Abstract
Escherichia coli (E. coli) consists of commensal (ComEC) and diarrhoeagenic (DEC) groups. ComEC are detected using traditional culture methods. Conformational steps are performed after culturing if it is required to test for the presence of DEC, increasing cost and time in obtaining the results. The aim of this study was to develop a single-step multiplex polymerase chain reaction (m-PCR) that can simultaneously amplify genes associated with DEC and ComEC, with the inclusion of controls to monitor inhibition. A total of 701 samples, taken from clinical and environmental water sources in South Africa, were analysed with the optimised m-PCR which targeted the eaeA, stx1, stx2, lt, st, ial, eagg, astA and bfp virulence genes. The mdh and gapdh genes were included as an internal and external control, respectively. The presence of the external control gapdh gene in all samples excluded any possible PCR inhibition. The internal control mdh gene was detected in 100 % of the environmental and 85 % of the clinical isolates, confirming the classification of isolates as E. coli PCR positive samples. All DEC types were detected in varying degrees from the mdh positive environmental and clinical isolates. Important gene code combinations were detected for clinical isolates of 0.4 % lt and eagg. However, 2.3 % of eaeA and ial, and 8.7 % of eaeA and eagg were reported for environmental water samples. The E. coli astA toxin was detected as positive at 35 and 17 % in environmental isolates and clinical isolates, respectively. Interestingly, 25 % of the E. coli astA toxin detected in environmental isolates and 17 % in clinical isolates did not contain any of the other virulence genes tested. In conclusion, the optimised single-step 11-gene m-PCR reactions could be successfully used for the identification of pathogenic and non-pathogenic E. coli types. The m-PCR was also successful in showing monitoring for PCR inhibition to ensure correct reporting of the results.
Keywords: Clinical/environmental samples, Diarrhoeagenic E. coli, Multiplex-PCR
Introduction
Escherichia coli (E. coli) consists of both commensal (ComEC) and diarrhoeagenic (DEC) types. DEC not only indicate the presence of intestinal pathogens or parasites but also constitute a human health risk in themselves (Grabow et al. 2003; Kaper et al. 2004). At present, seven groups of pathogenic E. coli have been identified, of which five were selected for this study based on their importance for surface-water pathogenicity. The DEC types have been classified into the following: entero-pathogenic E. coli (EPEC), entero-toxigenic E. coli (ETEC), entero-haemorrhagic E. coli (EHEC), entero-aggregative E. coli (EAEC) and entero-invasive E. coli (EIEC) (Ashbolt 2004; Kaper et al. 2004). There are media available for the detection of specific EHEC 0157:H7 but traditional culture methods for E. coli were not designed for the detection of DEC (Iijima et al. 2007) but rather ComEC. Further conformational steps are thus required after culturing to distinguish the DEC from the ComEC which increases cost and time in producing the results. Diarrhoeagenic bacteria such as Campylobacter jejuni, Salmonella enterica serovar, Shigella spp., and Vibrio spp., can be readily isolated using selective plating media, with the exception of STEC 0157. Serotyping is the predominant means of differentiating pathogenic strains of E. coli, and phenotypic assays based on virulence characteristics can also identify DEC. Genotypic assays targeting virulence genes, especially polymerase chain reaction (PCR), are becoming standard procedure (Iijima et al. 2007).
Diagnosis is currently recommended for cases of persistent diarrhoea, children with severe diarrhoea unresponsive to treatment and immunodeficient patients with moderate to severe diarrhoea, and in epidemic outbreaks of gastroenteritis (Vidal et al. 2005). Methods in molecular biology have progressed and offer significant increases in speed and specificity in identifying micro-organisms according to their specific genetic makeup encoded in the genomic DNA (Horokova et al. 2008). Technologies such as microarrays and PCR are used to explore the global virulence pattern of strains (Wu et al. 2007). However, for developing countries microarray is an expensive method which laboratories cannot afford for routine analysis. M-PCR is a rapid and cost-effective method for screening and identifying DEC. The targets selected for each category were: EHEC (stx1, stx2 and eaeA); Atypical EPEC (eaeA) and Typical EPEC (bfp); ETEC (st and lt); EIEC (ial); EAEC (eagg); Commensal E. coli (mdh); E. coli toxin (astA) and, for the external control, gapdh.
The major obstacle to using PCR for the detection and identification of pathogenic organisms from clinical or environmental water samples is the presence of substances that are inhibitory to PCR such as humic substances (Shieh et al. 1995; Wilson 1997). In order to monitor PCR inhibition sufficient laboratory controls are required in the m-PCR. The majority of published studies report the addition of 16s rRNA gene as the internal control to monitor for false negative results in m-PCR (Sabat et al. 2000; Grape et al. 2007). However, these are not sufficient to monitor false negative results for E. coli specifically, since 16s rRNA is amplified from the E. coli DNA. It would not be possible to determine whether a lack of PCR amplification of 16s rRNA is as a result of PCR inhibition in the sample or is because there is no E. coli in the sample. As reported by Hartman et al. (2005), the high level of PCR sensitivity creates an elevated risk of false positive and negative results.
Methodology
The aim of this study was to develop a single-step multiplex polymerase chain reaction (m-PCR) that distinguishes selected E. coli patho-types. Internal controls were included to monitor inhibition in each sample thereby indicating false positive or false negative results.
Growth and maintenance of bacterial strains
Thirty-eight bacterial strains, which included commensal and pathogenic E. coli strains, Shigella spp., Salmonella spp., Vibrio spp. [obtained from National Health laboratory services (NHLS); (Table 1)] and other strains of the Enterobacteriaceae family such as Klebsiella spp., Aeromonas spp., Pseudomonas aeruginosa, Bacillus subtilis, Bacillus cereus, Enterococcus spp. and Morganella morganni (obtained from undergraduate practical laboratory) were cultured on Plate Count Agar (PCA) (Oxoid, UK) and incubated under aerobic conditions at 37 °C for 16 h. Single colonies were enriched in nutrient broth (Oxoid, UK) and incubated under aerobic conditions at 37 °C for 16 h. The commensal E. coli strain was used as the positive control. Klebsiella pneumoniae (KLEPN 01) and Pseudomonas aeruginosa (PSEAE 01) were used as the negative controls for the Colilert® Quanti-Trays®/2000.
Table 1.
Bacterial strains used in molecular characterisation
| Bacterial strain | Reference nr | Genes present |
|---|---|---|
| Escherichia coli (Commensal)a | ATCC 25922 | mdh |
| Enterohaemorrhagic (EHEC) | ESCCO 21b | mdh, stx1, stx2 and eaeA |
| Enteroinvasive (EIEC) | ESCCOS ATCC 43893b | mdh and ial |
| Enterotoxigenic (ETEC) | ESCCO 22b | mdh, lt and st |
| Enteropathogenic (EPEC) | S-ESCCO 16 Plb | mdh, eaeA, bfp |
| Enteroaggregative (EAEC) | ESCCO 14b | mdh and eagg |
aEnvironmental isolate confirmed by API 20E (OMNIMED®) and PCR as commensal E. coli
bStrains purchased from National Health Laboratory Services (NHLS)
M-PCR testing on enriched environmental water samples and isolates
Once the m-PCR was developed it was tested on clinical, environmental isolates and environmental water samples.
Microbial analysis
Clinical isolates
239 clinical isolates were obtained from Ampath Laboratory (Pretoria). Single colonies which were confirmed E. coli positive by Ampath Laboratory were enriched as described above (growth and maintenance).
Environmental isolates
171 environmental water samples (container water, toilet seats, borehole, stream, river) were collected in 1 l sampling bottles and stored at 4 °C on route to the laboratory. The water samples (100 ml) were filtered onto 0.45 μm gridded nitro-cellulose membranes (NC) (Merck, Germany) using the standard membrane filtration technique, placed onto E. coli/Coliform Chromogenic Media (Oxoid, UK) and incubated under aerobic conditions at 37 °C for 16 h (Standard Methods 2005). Single colonies that appear purple on the selective E. coli media were enriched as described above in growth and maintenance.
Environmental water samples
291 water samples (Waste water: upstream, downstream and final effluent) were collected in 1 l sampling bottles and stored at 4 °C on route to the laboratory. The water samples were immediately analysed upon arrival at the laboratory for bacterial quality using the Colilert® Quanti-Tray®/2000 system (IDDEX). Enumeration of E. coli from water was done using 100 ml water according to the manufacturer’s instructions. The Quanti-Trays® were incubated for 18 h at 35 °C. After incubation, the Quanti-Trays®/2000 were examined under long wave (366 nm) ultraviolet light, and wells that turned both yellow and fluoresced were counted as E. coli positive (IDDEX).
DNA extraction
Clinical and environmental isolates
2 ml of the enriched single colony was centrifuged for 2 min at 13,000×g to pellet the cells and the supernatant was discarded. DNA was extracted from the collected bacterial cells using the silica/guanidium thiocyanate method reported by Boom et al. (1990) as well as adaptations of spin columns reported on by Borodina et al. (2003). The adjustments included the addition of 250 µl 100 % ethanol to the lysis buffer to enhance the binding of DNA to the Celite. The Celite containing the bound DNA was loaded onto a DNA binding membrane (Borodina et al. 2003) in the spin columns. DNA was eluted with 100 µl Qiagen elution buffer (Southern Cross Biotechnology®) [Omar et al. (2010)]. The extracted DNA was used as a template in all PCR reactions.
Colilert® Quanti-Trays®/2000 system
A total of 2 ml of the media was removed from up to ten positive E. coli wells of the Colilert® Quanti-Trays®/2000 using sterile 1 ml Neomedic disposable syringes with mounted needle (Kendon Medical Supplies) and aliquoted into 2 ml sterile Eppendorf tubes. The tubes were centrifuged for 2 min at 13,000×g to pellet the cells and the supernatant discarded. DNA was extracted from the collected bacterial cells as explained above and as reported by Omar et al. (2010). The extracted DNA was used as a template in all PCR reactions.
Multiplex polymerase chain reaction (m-PCR)
All m-PCR reactions were performed in a Biorad Mycycler™ thermal cycler in a total reaction volume of 20 μl. A hotstart multiplex PCR kit (Qiagen®) was used for the m-PCR protocol. Each reaction consisted of 1X Qiagen® PCR multiplex mix (containing HotstartTaq® DNA polymerase, multiplex PCR buffer and dNTP mix); 2 μl of the primer mixture [0.1 μM of mdh and lt primers [Forward (F) and reverse (R)], 0.2 μM of ial, eagg primers, astA primers, bfp primers and gapdh primers (F and R), 0.3 μM of eaeA and stx2 primers (F and R), 0.5 μm of stx1 and st primers [F and R (Table 2)]; 2 μl of sample DNA, 1 μl of gapdh cDNA and 5 μl PCR grade water. The reactions were subjected to an initial activation step at 95 °C for 15 min, followed by 35 cycles consisting of denaturing at 94 °C for 45 s, annealing at 55 °C for 45 s, extension at 68 °C for 2 min and final elongation at 72 °C for 5 min.
Table 2.
Primers used in the m-PCR reaction
| Pathogen | Primer | Sequence(5′-3′) | Size (bp) | Conc. (µM) | Reference |
|---|---|---|---|---|---|
| E. coli | mdh (F) | GGT ATG GAT CGT TCC GAC CT | 304 | 0.1 | Tarr et al. (2002) |
| mdh (R) | GGC AGA ATG GTA ACA CCA GAG T | ||||
| EIEC | ial (F) | GGT ATG ATG ATG ATG AGT CCA | 650 | 0.2 | López-Saucedo et al. (2003) |
| ial (R) | GGA GGC CAA CAA TTA TTT CC | ||||
| EHEC/Atypical EPEC | eaeA (F) | CTG AAC GGC GAT TAC GCG AA | 917 | 0.3 | Aranda et al. (2004) |
| eaeA (R) | CCA GAC GAT ACG ATC CAG | ||||
| Typical EPEC | bfpA (F) | AAT GGT GCT TGC GCT TGC TGC | 410 | 0.3 | Aranda et al. (2004) |
| bfpM (R) | TAT TAA CAC CGT AGC CTT TCG CTG AAG TAC CT | From this study | |||
| EAEC | eagg (F) | AGA CTC TGG CGA AAG ACT GTA TC | 194 | 0.2 | Pass et al. (2000) |
| eagg (R) | ATG GCT GTC TGT AAT AGA TGA GAA C | ||||
| EHEC | stx1 (F) | ACA CTG GAT GAT CTC AGT GG | 614 | 0.5 | Moses et al. (2006) |
| stx1 (R) | CTG AAT CCC CCT CCA TTA TG | ||||
| stx2 (F) | CCA TGA CAA CGG ACA GCA GTT | 779 | 0.3 | Moses et al. (2006) | |
| stx2 (R) | CCT GTC AAC TGA GCA CTT TG | ||||
| ETEC | lt (F) | GGC GAC AGA TTA TAC CGT GC | 360 | 0.1 | Pass et al. (2000) |
| lt (R) | CGG TCT CTA TAT TCC CTG TT | ||||
| st (F) | TTT CCC CTC TTT TAG TCA GTC AAC TG | 160 | 0.5 | Pass et al. (2000) | |
| st (R) | GGC AGG ATT ACA ACA AAG TTC ACA | ||||
| E. coli toxin | astA (F) | GCC ATC AAC ACA GTA TAT CC | 106 | 0.3 | Kimata et al. (2005) |
| astA (R) | GAG TGA CGG CTT TGT AGT C | ||||
| External control | gapdh (F) | GAG TCA ACG GAT TTG GTC GT | 238 | 0.3 | Mbene et al. (2009) |
| gapdh (R) | TTG ATT TTG GAG GGA TCT CG |
NB: F forward primer, R reverse primer
DNA was visualised using a 2.5 % (w/v) agarose gel in TAE buffer (40 mmol l−1 Tris acetate; 2 mmol l−1 EDTA, pH 8.3) with 0.5 μg ml−1 ethidium bromide. Electrophoresis was done for 1–2 h in electric field strength of 8 V cm−1 gel and the DNA visualized with UV light (Syngene, UK). This procedure was followed for all the experiments except where stated differently. The relative sizes of the DNA fragments were estimated by comparing their electrophoretic mobility with that of the standards run with the samples on each gel, either a 1 kB or 100 bp markers (Fermentas, US).
Specificity of the m-PCR
The specificity of the m-PCR was assessed by testing 38 bacterial strains which included commensal and pathogenic E. coli strains, Shigella spp., Salmonella spp. and serovar, Vibrio spp. and other strains of the Enterobacteriaceae family such as Klebsiella spp., Aeromonas spp., Pseudomonas aeruginosa, Bacillus subtilis, Bacillus cereus, Enterococcus spp. and Morganella morgannii (Table 3).
Table 3.
Specificity of the m-PCR
| Bacterial strain | Source | Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mdh | eaeA | bfp | stx1 | stx2 | ial | lt | gapdh | st | eagg | astA | ||
| Commensal E. coli | NHLS | + | − | − | − | − | − | + | − | − | − | |
| Enterohaemorrhagic E. coli | NHLS | + | + | − | + | + | − | − | + | − | − | − |
| Enteropathogenic E. coli | NHLS | + | + | + | − | − | − | − | + | − | − | + |
| Enteroaggregative E. coli | NHLS | + | − | − | − | − | − | − | + | − | + | − |
| Enterotoxigenic E. coli | NHLS | + | − | − | − | − | − | + | + | + | − | − |
| Enteroinvasive E. coli | NHLS | + | − | − | − | − | + | − | + | − | − | − |
| Shigella dysenteriae serovar type 1 | NHLS | + | − | − | − | − | + | − | + | − | − | − |
| Shigella dysenteriae serovar type 2 | NHLS | + | − | − | − | − | + | − | + | − | − | − |
| Shigella boydii serovar B | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Shigella flexneri | NHLS | + | − | − | − | − | − | − | + | − | − | − |
| Shigella sonnei | NHLS | + | + | − | − | − | + | − | + | − | − | − |
| Vibrio cholerae non-O1 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio cholerae O1 | NTCC | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio cholerae O1 | NTCC | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio parahaemolyticus | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio parahaemolyticus | NCTC | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio cholerae O139 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio cholerae Ogawa | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio mimicus | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio fluvialis | NCTC | − | − | − | − | − | − | − | + | − | − | − |
| Vibrio furnissii | ATCC | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Typhi salty O1 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Typhimurium saltm O1 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Typhimurium saltm O2 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Typhi salty O2 | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Paratyphi | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Paratyphi A | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Paratyphi C | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella Gallinarum | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Salmonella enterica serovar Enteritidis | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Pseudomonas aeruginosa | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Klebsiella pneumonia | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Bacillus subtilis | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Bacillus cereus | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Aeromonas veronii | ATCC | − | − | − | − | − | − | − | + | − | − | − |
| Enterococcus faecium | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Enterococcus faecalis | NHLS | − | − | − | − | − | − | − | + | − | − | − |
| Morganella morgannii | NHLS | − | − | − | − | − | − | − | + | − | − | − |
Results and discussion
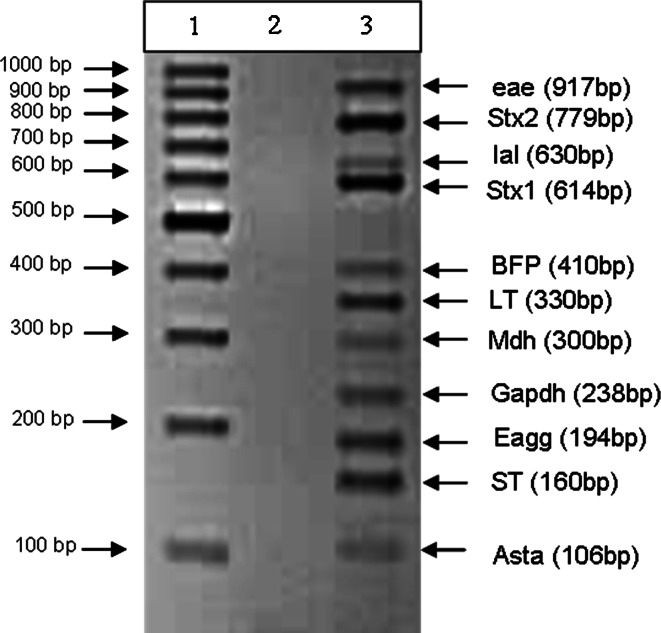
The main challenge of designing a multiplex PCR is the possibility of primer dimers and non-specific results which is a risk for false positive and negative results. Therefore, it is necessary to design and include primers with close annealing temperatures and to begin the program with a hotstart as reported by Vidal et al. (2005). The effect of the wide temperature range is overcome by the addition of Q-solution that is supplied by the manufacturer and that can be included with the enzyme. A wide variety of temperatures were tested before the final version of the multiplex PCR was optimized and tested. The results confirm that the single m-PCR was successfully compiled to detect all of the targeted genes in a single reaction even though primers with different melting temperatures ranging from 50 to 73 °C were used (Fig. 1). The PCR amplicons were confirmed as the correct target gene by sequencing (data not shown) showing the specific amplification of the genes in a mixture of DEC.
Fig. 1.
Agarose gel of the PCR products obtained for the E. coli multiplex PCR (lane 2). No template control (NTC) in (lane 2). The molecular weight marker is shown in (lane 1)
Specificity of the m-PCR
The specificity of the m-PCR was tested on 38 laboratory bacterial strains. Specificity was stated by Aldrich and Griffith (2003) as ‘the ability of the assay to detect a unique event to the exclusion of all other events’; that is, to what extent can the assay detect a specific pathobiologic effect that will exclude all other similar pathobiologic effects. Positive PCR results were only obtained for the E. coli and Shigella strains (Table 3). However, the mdh gene was not detected for Shigella boydii serotype B. Boerlin et al. (1999) state that Shigella is similar to EIEC and the stx1 is almost identical to the shiga toxin of Shigella dysenteriae in amino acid sequence and cannot be distinguished from serologically, yet ial and eaeA were detected for Shigella sonnei. No positive PCR results were obtained for the DNA from the other bacterial strains tested. Specific genes were detected for each patho-type as indicated in Table 1; there was no cross reactivity of genes between patho-types. No false positives and no PCR inhibition were obtained due to the external control gapdh gene that was detected in 100 % (38/38) of the samples.
Application
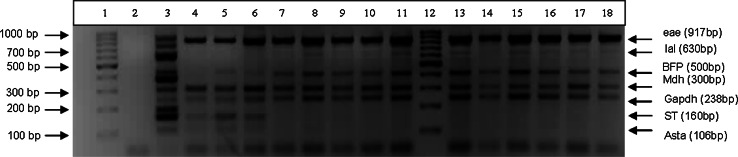
A total of 701 samples were analysed, samples composed of 239 clinical isolates, 171 environmental water isolates and 291 samples from the Colilert® Quanti-Tray®/2000 (Fig. 2); these samples were obtained from various provinces in South Africa. Isolates and water samples were subjected to the protocols described in the methodology, with 100 % (171/171) of environmental water isolates, 85 % (202/239) of the clinical isolates and 100 % (291/291) of the water samples testing positive for the mdh house-keeping gene (Fig. 2). For the 15 % (37/239) of clinical isolates in which the mdh gene was not detected, it is possible that these do not contain the malate dehydrogenase but the malic acid dehydrogenase gene, which is also a housekeeping enzyme of the citric acid cycle (Hsu and Tsen 2001). When the study was initiated Tarr et al. (2002) article was used, who included the malate dehydrogenase gene and indicated in their tests positive results for all the E. coli strains tested. Based on this the mdh gene was used as a control to confirm the microbiology results in case no pathogenic genes tested for were detected. It is only later that for a separate study the malic acid dehydrogenase gene was tested (also referred to as mdh by Hsu and Tsen 2001) that not all the E. coli strains present in the samples tested positive. The reason could be that the original work by the authors were done on strains that could not be present in South Africa or that we have strains that have different genetic characteristics. No false positives and no PCR inhibition were indicated in the m-PCR as the external control gene (gapdh) was detected in 100 % (701/701) of the samples. A supposedly negative test result for an infectious agent can influence therapeutic decisions, such as withholding antibiotic and antiviral drugs (Cone et al. 1992; Hartman et al. 2005). Therefore, the additions of the internal and external controls are important to ensure that there are no PCR inhibitors in the reaction as well as to validate the accuracy of the PCR in distinguishing false negative from true negative PCR results.
Fig. 2.
Agarose gel of the PCR products obtained from samples (lane 4–11, 13–18). No template control (NTC) in (lane 2). The molecular weight marker is shown in (lane 1 and 12). The positive reference control is shown in (lane 3)
Environmental water isolates
Of the E. coli positive environmental water isolates (171) tested, eagg gene (EAEC), ial gene (EIEC), st and lt genes (ETEC), stx1 gene and stx2 gene, eaeA gene (EHEC, Atypical-EPEC) tested positive (see Table 4 for the percentages of each gene). Positive gene combinations detected for eaeA and stx1 2.3, 0.6 % combination of eaeA, stx1 and stx2 (EHEC). Literature states that stx1 and/or stx2 can be detected individually or in combination due to being phage-encoded (Müller et al. 2001; Contreras et al. 2011; Feng et al. 2011). To discriminate between typical and atypical EPEC, 29.8 % tested positive for the eaeA and bfp gene (Typical-EPEC), 3.5 % bfp gene (Typical-EPEC) and 27 % eaeA gene (Atypical EPEC). For the astA gene (E. coli toxin) 25 % was detected without the combination of the virulence genes. The distribution of the astA toxin gene combined with the virulence genes is indicated in Table 6. Interesting results was 2.3 % combination of eaeA and ial as well as combination of eaeA and eagg 8.7 % (Table 5). Literature reports gene coding of eaeA with EHEC and EPEC (Presterl et al. 2003; Müller et al. 2001; Aranda et al. 2004; Moses et al. 2006) but not with eagg and ial. Published reports have described eaeA as the bacterial outer membrane protein intimin, which is essential in organizing host cytoskeletal rearrangements and generating the pedestal-like structure in which the bacteria reside. Intimin is required for full bacterial virulence and its expression is regulated by the per regulon. The per regulon comprises four reading frames (perA, B, C and D), and maximal expression requires all four gene products, however, expression of perC alone can induce intimin expression (Kenny et al. 1997). The question is has intimin been expressed from EAEC and EIEC? Or as Chen and Dubnau (2004) reported that DNA can be transferred from one organism to another via conjugation. They also reported DNA can be actively secreted by viable organisms. Hacker and Kaper (2000) reported that free DNA released from dead bacteria can be taken up by bacteria in the environment via natural transformation and may carry pathogenicity islands (PAIs). The majority of PAIs are located on the chromosome, but can also be part of bacterial plasmids and phages. More research has to be conducted to determine these gene-coding combinations.
Table 4.
PCR results obtained from the single isolates of the clinical and environmental isolates and water samples from the Colilert® Quanti-Tray®/2000
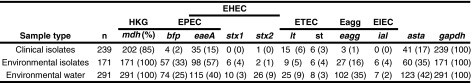
Table 6.
Distribution of the astA toxin gene combined with the virulence genes for isolates, environmental isolates and water samples
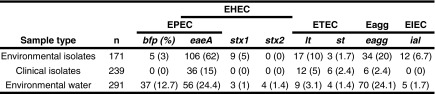
Table 5.
Gene combinations from clinical and environmental isolates
| Patho-type | Gene combinations | Clinical isolates (n) | Environmental isolates (n) | References |
|---|---|---|---|---|
| Atypical EPEC | eaeA | 32 | 46 |
Aranda et al. (2004); Botkin et al. (2012) |
| EHEC | eaeA + stx1 | 0 | 4 | Müller et al. (2001); Contreras et al. (2011); Feng et al. (2011) |
| eaeA + stx2 | 1 | 0 | ||
| eaeA + stx1 +stx2 | 0 | 1 | ||
| stx1 | 0 | 1 | ||
| stx2 | 0 | 1 | ||
| Typical EPEC | eaeA + bfp | 2 | 51 | Kaper et al. (2004); |
| bfb | 2 | 6 | Botkin et al. (2012) | |
| ETEC | lt + st | 0 | 3 | Presterl et al. (2003) |
| lt | 8 | 6 | ||
| st | 6 | 3 | ||
| lt + eagg | 1 | 0 | ||
| eaeA + ial | 0 | 4 | ||
| eaeA + eagg | 0 | 15 |
Clinical isolates
Of the clinical isolates (239) tested, eagg (EAEC), lt and st (ETEC), eaeA and stx2 (EHEC, Atypical-EPEC) tested positive (Table 4). Positive gene combinations were detected for 0.8 % eaeA and bfp (Typical-EPEC), 0.8 % bfp (Typical-EPEC) and 13.4 % eaeA gene (Atypical EPEC), 17 % astA (E. coli toxin). The significance of differentiating between typical and atypical EPEC is that atypical EPEC are more frequently isolated from diarrhoea cases than typical EPEC. However, while typical EPEC dominates in developing countries, atypical EPEC has also been shown to cause large outbreaks involving both children and adults (Kaper et al. 2004). For the astA gene (E. coli toxin) 78 % was detected without the combination of the virulence genes. The distribution of the astA toxin gene combined with the virulence genes is indicated in Table 6. This result is very important: Hidaka et al. (2009) reported that a 1996 outbreak of gastrointestinal illness was caused by E. coli 0166:H15 which possessed no enteropathogenicity-associated genes other than the astA gene. The astA gene was first identified in EAEC as a structural gene that encodes a distinct low-molecular-weight putative enterotoxin (Yatsuyanagi et al. 2003). Reports from Soto et al. (2009) indicate that the enteroaggregative heat stable toxin 1 (EAST-1) is encoded by the astA gene. This toxin is thought to play a role in EAEC pathogenicity. The toxin binds to the receptor and activates guanylate cyclise, which stimulates production of cyclic GMP (cGMP). High levels of cGMP in the cell inhibit the Na/Cl co-transport system, reduce the absorption of electrolytes and water from the intestine at villus tips and result in an elevated secretion of Cl− and water in crypt cells. The role of this toxin in the development of diarrhoea has yet to be defined (Soto et al. 2009). However, recently the astA gene has been detected not only in EAEC but also in EPEC, atypical EPEC, ETEC and EIEC strains (Yatsuyanagi et al. 2003). As discussed above, an interesting gene-coding combination was detected in the clinical isolates, 0.4 % lt and eagg genes.
Environmental water samples
Of the E. coli positive environmental water samples from the Colilert® Quanti-Tray®/2000 (291) tested, presence of eagg gene (EAEC), ial gene (EIEC), lt gene and st gene (ETEC) tested positive (Table 4). Positive gene combination detected for 0.3 % of eaeA and stx1, 5.8 % combination of eaeA and stx2 (EHEC), 3.1 % combination of eaeA, stx1 and stx2 (EHEC). To discriminate between typical and atypical EPEC 24.1 % tested positive for the eaeA and bfp gene (Typical-EPEC) and 1.4 % bfp gene (Typical-EPEC) 17 % eaeA gene (Atypical EPEC). For the astA gene (E. coli toxin) 40 % was detected without the combination of the virulence genes and the distribution of the astA toxin gene combined with the virulence genes are indicated in Table 6.
Conclusion
Both internal controls for m-PCR were used to monitor PCR inhibition that might occur due to the nature of the samples. The PCR was designed so that the gapdh gene would only be amplified in samples where no other PCR products were amplified. All the genes tested for could be detected using m-PCR with no non-specific amplification of genes. Atypical and typical EPEC could be successfully distinguished using single m-PCR reaction. The astA toxin gene was detected in both DEC and ComEC samples. Important gene combinations were detected. The m-PCR offers the user a fast and effective method to perform a simultaneous amplification not only for the detection of virulence genes from all categories of diarrhoeagenic E. coli (ETEC, typical or atypical EPEC, EIEC, EAEC, EHEC) but also commensal E. coli and internal controls to monitor for PCR inhibition. The m-PCR is easy to perform, sensitive, requires minimal specialized equipment or training, and provides same-day results necessary for rapid action in the case of diarrhoeal outbreaks.
Acknowledgments
Funding obtained from the University of Johannesburg and National Research Foundation (South Africa) to complete this study. Ampath Laboratories (Pretoria) for providing the clinical isolates. The authors would like to acknowledge the publication workshop arranged by the South African Young Water Professionals during which this manuscript was prepared. We would also like to convey our appreciation to the Department of Science and Technology, Water Institute of Southern Africa and the University of Johannesburg for covering the costs of the workshop.
Contributor Information
K. B. Omar, Phone: (+2711) 559-6324, Email: kousaro@uj.ac.za
T. G. Barnard, Email: tgbarnard@uj.ac.za
References
- Aldrich TE, Griffith J (2003) Environmental epidemiology and risk assessment. Wiley, New Jersey
- Aranda KRS, Fagundes- Neto U, Scaletsky ICA. Evaluation of multiplex PCR’s for diagnosis of infection with diarreagenic Escherichia coli and Shigella spp. J Clin Microbiol. 2004;42:5849–5853. doi: 10.1128/JCM.42.12.5849-5853.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt JH. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–238. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Jonson RP, Gyles CL. Association between virulence factors of the shiga-toxin producing Escherichia coli and disease in humans. J Clin Microbiol. 1999;37:497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom R, Sol CJA, Salimans MMM, Jansen CL, Wertheim-Van Dillen PME, Van Der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol . 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodina TA, Lehrach H, Soldatov AV. DNA purification on homemade silica spin columns. J Anal Biochem. 2003;321:135–137. doi: 10.1016/S0003-2697(03)00403-2. [DOI] [PubMed] [Google Scholar]
- Botkin DJ, Galli L, Sankarapani V, Soler M, Rivas M, Torres AG. Development of a multiplex PCR assay for detection of Shiga toxin-producing Escherichia coli, enterohemorrhagic E. coli, and enteropathogenic E. coli strains. Front Cell Infect Microbiol. 2012;2(8):1–10. doi: 10.3389/fcimb.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Dubnau D. DNA uptake during bacterial transformation. Nat Rev Microbiol. 2004;2:241–249. doi: 10.1038/nrmicro844. [DOI] [PubMed] [Google Scholar]
- Cone RW, Hobson AC, Huang MW. Co-amplified positive control detects inhibition of polymerase chain reactions. J Clin Microbiol. 1992;30:3185–3189. doi: 10.1128/jcm.30.12.3185-3189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras CA, Ochoa TJ, Ruiz J, Lacher DW, Rivera FP, Saenz Y, Chea-Woo E, Zavaleta N, Gil AI, Lanata CF, Huicho L, Maves RC, Torres C, DebRoy C, Cleary TG. Phylogenetic relationships of Shiga toxin-producing Escherichia coli isolated from Peruvian children. J Med Microbiol. 2011;60:639–646. doi: 10.1099/jmm.0.026666-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng PCH, Jinneman K, Scheutz F, Monday SR. Specificity of PCR and serological assays in the detection of Escherichia coli shiga toxin subtypes. Appl Environ Microbiol. 2011;77(18):6699–6702. doi: 10.1128/AEM.00370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabow WOK, Műller EE, Ehlers MM, de Wet CME, Uys M, Clay CG (2003) Occurrence in water sources of E. coli 0157:H7 and other pathogenic E. coli strains, Water Research Commission report No.: 1068/1/03, ISBN No.: 1-86845-988-8
- Grape M, Motakefi A, Pavuluri S, Kahlmeter G. Standard and real-time multiplex PCR methods for detection of trimethoprim resistance dfr genes in large collections of bacteria. Clin Microbiol Infect. 2007;13:1112–1118. doi: 10.1111/j.1469-0691.2007.01807.x. [DOI] [PubMed] [Google Scholar]
- Hacker J, Kaper JB. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol. 2000;54:641–679. doi: 10.1146/annurev.micro.54.1.641. [DOI] [PubMed] [Google Scholar]
- Hartman LJ, Coyne SR, Norwood DR. Development of a novel internal positive control for Taqman based assays®. Mol Cell Probes. 2005;19:51–59. doi: 10.1016/j.mcp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hidaka A, Hokyo T, Arikawa K, Fujihara S, Ogasawara J, Hase A, Hara-Kudo Y, Nishikawa Y. Multiplex real-time PCR for exhaustive detection of diarrhoeagenic Escherichia coli. J Appl Microbiol. 2009;106:410–420. doi: 10.1111/j.1365-2672.2008.04043.x. [DOI] [PubMed] [Google Scholar]
- Horokova K, Mlejnkova H, Mlejnek P. Specific detection of Escherichia coli isolated from water samples using polymerase chain reaction targeting four genes: cytochrome bd complex, lactose permease, ß-D-glucuronidase, and ß-D-galactosidase. J Appl Microbiol. 2008;105:970–976. doi: 10.1111/j.1365-2672.2008.03838.x. [DOI] [PubMed] [Google Scholar]
- Hsu S-C, Tsen H-Y. PCR primers designed from malic acid dehydrogenase gene and their use for detection of Escherichia coli in water and milk samples. Int J Food Microbiol. 2001;64:1–11. doi: 10.1016/S0168-1605(00)00425-6. [DOI] [PubMed] [Google Scholar]
- Iijima Y, Miki K, Kanamori S, Toyokawa M, Asari S. Evaluation of colony-based examinations of diarrhoeagenic E. coli in stool specimens: low probability of detection because of low concentrations, particularly during the early stage of gastroenteritis. Diagn Microbiol Infect Dis. 2007;58:303–308. doi: 10.1016/j.diagmicrobio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- Kenny B, Abe A, Stein M, Finlay BB. Enteropathogenic Escherichia coli protein secretion is induced in response to conditions similar to those in the gastrointestinal tract. Infect Immun. 1997;65(7):2606–2612. doi: 10.1128/iai.65.7.2606-2612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K, Shima T, Shimizu M, Tanaka D, Isobe J, Gyobu Y, Watahiki M, Nagai Y. Rapid categorization of pathogenic Escherichia coli by multiplex PCR. Microbiol Immunol. 2005;49(6):485–492. doi: 10.1111/j.1348-0421.2005.tb03752.x. [DOI] [PubMed] [Google Scholar]
- López-Saucedo C, Cerna JF, Villegas-Sepulveda N, Thompson R, Velazquez FR, Torres J, Tarr PI, Estrada-Garcia T. Single multiplex polymerase chain reaction to detect diverse loci associated with diarrheagenic Escherichia coli. Emerg Infect Dis. 2003;9(1):127–131. doi: 10.3201/eid0901.010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbene AB, Houreld NN, Abrahamse H. DNA damage after phototherapy in wounded fibroblast cells irradiated with 16 J/cm2. J Photochem Photobiol. 2009;94:131–137. doi: 10.1016/j.jphotobiol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Moses AE, Garbati MA, Egwu AO, Ameh EJ. Detection of E. coli 0157 and 026 serogroups in human immunodeficiency virus-infected patients with clinical manifestation of diarrhoea in Maiduguri, Nigeria. Research Journal of Medicine and Medical Sciences. 2006;1(4):140–145. [Google Scholar]
- Müller EE, Ehlers MM, Grabow WOK. The occurrence of E. coli 0157:H7 in South African water sources intended for direct and indirect human consumption. Water Res. 2001;35(13):3085–3088. doi: 10.1016/S0043-1354(00)00597-2. [DOI] [PubMed] [Google Scholar]
- Omar KB, Barnard TG, Jagals P. Development of a competitive PCR assay for the quantification of total Escherichia coli DNA in water. Afr J Biotechnol. 2010;9(4):564–572. [Google Scholar]
- Pass MA, Odedra R, Batt RM. Multiplex PCR for identification of Escherichia coli virulence genes. J Clin Microbiol. 2000;38:2001–2004. doi: 10.1128/jcm.38.5.2001-2004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presterl E, Zwick RH, Reichmann S, Aichelburg A, Winkler S, Kremsner PG, Graninger W. Frequency and virulence properties of diarrheagenic Escherichia coli in children with diarrhoea in Gabon. Am J Trop Med Hyg. 2003;69(4):406–410. [PubMed] [Google Scholar]
- Sabat G, Rose P, Hickey WJ, Harkin JM. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl Environ Microbiol. 2000;66(2):844–849. doi: 10.1128/AEM.66.2.844-849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh Y-SC, Wait D, Tai L, Sobsey MD. Methods to remove inhibitors in sewage and other faecal wastes for enterovirus detection by the polymerase chain reaction. J Virol Methods. 1995;54:51–66. doi: 10.1016/0166-0934(95)00025-P. [DOI] [PubMed] [Google Scholar]
- Soto SM, Guiral EG, Vila BJ. Prevalence of the set-1B and astA genes encoding enterotoxins in uropathogenic Escherichia coli clinical isolates. Microb Pathog. 2009;47:305–307. doi: 10.1016/j.micpath.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Standard Methods (2005) For the examination of water and wastewater, 21st edn, Edited by Eaton AD, Clesceri LS, Rice EW and Greenberg AE. Washington, USA
- Tarr CL, Large TM, Moeller CL, Lacher DW, Tarr PI, Acheson DW, Whittam TS. Molecular characterization of a serotype 0121:H19 clone, a distinct shiga toxin-producing clone of pathogenic Escherichia coli. Infect Immun. 2002;70(12):6853–6859. doi: 10.1128/IAI.70.12.6853-6859.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Kruger E, Durán C, Lagos R, Levine M, Prado V, Toto C, Vidal R. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43(10):5362–5365. doi: 10.1128/JCM.43.10.5362-5365.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AG. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63(10):3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XY, Chapman T, Trott DJ, Bettelheim K, Do TN, Driesen S, Walker MJ, Chin J. Comparative analysis of virulence genes, genetic diversity, and phylogeny of commensal and enterotoxigenic Escherichia coli isolates from weaned pigs. Appl Environ Microbiol. 2007;73(1):83–91. doi: 10.1128/AEM.00990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuyanagi J, Saito S, Miyajima Y, Amano KI, Enomoto K. Characterization of atypical Enteropathogenic Escherichia coli strains harbouring the astA gene that were associated with a waterborne outbreak of diarrhoea in Japan. J Clin Microbiol. 2003;41(5):2033–2039. doi: 10.1128/JCM.41.5.2033-2039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]