Abstract
The efficacy and safety of retreatment with varenicline in smokers attempting to quit were evaluated in this randomized, double-blind, placebo-controlled, multicenter trial (Australia, Belgium, Canada, the Czech Republic, France, Germany, the United Kingdom, and the United States). Participants were generally healthy adult smokers (≥10 cigarettes/day) with ≥1 prior quit attempt (≥2 weeks) using varenicline and no quit attempts in ≤3 months; they were randomly assigned (1:1) to 12 weeks' varenicline (n = 251) or placebo (n = 247) treatment, with individual counseling, plus 40 weeks' nontreatment follow-up. The primary efficacy end point was the carbon monoxide–confirmed (≤10 ppm) continuous abstinence rate for weeks 9–12, which was 45.0% (varenicline; n = 249) vs. 11.8% (placebo; n = 245; odds ratio: 7.08; 95% confidence interval: 4.34, 11.55; P < 0.0001). Common varenicline group adverse events were nausea, abnormal dreams, and headache, with no reported suicidal behavior. Varenicline is efficacious and well tolerated in smokers who have previously taken it. Abstinence rates are comparable with rates reported for varenicline-naive smokers.
Tobacco dependence continues to kill an estimated 5 million tobacco users each year worldwide.1 Most smokers would like to quit,2 and many make several quit attempts each year.3 However, few are able to achieve permanent or long-term abstinence (≥6 months) from smoking with a single quit attempt.4 This chronic cycling of periods of tobacco use followed by periods of abstinence and periods of relapse5 in highly dependent smokers has resulted in such tobacco dependence being defined as a chronic disease.2,6 Treatment guidelines encourage clinicians to prescribe/recommend medications plus counseling for each quit attempt.6 Repeated medication-assisted quit attempts can result in progressively greater abstinence over time.5 Even if increasing the frequency of quit attempts per year does not result in long periods of abstinence, these attempts may still be clinically important by reducing exposure to toxicants in tobacco smoke and improving lung function.4
In countries with long-standing tobacco control policies and declining prevalence of smoking, an increasing proportion of continuing smokers accessing smoking cessation medications have a history of previous quit attempts with these medications.7 In medical conditions for which multiple medication options are available for treatment, there may be more flexibility for the clinician to recommend/prescribe a drug different from the one used earlier, but smoking cessation medications approved by national regulatory agencies are generally limited to nicotine replacement therapies (NRTs), bupropion, and varenicline.6,8,9,10 Development and approval of new therapies have been slow. The most recent approvals were for bupropion (1997) and varenicline (2006).11
Switching from one form of NRT to another,12,13 or from NRT to bupropion,14 may increase the likelihood of abstinence with the next quit attempt, but there are few medications to try before retreatment with one of these medications is the only option remaining. There are limited data to guide clinical decisions for subsequent treatments with smoking cessation medications. Long-term abstinence rates (6 months) for previous nicotine patch users retreated with various forms of NRT range from 0 to 6.4%.15,16,17 The 6-month abstinence rate for the one bupropion retreatment study was 12%.18 A recent study tested repeated 6-month cycles of treatment with bupropion (7 weeks) or NRT (nicotine patches; 6 weeks) in smokers who failed to achieve abstinence at the end of initial treatment. Abstinence rates measured as 7-day point prevalence at the end of three consecutive retreatment cycles were 12.4, 16, and 15.9%, respectively. The report did not specify whether participants opted for repeated treatment with the same medication for each cycle.19 Although both NRT and bupropion have been shown to be efficacious for retreatment, abstinence rates are more than threefold lower for NRTs and twofold lower for bupropion compared with initial treatment with those drugs.6 With the approval of varenicline, an additional medication candidate for retreatment became available, but studies that provide clinician guidance for retreatment have not been conducted. This study evaluates the efficacy and safety of retreatment with varenicline in smokers who had taken varenicline for ≥2 weeks in a previous smoking cessation attempt.
Results
Participant disposition
Of the 593 participants screened, 498 were randomized to varenicline (n = 251) or placebo (n = 247). A total of 494 participants took at least one dose of varenicline (n = 249) or placebo (n = 245) and were included in both the main efficacy analyses and the safety analyses. Details of total participant disposition for the trial are provided in Figure 1.
Figure 1.
Participant disposition.
Baseline characteristics
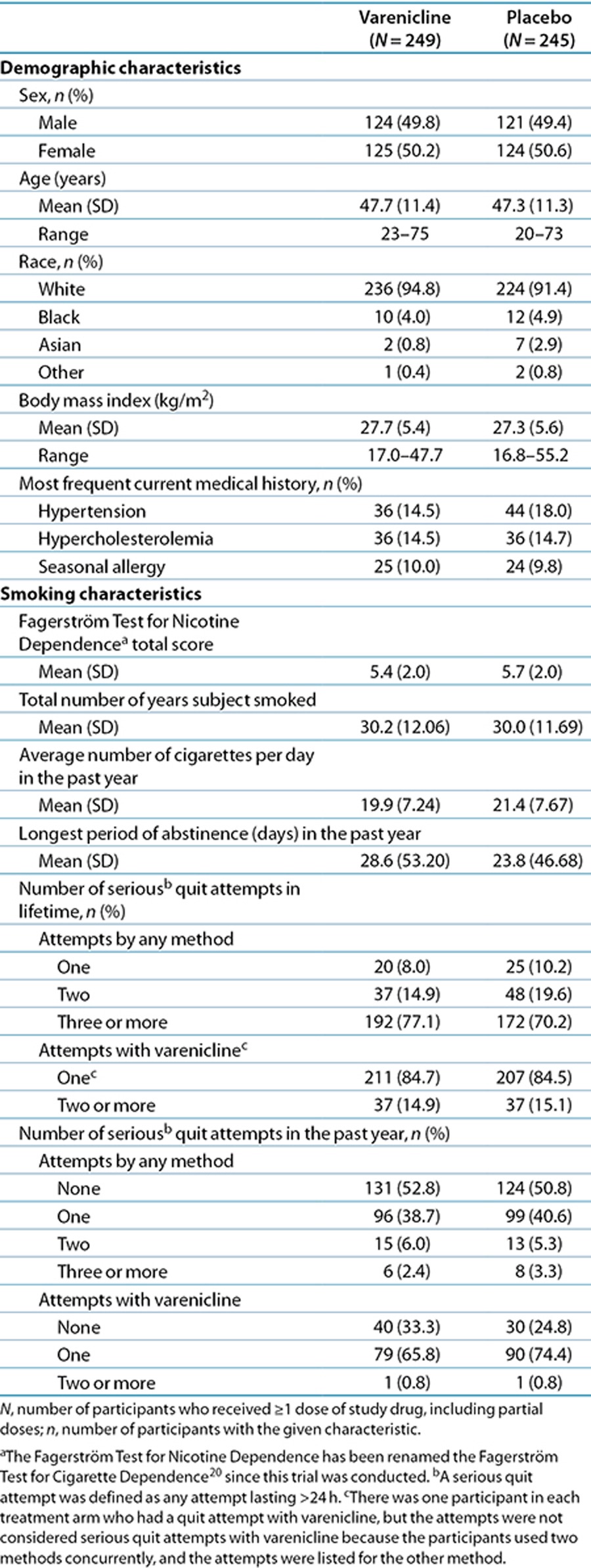
Participant baseline characteristics were similar for the two treatment groups (Table 1). Overall, 93% of participants were Caucasian, mean age was 47.5 years, participants had smoked a mean of 20 cigarettes/day for an average of 30 years, and mean Fagerström Test for Nicotine Dependence (now known as the Fagerström Test for Cigarette Dependence)20 scores were 5.6. Most participants (74%) reported three or more previous lifetime attempts to quit smoking, and all had made one or more quit attempts with varenicline.
Table 1. Participant characteristics at baseline.
Efficacy
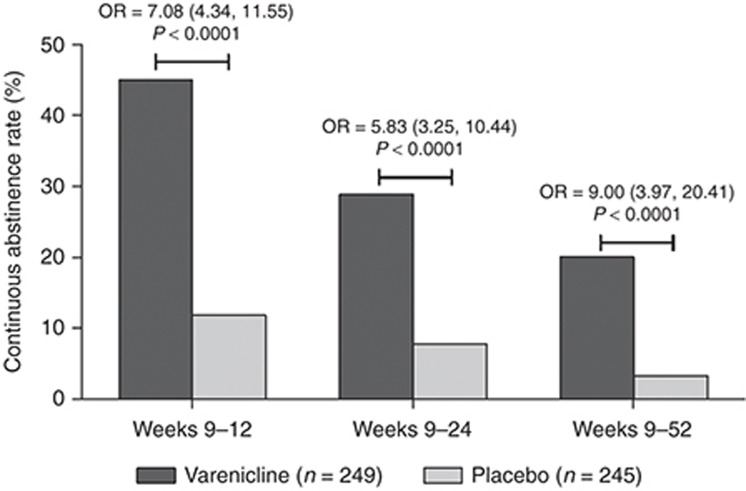
The following results are for those participants who took at least one dose of study drug (varenicline n = 249; placebo n = 245). Participants retreated with varenicline had significantly greater continuous abstinence rates (CARs) than those treated with placebo for weeks 9–12 (45.0 vs. 11.8%; odds ratio (OR) = 7.08 (95% confidence interval (CI): 4.34, 11.55), P < 0.0001), weeks 9–24 (28.9 vs. 7.8%; OR = 5.83 (95% CI: 3.25, 10.44), P < 0.0001), and weeks 9–52 (20.1 vs. 3.3%; OR = 9.00 (95% CI: 3.97, 20.41), P < 0.0001; Figure 2). For the primary end point of CAR for weeks 9–12, there was no interaction effect of treatment by pooled study center (P = 0.8407).
Figure 2.
Continuous abstinence rates (CARs), defined as the percentage of participants remaining continuously abstinent from week 9 to each in-clinic visit through week 52. ORs shown are for CAR during weeks 9–12 (primary end point) and for CARs during weeks 9–24 and 9–52 (secondary end points). CI, confidence interval; N, number of participants who received ≥1 dose, including partial doses, of randomized study drug; OR, odds ratio.
The CARs for weeks 9–12 for all the randomized (intent-to-treat) population were 44.6% for varenicline vs. 11.7% for placebo (OR = 6.96 (95% CI: 4.27, 11.33), P < 0.0001) and for the completers population (participants with >80% compliance with study medication), they were 56.4% for varenicline vs. 15.4% for placebo (OR = 8.99 (95% CI: 5.16, 15.67), P < 0.0001; Supplementary Table S1 online). More participants in the varenicline group (77.7%) vs. the placebo group (70.9%) complied with study medication (i.e., received any dose of study drug for >80% of the planned number of days in the study), and the median duration of treatment was 84 days in both treatment groups.
The primary efficacy end point of CAR for weeks 9–12 was further examined in exploratory subgroup analyses controlling separately for smoking cessation history and baseline characteristics. The analysis model included the main effect of treatment, one covariate (including one of the subgroup characteristics in the model separately), and the treatment by covariate interaction effect. There were no significant treatment by subgroup interactions in this exploratory analysis (all P > 0.09), and results can be generalized to all subgroups. CARs are listed by subgroup in (Supplementary Table S2 online).
In addition, the 7-day point prevalence abstinence rate was significantly higher for varenicline vs. placebo at week 12 (53.0 vs. 14.7%; OR = 7.85; 95% CI: 4.92, 12.51; P < 0.0001), week 24 (32.9 vs. 15.5%; OR = 2.94; 95% CI: 1.86, 4.64; P < 0.0001), and week 52 (28.9 vs. 12.2%; OR = 3.06; 95% CI: 1.88, 4.97; P < 0.0001).
Safety and tolerability
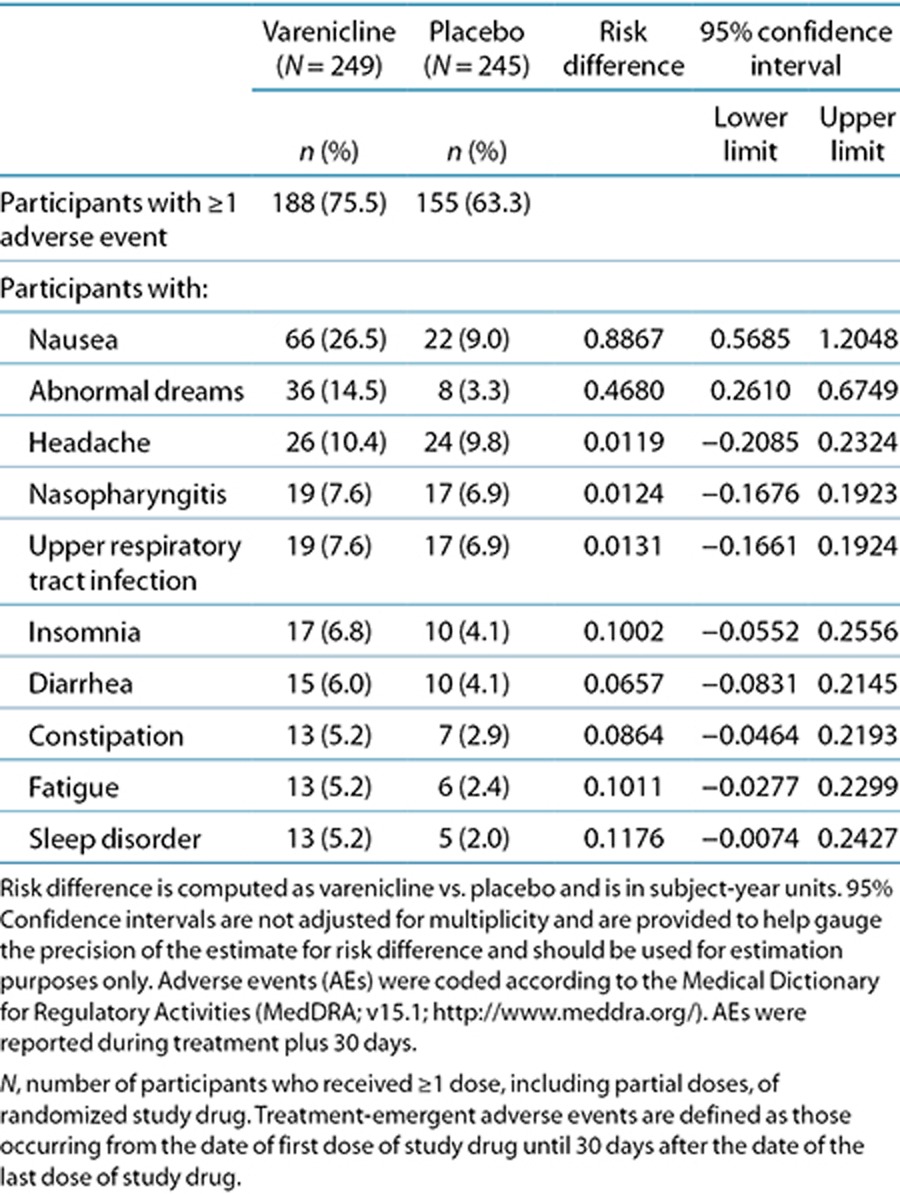
Adverse events (AEs) were similar to those reported in previous varenicline trials of participants who were naive to varenicline. AEs occurred in 188 (75.5%) of those retreated with varenicline and in 155 (63.3%) of those treated with placebo and were generally mild or moderate in both groups. The numbers of treatment discontinuations due to AEs were 18 (7.2%) and 7 (2.9%) for varenicline and placebo, respectively. Dose reductions or temporary discontinuations were 31 (12.4%) for varenicline and 11 (4.5%) for placebo. Common AEs occurring in ≥5% of those receiving varenicline vs. placebo were nausea (26.5 vs. 9.0%), abnormal dreams (14.5 vs. 3.3%), and headache (10.4 vs. 9.8%; Table 2). One cardiac AE (palpitations) occurred in one (0.4%) varenicline participant. Six cardiac AEs occurred in four (1.6%) placebo participants: acute coronary syndrome, angina pectoris, coronary artery disease, congestive cardiac failure, and palpitations (two participants).
Table 2. Participants with adverse events occurring in ≥5% of either treatment group.
The most frequent treatment-emergent psychiatric AEs, occurring in ≥2% of varenicline vs. placebo participants, were abnormal dreams (14.5 vs. 3.3%), insomnia (6.8 vs. 4.1%), sleep disorder (5.2 vs. 2.0%), depressed mood (3.2 vs. 0.4%), agitation (2.0 vs. 0.4%), depression (2.0 vs. 0.8%), and nightmare (2.0 vs. 0.8%). Suicidal ideation was reported on the Columbia Suicide Severity Rating Scale (C-SSRS)21 for three (1.2%) varenicline and no placebo participants during the treatment phase and for two (1.2%) varenicline and no placebo participants during follow-up. Of the three varenicline participants with suicidal ideation during the treatment phase, one had a positive lifetime history on the C-SSRS. Similarly, one of the two varenicline participants with suicidal ideation during the nontreatment follow-up period had a lifetime history of self-injurious behavior on the C-SSRS. At baseline, no participant in either group had any suicidal behavior or ideation. There were no reports of suicidal behavior on the C-SSRS during drug treatment or follow-up phases for either group. No AEs related to suicidal ideation or behavior were reported.
Treatment-emergent serious AEs (SAEs) were reported for 7 (2.8%) varenicline participants: knee arthroplasty, pyelonephritis, intervertebral disc protrusion, ankle fracture, chest pain, and drug hypersensitivity (1 reaction to amoxicillin and 1 reaction to hair dye). For placebo, 4 (1.6%) treatment-emergent SAEs were reported: acute coronary syndrome, ligament rupture, hyperventilation, and drug hypersensitivity. Of these, only chest pain reported in one varenicline participant (day 1 of dosing) was attributed to study drug. During post-drug follow-up, one death was reported for one varenicline participant (~31 weeks after the last dose of study drug). The cause of death on the death certificate was recorded as acute and chronic alcoholism. Death was not attributed to the study drug.
Discussion
In this first randomized trial of retreatment with varenicline for smoking cessation, varenicline was shown to be efficacious. The end-of-treatment CAR for varenicline (45%) was more than 3.8 times greater than that for placebo (11.8%) for both men and women. AEs reported were consistent with those reported in the varenicline label,22 and no suicidal behaviors were reported. Abstinence rates are consistent with varenicline abstinence rates across previous studies in diverse and varenicline-naive populations (Supplementary Table S3 online)23,24,25,26,27,28,29,30,31,32,33,34,35,36 and are in contrast to the lower retreatment abstinence rates with NRTs15,16,17 and bupropion.18
The relapse curve after varenicline retreatment was similar to relapse curves observed in other trials that have included supportive counseling.15,16,17,18,23 Seven-day point prevalence abstinence rates were significantly greater for varenicline vs. placebo at all points. Abstinence peaked for varenicline at week 12 (last week of dosing) and then declined steadily to week 52, as observed in treatment-naive trials. Placebo point prevalence rates peaked earlier, at week 7, and remained somewhat flat until the end of the study (Figure 3). The varenicline CAR was sixfold greater than that for placebo at 52 weeks. Our data suggest that although retreatment with varenicline is effective in attaining and maintaining smoking abstinence (remission), the risk of relapse and the need for additional courses of treatment will likely continue for many chronic smokers. This retreatment paradigm provides an additional treatment approach to that provided by varenicline maintenance therapy, which extends continuous varenicline treatment from 3 to 6 months to aid in maintaining abstinence.34
Figure 3.
Mean 7-day point prevalence of abstinence, defined as the percentage of participants remaining abstinent from cigarette smoking and use of other nicotine and/or tobacco products in the previous 7 days. CI, confidence interval; N, number of participants who received ≥1 dose, including partial doses, of randomized study drug; OR, odds ratio.
The study posed some unique challenges. All participants had earlier exposure to the medicine being investigated. Participants and site staff were not encouraged to guess treatment assignments, but some participants may have assumed that they were assigned placebo based on earlier varenicline experience and, consequently, discontinued study participation. However, the completion rate for placebo participants was similar to placebo completion rates for varenicline-naive participants in approval or pivotal trials.23,24 Because the trial recruited smokers who were willing to try varenicline again, our results may not be generalizable to all smokers who have tried varenicline previously.
This study has several strengths. The trial was designed to answer practical clinical questions about retreating patients with varenicline. Participants were generally healthy, but there were fewer exclusions for cardiovascular disease and cancers, resulting in a somewhat more diverse population than in pivotal varenicline trials.23,24 Although the study was not designed to assess the efficacy or safety of varenicline in smokers with a current psychiatric diagnosis, additional assessments to ensure participant safety and collect uniform data regarding suicidal ideation and behavior were conducted.
In summary, varenicline is efficacious and well tolerated in smokers who have previously taken varenicline. Clinicians can feel confident in recommending varenicline for patients who have failed to achieve abstinence or relapsed to smoking after previous treatment with varenicline.
Methods
Study design. This was a phase IV, double-blind, placebo-controlled, randomized, parallel-group, two-arm, multicenter clinical study with a 12-week drug treatment phase and a 40-week postdrug treatment follow-up phase. The trial was conducted in accordance with the Declaration of Helsinki37 and the International Conference on Harmonisation Good Clinical Practice Guidelines38 between 10 December 2010 and 2 November 2012 in Australia (four centers), Belgium (four centers), Canada (four centers), the Czech Republic (three centers), France (three centers), Germany (five centers), the United Kingdom (five centers), and the United States (seven centers). The institutional review board and/or independent ethics committee at each site approved the study protocol. Written informed consent was obtained from all participants before any procedures were performed.
Setting and participants. Eligible participants were 18 years of age or older, smoked 10 or more cigarettes/day during the 4 weeks before screening, had an exhaled carbon monoxide (CO) value >10 parts per million (ppm) at screening, had no quit attempts in the previous 3 months, had previously taken varenicline for 2 or more weeks, with the last dose taken ≥3 months before screening, and were motivated to stop smoking. Participants were recruited via advertising and patient lists. Key exclusion criteria included any previous significant adverse reaction to varenicline; previous participation in a varenicline study, severe chronic obstructive pulmonary disease; recent (<5 years) history of cancer (except cured basal cell or squamous cell carcinoma of the skin); clinically significant cardiovascular disease or cerebrovascular disease in the previous 2 months; history of a suicide attempt or any suicidal behavior in the past 2 years or current suicidal ideation identified by the C-SSRS21 at screening or baseline; current depression self-reported at screening or with a diagnosis of depression or treatment with antidepressants during the previous 12 months recorded in medical history; lifetime diagnosis of psychosis, panic disorder, other anxiety disorders, or bipolar disorder; active alcohol or substance abuse/dependence (except nicotine) within the past 12 months; or any severe medical or psychiatric condition or laboratory abnormality that would make the participant inappropriate for the study. Additional exclusions included use of NRT, bupropion, or other smoking cessation aids during treatment; use of investigational drugs in the 30 days before the baseline visit and during the study; and use of other tobacco products, electronic cigarettes, marijuana, or illegal or street drugs at any time during the study (those who used tobacco products, marijuana, or street drugs during the study were instructed not to, but were not discontinued from the study; a protocol deviation was recorded for such use). Women of childbearing potential were included provided that they were not pregnant or breastfeeding and agreed to avoid pregnancy and practice effective contraception from the start of the study drug treatment through 30 days after the last dose of the study drug.
Interventions and follow-up. Eligible participants were randomly assigned to receive either varenicline or placebo at a 1:1 ratio for 12 weeks of drug treatment using computer-generated block randomization within each site. Dosing was titrated: 0.5 mg/day for days 1–3, 0.5 mg twice daily for days 4–7, and 1 mg twice daily through the end of week 12. Compliance with dosing was obtained by pill counts and participant self-report at each visit during the drug treatment period.
Participants were then followed in a 40 week nondrug follow-up phase. Clinic visits were at screening, baseline, and weeks 1, 2, 3, 4, 6, 8, 9, 10, 11, and 12 during drug treatment; and at weeks 13, 16, 24, 32, 40, 48, and 52 during follow-up. Brief telephone visits occurred at weeks 5, 7, 14, 20, 28, 36, and 44. Individual counseling (≤10 min) based on the US Agency for Healthcare Research and Quality guideline6 was provided to all participants at clinic and telephone visits, beginning with the baseline visit, to assist in developing coping strategies for achieving and maintaining abstinence. Participants were to set a target quit date to coincide with the week 1 visit. Dosing could be reduced to 0.5 mg twice daily for participants who were unable to tolerate the recommended dose. Those who discontinued the study drug were encouraged to remain in the study and complete the remaining visits.
Suicidal ideation and behavior were assessed with the Suicidal Behaviors Questionnaire–Revised (SBQ-R)39 at screening and the C-SSRS21 at screening and at each clinic visit. No other standard psychiatric assessments were conducted by sites. Additional safety assessments were required for participants who were considered at risk based on their responses on the C-SSRS.
Efficacy measures. Smoking abstinence was assessed by self-report of no smoking (not even a puff) and no use of nicotine-containing products since the last study visit/last contact, confirmed by an exhaled CO value of ≤10 ppm at clinic visits. The primary efficacy end point was the CO-confirmed CAR for the last 4 weeks of treatment (weeks 9–12). The key secondary efficacy end point was CO-confirmed CAR for weeks 9–52. Other secondary end points were CO-confirmed CAR for weeks 9–24 and 7-day point prevalence abstinence (report of no smoking, not even a puff, for the previous 7 days) at weeks 12, 24, and 52.
AEs. All observed or participant-reported AEs were recorded in electronic case report forms and followed to resolution or to the end of the study. AEs that were determined to be life threatening, that resulted in death, hospitalization, significant disability/incapacity, or a congenital anomaly/birth defect, or that were considered important medical events were classified as SAEs. Summaries of AEs include events of all causalities that occurred during treatment and up to 30 days after treatment.
Statistical analysis. The primary efficacy (CAR weeks 9–12) and safety analyses were performed on the all-participants population, which included all randomized participants who took ≥1 dose of study drug, including partial doses. Additional analyses of CAR at weeks 9–12 were performed for the all-randomized (intent-to-treat) population and the completers population (participants with >80% compliance with study medication, as measured by having any dose of study drug for >80% of the planned number of days in the study) (Supplementary Table S1 online). A sample size of 490 participants randomized to varenicline or placebo in a 1:1 ratio was estimated to provide ≥90% power for a comparison of varenicline vs. placebo using a two-group continuity-corrected two-sided χ2 test at the 0.05 significance level for the primary end point (CAR for weeks 9–12), assuming an OR of 3.36 with a placebo CAR of 12% and a varenicline CAR of 31%. It was also estimated to provide 80% power for the treatment comparison in the key secondary end point (CAR for weeks 9–52) for an OR of at least 2.55 with a 6% CAR in the placebo group and 14% in the varenicline group.
In the case of a missed visit or visits during the primary evaluation period (weeks 9–12), a participant was defined as abstinent if the participant reported no smoking or use of nicotine products “since the last contact/visit” at the visit after the missing visit or visits. Missing CO measurements were imputed as negative (i.e., as ≤10 ppm), therefore not disqualifying the participant from being considered abstinent. However, those who missed all visits during weeks 9–12 were considered smokers. Numbers of participants with missing CO measurements during weeks 9–12 are shown (Supplementary Table S4 online).
Participants who discontinued the study were assumed to be smokers from the point of discontinuation through the end of the study. In computing abstinence rates, those who discontinued the study were included in the denominator, but not in the numerator, regardless of their last smoking status evaluation.
CARs for weeks 9–12, 9–24, and 9–52, and 7-day point prevalence abstinence at weeks 12, 24, and 52 were analyzed using a logistic regression model that included treatment and pooled study center as independent variables. Small study centers were pooled for model convergence. The final analysis results were based on the main effects model, regardless of the statistical significance of the treatment by pooled study center interaction.
For the C-SSRS, percentages of participants with “yes” answers for the Columbia Classification Algorithm of Suicide Assessment categories21 were calculated for screening (lifetime), baseline, and treatment phase (and 30 days after), and during follow-up (post-treatment) and by treatment group.
Author Contributions
D.G., P.H., L.P., K.N., L.-J.T, T.D.M., and J.T. wrote the manuscript. D.G., P.H., and J.T. designed the research. D.G., P.H., L.P., K.N., L.-J.T, T.D.M., and J.T. analyzed the data.
Study Highlights

Acknowledgments
The study was funded by Pfizer (clinicaltrials.gov identifier: NCT01244061). The authors thank all study site staff involved in this study. The principal investigators—in addition to the authors D.G., P.H., K.N., and L.P.—were involved in conducting the study at their sites and in collecting data. The principal investigators in each country were as follows: Australia: Ktut Arya (Australian Clinical Research Network), Mark Robert Daglish (Royal Brisbane and Women's Hospital), Ferdinandus De Looze (AusTrials Australia), and Stephen Hall (Emeritus Research); Belgium: Pierre Bartsch (Centre Hospitalier Universitaire de Liege), Wilfried A.K. De Backer (Universitair Ziekenhuis Antwerpen), and Laurence Galanti (Cliniques Universitaires U.C.L. de Mont-Godinne/Laboratoire); Canada: Ronald Collette (Burnaby), Claude Gagne (Clinique des Maladies Lipidiques de Quebec), and Randy O. Hart (White Hills Medical Clinic); the Czech Republic: Lucie Cwikova (Mestska Nemocnice Ostrava), Eva Kralikova (Vseobecna Fakultni Nemocnice v Praze), and Iva Tomaskova (Fakultni Nemocnice Brno); France, Francis Couturaud (CHU de la Cavale Blanche), Beatrice Le Maitre (CHU Côte de Nacre, Unité de Coordination de Tabacologie), and Xavier Quantin (Hopital Arnaud de Villeneuve); Germany: Grit Andersen (FOCUS Clinical Drug Development GmbH), Tobias Raupach (Universitaetsklinikum Goettingen Zentrum Innere Medizin Abteilung Kardiologie und Pneumologie), Tobias Ruether (Ludwig Maximilians-Universitaet Muenchen), Isabelle Schenkenberger (Klinische Forschung Berlin), and Katrin Zemke (Klinische Forschung Hamburg); the United Kingdom: Essam Eldin Ahmed Abdulhakim (Synexus Merseyside Clinical Research Centre), Hugh James Donnachie (Synexus Scotland Clinical Research Centre), Hana Hassanin (Synexus Lancashire Clinical Research Centre), Daniela Hristova (Synexus Thames Valley Clinical Research Centre), and Manish Saxena (William Harvey Research Institute, Barts and The London School of Medicine and Dentistry); and the United States: James Lewis Borders (Central Kentucky Research Associates), John E. Ervin (The Center for Pharmaceutical Research, PC), Elbert D. Glover (The University of Maryland), Bruce Glenn Rankin (Avail Clinical Research), Stephan Charles Sharp (Clinical Research Associates), and Donald P. Tashkin (University of California, Los Angeles David Geffen School of Medicine). Editorial and graphics support was provided by Abegale Templar and Alexandra Bound of Engage Scientific and funded by Pfizer.
D.G. reports grants, and nonfinancial and other support from Pfizer during the conduct of the study; and grants, stock ownership, and nonfinancial and other support from Pfizer, grants from Nabi Biopharmaceuticals, and personal fees and nonfinancial support from GlaxoSmithKline outside the submitted work. P.H. reports grants from Queen Mary University of London during the conduct of the study and grants and personal fees from Pfizer, McNeil, GlaxoSmithKline, and Novartis outside the submitted work. L.P. reports grants from Pfizer outside the submitted work. K.N. reports funding and nonfinancial support from Pfizer during the conduct of the study and personal fees from Pfizer (Belgium) outside the submitted work, for serving as an advisory board member and for lectures. L.-J.T, T.D.M., and J.T. are employees of Pfizer.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- World Health Organization Tobacco. Fact Sheet no. 339 . < http://www.who.int/mediacentre/factsheets/fs339/en/ > ( 2013. Accessed 24 October 2013.
- Joseph A.M., et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch. Intern. Med. 2011;171:1894–1900. doi: 10.1001/archinternmed.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene J.K., et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19:17–31. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- Partos T.R., Borland R., Yong H.H., Hyland A., Cummings K.M. The quitting rollercoaster: how recent quitting history affects future cessation outcomes (data from the International Tobacco Control 4-country cohort study) Nicotine Tob. Res. 2013;15:1578–1587. doi: 10.1093/ntr/ntt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbeck E.F., et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Ann. Intern. Med. 2009;150:437–446. doi: 10.7326/0003-4819-150-7-200904070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M.C., et al. Clinical Practice Guideline. Treating Tobacco Use and Dependence: 2008 Update . < http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf > ( 2008. Accessed 24 October 2013.
- Agboola S., McNeill A., Coleman T., Leonardi Bee J. A systematic review of the effectiveness of smoking relapse prevention interventions for abstinent smokers. Addiction. 2010;105:1362–1380. doi: 10.1111/j.1360-0443.2010.02996.x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency Guideline on the Development of Medicinal Products for the Treatment of Smoking (Doc. Ref. CHMP/EWP/369963/05) . < http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003509.pdf > ( 2008. Accessed 24 October 2013.
- National Institute for Health and Clinical Excellence Smoking Cessation Services. NICE Public Health Guidance 10 . < http://www.nice.org.uk/nicemedia/live/11925/39596/39596.pdf > ( 2008. Accessed 24 October 2013.
- Zwar N., et al. Supporting Smoking Cessation: a Guide for Health Professionals . < http://www.racgp.org.au/download/documents/Guidelines/smoking-cessation.pdf > ( 2011. Accessed 24 October 2013.
- Department of Health and Human Services, Food and Drug Administration Report to Congress. Innovative Products and Treatments to Achieve Abstinence From Tobacco Use, Reductions in Consumption of Tobacco, and Reductions in the Harm Associated With Continued Tobacco Use . < http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/UCM348930.pdf > ( 2013. Accessed 24 October 2013.
- Shiffman S., Dresler C.M., Rohay J.M. Successful treatment with a nicotine lozenge of smokers with prior failure in pharmacological therapy. Addiction. 2004;99:83–92. doi: 10.1111/j.1360-0443.2004.00576.x. [DOI] [PubMed] [Google Scholar]
- Tønnesen P., Mikkelsen K., Nørregaard J., Jørgensen S. Recycling of hard-core smokers with nicotine nasal spray. Eur. Respir. J. 1996;9:1619–1623. doi: 10.1183/09031936.96.09081619. [DOI] [PubMed] [Google Scholar]
- Durcan M.J., et al. Impact of prior nicotine replacement therapy on smoking cessation efficacy. Am. J. Health Behav. 2002;26:213–220. doi: 10.5993/ajhb.26.3.6. [DOI] [PubMed] [Google Scholar]
- Gourlay S.G., Forbes A., Marriner T., Pethica D., McNeil J.J. Double blind trial of repeated treatment with transdermal nicotine for relapsed smokers. BMJ. 1995;311:363–366. doi: 10.1136/bmj.311.7001.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R., Grass J.A., Pillitteri J.L. Treatment resistant smokers: a pilot study of nicotine nasal spray and inhaler. J. Addict. Dis. 2000;19:95–100. doi: 10.1300/J069v19n01_08. [DOI] [PubMed] [Google Scholar]
- Tønnesen P., Nørregaard J., Säwe U., Simonsen K. Recycling with nicotine patches in smoking cessation. Addiction. 1993;88:533–539. doi: 10.1111/j.1360-0443.1993.tb02060.x. [DOI] [PubMed] [Google Scholar]
- Gonzales D.H., et al. Bupropion SR as an aid to smoking cessation in smokers treated previously with bupropion: a randomized placebo-controlled study. Clin. Pharmacol. Ther. 2001;69:438–444. doi: 10.1067/mcp.2001.115750. [DOI] [PubMed] [Google Scholar]
- Cupertino A.P., Wick J.A., Richter K.P., Mussulman L., Nazir N., Ellerbeck E.F. The impact of repeated cycles of pharmacotherapy on smoking cessation: a longitudinal cohort study. Arch. Intern. Med. 2009;169:1928–1930. doi: 10.1001/archinternmed.2009.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res. 2012;14:75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Posner K., Oquendo M.A., Gould M., Stanley B., Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am. J. Psychiatry. 2007;164:1035–1043. doi: 10.1176/appi.ajp.164.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer CHANTIX® (Varenicline) Tablets . < http://www.labeling.pfizer.com/ShowLabeling.aspx?id=557 > ( 2013. Accessed 24 October 2013.
- Gonzales D., et al. Varenicline Phase 3 Study Group Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Jorenby D.E., et al. Varenicline Phase 3 Study Group Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Anthenelli R.M., et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann. Intern. Med. 2013;159:390–400. doi: 10.7326/0003-4819-159-6-201309170-00005. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Oshima A., Fujimoto Y., Maruyama N., Ishibashi T., Reeves K.R. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin. Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Rennard S., et al. Flexible Quit Date Study Group A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob. Res. 2012;14:343–350. doi: 10.1093/ntr/ntr220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti N.A., Pipe A.L., Benowitz N.L., Arteaga C., Garza D., Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin D.P., Rennard S., Hays J.T., Ma W., Lawrence D., Lee T.C. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139:591–599. doi: 10.1378/chest.10-0865. [DOI] [PubMed] [Google Scholar]
- Tsai S.T., et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin. Ther. 2007;29:1027–1039. doi: 10.1016/j.clinthera.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Garza D., Murphy M., Tseng L.J., Riordan H.J., Chatterjee A. A double-blind randomized placebo-controlled pilot study of neuropsychiatric adverse events in abstinent smokers treated with varenicline or placebo. Biol. Psychiatry. 2011;69:1075–1082. doi: 10.1016/j.biopsych.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Niaura R., et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr. Med. Res. Opin. 2008;24:1931–1941. doi: 10.1185/03007990802177523. [DOI] [PubMed] [Google Scholar]
- Oncken C., et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch. Intern. Med. 2006;166:1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- Tonstad S., Tønnesen P., Hajek P., Williams K.E., Billing C.B., Reeves K.R. Varenicline Phase 3 Study Group Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Wang C., Xiao D., Chan K.P., Pothirat C., Garza D., Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology. 2009;14:384–392. doi: 10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- Bolliger C.T., et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2011;33:465–477. doi: 10.1016/j.clinthera.2011.04.013. [DOI] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects . < http://www.wma.net/en/30publications/10policies/b3/index.html > ( 2008. Accessed 31 October 2012.
- International Conference on Harmonisation The ICH Process for Harmonisation of Guidelines . < http://www.ich.org/cache/compo/276-254-1.html > ( 2009. Accessed 31 October 2012.
- Osman A., Bagge C.L., Gutierrez P.M., Konick L.C., Kopper B.A., Barrios F.X. The Suicidal Behaviors Questionnaire–Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment. 2001;8:443–454. doi: 10.1177/107319110100800409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.