Abstract
Background
There is a higher prevalence of obesity in individuals with mental disorders compared to the general population. The results of several studies suggested that weight reduction in this population is possible following psycho-educational and/or behavioural weight management interventions. Evidence of the effectiveness alone is however inadequate for policy making. The aim of the current study was to evaluate the cost-effectiveness of a health promotion intervention targeting physical activity and healthy eating in individuals with mental disorders.
Methods
A Markov decision-analytic model using a public payer perspective was applied, projecting the one-year results of a 10-week intervention over a time horizon of 20 years, assuming a repeated yearly implementation of the programme. Scenario analysis was applied evaluating the effects on the results of alternative modelling assumptions. One-way sensitivity analysis was performed to assess the effects on the results of varying key input parameters.
Results
An incremental cost-effectiveness ratio of 27,096€/quality-adjusted life years (QALY) in men, and 40,139€/QALY in women was found in the base case. Scenario analysis assuming an increase in health-related quality of life as a result of the body mass index decrease resulted in much better cost-effectiveness in both men (3,357€/QALY) and women (3,766€/QALY). The uncertainty associated with the intervention effect had the greatest impact on the model.
Conclusions
As far as is known to the authors, this is the first health economic evaluation of a health promotion intervention targeting physical activity and healthy eating in individuals with mental disorders. Such research is important as it provides payers and governments with better insights how to spend the available resources in the most efficient way. Further research examining the cost-effectiveness of health promotion targeting physical activity and healthy eating in individuals with mental disorders is required.
Keywords: Mental disorders, Cost-effectiveness, Health promotion, Physical activity, Healthy eating
Background
The prevalence of overweight (Body Mass Index 25-29.9 kg/m2) and obesity (Body Mass Index ≥30 kg/m2) has increased in the last three decades and has become a serious global public health concern [1]. Obesity is a risk factor for the development of important non-communicable diseases including type 2 diabetes, coronary heart disease (CHD), stroke, and certain cancers such as colon cancer and breast cancer [2] leading to considerable healthcare expenditures [3]. Obesity is also associated with reduced health-related quality of life (HRQOL) [4] and with reduced life expectancy [5]. There is conclusive evidence that the burden of weight gain is even higher in individuals with mental disorders (MD) than in the general population [6, 7]. Important factors contributing to the high prevalence of overweight and obesity in people with MD are side effects of especially second generation antipsychotic drugs [8], a lack of regular physical activity (PA) and unhealthy eating behaviour [9].
Hence, health promotion interventions targeting PA and healthy eating should be integrated into the daily care of individuals with MD. The results of previous research suggested that weight loss following behavioural and/or psycho-educational programmes in MD patients is possible [10, 11]. Evidence on the effectiveness alone of such interventions is yet insufficient for policy making. Healthcare budgets are limited, hence policy makers are facing the problem how to set priorities in the allocation of healthcare resources to medical or public health interventions. Knowledge on this can be obtained by performing health economic evaluations of weight reduction programmes. The evidence derived from such research can assist regulatory bodies and health insurers establishing priorities within cost-constrained healthcare budgets. In the general population, health economic evaluations of health promotion programmes targeting PA [12] and healthy eating [13] yield mixed evidence on the cost-effectiveness of such interventions. In mental health care, such trials are lacking [14].
The aim of the current study was to evaluate the cost-effectiveness of a health promotion programme targeting PA and healthy eating in individuals with MD living in sheltered housing in the Flanders region in Belgium. Details on the design and results of the effectiveness study are described elsewhere [15]. In brief, the study design consisted of a cluster preference randomised controlled trial and was conducted in sheltered housing organisations (SHOs) in the Flanders region (Belgium). The intervention group included 16 SHOs (n = 201 individuals), while the control group included eight SHOs (n = 83 individuals). The health promotion intervention was based on an existing programme aimed at the general population developed by the Flemish Institute of Health Promotion and Disease Prevention [16]. As the target population of our study consisted of individuals with MD, some adjustments to the programme were made. The study consisted of a 10-week group-based and individually-based health promotion intervention, followed by a 6-month follow-up period. Individuals in the intervention group received the 10-week programme on top of their usual treatment, while those in the control group only received treatment as usual. The intervention was delivered by one or more mental health nurses working in the intervention SHOs. All participants in the intervention group received the same information in the same format comprising: (i) ten psycho-educational and behavioural group-based sessions in a 10-week period, (ii) group-based exercise in the same 10-week period (weekly 30’ supervised walking sessions), and (iii) individual support from the mental health nurses during the 10-week intervention. Data were collected at baseline, at ten weeks (end of the intervention) and after a six-month follow-up period.
Methods
Decision-analytic model
An age- and gender-dependent Markov decision-analytic model applying a public payer perspective was used to predict health outcomes and costs for the intervention and control arm. Health outcomes were expressed as quality-adjusted life years (QALYs) and calculated by multiplying the utility level (a HRQOL weight) for a given disease status with the number of years an individual is living with the disease. A utility of 1 equals perfect health, while 0 stands for death. In the cost dimension, both the programme costs and the disease costs were accounted for. Dividing the difference in costs between the intervention and the control group (=incremental costs) by the difference in QALYs between the two groups (=incremental QALYs) results in the incremental cost-effectiveness ratio (ICER) calculated as: ICER = (costI– costNI)/(QALYI-QALYNI), where ‘I’ stands for intervention and ‘NI’ for no intervention.
The Markov model was based on a published model [17] and further developed using ©Microsoft Excel (Microsoft Corporation, Redmond, WA, US) to account for the specific context and characteristics of the current study. Nine possible states were included in the model (Figure 1): (i) population at risk (‘at risk’), (ii) type 2 diabetes (‘diabetes’), (iii) CHD, first year (‘CHD 1’), (iv) CHD, following years (‘CHD 1+’), (v) stroke, first year (‘stroke 1’), (vi) stroke, following years (‘stroke 1+’), (vii) colon cancer, first year (‘colon cancer 1’), (viii) colon cancer, following years (‘colon cancer 1+’), and (ix) dead (‘dead’). The time horizon of the model was 20 years including 20 one-year periods (called ‘cycles’).
Figure 1.
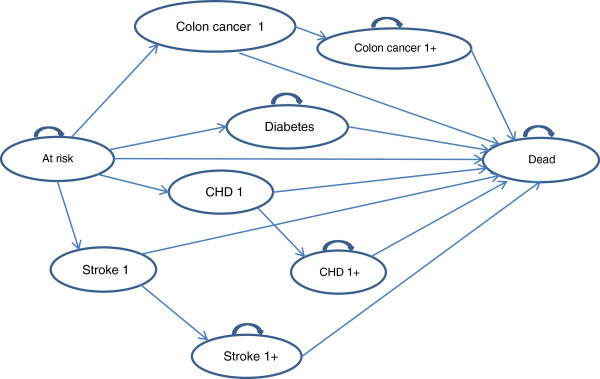
Markov decision-analytic model.
All individuals start in the ‘at risk’ state. During each cycle, an individual has a risk to move to one of the disease states or to ‘dead’. Once an individual is suffering from diabetes, he or she can only remain in that state or move to the ‘dead’ state. Patients suffering from stroke move to the ‘stroke 1’ state. Once a patient has had a stroke, he or she can only move to the ‘stroke 1+’ state or to ‘dead’. Patients who have had a fatal stroke move to the ‘dead’ state after being in the ‘stroke 1’ state for one cycle. Patients in the ‘stroke 1+’ state can only stay in that state or move to ‘dead’. Transitions throughout the model for patients suffering from CHD or colon cancer are analogous as for stroke. CHD includes myocardial infarction and stable or unstable angina. Once a patient enters the ‘dead’ state, no further transitions are possible, as this is the final state.
Clinical data inputs: disease and mortality transition probabilities
First, the risk of developing diabetes [18, 19], stroke [19, 20], CHD [18, 19], and colon cancer [21] for the general population was calculated. Second, the calculated transition probabilities were multiplied with a relative risk (RR) factor as it is known that individuals with MD are at a greater risk of having diabetes (RR 1.77) [22], stroke (RR 1.77) [23], CHD (RR 18-49 years: 1.42; RR 50-75 years: 1.01) [23], and colon cancer (RR 2.90) [24]. The transition probability from diabetes to colon cancer was calculated by multiplying the transition from ‘at risk’ to diabetes with a RR of 1.33 [25], since diabetic patients have a greater risk of developing colon cancer compared to those without diabetes. The mortality probabilities were obtained from the literature or they were calculated by multiplying national mortality probabilities [26] with the RR of dying from one of the diseases included in the model. Ubink-Veltmaat et al. [27] found a 40% mortality increase in people with diabetes compared to the general Dutch population. A twofold mortality risk was found among stroke patients compared to the Flemish general population [20]. Age- and sex-specific CHD mortality was derived from a study on CHD-mortality in the Netherlands [28]. Colon cancer mortality was obtained from the ‘Cancer survival in Belgium 2004-2008’ report [29]. An overview of the transition probabilities used in the model can be found in Table 1.
Table 1.
Age- and gender- dependent probabilities (%) for developing diabetes, stroke, coronary heart disease or colon cancer and associated mortality (%)
| Diabetes | Coronary heart disease | Stroke | Colon cancer | Overall mortality | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Case fatality rate | Incidence | Case fatality rate | |||||||||||||||||||
| Incidence | Case fatality rate | 1st year | Follow up | 1st year | Follow up | Incidence | Case fatality rate | |||||||||||||||
| Age (years) | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W | M | W |
| 20-24 | 0.06 | 0.05 | 0.12 | 0.04 | 0.01 | 0.01 | 5.50 | 7.30 | 4.87 | 7.74 | 0.02 | 0.02 | 13.00 | 25.00 | 0.16 | 0.06 | 0.01 | 0.01 | 6.62 | 5.67 | 0.08 | 0.03 |
| 25-29 | 0.10 | 0.08 | 0.13 | 0.06 | 0.02 | 0.02 | 5.50 | 7.30 | 4.87 | 7.74 | 0.03 | 0.03 | 13.00 | 25.00 | 0.18 | 0.08 | 0.01 | 0.01 | 6.62 | 5.67 | 0.09 | 0.04 |
| 30-34 | 0.17 | 0.14 | 0.13 | 0.06 | 0.05 | 0.03 | 5.50 | 7.30 | 4.87 | 7.74 | 0.05 | 0.04 | 13.00 | 25.00 | 0.18 | 0.09 | 0.01 | 0.01 | 6.62 | 5.67 | 0.09 | 0.04 |
| 35-39 | 0.28 | 0.23 | 0.19 | 0.11 | 0.10 | 0.05 | 5.50 | 7.30 | 4.87 | 7.74 | 0.07 | 0.06 | 13.00 | 25.00 | 0.26 | 0.15 | 0.03 | 0.03 | 6.62 | 5.67 | 0.12 | 0.07 |
| 40-44 | 0.48 | 0.39 | 0.26 | 0.16 | 0.19 | 0.08 | 5.50 | 7.30 | 4.87 | 7.74 | 0.11 | 0.09 | 13.00 | 25.00 | 0.36 | 0.23 | 0.04 | 0.04 | 6.62 | 5.67 | 0.15 | 0.10 |
| 45-49 | 0.78 | 0.63 | 0.44 | 0.27 | 0.35 | 0.13 | 5.50 | 7.30 | 4.87 | 7.74 | 0.16 | 0.13 | 13.00 | 25.00 | 0.61 | 0.38 | 0.09 | 0.09 | 6.62 | 5.67 | 0.24 | 0.16 |
| 50-55 | 1.18 | 0.96 | 0.74 | 0.44 | 0.41 | 0.16 | 5.50 | 7.30 | 4.87 | 7.74 | 0.25 | 0.20 | 36.00 | 18.00 | 1.03 | 0.63 | 0.16 | 0.16 | 7.70 | 6.89 | 0.39 | 0.27 |
| 56-59 | 1.65 | 1.34 | 1.19 | 0.64 | 0.63 | 0.27 | 15.30 | 17.90 | 13.56 | 18.97 | 0.37 | 0.30 | 36.00 | 18.00 | 1.65 | 0.90 | 0.26 | 0.26 | 7.70 | 6.89 | 0.61 | 0.37 |
| 60-64 | 2.08 | 1.69 | 1.82 | 0.98 | 0.89 | 0.41 | 15.30 | 17.90 | 13.56 | 18.97 | 0.55 | 0.44 | 24.00 | 23.00 | 2.53 | 1.39 | 0.37 | 0.38 | 7.70 | 6.89 | 0.90 | 0.54 |
| 65-69 | 2.46 | 2.00 | 2.58 | 1.36 | 1.17 | 0.62 | 15.30 | 17.90 | 13.56 | 18.97 | 0.83 | 0.67 | 24.00 | 23.00 | 3.59 | 1.93 | 0.60 | 0.60 | 9.77 | 8.91 | 1.28 | 0.74 |
Effect of the health promotion intervention
A simulation of the evolution of the cohort was made based on the change in Body Mass Index (BMI) between the intervention group and the control group and the age- and gender-dependent associated risk of developing BMI-related diseases. The effectiveness study [15] showed a small but significant difference in BMI change of 0.20 kg/m2 between the two study groups. The results of previous studies suggested that a reduction of one BMI unit results in a decreased risk of developing diabetes (men: 13.0%, women: 11.0%) [30], CHD (men: 4.7%, women: 5.7%) [31], stroke (men: 6.0%, women: 8.5%) [32, 33], and colon cancer (men: 5.2%, women: 2.0%) [34]. The risk reductions associated with BMI decrease applied in the Markov model were subsequently calculated using the data derived from these studies (Table 2).
Table 2.
Input parameters for the Markov decision-analytic model - base case
| Parameter | Base case | SE | Distribution | Reference |
|---|---|---|---|---|
| RR reduction (%) | ||||
| Diabetes - men | 2.60 | 0.003 | lognormal | [30] |
| Diabetes - women | 2.20 | 0.002 | lognormal | [30] |
| CHD - men | 0.94 | 0.001 | lognormal | [31] |
| CHD - women | 1.14 | 0.001 | lognormal | [31] |
| Stroke - men | 1.20 | 0.001 | lognormal | [32] |
| Stroke - women | 1.70 | 0.002 | lognormal | [33] |
| Colon cancer - men | 1.04 | 0.001 | lognormal | [34] |
| Colon cancer - women | 0.40 | 0.001 | lognormal | [34] |
| Cost/year data input (€) 1 | ||||
| Diabetes | 3,312 | 331 | gamma | [35] |
| CHD first year | 4,386 | 439 | gamma | [36–38] |
| CHD subsequent years | 1,183 | 118 | gamma | [36–38] |
| Stroke first year | 13,319 | 1,332 | gamma | [36] |
| Stroke subsequent years | 4,756 | 476 | gamma | [36] |
| Colon cancer | 9,575 | 958 | gamma | [36] |
| Intervention cost2 | 35 | 3.50 | gamma | [15] |
| Intervention cost3 | 21 | 2.13 | gamma | [15] |
| Utilities | ||||
| At risk | 0.71 | 0.04 | beta | [40] |
| Diabetes | 0.63 | 0.06 | beta | [17] |
| CHD 1 | 0.47 | 0.05 | beta | [17] |
| CHD 1+ | 0.56 | 0.06 | beta | [17] |
| Stroke 1 | 0.50 | 0.05 | beta | [17] |
| Stroke 1+ | 0.50 | 0.05 | beta | [17] |
| Colon cancer 1 | 0.64 | 0.06 | beta | [17] |
| Colon cancer 1+ | 0.64 | 0.06 | beta | [17] |
| Dead | 0 |
RR, relative risk; CHD, coronary heart disease.
1expressed in the year 2011 euros; 2intervention cost for the years 1,6,11,16.
3intervention cost for the other years.
Cost data input: disease costs and intervention costs
A public payer perspective was considered including only direct medical costs (Table 2). The costs are expressed in the year 2011 euros. The total diabetes cost was calculated accounting for the proportion of diabetic patients with no complications, micro vascular and/or macro vascular complications [35]. For stroke and CHD, first year costs and following year costs were taken separately into account as the cost related to a newly diagnosed cardiovascular disease was found to be different from the cost for those already suffering longer from stroke or CHD [36–38]. The colon cancer cost was obtained from a health economic evaluation of exercise in the prevention of cardiovascular and other prosperity diseases [36]. Future costs were discounted at 3% [39]. The intervention cost/patient included the use of a pedometer (13.70€), staff cost (20.57€), administrative costs (0.48€), and the intervention manual cost (0.25€). The staff cost/participant was calculated taking into account the total intervention duration time, the number of participating SHOs and individuals, and the time investment (hourly wages). An effective duration of five years was considered for the pedometers, so this cost was taken into account in the Markov model every five years.
Health-related quality of life (utilities)
The ‘at risk’ utility, i.e. the utility for patients without a history of one of the diseases included in the model was set at 0.71 [40]. The disease-specific utilities were derived from a health economic evaluation of a community-based PA intervention [17] (Table 2). QALYs were calculated by multiplying the utilities with the number of life years an individual is living with one of the diseases included in the model. Future QALYs were discounted at 1.5% [39].
Scenario analysis and sensitivity analyses
Health economic evaluations are frequently characterized by some degree of uncertainty or methodological considerations [41]. In the current study, scenario analyses and sensitivity analyses were performed to tackle this uncertainty. In the scenario analysis, four alternative modelling assumptions were assessed. First, full compliance with the intervention was assumed. For this analysis, a mean change of 0.33 kg/m2 (i.e. the mean BMI change of the participants who completed the programme) [15] was considered. In a second scenario, the effects on the costs and the QALYs of offering the programme twice a year maintaining a mean BMI change of 0.20 kg/m2 was analysed. In the base case analysis, no increase in HRQOL as a result of the BMI decrease was accounted for. So, in a third scenario, in the intervention group, a utility gain of 0.021 per unit BMI decrease was assumed based on the findings of a study of valuing HRQOL in diabetes patients [42]. In that study, a utility loss of 0.021 per unit BMI increase was accounted for. So, we assumed a utility gain of 0.021 per unit BMI decrease. In the base case analysis, a 20-year time horizon was used maintaining the same intervention effect. As a fourth scenario analysis, a more conservative analysis was conducted considering a five-year time horizon. One-way sensitivity analyses made it possible to assess the effects of key input parameters (intervention cost and effect, disease costs, and RR reductions of the diseases associated with a BMI decrease) on the ICER, by varying them separately. A probabilistic sensitivity analysis was performed to assess the uncertainty for the key input parameters by varying them concurrently. Cost data were assumed to follow a gamma distribution, utilities follow a beta distribution and risk reductions a lognormal distribution [43].
Ethics
The study was in compliance with the Helsinki Declaration and permission to perform the study was obtained from the Ethics Committee of the University Hospital of Ghent.
Results
Base case analysis
For the treatment as usual group, the average QALYs amounted to 11.59 and 12.04 with a cost of 8,352€ and 7,688€ for men and women respectively. The implementation of the health promotion intervention resulted in a limited QALY gain of 0.01 in both men and women. The total discounted costs in the intervention group were 8,579€ in men and 7,951€ in women, resulting in a delta cost between intervention and no intervention of 228€ and 263€ in men and women respectively. This resulted in an ICER of 27,096€/QALY in men and 40,139€/QALY in women (Table 3).
Table 3.
Cost-effectiveness results in men and women (base case and scenario analysis)
| Control | Intervention | ∆ QALY | ∆ Cost (€) | ICER (€/QALY) | |||
|---|---|---|---|---|---|---|---|
| QALYs | Cost (€) | QALYs | Cost (€) | ||||
| Men | |||||||
| Base case | 11.59 | 8,352 | 11.60 | 8,579 | 0.01 | 228 | 27,096 |
| Scenario 1 | 11.59 | 8,352 | 11.61 | 8,494 | 0.01 | 142 | 10,241 |
| Scenario 2 | 11.59 | 8,352 | 11.60 | 8,938 | 0.01 | 586 | 69,754 |
| Scenario 3 | 11.59 | 8,352 | 11.66 | 8,579 | 0.07 | 228 | 3,357 |
| Scenario 4 | 4.05 | 980 | 4.05 | 1,095 | 0.00 | 115 | 190,647 |
| Women | |||||||
| Base case | 12.04 | 7,688 | 12.05 | 7,951 | 0.01 | 263 | 40,139 |
| Scenario 1 | 12.04 | 7,688 | 12.05 | 7,881 | 0.01 | 193 | 17,857 |
| Scenario 2 | 12.04 | 7,688 | 12.05 | 8,320 | 0.01 | 632 | 96,567 |
| Scenario 3 | 12.04 | 7,688 | 12.11 | 7,951 | 0.07 | 263 | 3,766 |
| Scenario 4 | 4.08 | 807 | 4.08 | 926 | 0.00 | 119 | 266,700 |
Scenario 1, full compliance with the programme; scenario 2, offering the programme twice a year.
Scenario 3, increase in quality of life as a result of the BMI-decrease in the intervention group.
Scenario 4: time horizon of 5 years.
ICER, Incremental Cost-Effectiveness Ratio; QALY, Quality Adjusted Life Year.
Scenario analysis of alternative modelling assumptions
Applying the scenario of full compliance with the programme resulted in an ICER of 10,241€/QALY in men and 17,857€/QALY in women. An increase in HRQOL as a result of the BMI decrease resulted in an ICER of 3,357€/QALY in men and 3,766€/QALY in women. Worse results were found in the scenario offering the programme twice a year (Table 3). Taking into account a five-year time horizon resulted in an ICER of 190,647€/QALY in men and 266,700€/QALY in women.
Sensitivity analyses
The results of the one-way sensitivity analyses are shown using Tornado diagrams (Figure 2a and b). From this figure it can be concluded that the model is most sensitive to the intervention effect and to the intervention cost in both men and women. Varying other input parameters had less influence on the results. The findings of the probabilistic sensitivity analysis are shown in cost-effectiveness planes (Figure 3a and b). The points to the right of the threshold line refer to a cost-effectiveness ratio less than 30,000€/QALY. Based on 10,000 simulations, 95% credible intervals (CI) could be generated. The health promotion programme resulted in an average QALY gain of 0.008 (95% CI 0.003-0.014) at an average cost of 221€ (95% CI 168€-278€) in men and in an average QALY gain of 0.007 (95% CI 0.002-0.011) at an average cost of 256€ (95% CI 201€-316€) in women. In men, an average ICER of 26,336€/QALY (95% CI 14,439-83,209€/QALY) was found, while in women the ICER was 39,094€/QALY (95% CI 21,573-120,541€/QALY).
Figure 2.
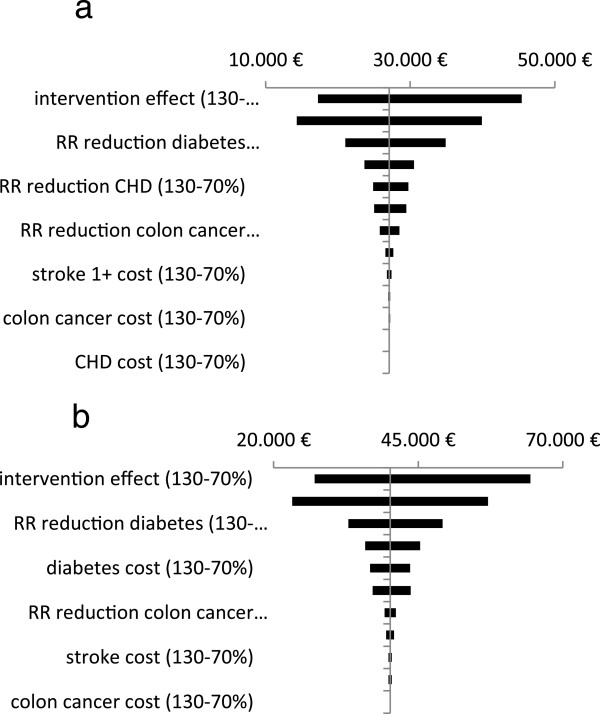
One-way sensitivity analysis: effects on cost/QALY. (a) Men. (b) Women.
Figure 3.
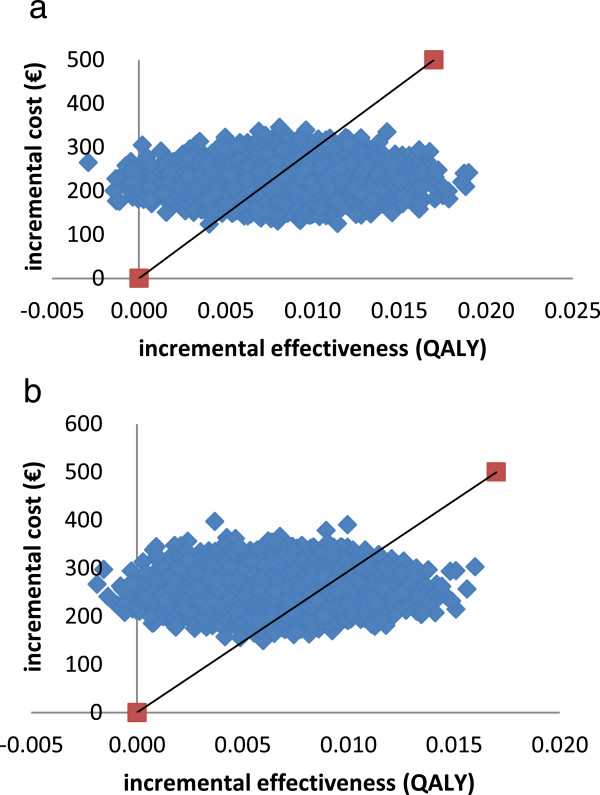
Probabilistic sensitivity analysis. (a) Men. (b) Women.
Discussion
The aim of the study was to evaluate the cost-effectiveness of a health promotion programme targeting PA and healthy eating in individuals with MD living in sheltered housing in the Flanders region (Belgium). A Markov decision-analytic model providing information on the costs and on the health effects (expressed as QALYs) related to the programme was used. The base case analysis showed an ICER of 27,096€/QALY in men and 40,139€/QALY in women. Assuming a cost-effectiveness threshold of about 30,000€/QALY in Belgium as recommended by the Belgian Healthcare Knowledge Centre [44], it can be concluded that the intervention was cost-effective in men but not in women. Mixed results were identified from the scenario analyses. The most promising outcome was found when an increase in HRQOL associated with a BMI decrease was assumed. The model was most sensitive to the input parameters ‘intervention effect’ and ‘intervention cost’ as demonstrated with the one-way sensitivity analyses.
In the base case, the health promotion programme was found to be borderline cost-effective in men and not cost-effective in women. The explanation for this result is likely the fact that a limited, although statistically significant, BMI change of 0.20 kg/m2, drawn from the effectiveness study [15], was used to calculate the risk reductions for the diseases included in the model. This was confirmed by the one-way sensitivity analysis results, identifying the ‘intervention effect’ as the input parameter most influencing the outcome. The limited change in BMI may be explained by the relatively short intervention duration of ten weeks. A more promising result was found if a BMI change of 0.33 kg/m2 assuming full compliance with the programme was considered. It can yet be discussed to what extent full compliance with the programme is achievable in populations with MD. Previous qualitative research identified a number of barriers hampering the participation of psychiatric patients in health promotion interventions [45]. Applying the scenario in which an increase in HRQOL as a result of BMI decrease was assumed, resulted in a conclusive cost-effective outcome in both men and women. This result must however be interpreted cautiously. Research on the effects of weight loss on HRQOL in people with MD is scarce providing no conclusive evidence that weight loss is associated with an increase in HRQOL [14]. A clearly not cost-effective result was observed considering a five-year time horizon. If the health effects are expressed as life years(LY) gained, the intervention resulted in 0.01 LY gained in men (intervention: 16.62 LY, control: 16.61 LY) and in women (intervention: 17.18 LY, control: 17.17 LY) (data not shown).
In the current healthcare environment, there is growing need on health economic evaluations of such programmes because this can assist regulatory bodies and health insurers establishing priorities within cost-constrained healthcare budgets. In mental health care, health economic research predominantly focused on cost-effectiveness analyses of psychopharmacological drugs [46] and mental health promotion and MD prevention [47]. Although the need for health economic evaluations of health promotion programmes targeting PA and healthy eating in mental health care was already addressed [48], such studies are lacking [14]. A uniform 70% to 130% uncertainty was used in the one-way sensitivity analysis, since the main reason to perform this analysis was to gain insight in those parameters most influencing the study outcome and not to assess the implications on the study outcome of uncertainty in the parameters. The full uncertainty around the input parameters was reflected with the probabilistic sensitivity analysis [41].
Some limitations and considerations need to be addressed. First, a 20-year time horizon assuming that the 10-week health promotion programme was repeated every year maintaining the same BMI change was considered. Studies evaluating the long-term effects of health promotion targeting PA and healthy eating in individuals with MD are yet lacking [14]. So, uncertainty exists concerning the long-term effects on BMI of offering such a programme once a year. The time horizon used in the current study was based on the time horizon used in a previous health economic evaluation of a community-based PA intervention [17]. We are aware that the time horizon is excessive on the basis of evidence found following a 10-week intervention the benefits of which disappeared at six months [15]. There is only limited evidence suggesting that health promotion targeting PA and healthy eating can result in longer-term weight loss. Unick et al. [49] found that weight loss was possible in overweight and obese diabetes patients following a repeated health promotion intervention targeting PA and healthy eating during a 4-year time period. Nevertheless, there is no evidence supporting that a repeated health promotion programmme would maintain the same effect during a 20-year time period. So, as a more conservative approach, an additional analysis was performed considering a five-year time period resulting in a clearly not cost-effective outcome in both men and women. On the other hand, the use of extensive time horizons in health economic evaluations is quite common [17, 36, 50]. Second, the model is a simplification of real life because model complexity prevented us to for example allow some combinations of disease states. Efforts to approximate the reality were yet applied, such as the inclusion of a transition probability from diabetes to colon cancer. No transitions from diabetes to CHD and stroke were possible, but the proportion of macro vascular complications such as CHD and stroke [51] was accounted for in the calculation of the diabetes cost. Costs related to follow-up after being diagnosed with one of the diseases included in the model were accounted for in the follow-up states (the states ‘1+’ in the Markov model). Third, the disease transition and mortality probabilities were retrieved from the literature and nationally available data. For some probabilities, national data were absent, so data from other countries was used. This may have resulted in an underestimation or overestimation of some probabilities included in the model. A utility of 0.71 [40] found in a sample of schizophrenia and major depressive disorder patients was used for the ‘at risk’ state. We are aware that utilities may differ according to the psychiatric diagnosis [52]. Nevertheless, the use of the 0.71 utility in our study is likely to be a reasonable reflection of the reality as about two-thirds of our study population consisted of schizophrenia and mood disorder patients. It is also important to note that utility weights may vary according to the measurement instrument being used. Lamers et al. [53] compared the use of the EQ-5D and the SF-6D questionnaires in a MD patients sample. It was found that the use of the EQ-5D resulted in larger health gains and consequent lower cost-utility ratios compared with the SF-6D. In one of the scenario analyses, an increase in HRQOL due to a BMI decrease was assumed. It can be argued that there is a risk of double counting of the utility benefit since the risk reduction of disease already incorporates a gain in QALYs. In the scenario analysis, the utility increase was only accounted for in the intervention group in the ‘at risk’ state and not in the different disease states.
Conclusions
In conclusion, as far as is known to the authors, this is the first study assessing the cost-effectiveness of a health promotion intervention targeting PA and healthy eating in individuals with MD. It was found that the health promotion intervention is likely to be cost-effective on the long-term if the programme would be repeated every year maintaining the same effect. This rather optimistic finding must be cautiously interpreted since there is no evidence supporting the long-term effectiveness of such interventions on BMI. Further research examining the cost-effectiveness of health promotion interventions in populations with MD is required. Further health economic evaluations of health promotion programmes is also required accounting for other study conditions such as individually-based programmes, programmes with longer duration and/or delivered in other settings. Such research has a substantial social value because healthcare budgets are limited, hence policy makers are facing the problem how to set priorities in the allocation of healthcare resources to medical or public health interventions.
Funding
The research was funded by a scholarship from the Ghent University Special Research Fund.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NV carried out the main part of the health economic modelling and drafting of the manuscript. DDS and LA participated in the development of the Markov decision-analytic model. JDM, LM and CVH revised the manuscript. All authors read an approved the final manuscript.
Contributor Information
Nick Verhaeghe, Email: nick.verhaeghe@ugent.be.
Delphine De Smedt, Email: delphine.desmedt@ugent.be.
Jan De Maeseneer, Email: jan.demaeseneer@ugent.be.
Lea Maes, Email: lea.maes@ugent.be.
Cornelis Van Heeringen, Email: cornelis.vanheeringen@ugent.be.
Lieven Annemans, Email: lieven.annemans@ugent.be.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Muller-Riemenschneider F, Reinhold T, Berghofer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol. 2008;23:499–509. doi: 10.1007/s10654-008-9239-1. [DOI] [PubMed] [Google Scholar]
- 4.Ul-Haq Z, Mackay DF, Pell JP. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity. 2013;21:E322–E327. doi: 10.1002/oby.20107. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316:104–108. doi: 10.1016/j.mce.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Mackin P, Bishop D, Watkinson H, Gallagher P, Ferrier IN. Metabolic disease and cardiovascular risk in people treated with antipsychotics in the community. Br J Psychiatry. 2007;191:23–29. doi: 10.1192/bjp.bp.106.031716. [DOI] [PubMed] [Google Scholar]
- 8.Parsons B, Allison DB, Loebel A, Williams K, Giller E, Romano S, Siu C. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110:103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Osborn DP, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. 2007;42:787–793. doi: 10.1007/s00127-007-0247-3. [DOI] [PubMed] [Google Scholar]
- 10.Ball MP, Coons VB, Buchanan RW. A program for treating olanzapine-related weight gain. Psychiatr Serv. 2001;52:967–969. doi: 10.1176/appi.ps.52.7.967. [DOI] [PubMed] [Google Scholar]
- 11.Mauri M, Simoncini M, Castrogiovanni S, Iovieno N, Cecconi D, Dell’Agnello G, Quadrigli M, Rossi A, Donda P, Fagiolini P, Cassano GB. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry. 2008;41:17–23. doi: 10.1055/s-2007-992148. [DOI] [PubMed] [Google Scholar]
- 12.Garrett S, Elley CR, Rose SB, O’Dea D, Lawton BA, Dowell AC. Are physical activity interventions in primary care and the community cost-effective? A systematic review of the evidence. Br J Gen Pract. 2011;61:e125–e133. doi: 10.3399/bjgp11X561249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Baal PH, van den Berg M, Hoogenveen RT, Vijgen SM, Engelfriet PM. Cost-effectiveness of a low-calorie diet and orlistat for obese persons: modeling long-term health gains through prevention of obesity-related chronic diseases. Value Health. 2008;11:1033–1040. doi: 10.1111/j.1524-4733.2008.00328.x. [DOI] [PubMed] [Google Scholar]
- 14.Verhaeghe N, De MJ, Maes L, Van HC, Annemans L. Effectiveness and cost-effectiveness of lifestyle interventions on physical activity and eating habits in persons with severe mental disorders: a systematic review. Int J Behav Nutr Phys Act. 2011;8:28. doi: 10.1186/1479-5868-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhaeghe N, Clays E, Vereecken C, De Maeseneer J, Maes L, Van Heeringen C, De Bacquer D, Annemans L. Health promotion in individuals with mental disorders: a cluster preference randomized controlled trial. BMC Public Health. 2013;13:657. doi: 10.1186/1471-2458-13-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemish Institute of Health Promotion and Disease Prevention . Manual Health Promotion: Healthy eating and physical activity. 2007. [Google Scholar]
- 17.De Smedt D, De Cocker K, Annemans L, De Bourdeaudhuij I, Cardon G. A cost-effectiveness study of the community-based intervention ‘10 000 Steps Ghent’. Public Health Nutr. 2012;15:442–451. doi: 10.1017/S1368980011001716. [DOI] [PubMed] [Google Scholar]
- 18.Flemish Agency for Care and Health . Chronic Diseases: Incidence and Prevalence. Brussels: Flemish Agency for Care and Health; 2010. [Google Scholar]
- 19.Rijksinstituut voor Volksgezondheid en Milieu . Nationaal Kompas Volksgezondheid. 2007. [Google Scholar]
- 20.Intego . Registratienetwerk van huisartsen in Vlaanderen. 2010. [Google Scholar]
- 21.Belgian Cancer Registry . Tabellen op jaarbasis. 2010. [Google Scholar]
- 22.Truyers C, Buntinx F, De LJ, De HM, van Winkel R, Aertgeerts B, Bartholomeeusen S, Lesaffre E. Incident somatic comorbidity after psychosis: results from a retrospective cohort study based on Flemish general practice data. BMC Fam Pract. 2011;12:132. doi: 10.1186/1471-2296-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborn DP, Levy G, Nazareth I, Petersen I, Islam A, King MB. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Rsearch Database. Arch Gen Psychiatry. 2007;64:242–249. doi: 10.1001/archpsyc.64.2.242. [DOI] [PubMed] [Google Scholar]
- 24.Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry. 2007;64:1368–1376. doi: 10.1001/archpsyc.64.12.1368. [DOI] [PubMed] [Google Scholar]
- 25.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 26.Statistics Belgiumhttp://statbel.fgov.be/nl/statistieken/cijfers/. 2010. Brussels, Statistics Belgium. 2012
- 27.Ubink-Veltmaat LJ, Bilo HJ, Groenier KH, Houweling ST, Rischen RO, Meyboom-de JB. Prevalence, incidence and mortality of type 2 diabetes mellitus revisited: a prospective population-based study in The Netherlands (ZODIAC-1) Eur J Epidemiol. 2003;18:793–800. doi: 10.1023/A:1025369623365. [DOI] [PubMed] [Google Scholar]
- 28.Vaartjes I, van Dis I, Grobbee DE, Bots ML. The dynamics of mortality in follow-up time after an acute myocardial infarction, lower extremity arterial disease and ischemic stroke. BMC Cardiovasc Disord. 2010;10:57. doi: 10.1186/1471-2261-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belgian Cancer Registry . Cancer Survival in Belgium 2004-2008. Brussels: Belgian Cancer Registry; 2012. [Google Scholar]
- 30.Schienkiewitz A, Schulze MB, Hoffmann K, Kroke A, Boeing H. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2006;84:427–433. doi: 10.1093/ajcn/84.1.427. [DOI] [PubMed] [Google Scholar]
- 31.McGee DL. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Ann Epidemiol. 2005;15:87–97. doi: 10.1016/j.annepidem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162:2557–2562. doi: 10.1001/archinte.162.22.2557. [DOI] [PubMed] [Google Scholar]
- 33.Rosengren A, Wilhelmsen L, Lappas G, Johansson S. Body mass index, coronary heart disease and stroke in Swedish women. A prospective 19-year follow-up in the BEDA study. Eur J Cardiovasc Prev Rehabil. 2003;10:443–450. doi: 10.1097/01.hjr.0000085253.65733.ef. [DOI] [PubMed] [Google Scholar]
- 34.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 35.Williams R, Van GL, Lucioni C. Assessing the impact of complications on the costs of Type II diabetes. Diabetologia. 2002;45:S13–S17. doi: 10.1007/s00125-002-0859-9. [DOI] [PubMed] [Google Scholar]
- 36.Annemans L, Lamotte M, Clarys P, Van den Abeele E. Health economic evaluation of controlled and maintained physical exercise in the prevention of cardiovascular and other prosperity diseases. Eur J Cardiovasc Prev Rehabil. 2007;14:815–824. doi: 10.1097/HJR.0b013e3282ef514f. [DOI] [PubMed] [Google Scholar]
- 37.Annemans L, Marbaix S, Webb K, Van GL, Scheen A. Cost effectiveness of atorvastatin in patients with type 2 diabetes mellitus: a pharmacoeconomic analysis of the collaborative atorvastatin diabetes study in the belgian population. Clin Drug Investig. 2010;30:133–142. doi: 10.2165/11531910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 38.Lamotte M, Caekelbergh K, Annemans L. Assessment of the cost of cardiovascular death. Value Health. 2003;6:658. doi: 10.1016/S1098-3015(10)61684-1. [DOI] [Google Scholar]
- 39.Cleemput I, Crott R, Vrijens F, Huybrechts M, Van Wilder P, Ramaekers D. Voorlopige Richtlijnen voor Farmaco-economisch Onderzoek in België. Health Technology Assessment (HTA), KCE Report 28A (D/2006/10.273/10) edn. Brussels: Belgian Health Care Knowledge Centre (KCE); 2006. [Google Scholar]
- 40.Saarni SI, Viertio S, Perala J, Koskinen S, Lonnqvist J, Suvisaari J. Quality of life of people with schizophrenia, bipolar disorder and other psychotic disorders. Br J Psychiatry. 2010;197:386–394. doi: 10.1192/bjp.bp.109.076489. [DOI] [PubMed] [Google Scholar]
- 41.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford: Oxford University Press; 2005. [Google Scholar]
- 42.Coffey JT, Brandle M, Zhou H, Marriott D, Burke R, Tabaei BP, Engelgau MM, Kaplan RM, Herman WH. Valuing health-related quality of life in diabetes. Diabetes Care. 2002;25:2238–2243. doi: 10.2337/diacare.25.12.2238. [DOI] [PubMed] [Google Scholar]
- 43.Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 44.Cleemput I, Neyt M, Thiry N, De Laet C, Leys M. Threshold Values for Cost-Effectiveness in Health Care. Health Technology Assessment (HTA). KCE Report 100C (D/2008/10.273/96) edn. Brussels: Belgian Health Care Knowledge Centre (KCE); 2008. [Google Scholar]
- 45.McDevitt J, Snyder M, Miller A, Wilbur J. Perceptions of barriers and benefits to physical activity among outpatients in psychiatric rehabilitation. J Nurs Scholarsh. 2006;38:50–55. doi: 10.1111/j.1547-5069.2006.00077.x. [DOI] [PubMed] [Google Scholar]
- 46.Polsky D, Doshi JA, Bauer MS, Glick HA. Clinical trial-based cost-effectiveness analyses of antipsychotic use. Am J Psychiatry. 2006;163:2047–2056. doi: 10.1176/appi.ajp.163.12.2047. [DOI] [PubMed] [Google Scholar]
- 47.Zechmeister I, Kilian R, McDaid D. Is it worth investing in mental health promotion and prevention of mental illness? A systematic review of the evidence from economic evaluations. BMC Public Health. 2008;8:20. doi: 10.1186/1471-2458-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menza M, Vreeland B, Minsky S, Gara M, Radler DR, Sakowitz M. Managing atypical antipsychotic-associated weight gain: 12-month data on a multimodal weight control program. J Clin Psychiatry. 2004;65:471–477. doi: 10.4088/JCP.v65n0404. [DOI] [PubMed] [Google Scholar]
- 49.Unick JL, Beavers D, Bond DS, Clark JM, Jakicic JM, Kitabchi AE, Knowler WC, Wadden TE, Wagenknecht LE, Wing RR. The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med. 2013;126:236–242. doi: 10.1016/j.amjmed.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hersey JC, Khavjou O, Strange LB, Atkinson RL, Blair SN, Campbell S, Hobbs CL, Kelly B, Fitzgerald TM, Kish-Doto J, Koch MA, Munoz B, Peele E, Stockdale J, Augustine C, Mitchell G, Arday D, Kugler J, Dorn P, Ellzy J, Julian R, Grissom J, Britt M. The efficacy and cost-effectiveness of a community weight management intervention: a randomized controlled trial of the health weight management demonstration. Prev Med. 2012;54:42–49. doi: 10.1016/j.ypmed.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diab. 2008;26:77–82. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 52.van Asselt AD, Dirksen CD, Arntz A, Giesen-Bloo JH, Severens JL. The EQ-5D: A useful quality of life measure in borderline personality disorder? Eur Psychiatry. 2009;24:79–85. doi: 10.1016/j.eurpsy.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Lamers LM, Bouwmans CA, van Straten A, Donker MC, Hakkaart L. Comparison of EQ-5D and SF-6D utilities in mental health patients. Health Econ. 2006;15:1229–1236. doi: 10.1002/hec.1125. [DOI] [PubMed] [Google Scholar]
Pre-publication history
- The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2458/14/856/prepub


