Abstract
Cardiac arrest is a leading cause of death and permanent disability. Most victims succumb to the oxidative and inflammatory damage sustained during cardiac arrest/resuscitation, but even survivors typically battle long-term neurocognitive impairment. Although extensive research has delineated the complex mechanisms that culminate in neuronal damage and death, no effective treatments have been developed to interrupt these mechanisms. Of importance, many of these injury cascades are also active in the aging brain, where neurons and other cells are under persistent oxidative and inflammatory stress which eventually damages or kills the cells. In light of these similarities, it is reasonable to propose that the brain essentially ages the equivalent of several years within the few minutes taken to resuscitate a patient from cardiac arrest. Accordingly, cardiac arrest-resuscitation models may afford an opportunity to study the deleterious mechanisms underlying the aging process, on an accelerated time course. The aging and resuscitation fields both stand to gain pivotal insights from one another regarding the mechanisms of injury sustained during resuscitation from cardiac arrest and during aging. This synergism between the two fields could be harnessed to foster development of treatments to not only save lives but also to enhance the quality of life for the elderly.
Keywords: Caspases, Glutathione, Inflammation, Ischemia, Neurodegeneration, Oxidative Stress
Introduction
Cardiac arrest remains a leading cause of death and persistent disability in the USA. In its 2014 update on heart disease and stroke statistics, the American Heart Association estimated that approximately 380,000 out of 424,000 (~90%) Americans who experience out-of-hospital cardiac arrest annually do not survive (Go et al. 2014). Only 23% (97,520) of all cardiac arrest victims present to emergency medical services personnel with a shockable cardiac rhythm, and most who are initially resuscitated later succumb to extensive ischemia-reperfusion injury to the brain and other vital organs (Dezfulian et al. 2009; Heron 2012; Nolan et al. 2012; Young 2009; Go et al. 2014). Moreover, approximately half of the c. 10 % of cardiac arrest victims who do survive to hospital discharge experience persistent neurocognitive impairment manifested as memory and sensorimotor deficits that profoundly impact their quality of life (Adrie et al. 2004; Moulaert et al. 2009; Wachelder et al. 2009; Young 2009; Go et al. 2014).
While many studies have examined the complex mechanisms of brain damage following cardiac arrest-initiated ischemia-reperfusion injury, the precise cascade of events culminating in neurocognitive impairment remains to be completely delineated. It is known that ATP depletion, intracellular Ca2+ overload (Bano and Nicotera 2007; Li et al. 2007), reactive oxygen and nitrogen species (Calapai et al. 2000), inflammation, and glutamate-induced excitotoxicity (Conroy et al. 1999; Backstrom et al. 2003) initiated by cardiac arrest and resuscitation collectively inflict lethal damage to neurons, oligodendrocytes, microglia, and the cerebrovascular endothelium and disrupt the blood–brain barrier (BBB). Despite mounting knowledge of the mechanisms of brain injury, currently there are no clinically proven pharmacological treatments to protect the brain during cardiac arrest and cardiopulmonary resuscitation (CPR) (Dezfulian et al. 2009).
Similar to long-term recovery from cardiac arrest and CPR, the principal mechanisms of neurocognitive impairment in the aging brain have yet to be assembled into a coherent cascade of events that would allow for development of efficacious preventative therapies. However, it is becoming increasingly evident that many age-related changes leading to neuronal damage and death parallel those observed during and following cardiac arrest. Specifically, accumulation of reactive oxygen and nitrogen species as well as proinflammatory cytokines and markers of inflammation have all been observed in brain aging studies (Hagen 2003; Kregel and Zhang 2007; Cortese et al. 2011). Moreover, as the brain ages, calcium mismanagement and mitochondrial dysfunction also contribute to the death and dysfunction of neurons and other cells within the most vulnerable regions such as the hippocampus (Landfield 1988; Foster and Norris 1997; Toescu et al. 2004). The progression of neuronal impairment and cell death observed during aging is, however, a much slower process than the injury cascade that follows cardiac arrest, CPR, and post-arrest recovery. The purpose of this review is to highlight parallel mechanisms of brain damage and neuronal death that ensue following cardiac arrest and in the aging brain. Despite their different time courses, mechanistic information gained from studying the two conditions could be harnessed to synergistically advance both fields and to develop treatments targeting specific components in these neurodegenerative pathways to provide more robust protection of patients from neurocognitive impairment and/or death.
Oxidative stress
Oxidative injury during cardiac arrest and cardiopulmonary resuscitation
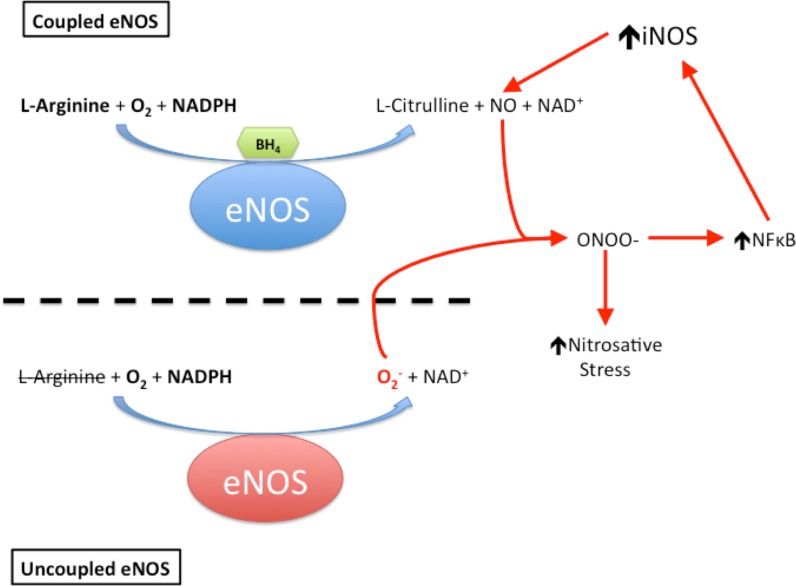
A major culprit in the ischemia-reperfusion injury sustained following cardiac arrest and CPR is the oxidative stress imposed on the brain (Idris et al. 2005; Wang et al. 2007). Intense formation and accumulation of reactive oxygen (Opie 1991; Cerchiari et al. 1987; Becker 2004; Idris et al. 2005) and nitrogen species, i.e., ROS/RNS (Lipton 1999; Love 1999; White et al. 2000; Dohi et al. 2003; Keynes and Garthwaite 2004; Thiyagarajan et al. 2004; Zhu et al. 2004), within the affected tissue leads to lipid peroxidation, inactivation of metabolic enzymes, and mitochondrial dysfunction, which collectively ignite a cascade of cell death that manifests as neurocognitive impairment once brain regions such as the hippocampus and cerebellum—which are highly susceptible to oxidative damage—are substantially impacted (Cerchiari et al. 1987; Brown and Borutaite 1999; White et al. 2000; Dohi et al. 2003; Becker 2004; Zhu et al. 2004). When its co-factor, tetrahydrobiopterin (BH4), is oxidized by superoxide and hydrogen peroxide, the endothelial isoform of nitric oxide synthase (eNOS) becomes uncoupled and no longer generates nitric oxide (NO), instead producing the superoxide anion (Fig. 1) (Manukhina et al. 2006; Kalyanaraman 2013). The resultant excess of reactive oxygen species (ROS) activates the inducible NOS isoform (iNOS), which then overproduces NO (Manukhina et al. 2006; Kalyanaraman 2013). iNOS-generated NO combines with superoxide from uncoupled eNOS to form peroxynitrite, which nitrosylates tyrosine residues, thereby inactivating proteins essential for cellular energy metabolism and function, and nitrosylates the pivotal intracellular antioxidant glutathione to form non-antioxidant S-nitrosyl glutathione (Manukhina et al. 2006; Kalogeris et al. 2012; Kalyanaraman 2013). Figure 1 summarizes this vicious cycle of peroxynitrite generation.
Fig. 1.
Uncoupling eNOS initiates a vicious cycle of nitrosative stress. Oxidation of its cofactor, tetrahydrobiopterin (BH4), uncouples the endothelial isoform of nitric oxide synthase (eNOS), which then generates the superoxide anion (O2 −). Superoxide then combines with nitric oxide (NO) produced by adjacent, still-coupled eNOS to form the powerful oxidant peroxynitrite (ONOO−), which in turn upregulates nuclear factor kappa B (NFκB). NFκB activates expression of the inducible nitric oxide synthase (iNOS), which produces massive amounts of NO that combine with eNOS-generated O2 − to intensify ONOO− formation.
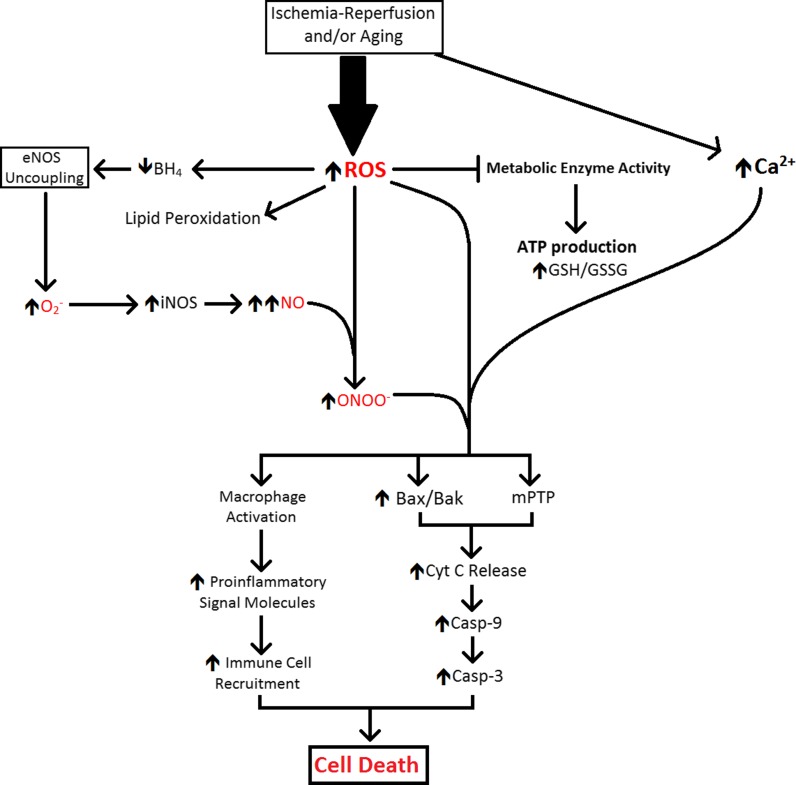
During ischemia, the intracellular accumulation of protons from anaerobic glycolysis and ATP hydrolysis causes an abrupt drop in cytosolic pH (Kalogeris et al. 2012). To minimize intracellular acidification, the Na+/H+ exchanger expels H+ from cells in exchange for Na+ (Baines 2010). Intracellular Na+ ions are then exchanged for extracellular Ca2+ by Na+/Ca2+ countertransport (Kalogeris et al. 2012). Upon reperfusion, washout of extracellular H+ by restored circulation increases the H+ gradient across the cell membrane and accelerates the actions of the Na+/H+ and Na+/Ca2+ exchangers, exacerbating the intracellular Ca2+ overload (Baines 2010; Kalogeris et al. 2012). In combination with excess Ca2+, the reperfusion burst of ROS/RNS triggers integration of the pro-apoptotic Bcl2 family proteins, Bax and Bak, into the outer mitochondrial membrane (Baines 2010). The pore formed by Bax/Bak enables efflux of small mitochondrial proteins such as cytochrome c, second mitochondria-derived activator of caspases (SMAC), and endonuclease-G (Baines 2010). In the cytosol, SMAC and cytochrome c combine with apoptotic protease activating factor 1 (APAF1), forming an apoptosome which activates caspase-9 and caspase-3 (Baines 2010). In concert with caspase-mediated pro-apoptotic signaling, Bax/Bak permits endonuclease-G efflux from mitochondria; this enzyme enters the nucleus and fragments genomic DNA, a pivotal event in apoptotic cell death (Baines 2010). The post-ischemic Ca2+ overload and ROS/RNS burst in the mitochondrial matrix also open a large, non-selective channel in the inner mitochondrial membrane, the mitochondrial permeability transition pore (mPTP). Opening of mPTP collapses the proton electrochemical gradient required for oxidative phosphorylation, further draining cellular ATP reserves already depleted by ischemia (Baines 2010; Halestrap 2010; Kalogeris et al. 2012). Figure 2 summarizes the cascade by which this intense oxidative insult ultimately opens both the Bax/Bak and mPTP pores, thereby activating caspase-9 and caspase-3, DNA fragmentation and mitochondrial rupture culminating in apoptosis of neurons and astroglia (Kirkland and Franklin 2003; Baines 2010; Halestrap 2010; Franklin 2011; Martin et al. 2011).
Fig. 2.
Mechanisms of oxidative and inflammatory injury during aging and recovery from cardiac arrest. The figure summarizes mechanisms of brain injury common to cardiac arrest-resuscitation and aging, albeit over entirely different time courses. Casp-9 caspase-9, Casp-3 caspase-3, Cyt C cytochrome c, GSH/GSSG concentration ratio of reduced (GSH) to oxidized (GSSG) glutathione, iNOS incucible isoform of nitric oxide synthase, mPTP mitochondrial permeability transition pore, NO nitric oxide, ROS reactive oxygen species, ONOO − peroxynitrite
Oxidative injury during aging
The “oxidative stress theory” of aging identifies the accumulation of oxidative damage caused by ROS/RNS over the course of the aging process as pivotal to the progressive decline of biological function and shorter lifespan (Kregel and Zhang 2007). According to this paradigm, ROS as well as RNS accumulate due to an imbalance between their production and detoxification by endogenous redox systems, e.g., the glutathione peroxidase/reductase and thioredoxin/peroxiredoxin systems (Hagen 2003; Kregel and Zhang 2007). This oxidative imbalance potentiates deleterious protein oxidation, lipid peroxidation, and apoptotic cell death (Blumberg 2004; Stadtman 2004; Matsuzawa and Ichijo 2005).
One line of evidence supporting the “oxidative stress theory” of aging stems from measurements of the common biomarkers of antioxidative capacity—that is, the degree to which the body is able to neutralize existing and de novo oxidative stress via its endogenous antioxidant defense mechanisms. Among the most widely accepted measures of in vivo redox state are the ratios of reduced to oxidized glutathione (GSH/GSSG) and nicotinamide adenine dinucleotide phosphate (NADPH/NAPD+) (Kregel and Zhang 2007). Of these redox systems, GSH/GSSG can be taken as a global measure of the collective poise of endogenous antioxidant defenses, as the glutathione reductase/peroxidase system is linked, via redox cycles, to the other cellular antioxidant systems (Schafer and Buettner 2001). A progressive decline in GSH/GSSG with advancing age has been identified (Droge 2002), suggesting either a decreased ability of cells to neutralize oxidative stress, increased formation of ROS/RNS, or perhaps impaired GSH generation as the result of decreased glutathione reductase activity. In support of the latter possibility, several studies have reported oxidative inactivation of metabolic enzymes and membrane lipid peroxidation in aging brain (Beckman and Ames 1998; Bokov et al. 2004; Poon et al. 2004; Rodrigues Siqueira et al. 2005; Kregel and Zhang 2007). A major source of oxidant accumulation with age is mitochondrial dysfunction leading to overproduction and release of ROS/RNS into the cytosol (Sohal et al. 1995; Giulivi 1998). The ROS/RNS then uncouple eNOS, causing the enzyme to overproduce superoxide (O2−), which activates iNOS and drives the production of cytotoxic peroxynitrite in a manner similar to that of cardiac arrest induced brain ischemia-reperfusion (Manukhina et al. 2006; Ungvari et al. 2010; Kalyanaraman 2013). Collectively, cytosolic ROS/RNS accumulation activates death cascades mediated by caspase-9 and caspase-3 (Kirkland and Franklin 2003; Franklin 2011; Martin et al. 2011). Finally, it is important to note that chronic oxidative stress with advancing age has been implicated in the pathogenesis of age-related neurodegenerative disorders (Volicer and Crino 1990; Dexter et al. 1994).
Oxidative damage to mitochondrial DNA
According to the mitochondrial theory of aging, the accumulation of mutations in mitochondrial DNA (mtDNA) over the lifetime leads to bioenergetic impairment and contributes substantially to aging (Linnane et al. 1989; Lee and Wei 2007). Because of its close proximity to the respiratory chain, the mitochondrial genome is particularly susceptible to oxidative damage from excessive ROS/RNS produced during ischemia-reperfusion (Richter 1995; Chen et al. 2001) and accumulated during aging (Mecocci et al. 1993; Ozawa 1995; Barja and Herrero 2000). Moreover, mtDNA is much more vulnerable to oxidative modification than nuclear DNA (Ames et al. 1993; Richter 1995), due to its lack of protection by histones and the limited ability to repair mtDNA, compared with nuclear DNA (Croteau et al. 1999; Lee and Wei 2007). Modification of mtDNA by ROS/RNS may alter genes expressing protein components of the respiratory chain, which creates a vicious cycle of ROS/RNS over-production and further cell damage, eventually culminating in apoptosis (Murakami et al. 1998; Chen et al. 2001; Lee and Wei 2007). Indeed, disturbances of mitochondrial gene expression which disable oxidative phosphorylation within the CA1 neurons of the hippocampus have been demonstrated during reperfusion, and ultimately culminate in cell death (Abe et al. 1996).
Neurocognitive impairment from oxidative stress
Survivors of cardiac arrest often endure cognitive and behavioral impairments such as deficits in long-term memory and executive function (Parnia et al. 2007). For example, between 2 months to 1 year after resuscitation, patients’ ability to recall memory is impaired (Grubb et al. 1996). Computed tomography and magnetic resonance imaging revealed that oxidant-induced tissue atrophy following cardiac arrest extended beyond the hippocampus to involve the frontal and temporal lobes, which relates to the disrupted executive function in cardiac arrest survivors (Grubb et al. 2000; Nunes et al. 2003). In a longer-term study that compared cardiac arrest survivors to patients who survived myocardial infarction without cardiac arrest, memory scores in both groups declined with age, but the cardiac arrest survivors performed significantly worse on recall memory assessments vs. myocardial infarction survivors 3 years after the ischemic event (Drysdale et al. 2000). This impairment demonstrates that neurocognitive function may be sufficiently compromised to severely impact quality of life (O’Reilly et al. 2003). Similarly, memory and executive function become impaired during aging. As oxidative stress accumulates with advancing age, the brain deterioration eventually causes neurocognitive impairments resembling to those that follow cardiac arrest-resuscitation (Mahncke et al. 2006; Kim and Oh 2013).
Oxidative stress has been implicated in the pathogenesis of neurodegeneration and neurocognitive impairment after cardiac arrest (Liu et al. 1998; Parnia et al. 2007; Fiskum et al. 2008) and during aging (Dexter et al. 1994; Volicer and Crino 1990). To test the hypothesis that overproduction of ROS/RNS produces neurocognitive impairment, Vereczki et al. compared resuscitation of dogs with 100 % oxygen vs. room air (ca. 21 % oxygen) and found that hyperoxic resuscitation increased hippocampal tyrosine nitration—a marker of oxidative cell injury—and intensified post-ischemic impairment of hippocampus-dependent functions (Matsuzawa and Ichijo 2005; Vereczki et al. 2006; Kregel and Zhang 2007).
Summary
A common mechanism underlying brain deterioration both following cardiac arrest-resuscitation and during aging is the accumulation of ROS/RNS, which act to modify mtDNA, disrupt cellular function, initiate apoptosis, and ultimately impair neurocognitive function. An important difference between aging vs. post-cardiac arrest is the time course of ROS/RNS accumulation—that is, alterations in ROS/RNS concentrations occur within minutes following resuscitation from cardiac arrest, but develop over many years during aging. In both cases, the accumulated ROS/RNS attack mitochondrial and nuclear DNA, inactivate enzymes catalyzing energy metabolism, compromise ATP production, and impair the glutathione peroxidase/reductase and thioredoxin/peroxiredoxin antioxidant systems. Additionally, oxidative stress in both settings provokes opening of the Bax/Bak and mitochondrial permeability transition pores, which respectively release cytochrome c into the cytosol and dissipate the electrochemical gradient required for oxidative phosphorylation. Cytochrome-c release initiates activation of caspase-9 and caspase-3 and eventually apoptotic cell death within the affected brain tissue. Cell death caused by oxidative stress in both post-resuscitation and aging results in recall memory loss and deficits in executive function. Thus, aging and cardiac arrest-resuscitation produce remarkably similar cascades of oxidative stress, cell death, and neurocognitive impairment, albeit over vastly different time courses.
Immune response
Immune response to cardiac arrest and resuscitation
The sterile inflammatory response—i.e., that in the absence of microorganisms—to ischemia and reperfusion during cardiac arrest and resuscitation is initiated in an effort to repair damaged tissue. In a manner similar to the response directed against invading pathogens, ischemia increases neutrophil recruitment and production of cytokines, chemokines, and other pro-inflammatory stimuli with in the brain (Kalogeris et al. 2012; Kvietys and Granger 2012). Activated neutrophils infiltrate the ischemic brain parenchyma and initiate damage by releasing ROS/RNS, hydrolytic enzymes, and pore-forming molecules onto targeted cells (Kalogeris et al. 2012). The neutrophil-generated ROS/RNS promote leukocyte adhesion to post-capillary venules and their infiltration of the tissue, intensifying post-ischemic injury (Kalogeris et al. 2012; Kvietys and Granger 2012). In the brain capillary endothelium, xanthine oxidase and other ROS-forming enzymes perturb nitric oxide production and induce endothelial expression of leukocyte-specific adhesion molecules to promote adhesion of innate immune cells (Kalogeris et al. 2012; Kvietys and Granger 2012). Moreover, during this time, other perivascular cells in the brain including macrophages and mast cells are activated and begin to release inflammatory mediators such as TNF-α, platelet-activating factor, leukotriene B4, and other cytokines to promote leukocyte adhesion to the post-capillary endothelium (Kalogeris et al. 2012). Collectively, these maladaptive responses to ischemia and reperfusion of the brain and capillary endothelium exacerbate the oxidative injury inflicted by cardiac arrest and provoke endothelium-dependent microcirculatory dysfunction, which disrupts delivery of nutrients and clearance of waste products after resuscitation (Jerome et al. 1995; Kalogeris et al. 2012; Kvietys and Granger 2012).
Release of TNF-α triggers an extrinsic apoptotic pathway in post-ischemic tissue that activates caspase-8, which then cleaves and activates caspase-3, leading to cleavage of cellular proteins and death of affected cells (Kroemer et al. 2007; Broughton et al. 2009). An intrinsic pathway is activated by oxidant-induced Bax and Bak integration into the outer mitochondrial membrane, allowing the aforementioned cytochrome-c release and activation of the caspase-9 and caspase-3 cell death cascade (Kroemer et al. 2007; Broughton et al. 2009). Moreover, necrosis induced by ischemia-reperfusion also activates the complement system (Hill and Ward 1971; Rossen et al. 1994; Frangogiannis et al. 2002; Ioannou et al. 2011). The classical, alternative, and mannose-binding lectin complement pathways have all been implicated in ischemia-reperfusion injury (Kalogeris et al. 2012). The activated complement system recruits neutrophils and macrophages to the site of injury and also causes direct cell lysis by formation of a plasma membrane attack complex (Kalogeris et al. 2012). Thus, cardiac arrest leads to brain damage through a multifaceted mechanism of inflammation and cytotoxic pore formation.
Immune response to aging
Increased basal inflammation is considered an underlying mechanism of aging (Sierra et al. 2014). There is an age-related increase in proinflammatory cytokines in the aging brain, including IL-1β, IL-6, and TNFα (Krabbe et al. 2004; Diniz et al. 2010). Additionally, the chronic oxyradical burden that accompanies aging causes stress to the endoplasmic reticulum of microglia, which in turn provokes NF-κB activity to exacerbate the inflammatory response to aging (Hasnain et al. 2012). In accordance with the “oxidative stress theory” of aging, it is conceivable that the chronic accumulation of oxidants would potentiate an immune response similar to that ensuing after the acute, rapid accumulation of ROS/RNS following cardiac arrest-induced ischemia-reperfusion.
As endogenous antioxidant defenses are gradually depleted in the aging brain, sustained activation of perivascular macrophages by ROS/RNS would provoke these cells to infiltrate the brain parenchyma and cause damage and cell death. Specifically, by releasing ROS, proteolytic enzymes, and inflammatory cytokines (e.g., IL-1β, IL-6, and TNFα), these activated macrophages initiate mechanisms that incorporate Bax and Bak into the outer mitochondrial membrane (Kroemer et al. 2007; Broughton et al. 2009). The resulting cytochrome c release activates the intrinsic, caspase-mediated apoptotic pathway which eventually destroys the affected neurons, astrocytes, and microglia (Kroemer et al. 2007; Broughton et al. 2009). When TNF-α released by the macrophages triggers the extrinsic apoptotic pathway, caspase-8 and caspase-3 are activated, leading to further cell death (Kroemer et al. 2007; Broughton et al. 2009). The microcirculatory dysfunction summarized above exacerbates these intrinsic and extrinsic apoptotic pathways (Jerome et al. 1995; Kalogeris et al. 2012; Kvietys and Granger 2012). During aging, chronic apoptosis of this nature would lead to degeneration of brain tissue in the regions that are particularly susceptible to oxidative stress, culminating in neurocognitive impairment. Additionally, recent studies have revealed that triggering of the innate immune system provokes an exaggerated local immune response within the hippocampus of aged rats (Barrientos et al. 2006; Cortese et al. 2011). In this experimental model, Escherichia coli were injected into the peritoneum of aged and young rats in order to activate the innate immune system. In response to signals triggered by this immune activation, aged rats showed a more intense inflammation within the brain than the young rats, exemplified by persistently increased hippocampal production of the pro-inflammatory cytokine interleukin-1β (Barrientos et al. 2006). This exaggerated inflammatory response did not affect short-term memory, but did produce substantial deficits in hippocampus-dependent long-term memory (Barrientos et al. 2006).
Summary
Cardiac arrest-resuscitation and aging trigger an immune response that provokes brain degeneration, particularly in the regions most susceptible to oxidative stress and oxidant-induced inflammation. In both cases, and by similar mechanisms, this response is exaggerated by excessive production of ROS/RNS and leads to activation of perivascular inflammatory macrophages. Once activated, these cells release pro-inflammatory cytokines to recruit neutrophils to the site of injury and provoke leukocyte adhesion to the post-capillary endothelium. The neutrophils release proteolytic enzymes and ROS/RNS to cause apoptosis of the injured cells, and the accumulated leukocytes then clear the cellular remnants, leading to degeneration of the regions affected by ischemia-reperfusion and aging. One susceptible region—the hippocampus—is pivotal in neurocognitive functions such as learning and memory. Ischemia-reperfusion and aging impose oxidative stress, initiating an inflammatory response leading to tissue degeneration and neurocognitive impairment (Fig. 2).
Antioxidant therapies
Numerous preclinical and clinical studies have examined the potential neuroprotective effects of various antioxidants during both cardiac arrest (Table 1) and aging (Table 2). However, many agents that exerted robust neuroprotection in preclinical studies failed to protect when used in clinical trials. Thus, clinically effective pharmacological treatments to mitigate oxidative stress and brain injury from cardiac arrest-resuscitation and/or aging remain elusive.
Table 1.
Preclinical and clinical studies of interventions to protect the brain from cardiac arrest-resuscitation
| Reference | Trial type | Species | Treatment | Factor(s) tested | Findings |
|---|---|---|---|---|---|
| Undén et al. (2013) | Preclinical | Rat | Erythropoietin | Ischemia-reperfusion injury of brain | Post-ischemic treatment with erythropoietin is not neuroprotective in a cardiac arrest model. |
| Ostadal et al. (2013) | Preclinical | Swine | Hypothermia | Oxidative stress | Therapeutic hypothermia aided in maintenance of blood pressure and cerebral oxygenation, and prevented oxidant-induced organ damage after cardiac arrest |
| Dohi et al. (2013) | Clinical | Human | Hypothermia | Oxidative stress | Hypothermia downregulated ROS production and fortified endogenous antioxidant systems during resuscitation |
| Motl et al. (2012) | Preclinical | Rat | Vitamin C | Resuscitability | Vitamin C failed to preserve ventricular distensibility and impaired resuscitability |
| Gong et al. (2012) | Preclinical | Swine | Hypothermia | Oxidative stress | Hypothermia decreased production of ROS, preserved function of mitochondrial respiratory enzymes and upregulated the antioxidant MnSOD and Nrf2 |
| Tsai et al. (2011) | Preclinical | Rat | Vitamin C | Oxidative stress | Vitamin C (100 mg/kg body wt) decreased lipid peroxidation and respiratory dysfunction following cardiac arrest |
Table 2.
Preclinical and clinical studies of interventions to slow the neurodegenerative effects of aging
| Reference | Stage | Subject | Treatment | Factor(s) tested | Findings |
|---|---|---|---|---|---|
| Dysken et al. (2014) | Clinical | Human | Vitamin E | Cognitive function in AD | Among patients with mild to moderate AD, those who received vitamin E (2,000 IU/day) showed slower decline in cognitive function |
| Shetty et al. (2013) | Preclinical | Mouse | CoQ | Cognitive function | Protein oxidation was decreased and spatial learning impairment was not as severe in aged mice supplemented with high-dose CoQ |
| von Arnim et al. (2012) | Clinical | Human | N/A | Serum antioxidant concentrations | Vitamin C and β-carotene concentrations were lower in demented vs control subjects |
| Galasko et al. (2012) | Clinical | Human | Vitamin C + vitamin E + α-lipoic acid coenzyme-Q | CSF biomarkers of AD and OxS | Vitamin C (500 mg) + vitamin E (800 IU) + α-lipoic acid (900 mg) administered daily for 16 weeks did not influence biomarkers of AD, but did reduce oxidative stress. However, this treatment may accelerate cognitive decline |
| Lloret et al. (2009) | Clinical | Human | Vitamin E | Cognitive function | Vitamin E lowers oxidative stress and maintains preserves function in some AD patients, but in patients for whom vitamin E did not prevent oxidative stress, supplementation caused detrimental effects to cognition |
| Pérez et al. (2009) | Preclinical | Mouse | N/A | Overexpression of antioxidant enzymes | Overexpression of copper zince superoxide dismutase, catalase, and/or manganese superoxide dismutase was insufficient to extend lifespan |
| Mcdonald et al. (2005) | Preclinical | Mouse | Coenzyme-Q + vitamin E | Cognitive function | Aged mice given daily supplements of CoQ (123 mg/kg body wt) with (+)-α-tocopherol (200 mg/kg body wt) showed enhanced learning |
| Maxwell et al. (2005) | Prospective analysis | Human | Vitamin C and vitamin E | Risk of cognitive decline | Population-based prospective 5-year study shows that patients who take antioxidant vitamins were less likely to develop significant cognitive decline |
| Arzi et al. (2004) | Preclinical | Mouse | Vitamin C and vitamin E | Cognitive function | Separately, vitamin C and E had no effect on cognitive function in aged mice. Combined, improved cognitive function in aged but not young mice. Synergistic effect of combined administration proposed to be regeneration of α-tocopherol by vitamin C |
| Sumien et al. (2004) | Preclinical | Mouse | Vitamin E | Cognitive function | Short-term supplementation of vitamin E (1.65 g/kg body wt) did not reverse preexisting age-related impairments in cognitive function |
| Sumien et al. (2003) | Preclinical | Mouse | Vitamin E | Oxidative damage | Supplementation with vitamin E had little or no impact on the steady-state degree of cellular oxidative damage |
AD Alzheimer’s disease, OxS oxidative stress
Antioxidant therapy following cardiac arrest-resuscitation
In rats subjected to ventricular fibrillation and CPR, treatment with ascorbic acid (vitamin C) following cardiac arrest reduced lipid peroxidation and mitochondrial oxidative stress (Tsai et al. 2011), but failed to preserve left ventricular distensibility during CPR and negatively impacted resuscitability (Motl et al. 2012). The study of Motl et al. (2012) suggests that scavenging ROS may disrupt protective oxidant-mediated signaling.
Erythropoietin minimizes ischemia-reperfusion injury of brain by stabilizing mitochondrial function and preventing formation of ROS (Nguyen et al. 2014). Erythropoietin induces key components of the brain’s antioxidant defenses, such as glutathione S-transferase, NAD(P)H:quinone oxidoreductase-1, and heme oxygenase-1 (Zhang et al. 2010). Erythropoietin, however, does not readily traverse the blood brain barrier, so massive doses are required for neuroprotection, greatly increasing the risk for thrombosis and stroke (McPherson and Juul 2008).
Therapeutic hypothermia is the only intervention that has proven to be clinically effective in minimizing brain injury from cardiac arrest-induced ischemia-reperfusion. By slowing cellular metabolism, hypothermia dampens production of ROS and fortifies endogenous antioxidant defenses during rewarming (Dohi et al. 2013). In a swine model of cardiac arrest-resuscitation, therapeutic hypothermia maintained blood pressure and cerebral oxygenation after ROSC and prevented organ damage by suppressing oxidative stress (Ostadal et al. 2013). This antioxidant action of hypothermia during cardiac arrest is partly attributed to protection of respiratory enzymes and upregulation of an antioxidant enzyme, manganese superoxide dismutase (Gong et al. 2012).
Antioxidant therapy in aging
Ascorbic acid content of brain is lower in demented elderly individuals, and an analysis of 894 patient records revealed that dementia patients taking pharmacological dosages of vitamin C were less likely to develop significant cognitive decline (von Arnim et al. 2012). However, supplementation with vitamin C has failed to prevent cognitive decline with aging (Arzi et al. 2004; Galasko et al. 2012). Similarly, α-tocopherol (vitamin E) had no impact on steady-state oxidative damage (Sumien et al. 2003) and short-term supplementation with vitamin E did not reverse preexisting age-related cognitive impairments in mice (Sumien et al. 2004), but did slow the decline in cognitive function in Alzheimer’s disease patients (Dysken et al. 2014). On the other hand, co-administration of vitamin E with other antioxidant vitamins (vitamin C, coenzyme Q, α-lipoic acid) improved cognitive function in aged mice (Arzi et al. 2004; Mcdonald et al. 2005) and elderly human subjects (Galasko et al. 2012). Finally, high-dose coenzyme Q10 was shown to preserve spatial learning and decrease protein oxidation in brain mitochondria of aged mice when given for a short duration (Shetty et al. 2013).
Summary
Although antioxidant therapies have not proven unequivocally effective against oxidant-induced damage and neurological impairment following cardiac arrest-resuscitation and during aging, treatments are still being developed. Therapeutic hypothermia greatly reduces post-resuscitation oxidative stress, and coenzyme Q administration has shown promise in preservation of cognitive function during aging, but it has been recently suggested by Ghosh et al. that the reactive oxygen species generated during both cardiac arrest-resuscitation and aging may be formed downstream of more impactful therapeutic targets. Accordingly, induction of antioxidant defenses upstream of RONS production, e.g., by activating NAD(P)H production or induction of antioxidant gene expression may afford more robust protection of cognitive function than simple antioxidant treatments (Ghosh et al. 2014a, b).
Conclusions and commentary
In accordance with the “oxidative stress theory” of aging, it is apparent that many components of the pathogenesis of damage and death in the aging brain are common to the mechanisms of brain injury following cardiac arrest-resuscitation, particularly those mechanisms mediated by ROS/RNS. The principal difference between these two forms of neurodegeneration and neurocognitive impairment is their respective time courses. That is, the years of ROS/RNS accumulation and the resulting mitochondrial and cellular dysfunction that provoke inflammatory responses and cell death in the aged brain occur within a matter of minutes following cardiac arrest and resuscitation. Accordingly, a collaborative effort to resolve the mechanisms of injury in these neurodegenerative scenarios could potentially enhance our understanding of both the pathobiology of aging and of brain resuscitation following cardiac arrest. Indeed, cardiac arrest-resuscitation may provide an accelerated model of the brain aging process, affording more efficient development of treatments to target the common elements of these injury cascades to ultimately promote patient survival and quality of life.
Acknowledgments
This work was supported by research grant R01 NS076975 from the U.S. National Institute of Neurological Disorders and Stroke and by research grant P01 AG22550 from the National Institute on Aging. BHC was supported by a pre-doctoral fellowship from the National Institute of Aging, Training in the Neurobiology of Aging, grant T31 AG020494. This work was conducted in partial fulfillment of the requirements for the Ph.D. degree for BHC.
Abbreviations
- AD
Alzheimer’s disease
- Bak
Bcl-2 homologous antagonist killer
- Bax
Bcl-2-associated X protein
- BBB
Blood–brain barrier
- Bcl-2
B-cell lymphoma 2 family of proteins
- BH4
Tetrahydrobiopterin
- Casp-3
Caspase-3
- Casp-9
Caspase-9
- CoQ
Coenzyme-Q
- CPR
Cardiopulmonary resuscitation
- Cyt C
Cytochrome C
- eNOS
Endothelial isoform of nitric oxide synthase
- GSH
Reduced form of glutathione
- GSH/GSSG
Concentration ratio of reduced to oxidized glutathione
- iNOS
Inducible isoform of nitric oxide synthase
- mPTP
Mitochondrial permeability transition pore
- mtDNA
Mitochondrial DNA
- NFκB
Nuclear factor-kappa B
- NO
Nitric oxide
- O2−
Superoxide
- ONOO−
Peroxynitrite
- OxS
Oxidative stress
- ROS/RNS
Reactive oxygen and nitrogen species
- SMAC
Second mitochondria-derived activator of caspases
- TNF-α
Tumor necrosis factor alpha
References
- Abe K, Kawagoe J, Itoyama Y, Kogure K. Isolation of an ischemia-induced gene and early disturbance of mitochondrial DNA expression after transient forebrain ischemia. Adv Neurol. 1996;71:485–503. [PubMed] [Google Scholar]
- Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care. 2004;10:208–212. doi: 10.1097/01.ccx.0000126090.06275.fe. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 90:7915–7922 [DOI] [PMC free article] [PubMed]
- Arzi A, Hemmati AA, Razian AA. Effect of Vitamins C and E on cognitive function in mouse. Pharm Res. 2004;49:249–252. doi: 10.1016/j.phrs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Backstrom T, Goiny M, Lockowandt U, Liska J, Franco-Cereceda A. Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J Appl Physiol. 2003;94:1122–1128. doi: 10.1152/japplphysiol.00138.2002. [DOI] [PubMed] [Google Scholar]
- Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- Bano D, Nicotera P. Ca2+ signals and neuronal death in brain ischemia. Stroke. 2007;38:674–676. doi: 10.1161/01.STR.0000256294.46009.29. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF (2006) Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging 27:723–732 [DOI] [PubMed]
- Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14:312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Blumberg J. Use of biomarkers of oxidative stress in research studies. J Nutr. 2004;134:3188S–3189S. doi: 10.1093/jn/134.11.3188S. [DOI] [PubMed] [Google Scholar]
- Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide, cytochrome c and mitochondria. Biochem Soc Symp. 1999;66:17–25. doi: 10.1042/bss0660017. [DOI] [PubMed] [Google Scholar]
- Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharm. 2000;401:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- Cerchiari EL, Hoel TM, Safar P, Sclabassi RJ. Protective effects of combined superoxide dismutase and deferoxamine on recovery of cerebral blood flow and function after cardiac arrest in dogs. Stroke. 1987;18:869–878. doi: 10.1161/01.str.18.5.869. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu CJ, He YY, Yang DI, Xu J, Hsu CY. Reduction and restoration of mitochondrial dna content after focal cerebral ischemia/reperfusion. Stroke. 2001;32:2382–2387. doi: 10.1161/hs1001.097099. [DOI] [PubMed] [Google Scholar]
- Conroy BP, Black D, Lin CY, Jenkins LW, Crumrine RC, DeWitt DS, Johnston WE. Lamotrigine attenuates cortical glutamate release during global cerebral ischemia in pigs on cardiopulmonary bypass. Anesthesiology. 1999;90:844–854. doi: 10.1097/00000542-199903000-00028. [DOI] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau DL, Stierum RH, Bohr VA. Mitochondrial DNA repair pathways. Mutat Res. 1999;434:137–148. doi: 10.1016/s0921-8777(99)00025-7. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Holley AE, Flitter WD, Slater TF, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Talib L, Gattaz WF, Forlenza OV. Interleukin-1β serum levels is increased in antidepressant-free elderly depressed patients. Am J Geriatr Psychiatr. 2010;18:172–176. doi: 10.1097/JGP.0b013e3181c2947f. [DOI] [PubMed] [Google Scholar]
- Dohi K, Ohtaki H, Inn R, Ikeda Y, Shioda HS, Aruga T. Peroxynitrite and caspase-3 expression after ischemia/reperfusion in mouse cardiac arrest model. Acta Neurochir Suppl. 2003;86:87–91. doi: 10.1007/978-3-7091-0651-8_20. [DOI] [PubMed] [Google Scholar]
- Dohi K, Miyamoto K, Fukuda K, Nakamura S, Hayashi M, Ohtaki H, Shioda S, Aruga T. Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxidative Med Cell Longev. 2013;2013:562429. doi: 10.1155/2013/562429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Drysdale EE, Grubb NR, Fox KA, O’Carroll RE. Chronicity of memory impairment in long-term out-of-hospital cardiac arrest survivors. Resuscitation. 2000;47:27–32. doi: 10.1016/s0300-9572(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Dysken MW, Sano M, Asthana S, Sano M, Asthana S, Vertrees JE, Pallaki M, Llorente M, Love S, Schellenberg GD, McCarten JR, Malphurs J, Preto S, Chen P, Loreck DJ, Trapp G, Bakshi RS, Mintzer JE, Heidebrink JL, Vidal-Cardona A, Arroyo LM, Cruz AR, Zachariah S, Kowall NW, Chopra MP, Craft S, Thielke S, Turvey CL, Woodman C, Monnell KA, Gordon K, Tomaska J, Segal Y, Peduzzi PN, Guarino PD. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VAcooperative randomized trial. JAMA. 2014;311:33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, Hopkins I, Richards EM, Rosenthal RE. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008;1147:129–138. doi: 10.1196/annals.1427.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Franklin JL. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal. 2011;14:1437–1448. doi: 10.1089/ars.2010.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, Cotman C, Cottrell B, Montine TJ, Thomas RG, Aisen P. Antioxidants for alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarkers. Arch Neurol. 2012;60:836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Brewer GJ. Dual-energy precurser and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species. Neurobiol Aging. 2014;35:179–190. doi: 10.1016/j.neurobiolaging.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, LeVault KR, Brewer GJ (2014b) Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell 1–10. doi: 10.1111/acel.12216 [DOI] [PMC free article] [PubMed]
- Giulivi C. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem J. 1998;332:673–679. doi: 10.1042/bj3320673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, 3rd, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S Heart disease and stroke statistics–2014 update: a report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei X, Zhang MY, Cui J. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating MnSOD in a pig model of cardiac arrest. PLoS One. 2012;7:e35313. doi: 10.1371/journal.pone.0035313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb NR, O’Carroll R, Cobbe SM, Sirel J, Fox KA. Chronic memory impairment after cardiac arrest outside hospital. Br Med J. 1996;313:143–146. doi: 10.1136/bmj.313.7050.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb NR, Fox KA, Smith K, et al. Memory impairment in out-of-hospital cardiac arrest survivors is associated with global reduction in brain volume, not focal hippocampal injury. Stroke. 2000;31:1509–1514. doi: 10.1161/01.str.31.7.1509. [DOI] [PubMed] [Google Scholar]
- Hagen TM. Oxidative stress, redox imbalance, and the aging process. Antioxid Redox Signal. 2003;5:503–506. doi: 10.1089/152308603770310149. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- Hasnain SZ, Lourie R, Das I, Chen CH, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M. Deaths: leading causes for 2008. Natl Vital Stat Rep. 2012;60:1–94. [PubMed] [Google Scholar]
- Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900. doi: 10.1084/jem.133.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idris AH, Roberts LJ, 2nd, Caruso L, Showstark M, Layon AJ, Becker LB, Vanden Hoek T, Gabrielli A. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med. 2005;33:2043–2048. doi: 10.1097/01.ccm.0000174104.50799.bd. [DOI] [PubMed] [Google Scholar]
- Ioannou A, Dalle Lucca J, Tsokos GC. Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol. 2011;141:3–14. doi: 10.1016/j.clim.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Jerome SN, Akimitsu T, Gute DC, Korthuis RJ. Ischemic preconditioning attenuates capillary no-reflow induced by prolonged ischemia and reperfusion. Am J Physiol. 1995;268:H2063–H2067. doi: 10.1152/ajpheart.1995.268.5.H2063. [DOI] [PubMed] [Google Scholar]
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B. Teaching the basics of redox biology to medical and graduate students: oxidants, antioxidants and disease mechanisms. Redox Biol. 2013;1:244–257. doi: 10.1016/j.redox.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4:179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Oh S. Age-related changes in cognition and speech perception. Korean J Audiol. 2013;17:54–58. doi: 10.7874/kja.2013.17.2.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxid Redox Signal. 2003;5:589–596. doi: 10.1089/152308603770310257. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556–592. doi: 10.1016/j.freeradbiomed.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW. Hippocampal neurobiological mechanisms of age-related memory dysfunction. Neurobiol Aging. 1988;9:571–579. doi: 10.1016/s0197-4580(88)80116-7. [DOI] [PubMed] [Google Scholar]
- Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 2007;232:592–606. [PubMed] [Google Scholar]
- Li XM, Yang JM, Hu DH, Hou FQ, Zhao M, Zhu XH, Wang Y, Li JG, Hu P, Chen L, Qin LN, Gao TM. Contribution of downregulation of L-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J Neurosci. 2007;27:5249–5259. doi: 10.1523/JNEUROSCI.0802-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet. 1989;1(8639):642–645. doi: 10.1016/s0140-6736(89)92145-4. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu T, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- Lloret A, Badía MC, Mora NJ, Pallardó FV, Alonso MD, Viña J. Vitamin E paradox in Alzheimer’s desease: it does not prevent loss of cognittion and may even be detrimental. J Alzheimers Dis. 2009;17:143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. Prog Brain Res. 2006;157:81–109. doi: 10.1016/S0079-6123(06)57006-2. [DOI] [PubMed] [Google Scholar]
- Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med (Maywood) 2006;231:343–365. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Adams NA, Pan Y, Price A, Wong M. The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. J Neurosci. 2011;31:359–370. doi: 10.1523/JNEUROSCI.2225-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid Redox Signal. 2005;7:472–481. doi: 10.1089/ars.2005.7.472. [DOI] [PubMed] [Google Scholar]
- Maxwell CJ, Hicks MS, Hogan DB, Basran J, Ebly EM. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement Geriatr Cogn Disord. 2005;20:45–51. doi: 10.1159/000085074. [DOI] [PubMed] [Google Scholar]
- Mcdonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and α-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- Motl J, Radhakrishnan J, Ayoub IM, Grmec S, Gazmuri RJ (2012) Vitamin C Compromises Cardiac Resuscitability in a Rat Model of Ventricular Fibrillation. Am J Ther. doi:10.1097/MJT.0b013e31824e2b9f. http://www.ncbi.nlm.nih.gov/pubmed/?term=Motl+J%2C+Radhakrishnan+J%2C+Ayoub+IM%2C+Grmec+S%2C+Gazmuri+RJ+(2012)+Vitamin+C+Compromises+Cardiac+Resuscitability%C2%A0%C2%A0in+a+Rat+Model+of+Ventricular+Fibrillation.+Am+J+Ther+(in+press) [DOI] [PubMed]
- Moulaert VRMP, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: a systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AQ, Cherry BH, Scott GF, Ryou M, Mallet RT (2014) Erythropoietin: powerful protection of ischemic and post-ischemic brain. Exp Biol Med. doi: 10.1177/1535370214523703 [DOI] [PMC free article] [PubMed]
- Nolan JP, Lyon RM, Sasson C, Rossetti AO, Lansky AJ, Fox KA, Meier P. Advances in the hospital management of patients following an out of hospital cardiac arrest. Heart. 2012;98:1201–1206. doi: 10.1136/heartjnl-2011-301293. [DOI] [PubMed] [Google Scholar]
- Nunes B, Pais J, Garcia R, Magalhães Z, Granja C, Silva MC. Cardiac arrest: long-term cognitive and imaging analysis. Resuscitation. 2003;57:287–297. doi: 10.1016/s0300-9572(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Opie LH. Reperfusion injury—fad, fashion, or fact? Cardiovasc Drugs Ther. 1991;5(Suppl 2):223–224. doi: 10.1007/BF00054744. [DOI] [PubMed] [Google Scholar]
- O’Reilly SM, Grubb NR, O’Carroll RE (2003) In-hospital cardiac arrest leads to chronic memory impairment. Resuscitation 58:73–79 [DOI] [PubMed]
- Ostadal P, Mlcek M, Kruger A, Horakova S, Skabradova M, Holy F, Svoboda T, Belohlavek J, Hrachovina V, Taborsky L, Dudkova V, Psotova H, Kittnar O, Neuzil P. Mild therapeutic hypothermia is superior to controlled normothermia for the maintenance of blood pressure and cerebral oxygenation, prevention of organ damage and suppression of oxidative stress after cardiac arrest in a porcine model. J Transl Med. 2013;11:124. doi: 10.1186/1479-5876-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T. Mitochondrial DNA mutations associated with aging and degenerative diseases. Exp Gerontol. 1995;30:269–290. doi: 10.1016/0531-5565(94)00057-a. [DOI] [PubMed] [Google Scholar]
- Parnia S, Spearpoint K, Fenwick PB (2007) Near death experiences, cognitive function and psychological outcomes of surviving cardiac arrest. Resuscitation 74:215–221. doi:10.1016/j.resuscitation.2007.01.020 [DOI] [PubMed]
- Pérez VI, Remmen HV, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespand of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals and brain aging. Clin Geriatr Med. 2004;20:329–359. doi: 10.1016/j.cger.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27:647–653. doi: 10.1016/1357-2725(95)00025-k. [DOI] [PubMed] [Google Scholar]
- Rodrigues Siqueira I, Fochesatto C, da Silva Torres IL, Dalmaz C, Alexandre Netto C. Aging affects oxidative state in hippocampus, hypothalamus and adrenal glands of Wistar rats. Life Sci. 2005;78:271–278. doi: 10.1016/j.lfs.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Rossen RD, Michael LH, Hawkins HK, Youker K, Dreyer WJ, Baughn RE, Entman ML. Cardiolipin-protein complexes and initiation of complement activation after coronary artery occlusion. Circ Res. 1994;75:546–555. doi: 10.1161/01.res.75.3.546. [DOI] [PubMed] [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Shetty RA, Forster MJ. Coenzyme Q10 supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. AGE. 2013;35:1821–1834. doi: 10.1007/s11357-012-9484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Trembley MЀ. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Sohal BH. Oxidative stress and aging in the Mongolian gerbil (Meriones unguiculatus) Mech Ageing Dev. 1995;81:15–25. doi: 10.1016/0047-6374(94)01578-a. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Role of oxidant species in aging. Curr Med Chem. 2004;11:1105–1112. doi: 10.2174/0929867043365341. [DOI] [PubMed] [Google Scholar]
- Sumien N, Forster MJ, Sohal RS. Supplementation with vitamin E fails to attenuate oxidative damage in aged mice. Exp Gerontol. 2003;38:699–704. doi: 10.1016/s0531-5565(03)00068-8. [DOI] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Thiyagarajan M, Kaul CL, Sharma SS. Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol. 2004;142:899–911. doi: 10.1038/sj.bjp.0705811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Huang CH, Tsai CY, Chen HW, Lee HC, Cheng HJ, Hsu CY, Wang TD, Chang WT, Chen WJ. Ascorbic acid mitigates the myocardial injury after cardiac arrest and electrical shock. Intensive Care Med. 2011;37:2033–2040. doi: 10.1007/s00134-011-2362-6. [DOI] [PubMed] [Google Scholar]
- Undén J, Sjölund C, Länsberg JK, Wieloch T, Ruscher K, Romner B. Post-ischemic continuous infusion of erythropoeitin enhances recovery of lost memory function after global cerebral ischemia in the rat. BMC Neurosci. 2013;12:14–27. doi: 10.1186/1471-2202-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G (2006) Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab 26:821–835. doi:10.1038/sj.jcbfm.9600234 [DOI] [PMC free article] [PubMed]
- Volicer L, Crino PB. Involvement of free radicals in dementia of the Alzheimer type: a hypothesis. Neurobiol Aging. 1990;11:567–571. doi: 10.1016/0197-4580(90)90119-k. [DOI] [PubMed] [Google Scholar]
- von Arnim CA, Herbolsheimer F, Nikolaus T, Peter R, Biesalski HK, Ludolph AC, Riepe M, Nagel G. Dietary antioxidants and dementia in a population-based case–control study among older people in South Germany. J Alzheimers Dis. 2012;31:717–724. doi: 10.3233/JAD-2012-120634. [DOI] [PubMed] [Google Scholar]
- Wachelder EM, Moulaert VRMP, van Heugten C, Verbunt JA, Bekkers SCAM, Wade DT. Life after survival: long-term daily functioning and quality of life after an out-of-hospital cardiac arrest. Resuscitation. 2009;80:517–522. doi: 10.1016/j.resuscitation.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Wang X, Perez E, Liu R, Yan LJ, Mallet RT, Yang SH. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O'Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. N Engl J Med. 2009;361:605–611. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu Y, Zhou D, Want Z, Chen G. Recombinant human erythropoietin (rhEPO) alleviates early brain injury following sub-arachnoid hemorrhage in rats: possible involvement of Nrf2-ARE pathway. Cytokine. 2010;52:252–257. doi: 10.1016/j.cyto.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang X, Qiu L, Peeters-Scholte C, Hagberg H, Blomgren K. Nitrosylation precedes caspase-3 activation and translocation of apoptosis-inducing factor in neonatal rat cerebral hypoxia-ischaemia. J Neurochem. 2004;90:462–471. doi: 10.1111/j.1471-4159.2004.02500.x. [DOI] [PubMed] [Google Scholar]