Abstract
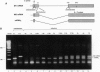
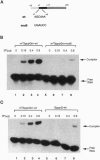
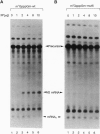
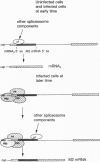
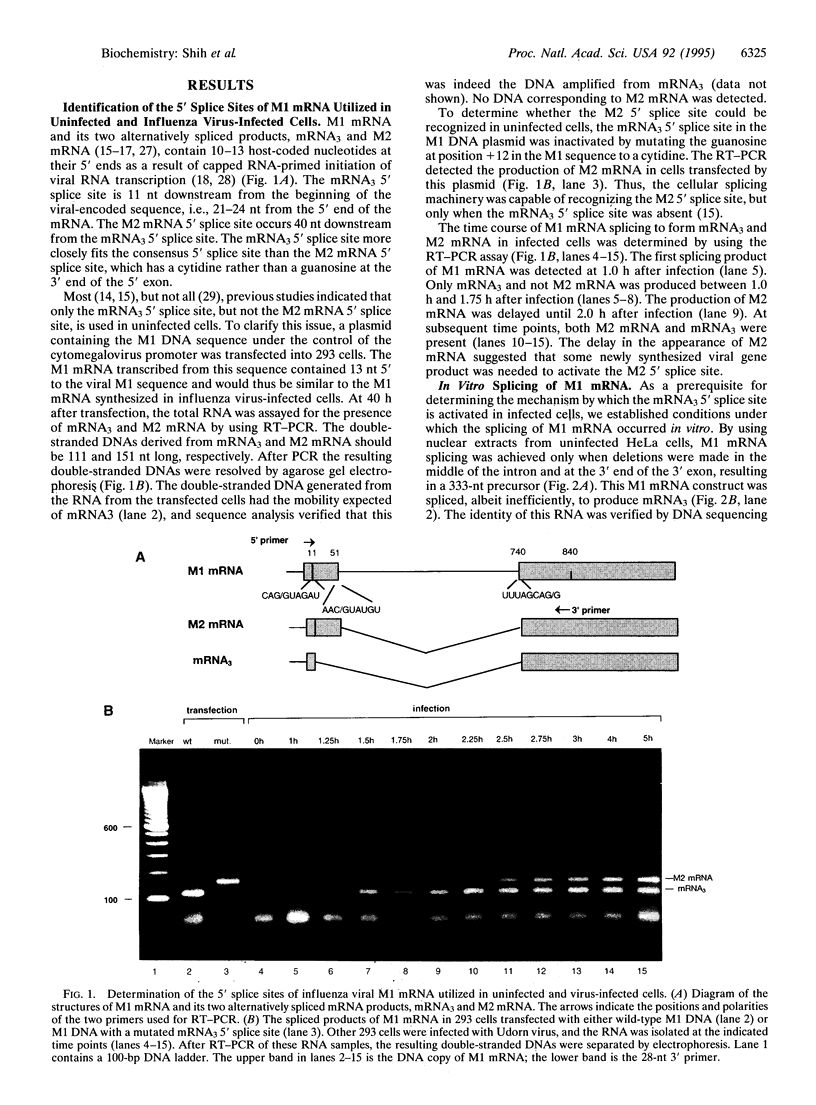
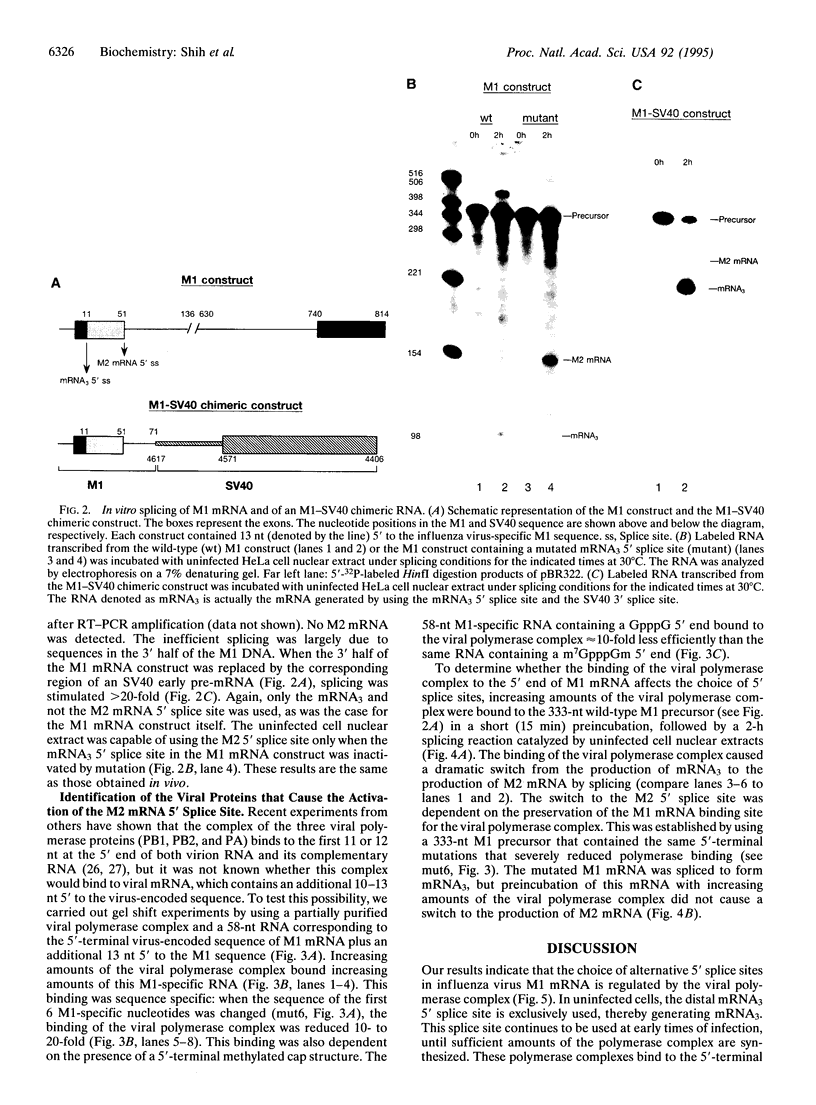
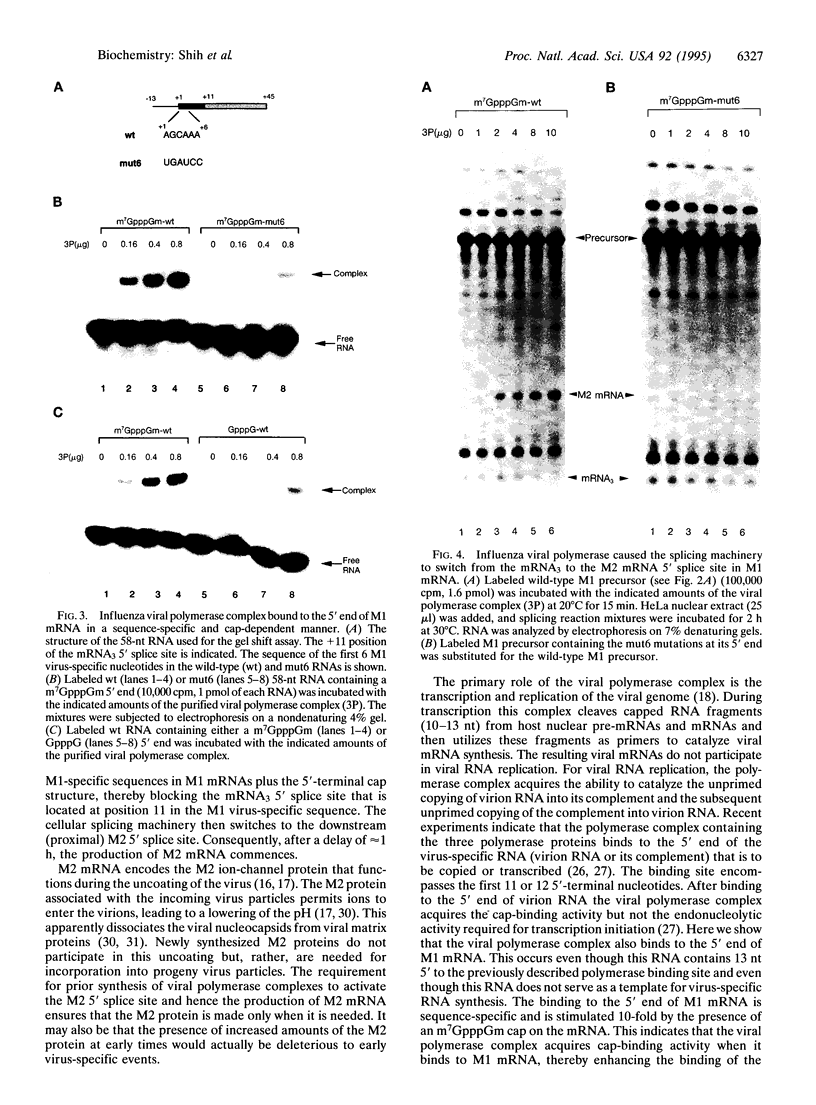
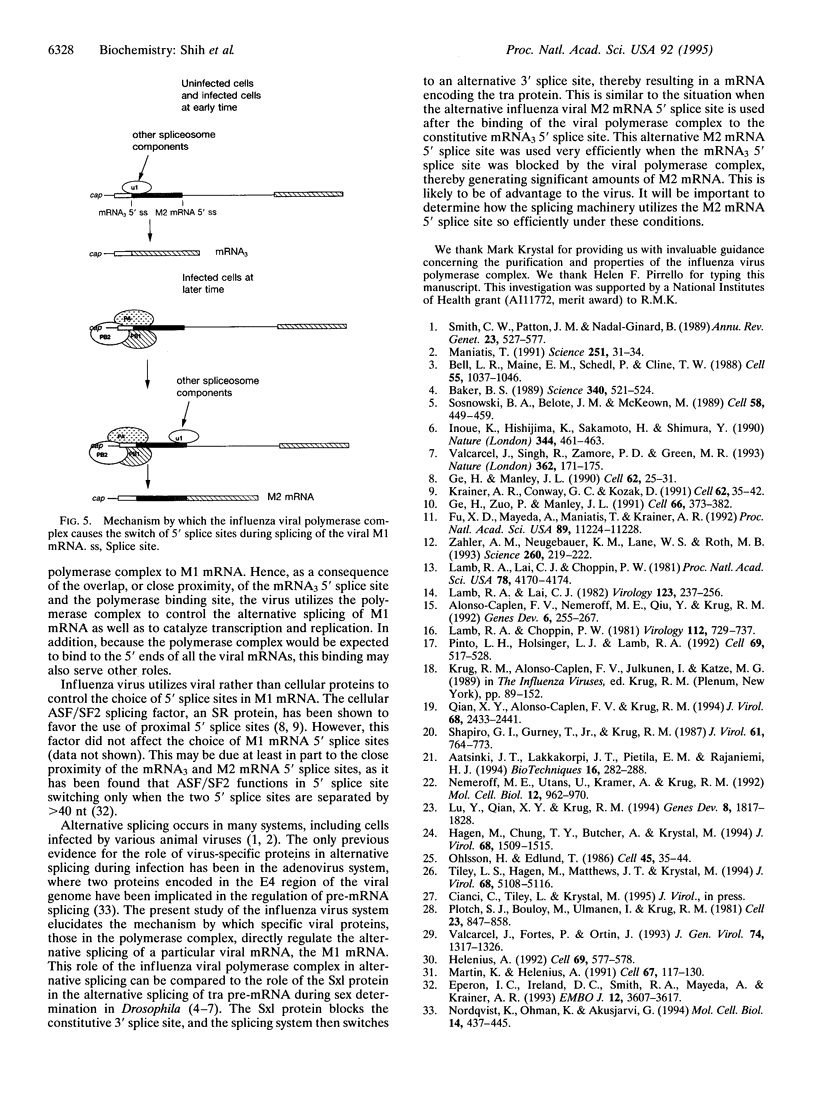
The influenza virus M1 mRNA has two alternative 5' splice sites: a distal 5' splice site producing mRNA3 that has the coding potential for 9 amino acids and a proximal 5' splice site producing M2 mRNA encoding the essential M2 ion-channel protein. Only mRNA3 was made in uninfected cells transfected with DNA expressing M1 mRNA. Similarly, using nuclear extracts from uninfected cells, in vitro splicing of M1 mRNA yielded only mRNA3. Only when the mRNA3 5' splice site was inactivated by mutation was M2 mRNA made in uninfected cells and in uninfected cell extracts. In influenza virus-infected cells, M2 mRNA was made, but only after a delay, suggesting that newly synthesized viral gene product(s) were needed to activate the M2 5' splice site. We present strong evidence that these gene products are the complex of the three polymerase proteins, the same complex that functions in the transcription and replication of the viral genome. Gel shift experiments showed that the viral polymerase complex bound to the 5' end of the viral M1 mRNA in a sequence-specific and cap-dependent manner. During in vitro splicing catalyzed by uninfected cell extracts, the binding of the viral polymerase complex blocked the mRNA3 5' splice site, resulting in the switch to the M2 mRNA 5' splice site and the production of M2 mRNA.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aatsinki J. T., Lakkakorpi J. T., Pietilä E. M., Rajaniemi H. J. A coupled one-step reverse transcription PCR procedure for generation of full-length open reading frames. Biotechniques. 1994 Feb;16(2):282-4, 286-8. [PubMed] [Google Scholar]
- Alonso-Caplen F. V., Nemeroff M. E., Qiu Y., Krug R. M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992 Feb;6(2):255–267. doi: 10.1101/gad.6.2.255. [DOI] [PubMed] [Google Scholar]
- Baker B. S. Sex in flies: the splice of life. Nature. 1989 Aug 17;340(6234):521–524. doi: 10.1038/340521a0. [DOI] [PubMed] [Google Scholar]
- Bell L. R., Maine E. M., Schedl P., Cline T. W. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988 Dec 23;55(6):1037–1046. doi: 10.1016/0092-8674(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Ireland D. C., Smith R. A., Mayeda A., Krainer A. R. Pathways for selection of 5' splice sites by U1 snRNPs and SF2/ASF. EMBO J. 1993 Sep;12(9):3607–3617. doi: 10.1002/j.1460-2075.1993.tb06034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Mayeda A., Maniatis T., Krainer A. R. General splicing factors SF2 and SC35 have equivalent activities in vitro, and both affect alternative 5' and 3' splice site selection. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11224–11228. doi: 10.1073/pnas.89.23.11224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge H., Manley J. L. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990 Jul 13;62(1):25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- Ge H., Zuo P., Manley J. L. Primary structure of the human splicing factor ASF reveals similarities with Drosophila regulators. Cell. 1991 Jul 26;66(2):373–382. doi: 10.1016/0092-8674(91)90626-a. [DOI] [PubMed] [Google Scholar]
- Hagen M., Chung T. D., Butcher J. A., Krystal M. Recombinant influenza virus polymerase: requirement of both 5' and 3' viral ends for endonuclease activity. J Virol. 1994 Mar;68(3):1509–1515. doi: 10.1128/jvi.68.3.1509-1515.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. Unpacking the incoming influenza virus. Cell. 1992 May 15;69(4):577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- Inoue K., Hoshijima K., Sakamoto H., Shimura Y. Binding of the Drosophila sex-lethal gene product to the alternative splice site of transformer primary transcript. Nature. 1990 Mar 29;344(6265):461–463. doi: 10.1038/344461a0. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Conway G. C., Kozak D. The essential pre-mRNA splicing factor SF2 influences 5' splice site selection by activating proximal sites. Cell. 1990 Jul 13;62(1):35–42. doi: 10.1016/0092-8674(90)90237-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Identification of a second protein (M2) encoded by RNA segment 7 of influenza virus. Virology. 1981 Jul 30;112(2):729–737. doi: 10.1016/0042-6822(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J., Choppin P. W. Sequences of mRNAs derived from genome RNA segment 7 of influenza virus: colinear and interrupted mRNAs code for overlapping proteins. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4170–4174. doi: 10.1073/pnas.78.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Lai C. J. Spliced and unspliced messenger RNAs synthesized from cloned influenza virus M DNA in an SV40 vector: expression of the influenza virus membrane protein (M1). Virology. 1982 Dec;123(2):237–256. doi: 10.1016/0042-6822(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Lu Y., Qian X. Y., Krug R. M. The influenza virus NS1 protein: a novel inhibitor of pre-mRNA splicing. Genes Dev. 1994 Aug 1;8(15):1817–1828. doi: 10.1101/gad.8.15.1817. [DOI] [PubMed] [Google Scholar]
- Maniatis T. Mechanisms of alternative pre-mRNA splicing. Science. 1991 Jan 4;251(4989):33–34. doi: 10.1126/science.1824726. [DOI] [PubMed] [Google Scholar]
- Martin K., Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991 Oct 4;67(1):117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- Nemeroff M. E., Utans U., Krämer A., Krug R. M. Identification of cis-acting intron and exon regions in influenza virus NS1 mRNA that inhibit splicing and cause the formation of aberrantly sedimenting presplicing complexes. Mol Cell Biol. 1992 Mar;12(3):962–970. doi: 10.1128/mcb.12.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordqvist K., Ohman K., Akusjärvi G. Human adenovirus encodes two proteins which have opposite effects on accumulation of alternatively spliced mRNAs. Mol Cell Biol. 1994 Jan;14(1):437–445. doi: 10.1128/mcb.14.1.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson H., Edlund T. Sequence-specific interactions of nuclear factors with the insulin gene enhancer. Cell. 1986 Apr 11;45(1):35–44. doi: 10.1016/0092-8674(86)90535-0. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Plotch S. J., Bouloy M., Ulmanen I., Krug R. M. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981 Mar;23(3):847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- Qian X. Y., Alonso-Caplen F., Krug R. M. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J Virol. 1994 Apr;68(4):2433–2441. doi: 10.1128/jvi.68.4.2433-2441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro G. I., Gurney T., Jr, Krug R. M. Influenza virus gene expression: control mechanisms at early and late times of infection and nuclear-cytoplasmic transport of virus-specific RNAs. J Virol. 1987 Mar;61(3):764–773. doi: 10.1128/jvi.61.3.764-773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Sosnowski B. A., Belote J. M., McKeown M. Sex-specific alternative splicing of RNA from the transformer gene results from sequence-dependent splice site blockage. Cell. 1989 Aug 11;58(3):449–459. doi: 10.1016/0092-8674(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Tiley L. S., Hagen M., Matthews J. T., Krystal M. Sequence-specific binding of the influenza virus RNA polymerase to sequences located at the 5' ends of the viral RNAs. J Virol. 1994 Aug;68(8):5108–5116. doi: 10.1128/jvi.68.8.5108-5116.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcárcel J., Fortes P., Ortín J. Splicing of influenza virus matrix protein mRNA expressed from a simian virus 40 recombinant. J Gen Virol. 1993 Jul;74(Pt 7):1317–1326. doi: 10.1099/0022-1317-74-7-1317. [DOI] [PubMed] [Google Scholar]
- Valcárcel J., Singh R., Zamore P. D., Green M. R. The protein Sex-lethal antagonizes the splicing factor U2AF to regulate alternative splicing of transformer pre-mRNA. Nature. 1993 Mar 11;362(6416):171–175. doi: 10.1038/362171a0. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Neugebauer K. M., Lane W. S., Roth M. B. Distinct functions of SR proteins in alternative pre-mRNA splicing. Science. 1993 Apr 9;260(5105):219–222. doi: 10.1126/science.8385799. [DOI] [PubMed] [Google Scholar]