups A (p < 0.001), B (p < 0.05), and C (p < 0.05) following yogic practice (Table 4). The ratio of the low- to high-frequency components of HRV (LF/HF) showed a significant positive correlation with age before (p < 0.05, r = 0.314) and after (p < 0.001, r = 0.433) yogic practice (Fig. 4c). LF/HF was significantly (p < 0.05) higher in group C as compared to group A, and LF/HF was found to be decreased significantly (p < 0.05) in groups A, B, and C following yogic practice (Table 4). Significant negative correlation was found between age and total power of HRV before (p < 0.001, r = −0.420) and after (p < 0.001, r = −0.479) yogic practice (Fig. 4d). It was significantly (p < 0.01) lower in group C as compared to group A (Table 4). Total power increased significantly (p < 0.01) in groups A, B, and C following yogic practice (Table 4). Significant negative correlation was found between age and SDNN before (p < 0.01, r = −0.353) and after (p < 0.01, r = −0.331) yogic practice (Fig. 4e). It was increased significantly (p < 0.01) in all the age groups following yogic practice as shown in Table 4. Significant negative correlation was found between age and RMSSD before (p < 0.001, r = −0.473) and after (p < 0.01, r = −0.383) yogic practice (Fig. 4f). Values of RMSSD increased significantly (p < 0.05) in groups B and C, but there was insignificant increment of 32.02 % in group A following yogic practice (Table 4). Negative and insignificant correlation was found between mean RR with age before (p = 0.42, r = −0.105) and after (p = 0.215, r = −0.162) yogic practice (Fig. 4g). It increased significantly in groups A (p < 0.001), B (p < 0.01), and C (p < 0.05) following 3 months of yogic practice as shown in Table 4. Significant negative correlation was found between age and pNN50 before (p < 0.05, r = −0.289) and after (p < 0.05, r = −0.414) yogic practice (Fig. 4h). It increased significantly in groups A (p < 0.001), B (p < 0.05), and C (p < 0.05) following yogic practice (Table 4). Electromagnetic activity was measured in terms of SC, which was significantly and negatively correlated (p < 0.01, r = −0.350) with age before yogic practice (Fig. 4i). Insignificant negative correlation (p = 0.146, r = −0.189) was found between age and SC after yogic practice (Fig. 4i). It increased significantly following yogic practice in groups A (p < 0.01), B (p < 0.01), and C (p < 0.001) as shown in Table 4.
Fig. 4.
Correlation between age with heart rate variability (HRV) before and after yogic practice. a Correlation of age with low-frequency component (LF) of HRV, b correlation of age with high-frequency components (HF) of HRV, c correlation of age with LF/HF, d correlation of age with total power, e correlation of age with SDNN, f correlation of age with RMSSD, g correlation of age with mean RR, h correlation of age with pNN50, i correlation of age with skin conductance
Table 4.
Effect of yogic practice on cardio metabolic risk factors of different age groups
| Parameters | 20–29 years (A) | 30–39 years (B) | 40–50 years (C) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| HF (nu) | 58.7 ± 3.29 | 84.2 ± 6.56** | 47.5 ± 3.12 | 57.2 ± 3.58 | 38.9 ± 2.78¥¥¥ | 55.9 ± 3.27*** |
| LF (nu) | 41.5 ± 3.29 | 25.5 ± 1.59*** | 51.4 ± 3.57 | 38.6 ± 2.73** | 56.7 ± 3.69¥¥ | 41.7 ± 2.95** |
| LF/HF | 0.7 ± 0.15 | 0.3 ± 0.03 | 1.1 ± 0.32 | 0.7 ± 0.08 | 1.5 ± 0.23¥ | 0.7 ± 0.17* |
| Total power (ms2) | 4,371.9 ± 459.4 | 6,208.7 ± 325.0** | 3,548.4 ± 422.3** | 5,152.2 ± 422.4 | 2,421.2 ± 257.4¥¥ | 3,590.8 ± 311.4** |
| Mean RR (ms) | 984.9 ± 23.38 | 1,322.1 ± 95.07*** | 903.2 ± 33.0 | 1,209.2 ± 87.8** | 897.0 ± 27.53 | 1,171.2 ± 95.4* |
| SDNN (ms) | 84.2 ± 7.18 | 120.7 ± 10.59** | 72.4 ± 7.33 | 107.5 ± 8.42** | 54.9 ± 5.17 | 88.1 ± 7.68** |
| RMSSD (ms) | 111.0 ± 20.52 | 146.6 ± 34.91 | 61.1 ± 7.22 | 79.6 ± 7.38* | 36.4 ± 4.52 | 52.7 ± 5.63* |
| pNN50 (%) | 36.2 ± 4.69 | 56.8 ± 2.98*** | 31.9 ± 4.08 | 44.0 ± 3.18** | 22.9 ± 4.15 | 40.8 ± 3.66* |
| SC (US) | 0.4 ± 0.03 | 0.74 ± 0.01** | 0.35 ± 0.03 | 0.71 ± 0.09** | 0.27 ± 0.02 | 0.64 ± 0.08*** |
Values are expressed as mean±SEM
HF (nu) high-frequency power (numeric unit), LF (nu) low-frequency power (numeric unit), LF/HF ratio of low to high frequency, SDNN standard deviation of all NN interval, RMSSD square root of the mean of the sum of the squares of different between adjustment NN interval, pNN50 percent difference between adjuscent NN intervals that are greater than 50 ms, SC skin conductance
*p < 0.05; **p < 0.01; and ***p < 0.001; comparisons were made between initial baseline values and the values obtained after yogic practice using paired “t” test. ¥ p < 0.05; ¥¥ p < 0.01; and ¥¥¥ p < 0.001, as compared to pre exposure values of group A; one-way ANOVA followed by Tukey–Kramer’s multiple comparison post hoc test were used to find out the difference between different age groups
Catecholamines, serotonin, and stress hormones
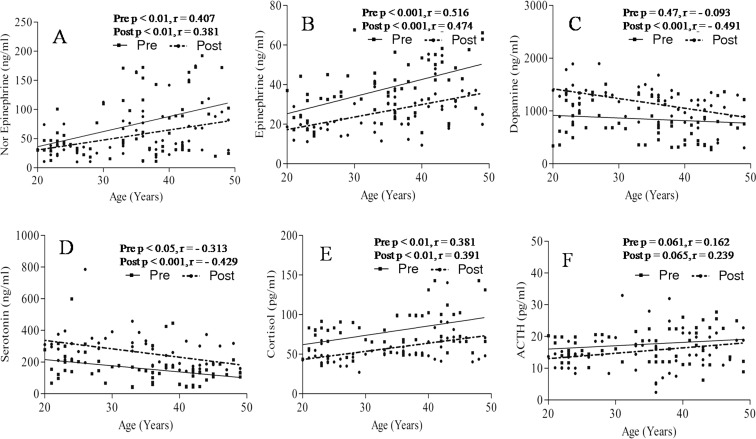
Levels of norepinephrine showed a significant positive correlation with age before (p < 0.01, r = 0.407) and after (p < 0.01, r = 0.381) yogic practice (Fig. 5a). Levels of norepinephrine were significantly (p < 0.01) higher in group C as compared to group A (Table 5). There was insignificant reduction of 22.42 and 22.16 % in norepinephrine level in groups A and B, respectively, following yogic practice (Table 5). Significant reduction (p < 0.05) of levels of norepinephrine was observed in group C following yogic practice (Table 5). Significant positive correlation was found between levels of epinephrine and age before (p < 0.001, r = 0.516) and after (p < 0.001, r = 0.474) yogic practice (Fig. 5b). Levels of epinephrine were significantly (p < 0.001) higher in group C as compared to group A (Table 5). Levels of epinephrine decreased significantly following yogic practice in groups A (p < 0.05), B (p < 0.05), and C (p < 0.01) as shown in Table 5. No correlation was observed between dopamine and age before yogic practice (p = 0.47, r = −0.093) as shown in Fig. 5c. Significant negative correlation (p < 0.001, r = −0.491) was found between levels of dopamine and age after yogic practice (Fig. 5c). It increased significantly in groups A (p < 0.001) and B (p < 0.05). An insignificant increment of 21.23 % was noted in group C following yogic practice (Table 5). Significant negative correlation was found between levels of serotonin with age before (p < 0.05, r = −0.313) and after (p < 0.001, r = −0.429) yogic practice (Fig. 5d). Levels of serotonin were significantly (p < 0.05) low in group C as compared to group A (Table 5). Levels of serotonin increased significantly in groups A (p < 0.05), B (p < 0.05), and C (p < 0.05) following yogic practice (Table 5). Significant positive correlation was found between levels of cortisol with age before (p < 0.01, r = 0.381) and after (p < 0.01, r = 0.391) yogic practice (Fig. 5e). Levels of cortisol was significantly higher in group C as compared to groups A (p < 0.01) and B (p < 0.05) as shown in Table 5. Cortisol level decreased significantly (p < 0.001) in groups A and B following yogic practice (Table 5). Insignificant reduction of 23.5 7 % was noted in cortisol level in group C following yogic practice (Table 5). We did not found any correlation between levels of ACTH with age before (p = 0.061, r = 0.162) and after (p = 0.065, r = 0.239) yogic practice (Fig. 5f). ACTH was decreased significantly (p < 0.01) in group A following yogic practice (Table 5).
Fig. 5.
Correlation between age with catecholamines, serotonin, stress hormones before and after yogic practice. a Correlation of age with nor epinephrine, b correlation of age with epinephrine, c correlation of age with dopamine, d correlation of age with serotonin, e correlation of age with cortisol, f correlation of age with adrenocorticotropic hormone
Table 5.
Effect of yogic practice on catecholamines, serotonin, and stress hormones of different age groups
| Parameters | 20–29 years (A) | 30–39 years (B) | 40–50 years (C) | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Epinephrine | 29.0 ± 2.26 | 18.7 ± 0.99** | 36.9 ± 2.69 | 26.3 ± 1.97** | 47.1 ± 3.32¥¥¥ | 33.9 ± 3.04 |
| Norepinephrine | 41.8 ± 4.54 | 32.5 ± 3.97 | 80.2 ± 13.00 | 62.4 ± 8.33 | 98.1 ± 13.62¥¥ | 70.3 ± 10.15* |
| Dopamine | 910.4 ± 79.07 | 1,321.9 ± 64.64*** | 863.5 ± 108.30 | 1,218.8 ± 54.11* | 760.7 ± 116.35 | 922.1 ± 65.92 |
| Serotonin | 209.6 ± 26.88 | 315.8 ± 27.84* | 163.7 ± 25.04 | 261.4 ± 20.23** | 105.32 ± 11.17¥ | 203.4 ± 16.9*** |
| Cortisol | 68.5 ± 4.43 | 47.4 ± 2.74*** | 73.2 ± 4.38 | 53.9 ± 2.48*** | 95.0 ± 7.09¥¥ ϕ | 72.7 ± 6.91 |
| ACTH | 16.4 ± 0.75 | 13.25 ± 0.72** | 17.1 ± 0.94 | 15.5 ± 1.97 | 19.18 ± 1.78 | 17.6 ± 0.90 |
Values are expressed as mean±SEM
Epinephrine, norepinephrine, dopamine, and serotonin are expressed as nanogram per milliliter; cortisol and adrenocorticotropic hormone (ACTH) are expressed as picogram per milliliter
*p < 0.05; **p < 0.01; and ***p < 0.001; comparisons were made between initial baseline values and the values obtained after yogic practice using paired “t” test. ¥ p < 0.05; ¥¥ p < 0.01; and ¥¥¥ p < 0.001, as compared to pre-exposure values of group A; one-way ANOVA followed by Tukey–Kramer’s multiple comparison post hoc test to find out difference between different age group. ϕ p < 0.05; as compared to pre-exposure values of group B; one-way ANOVA followed by Tukey–Kramer’s multiple comparison post hoc test were used to find out the difference between different age groups
Brain-derived neurotropic factor
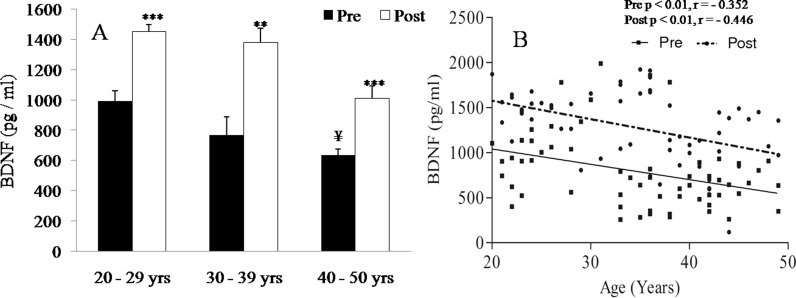
Levels of BDNF were significantly (p < 0.05) low in group C as compared to group A (Fig. 6a). It was increased significantly following yogic practice in groups A (p < 0.001), B (p < 0.01), and C (p < 0.001) as shown in Fig. 6a. Levels of BDNF were significantly and negatively correlated with age before (p < 0.01, r = −0.352) and after (p < 0.01, r = −0.446) yogic practice (Fig. 6b).
Fig. 6.
Effects of age and yogic practice on brain-derived neurotropic factor (BDNF). a Effects of yogic practice on levels of BDNF of different age group, b correlation between age and BDNF before and after yogic practice
Discussion
In this study, BW and BMI increased significantly with advancement of age, though there was a trend of reduction in mean values of these two parameters following yogic practice in groups B and C; these increased in group A. Due to more physical activity, fat deposition was prevented in younger age, whereas less physical activity in advanced age may be associated with fat deposition and increases body surface area, which causes an increment of BW, and BMI with advancement of age. Ray et al. (2001) stated that body weight was decreased; in contrast, Selvamurthy et al. (1988) reported increment of body weight following yogic practice. Sahay (2007) has also reported a significant decrease in the body fat and increase in lean body mass in type 2 diabetics following yogic intervention. A significant reduction in the skin fold thickness in normal healthy volunteers was also reported in the literature (Bera and Rajapurkar 1993).
Significant increase in HR, SBP, DBP, and MBP was noted in the present study with advancement of age. HR, SBP, DBP, and MBP was decreased significantly in senior age groups following yogic practice; however, insignificant change was noted in all components of BP in group A. Environmental conditions and variety of behavioral factors such as stress and anxiety may increase HR, SBP, DBP, and MBP of the individual. In developed or developing countries, day-to-day stress and tension is a major factor to increase BP with advancement of age. As yoga is known as a stress reliever, it has the beneficial role to decrease stress and anxiety as well as HR, SBP, DBP, and MBP (Chung et al. 2012). Stress-induced cortisol levels have been found to have positive correlation with blood pressure (Fraser et al. 1999). The present study showed that there was a positive correlation of ACTH and cortisol levels with age. Thus, it may be stated that increase in cortisol level with advancement of age directly increase HR and blood pressure (SBP, DBP, and MBP). It was also noticed that levels of cortisol decreased significantly following yogic practice and this may reduce heart rate and blood pressure (SBP, DBP, and MBP) significantly. Apart from that, a strong relationship between BMI and blood pressure is well known, and weight reduction is associated with a mean decrease in blood pressure (Fraser et al. 1999; Stamier et al. 1978 and Relsln et al. 1978). Advancement of age associated with increment of TC, TG, LDL-cholesterol and decrement of HDL-cholesterol may increase peripheral resistance as well as BP (Fraser et al. 1999). As yogic practice decrease TC, TG, LDL-cholesterol and increase HDL-cholesterol may decrease BP. Yogic practices have beneficial role to decrease BP and HR by changing homeostasis, and practitioner goes towards parasympathodominance (Murugesan et al. 2000; Selvamurthy et al. 1998). The ratio of low- to high-frequency spectral powers has been used as an index of sympathovagal balance (Eckberg 1997); increase with advancement of age also increase BP and HR, and decrease following yogic practice shifted the individual towards parasympathodominance also decrease BP and HR. Increment of epinephrine and norepinephrine resulting in advancement of age may have an important role to increase BP. Increased BP with advancement of age, in this study, may be due to increase in total peripheral resistance. Resistance may be due alteration of fluid composition and volume with progression of age. Prevalence of declining renal function in aging is recognized as renal mass decreases by 20 to 25 % between the ages of 30 and 80 years (Beck 1998). Age-related accumulation of extracellular matrix, expansion of glomerular mesangium, and alteration of tubular epithelial transporters are also commonly reported (Muhlberg and Platt 1999). These factors contribute to an overall decline in glomerular filtration rate and impairment of tubular re-absorptive function that compromises the ability of kidneys to maintain proper extracellular fluid volume and composition. Alteration of extracellular fluid volume and composition may increase blood pressure. Selvamurthy et al. (1998) stated that yogic practices have the ability to decrease sodium and potassium concentration and also decrease rennin activity in the blood to decrease BP towards normal in the hypertensive patient. Selvamurthy et al. (1998) showed progressive decrement of norepinephrine and epinephrine, which decreased systolic and diastolic blood pressure following selected yogic practice in the hypertensive patient. Plasma epinephrine and norepinephrine concentration was decreased in this study and may decrease BP.
RPP is an index of myocardial oxygen consumption, and DoP indicates load on heart (Gobel et al. 1978). RPP and DoP were higher in the higher age group. These two indices showed a decrement following yogic practices due to decrease myocardial oxygen consumption and load on the heart. RPP is derived from SBP multiplied by HR, and DoP is derived from MBP multiplied by HR (Gobel et al. 1978). Findings of this study show that HR and BP is positively correlated with age and may increase RPP and DoP. RPP and DoP decrease following yogic practice may be due to decrease in BP and HR. Madanmohan et al. (2005) showed that pranayama have the role to decrease RPP and DoP.
TC increased significantly with advancement of age, and it may be due to increase in fat deposition. Although visceral fat was not specifically assessed in this study, body weight and body mass index was higher in elderly subjects compared with their younger mates as reported in earlier studies also (Malaguarnera et al. 1998). Essig et al. (2004) also found body fat content, TG, TC, and LDL-cholesterol to increase in men and women with age. HDL-cholesterol was decreased with advancement of age in this study. TC and LDL-cholesterol decreased significantly following yogic practice. This may be due to increased activity of lipase enzyme at the cellular level, which affects the metabolism of lipoprotein and thus increase uptake of triglyceride by adipose tissues (Delmonte 1985; Tulpule et al. 1971). Apart from that, yogic practice may decrease the absorption of fatty acid from gastrointestinal tract, which may decrease TC, LDL-cholesterol, and BMI. Singh et al. (2008) and Gordon et al. (2008) reported a significant reduction in free fatty acids, LDL cholesterol, very low density lipoprotein cholesterol, and an increase in HDL cholesterol following yogic practice.
The results of this study revealed that aging was accompanied by an overall decline in autonomic nervous system activity. Components of HRV such as LF, HF, LF/HF, TP, SDNN, RMSSD, and pNN50 are translated as benchmarks to test the autonomic functions. Decreased values of HF, TP, SDNN, RMSSD, mean RR, and pNN50 and increased values of LF and LF/HF was noted with advancement of age. Jensen-Ustad et al. (1997) observed that progression of age lowered the values of heart rate variability and shifted the individual towards sympathodominance. TP, the principal index of general sympathovagal tone, exhibited a significant negative correlation with age. Age-associated decline in TP undergird previous findings (Kuo et al. 1999; Zhang 2007). Based on striking resemblance between LF, an index of sympathovagal modulation, TP, and LF was seen as a potential indicator of ANS activity as a whole. The present study showed that a significant positive correlation of LF with age might decrease sympathovagul modulation. However, opinions on LF as a marker of sympathetic activity are diverse in reported literatures (Fagard 2001; Zhang 2007). As LF is translated as a benchmark of sympathetic activity, sympathetic capacity was observed to increase with age in the current study. SDNN and HF, the two indices of vagal modulation, demonstrated negative correlations with age. Consequently, parasympathetic activity declined with age. These finding is in the same line of the previous study (Kuo et al. 1999; Zhang 2007). Autonomic balance is predominated by sympathetic activity with increasing age as indicated by normalized values of LF and HF. Ratio of low- to high-frequency spectral powers has been used as an index of sympathovagal balance (Eckberg 1997). The present study showed that significant enhancement of LF/HF may shift the individual towards sympathodominance with advancement of age. LF in powers, quantifying the ratio of sympathetic to parasympathetic activity, indicates lower sympathetic activity following yogic practice. LF, the quantitative marker of sympathetic activity expressed in normalized units, decreased following yogic practice, indicating lower sympathetic activity (Malliani et al. 1997). Yogic practice have the ability to decrease LF/HF and may be associated with sympathovagul balance and shift the individual towards parasympathodominance. Observed decrease in LF power (normalized units) and corresponding increase in HF power (normalized units) are similar to that observed in men following the practice of yoga (Patil and Telles 2006). Time domain index of HRV, SDNN, square root of variance, and a measure of overall HRV increased following yogic practice. Increased HRV signifies extent of the ability of the cardiac ANS to accommodate wide variations, but not the rate of response. Time domain measures (i.e., mean R-R interval, SDNN, RMSSD, and pNN50) are recognized to be strongly dependent on the vagal modulation (Massin et al. 1999). pNN50 increased following yogic practice for an increased complexity in the dynamics of the heart. Moreover, there are significant increase of parasympathetic activity, as evidenced by increase in HF component, and a calming effect on the heart, as evidenced by increased variation in inter-RR intervals.
Bioelectromagnetism, as SC, showed a significant negative correlation with age. Earlier studies support this fact (Barontini et al. 1997). A total of 3 months yogic practice increased SC. SC is directly related to wound healing and any age-related emotions. SC is under the control of sympathetic nervous system and reflects activity of eccrine sweat glands and release of neurotransmitter acetylcholine (Cacioppo et al. 1997). Normally, the sweat gland offers low resistance to current when passing through the electrodes. Inactivity of the glands increase resistance of the membrane. The activity of the different areas of the central nervous system like the hypothalamus, reticular formation, and cerebral cortex is being reflected on the sweat gland. Hence, changes in the activity of the sweat gland are closely related to one’s arousal, awareness, levels of tension, and relaxation (Woodworth and Schlosberg 1971). This results were also supported by other observations (Kumar and Joshi 2009; Panjwani et al. 1995).
Epinephrine and norepinephrine are catecholamines secreted from adrenal medulla and acts through the alpha and beta adrenergic receptors. These two catecholamines increased significantly with advancement of age and may be due to increase sympathetic activity. Levels of plasma epinephrine and norepinephrine was decreased following yogic practice. Earlier study also supports this fact (Selvamurthy et al. 1998). Physical and or mental stress is responsible for the activation of the hypothalamopituitary adrenal axis producing epinephrine and norepinephrine and increase sympathetic activity. Decreased stress as measured by ACTH, cortisol, and HRV following yogic practice may decrease the levels of epinephrine and norepinephrine; discharge of alpha and beta receptors decrease BP and HR.
Serotonine is a local hormone responsible for moods and behavior. It decreased with advancement of age and may be due to alteration of autonomic function, and aging might make people prone to stress (increase cortisol level with advancement of age) and anxiety. Yogic practice modulate the levels of stress (cortisol and HRV), and decrease anxiety may increase levels of serotonin in the practitioner. Levels of serotonin has not been assessed earlier following yogic practice. Change in the levels of serotonin may be due to relaxation of central and or autonomic nervous system being reflected by levels of cortisol and components of HRV and BP. Apart from that, yoga has substantial antidepressant effects that correlated with elevation of plasma BDNF levels, and this is agreement with other observations (Naveen et al. 2013).
From the present study, it has been observed that aging has negative correlation with BDNF—a key mediator of neuronal plasticity in adult, adult neurogenesis, and brain aging. BDNF has survival- and growth-promoting actions on a variety of neurons, including dorsal root ganglion cells (Acheson 1995) and hippocampal and cortical neurons (Huang and Reichardt 2001). A significant improvement in the BDNF level has been noticed for the entire groups in response to yogic practice. Decrement of BDNF level with age may be due to moods and loneliness with advancement age. Another possible cause of decrement of BDNF level with advancement of age is associated with prevalence of some neuro-endocrine or brain disorders like Parkinson’s disease, which is also related to decrease dopamine concentration with age. Earlier studies also showed that BDNF has a negative correlation with age (Lommatzsch et al. 2005). Improvement in levels of BDNF following yogic practice may be due to decrease stress level, shown by decrease cortisol levels, and improved HRV variables. Except that, yogic practice has a direct role on improvement of moods and also have beneficial role to make well neuro- or psychological disorders as measured by increase serotonin and dopamine levels. Previous researcher showed that yoga has the beneficial role to improve cognition and BDNF levels (Xiong, and Doraiswamy 2009, Naveen et al. 2013). Yogic practices also have potentially beneficial effects on lipid profiles and lower oxidative stress, both of which could in turn reduce the risk for cerebrovascular disease- and age-related neurodegeneration (Singh et al. 2008; Sinha et al. 2007). Further, meditation may potentially strengthen neuronal circuits and enhance cognitive performance (Froeliger et al. 2012). Increment of levels of serotonin and dopamine following 3 months of yogic practice also support the above-mentioned fact. Another important mechanism revealed from the present study that yogic practice lowers the age-related enhancement of epinephrine and no epinephrine level. Along with these, it shifts the individuals towards parasympathodominance by improving HRV parameters. This observation is in corroboration with previous studies of other researchers (Selvamurthy et al. 1998).
Age-associated changes in hypothalamo-pituitary-adrenal axis has been well accepted (Kuzina et al. 2010). The present study also showed higher cortisol level in the older age group than their younger mates. Reduction in heart rate, blood pressure, oxygen consumption, and improvement in HRV parameters are associated with physical relaxation. This may decrease cortisol and ACTH levels. Apart from serotonin, dopamine showed an improvement and is directly related to mental relaxation and may decrease cortisol and ACTH. Moreover, yogic practice has a beneficial role to shift individuals towards parasympathodominance and make practitioner calm and quiet, which may decrease cortisol level significantly in yoga practitioner. Previous researchers showed that yogic practice have beneficial role in decreasing cortisol level and making architectural change on the EEG alpha wave with the practitioner going towards the relaxation state (Field 2011).
The present study suffers from some limitations that need to be acknowledged and addressed. One of these is the relatively small sample size in each group. An additional limitation is that this study is a single-arm study without any separate control group. Except the above limitations, the study has a greater applicability in scientific yoga research. Studies of yogic intervention and its therapeutic application over many patients have been accepted and well documented in earlier research but, effects of yogic practice on physically as well as mentally healthy and active service personnel as reported in the present research are very few. Yogic practice has effects on levels of BDNF, serotonin, dopamine, epinephrine, and norepinephrine. So, the focus of this study, the effect of yoga on age-related degenerative changes, has greater applicability in the field of yoga and/or physical activity research.
Conclusion
Based on the results of the present study, it may be concluded that the aging process has an active role on degenerative changes in cardiometabolic risk factors, autonomic functions, and monoamines as well as levels of BDNF, which may revert back towards normal or near-normal levels through yogic practice in healthy active males.
Acknowledgments
The authors express their sincere gratitude to the Director, Defence Institute of Physiology and Allied Sciences (DIPAS), Delhi, for giving the necessary permission to conduct this study. Additionally, the authors are grateful to the Director, Morarji Desai National Institute of Yoga (MDNIY), Delhi, for the continuous support to the study. Moreover, the authors also acknowledge the support of the Project Director, CARTY for giving necessary permission, encouragement, and moral support. The authors acknowledge the support of a yoga instructor. A warm thanks is due to all volunteers who participated in the study, without whose willing and wholehearted cooperation this study would not have been possible, and, finally, to the concerned officials for providing all facilities required for a smooth conduction of the trial.
Conflict of interest
The authors declare that there is no actual or potential conflict of interest regarding this study.
Ethical standards
All the experiments comply with the current laws of the country and approved by the Institutional Ethical Committee, DIPAS, Delhi, India.
References
- Acheson A. BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci. 2004;107:355–364. doi: 10.1042/CS20040148. [DOI] [PubMed] [Google Scholar]
- Barontini M, Lázzari JO, Levin G, Armando I, Basso SJ. Age-related changes in sympathetic activity: biochemical measurements and target organ responses. Arch Gerontol Geriatr. 1997;25(2):175–186. doi: 10.1016/S0167-4943(97)00008-3. [DOI] [PubMed] [Google Scholar]
- Beck LH. Changes in renal function with aging. Clin Geriatr Med. 1998;14:199–209. [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Res. 2005;25:3011–3021. [PubMed] [Google Scholar]
- Bera TK, Rajapurkar MV. Body composition, cardiovascular endurance and anaerobic power of yogic practitioner. Indian J Physiol Pharmacol. 1993;37:225–228. [PubMed] [Google Scholar]
- Botwinick J, Kornetsky C. Age differences in the acquisition and extinction of the GSR. J Gerontol. 1960;15:83–84. doi: 10.1093/geronj/15.1.83. [DOI] [PubMed] [Google Scholar]
- Bowman AJ, Clayton RH, Murray A, et al. Effects of aerobic exercise training and yoga on the baroreflex in healthy elderly persons. Eur J Clin Investig. 1997;27:443–449. doi: 10.1046/j.1365-2362.1997.1340681.x. [DOI] [PubMed] [Google Scholar]
- Burzynski SR. Aging: gene silencing or gene activation. Med Hypotheses. 2005;64:201–208. doi: 10.1016/j.mehy.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Klein DJ, Poehlmann KM. The psychophysiology of emotion across the lifespan. Annu Rev Gerontol Geriatr. 1997;17:27–74. [Google Scholar]
- Chung SC, Brooks MM, Rai M, Balk JL, Rai S. Effect of sahaja yoga meditation on quality of life, anxiety, and blood pressure control. J Altern Complement Med. 2012;18:589–596. doi: 10.1089/acm.2011.0038. [DOI] [PubMed] [Google Scholar]
- Delmonte MM. Biochemical indices associated with meditation practice. A literature review. Neurosci Biobehav Rev. 1985;9:557–561. doi: 10.1016/0149-7634(85)90002-8. [DOI] [PubMed] [Google Scholar]
- Dreher J, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. PNAS. 2008;105:3915106–3915111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg D. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–3232. doi: 10.1161/01.CIR.96.9.3224. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Heo S, McLaren M, Pence BD, Martin SA, Vieira VJ, Woods JA, McAuley E, Kramer AF. Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J Neurosci. 2010;30(15):5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essig F, Sinclair D, Hare J, Moreillon J, Funk D, Swank AM. Cross-sectional analysis of cardiovascular factors for participants of a university faculty and staff wellness program. J Exerc Physiol. 2004;7:37–43. [Google Scholar]
- Fagard RH. A population-based study on the determinants of heart rate and heart rate variability in the frequency domain. Verh K Acad Geneeskd Belg. 2001;63(1):57–89. [PubMed] [Google Scholar]
- Fahri A. Changes in serum lipid profile following moderate exercise. Afr J Pharm Pharmacol. 2010;4(11):829–833. [Google Scholar]
- Farley A, McLafferty E, Hendry C. The physiological effects of ageing on the activities of living. Nurs Stand. 2006;20:46–52. doi: 10.7748/ns2006.07.20.45.46.c4468. [DOI] [PubMed] [Google Scholar]
- Field T. Yoga clinical review. Complement Ther Clin Pract. 2011 doi: 10.1016/j.ctcp.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JMC. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33:1364–1368. doi: 10.1161/01.HYP.33.6.1364. [DOI] [PubMed] [Google Scholar]
- Frisard M, Ravussin E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine. 2006;29:27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- Froeliger BE, Garland EL, McClernon FJ. Yoga meditation practitioners exhibit greater gray matter volume and fewer reported cognitive failures: results of a preliminary voxel-based morphometric analysis. Evid Based Complement Alternat Med. 2012 doi: 10.1155/2012/821307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garau C, Aparicio S, Rial RV, Nicolau MC, Esteban S. Age-related changes in circadian rhythm of serotonin synthesis in ring doves: effects of increased tryptophan ingestion. Exp Gerontol. 2006;41(1):40–48. doi: 10.1016/j.exger.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Gobel FL, Nordstrom LA, Nelson RR, Jorgenson CR, Wang Y. The rate pressure product as an index of myocardial oxygen consumption during exercise in patient with angina pectoris. Circulation. 1978;57:549–556. doi: 10.1161/01.CIR.57.3.549. [DOI] [PubMed] [Google Scholar]
- Gordon LA, Morrison EY, McGrowder DA, Young R, Fraser YT, Zamora EM, et al. Effect of exercise therapy on lipid profile and oxidative stress indicators in patients with type 2 diabetes. BMC Complement Alternat Med. 2008;8:21. doi: 10.1186/1472-6882-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe N, Pontifex MB, Hillman C, McAuley E. The acute effects of yoga on executive function. J Phys Act Health. 2013;10:488–495. doi: 10.1123/jpah.10.4.488. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FJ, Chien DK, Chung UL. Effects of Hatha yoga on stress in middle-aged women. J Nurs Res. 2013;21(1):59–66. doi: 10.1097/jnr.0b013e3182829d6d. [DOI] [PubMed] [Google Scholar]
- Jensen-Ustad K, Strock N, Bouvier F, Ericson M, Lindblad LE, Jensen-Ustad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiol Scand. 1997;160:235–241. doi: 10.1046/j.1365-201X.1997.00142.x. [DOI] [PubMed] [Google Scholar]
- Kirk-Sanchez NJ, McGough EL. Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging. 2014;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Joshi B. Study on the effect of Pranakarshan pranayama and Yoga nidra on alpha EEG & GSR. Indian J Tradit Knowl. 2009;8:453–454. [Google Scholar]
- Kuo TB, et al. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- Kuzina IN, Kilikovskiĭ VV, Smirnova OV. Age-related changes in blood concentration of hypothalamic-pituitary-adrenal axis hormones, their central and peripheral regulators in healthy men. Fiziol Cheloveka. 2010;36(5):101–109. [PubMed] [Google Scholar]
- Landahl S, Bengtsson C, Sigurdsson JA, Svanborg A, Svärdsudd K. Age-related changes in blood pressure. Hypertension. 1986;8:1044–1049. doi: 10.1161/01.HYP.8.11.1044. [DOI] [PubMed] [Google Scholar]
- Leosco D, Parisi V, Femminella GD, Formisano R, Petraglia L, Allocca E, Bonaduce D. Effects of exercise training on cardiovascular adrenergic system. Front Physiol. 2013;4:348. doi: 10.3389/fphys.2013.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Madanmohan, Kaviraja U, Bhavanani AB, Vijayalakshmi P, Surendiran A. Effects of slow and fast pranayams on reaction time and Cardiorespiratory variables. Indian J Physiol Pharmacol. 2005;49:313–318. [PubMed] [Google Scholar]
- Malaguarnera M, Giugno I, Ruello P, Rizzo M, Panebianco MP, Pistone G, Omasello FB. Lipid profile variations in a group of healthy elderly and centenarians. Eur Rev Med Pharmacol Sci. 1998;2:75–79. [PubMed] [Google Scholar]
- Malliani A, Pagani M, Furlan R, Guzzetti S, Lucini D, Montano N, et al. Individual recognition by heart rate variability of two different autonomic profiles related to posture. Circulation. 1997;96:4143–4145. doi: 10.1161/01.CIR.96.12.4143. [DOI] [PubMed] [Google Scholar]
- Massin MM, Derkenne B, von Bernuth G. Correlations between indices of heart rate variability in healthy children with congenital heart disease. Cardiology. 1999;91:109–113. doi: 10.1159/000006889. [DOI] [PubMed] [Google Scholar]
- Muhlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45:243–253. doi: 10.1159/000022097. [DOI] [PubMed] [Google Scholar]
- Murugesan R, Govindarajulu N, Bera TK. Effect of selected yogic practices on the management of hypertension. Indian J Physiol Pharmacol. 2000;44:207–210. [PubMed] [Google Scholar]
- Naveen GH, Thirthalli J, Rao MG, Varambally S, Christopher R, Gangadhar BN (2013) Positive therapeutic and neurotropic effects of yoga in depression: a comparative study. Indian J Psychiatry 55(Suppl3):S400–S404. doi:10.4103/0019-5545.116313 [DOI] [PMC free article] [PubMed]
- Oken BS, Kishiyama S, Zajdel D, et al. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology. 2004;62:2058–2064. doi: 10.1212/01.WNL.0000129534.88602.5C. [DOI] [PubMed] [Google Scholar]
- Oken BS, Zajdel D, Kishiyama S, et al. Randomized controlled, six-month trial of yoga in healthy seniors: effects on cognition and quality of life. Altern Ther Health Med. 2006;12:40–47. [PMC free article] [PubMed] [Google Scholar]
- Panjwani U, Gupta H, Singh SH, Selvamurthy W, Rai UC. Effect of Sahaja Yoga practice on stress management in patients of epilepsy. Indian J Physiol Pharmacol. 1995;39:111–116. [PubMed] [Google Scholar]
- Papp ME, Lindfors P, Storck N, Wändell PE. Increased heart rate variability but no effect on blood pressure from 8 weeks of hatha yoga—a pilot study. BMC Res Notes. 2013 doi: 10.1186/1756-0500-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil SP, Telles S. Changes in heart rate variability during and after two yoga based relaxation techniques. Int J Stress Manag. 2006;13:460–473. doi: 10.1037/1072-5245.13.4.460. [DOI] [Google Scholar]
- Ray US, Mukhopadhyaya S, Purkayastha SS, Asnani V, Tomer OS, Prashad R, Thakur L, Selvamurthy W (2001) Effect of yogic exercises on physical and mental health of young fellowship course trainees. Indian J Physiol Pharmacol 45:37–53 [PubMed]
- Reardon M, Malik M. Changes in heart rate variability with age. Pacing Clin Electrophysiol. 1996 doi: 10.1111/j.1540-8159.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- Relsln E, Abe R, Modan M, Sllverberg OS, Ellahou HE, Modan B. Effect of weight loss without salt restriction on the reduction of blood pressure in overweight hypertensive patients. N Engl J Med. 1978;298:1–6. doi: 10.1056/NEJM197801052980101. [DOI] [PubMed] [Google Scholar]
- Sahay BK. Role of yoga in diabetes. J Assoc Physicians India. 2007;55:121–126. [PubMed] [Google Scholar]
- Selvamurthy W, Ray US, Hedge KS, Sharma RP. Physiological responses to cold (100 °C) in men after six months’ practice of yoga exercises. Int J Biometeorol. 1988;32:188–193. doi: 10.1007/BF01045278. [DOI] [PubMed] [Google Scholar]
- Selvamurthy W, Sridharan K, Ray US, Tiwary RS, Hegde KS, Radhakrishan U, Sinha KC. A new physiological approach to control essential hypertension. Indian J Physiol Pharmacol. 1998;42:205–213. [PubMed] [Google Scholar]
- Shimazu T, Tamura N, Shimazu K. Aging of the autonomic nervous system. Nihon Rinsho. 2005;63(6):973–977. [PubMed] [Google Scholar]
- Singh S, Kyizom T, Singh KP, Tandon OP, Madhu SV. Influence of pranayamas and yoga—asanas on serum insulin, blood glucose and lipid profile in type 2 diabetes. Indian J Clin Biochem. 2008;23:365–368. doi: 10.1007/s12291-008-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Singh SN, Monga YP, Ray US (2007) Improvement of glutathione and total antioxidant status with yoga. J Altern Complement Med 13(10):1085–1090. doi:10.1089/acm.2007.0567 [DOI] [PubMed]
- Smith C, Hancock H, Blake Mortimer J, Eckert K. A randomized comparative trial of yoga and relaxation to reduce stress and anxiety. Complement Ther Med. 2007;15:77–83. doi: 10.1016/j.ctim.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Smith JA, Greer T, Sheets T, Watson S. Is there more to yoga than exercise? Altern Ther Health Med. 2011;17(3):22–29. [PubMed] [Google Scholar]
- Stamier R, Stamler J, Rledllnger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA. 1978;240:1607–1610. doi: 10.1001/jama.1978.03290150053024. [DOI] [PubMed] [Google Scholar]
- Steen B. Biological aging—a mini review. Lakartidningen. 2001;98:1924–1928. [PubMed] [Google Scholar]
- Tapp LR, Signorile JF. Efficacy of WBV as a modality for inducing changes in body composition, aerobic fitness, and muscular strength: a pilot study. Clin Interv Aging. 2014;9:63–72. doi: 10.2147/CIA.S30048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman A. Catabolic insufficiency and aging. Ann N Y Acad Sci. 2006;1067:27–36. doi: 10.1196/annals.1354.005. [DOI] [PubMed] [Google Scholar]
- Tulpule TH, Shah HM, Shah SJ, Haveliwala HK. Yogic exercises in the management of ischaemic heart disease. Indian Heart J. 1971;23(4):259–264. [PubMed] [Google Scholar]
- Viña J, Borrás C, Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- Woodworth RS, Schlosberg H. In: Experimental psychology. Kling JW, Rigg LA, editors. New York: Rinehart and Winston; 1971. [Google Scholar]
- Xiong GL, Doraiswamy PM. Does meditation enhance cognition and brain plasticity? Ann N Y Acad Sci. 2009;1172:63–69. doi: 10.1196/annals.1393.002. [DOI] [PubMed] [Google Scholar]
- Yonezawa Y, Kondo H, Nomaguchi TA. Age-related changes in serotonin content and its release reaction of rat platelets. Mech Ageing Dev. 1989;47(1):65–75. doi: 10.1016/0047-6374(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Yurkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga based exercise program in hemodialysis patients: A randomized control study. Complement Ther Med. 2007;15:164–171. doi: 10.1016/j.ctim.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Zhang J. Effect of age and sex on heart rate variability in healthy subjects. J Manipulative Physiol Ther. 2007;30(5):374–379. doi: 10.1016/j.jmpt.2007.04.001. [DOI] [PubMed] [Google Scholar]