Abstract
Previous studies have indirectly shown that type 1 gonococci are more resistant to phagocytosis by human neutrophils (PMN) than type 3 gonococci. Using phase contrast, fluorescent, and light microscopy, we directly quantitated PMN-gonococcal interaction, with emphasis on separating ingestion from attachment. PMN monolayers were incubated on slides with type 1 or type 3 gonococcal fluorescent antibody (FA). After methanol fixation, the FA-stained gonococci associated with PMN were cointed. Since the live PMN excludes FA, the FA-stained gonococci represent only extracellular gonococci. Methylene blue was then added to the smae slide to stain both ingested and surface attached gonococci. Using these methods, intracellular and extracellular cell-associated gonococci were quantitated under varying conditions. The numbers of methylene blue-stained cell-associated gonococci that were ingested were: with normal serum, 3.7 plus or minus 4.1 per cent for type 1 and 56.2 plus or minus 3.7 percent for type 3 (P smaller than 0.001); with heat-inactivated serum, 1.0 plus or minus 3.0 per cent for type 1 and 52.6 plus or minus 3.7 per cent for type 3 (P smaller than 0.001); with higher-titer anti-gonococcal antibody serum, 4.8 plus or minus 4.3 percent for type 1 and 64.0 plus or minus 1.6 per cent for type 3 (P smaller than 0.001). Thus, most type 3 organisms were ingested, but most type 1 gonococci were bound on the PMN surface.
Full text
PDF


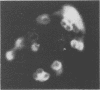
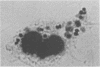
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang Y. H. Studies on phagocytosis. I. Uptake of radio-iodinated (131-I) human serum albumin as a measure of the degree of phagocytosis in vitro. Exp Cell Res. 1969 Jan;54(1):42–48. doi: 10.1016/0014-4827(69)90290-0. [DOI] [PubMed] [Google Scholar]
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus S. J., Glassman L. H. Scanning electron microscope study of Neisseria gonorrhoeae. Appl Microbiol. 1974 Mar;27(3):584–592. doi: 10.1128/am.27.3.584-592.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Endocytosis. Semin Hematol. 1970 Apr;7(2):161–171. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFFER J. M., KUCERA C. J., SPINK W. W. The protection of intracellular brucella against therapeutic agents and the bactericidal action of serum. J Exp Med. 1953 Jan;97(1):77–90. doi: 10.1084/jem.97.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Zeligs B. Studies on gonococcus infection. VI. Electron microscopic study on in vitro phagocytosis of gonococci by human leukocytes. Infect Immun. 1974 Sep;10(3):645–656. doi: 10.1128/iai.10.3.645-656.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972 Dec;126(6):601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. The preservation of gonococci in liquid nitrogen. J Clin Pathol. 1971 Mar;24(2):122–123. doi: 10.1136/jcp.24.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. J. The fate of gonococci in polymorphonuclear leucocytes. J Med Microbiol. 1970 Aug;3(3):501–509. doi: 10.1099/00222615-3-3-501. [DOI] [PubMed] [Google Scholar]