Abstract
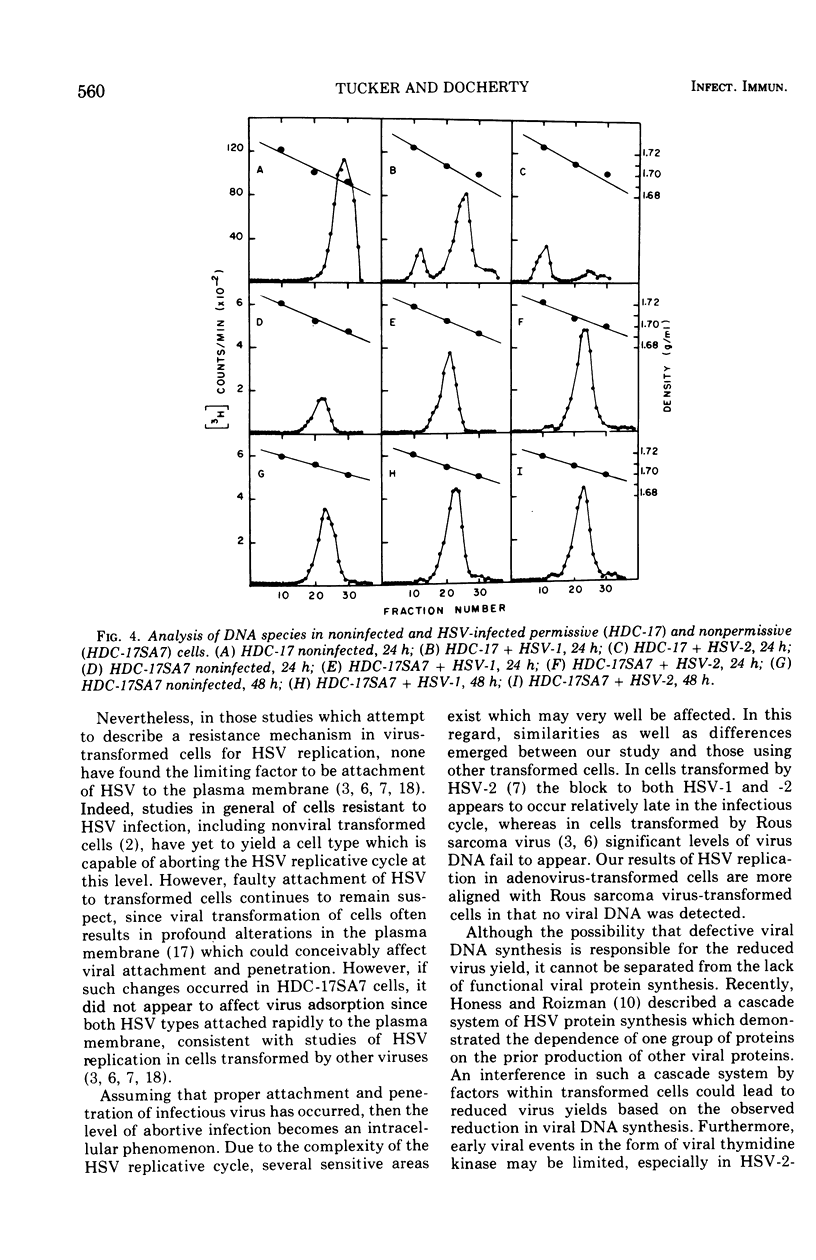
A cell line that normally supports the replication of herpes simplex virus types 1 and 2 became resistant to these viruses after transformation by simian adenovirus 7. Kinetic studies of the mechanism of resistance demonstrated that both herpesviruses were able to attach to the transformed cells and express some early genomic functions, as demonstrated by the presence of low levels of viral thymidine kinase. However, isopycnic centrifugation studies of the abortive system failed to detect viral deoxyribonucleic acid synthesis, whereas indirect immunofluorescent studies of viral proteins revealed that less than 10 per cent of the cells contained these viral macromolecules at any given time. Collectively the data suggest that after transformation by simian adenovirus 7 these cells are altered so as to render them resistant or incapable of supporting the growth of herpes simplex virus types 1 and 2. The results further suggest that the block occurs after viral absorption and prior to viral deoxyribonucleic acid synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURELIAN L., ROIZMAN B. THE HOST RANGE OF HERPES SIMPLEX VIRUS; INTERFERON, VIRAL DNA, AND ANTIGEN SYNTHESIS IN ABORTIVE INFECTION OF DOG KIDNEY CELLS. Virology. 1964 Apr;22:452–461. doi: 10.1016/0042-6822(64)90066-2. [DOI] [PubMed] [Google Scholar]
- Albert D. M., Rabson A. S. Inhibition of ocular herpes simples infection in rabbits by extracts of Burkitt's lymphoma cell cultures. Proc Soc Exp Biol Med. 1971 Oct;138(1):108–111. doi: 10.3181/00379727-138-35841. [DOI] [PubMed] [Google Scholar]
- Campbell W. F., Murray B. K., Biswal N., Benyesh-Melnick M. Restriction of herpes simplex virus type 1 replication in oncornavirus-transformed cells. J Natl Cancer Inst. 1974 Mar;52(3):757–761. doi: 10.1093/jnci/52.3.757. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Goldberg R. J., Rapp F. Differential effect of 7,12-dimethylbenz(a)anthracene on infectivity of herpes simplex virus type 2. Proc Soc Exp Biol Med. 1971 Jan;136(1):328–333. doi: 10.3181/00379727-136-35258. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Mitchell W. R., Thompson C. J., Anthony A. Abortive herpes simplex virus replication in Rous sarcoma virus transformed cells. Proc Soc Exp Biol Med. 1973 Nov;144(2):697–704. doi: 10.3181/00379727-144-37665. [DOI] [PubMed] [Google Scholar]
- Docherty J. J., Mäntyjärvi R. A., Rapp F. Mechanism of the restricted growth of herpes simplex virus type 2 in a hamster cell line. J Gen Virol. 1972 Sep;16(3):255–264. doi: 10.1099/0022-1317-16-3-255. [DOI] [PubMed] [Google Scholar]
- Doller E., Duff R., Rapp F. Resistance of hamster cells transformed by herpes simplex virus type 2 to superinfection by herpes simplex viruses. Intervirology. 1973;1(3):154–167. doi: 10.1159/000148842. [DOI] [PubMed] [Google Scholar]
- Fletcher R. D., Jayavasu C. Interaction of herpes simplex virus and rhinovirus in cell culture. Brief report. Arch Gesamte Virusforsch. 1972;38(1):105–107. doi: 10.1007/BF01241361. [DOI] [PubMed] [Google Scholar]
- Floyd R., Glasser R., Vonka V., Benyesh-Melnick M. Studies on the growth of herpes simplex virus in lymphoblastoid cells. Acta Virol. 1971 Mar;15(2):133–142. [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogino T., Rapp F. Differences in thermal stability of deoxythymidine kinase activity in extracts from cell infected with herpes simplex virus type 1 or type 2. Virology. 1971 Dec;46(3):953–955. doi: 10.1016/0042-6822(71)90094-8. [DOI] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Henson D., Bowling C. P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology. 1969 Sep;39(1):134–137. doi: 10.1016/0042-6822(69)90355-9. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., Tyrrell S. A., Legallais F. Y. An inhibitor of herpesvirus hominis in extracts of cultures of Burkitt's lymphoma cells. Proc Soc Exp Biol Med. 1971 May;137(1):264–267. doi: 10.3181/00379727-137-35557. [DOI] [PubMed] [Google Scholar]
- Saxton R. E., Stevens J. G. Restriction of herpes simplex virus replication by poliovirus: a selective inhibition of viral translation. Virology. 1972 Apr;48(1):207–220. doi: 10.1016/0042-6822(72)90128-6. [DOI] [PubMed] [Google Scholar]
- Szántó J., Lesso J. Reproduction of herpes simplex virus in myxovirus-infected cell cultures. Acta Virol. 1971 Jan;15(1):47–57. [PubMed] [Google Scholar]
- van der Noordaa J., Enders J. F., Diamandopoulos G. T. Increased resistance to herpes simplex virus of hamster and human cells transformed by SV40. Proc Soc Exp Biol Med. 1966 Jul;122(3):915–920. doi: 10.3181/00379727-122-31289. [DOI] [PubMed] [Google Scholar]