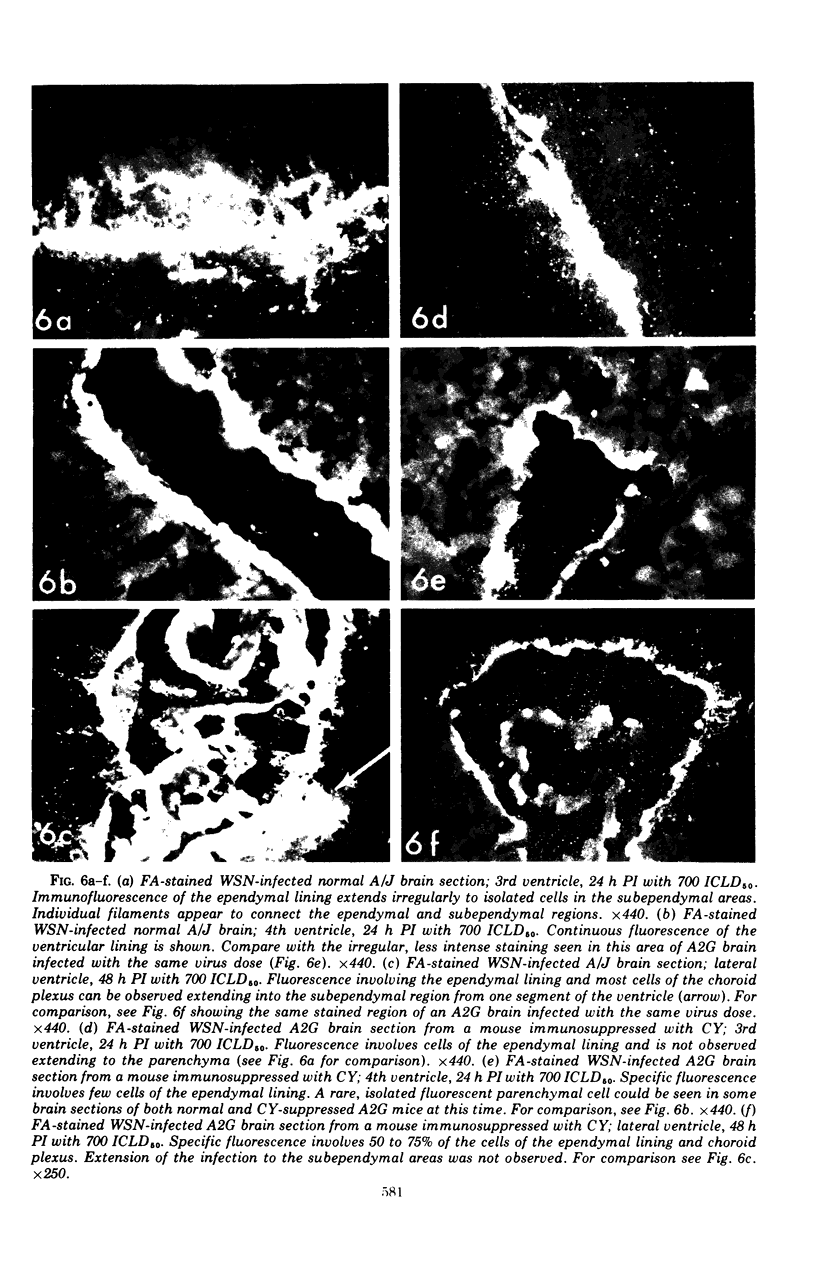
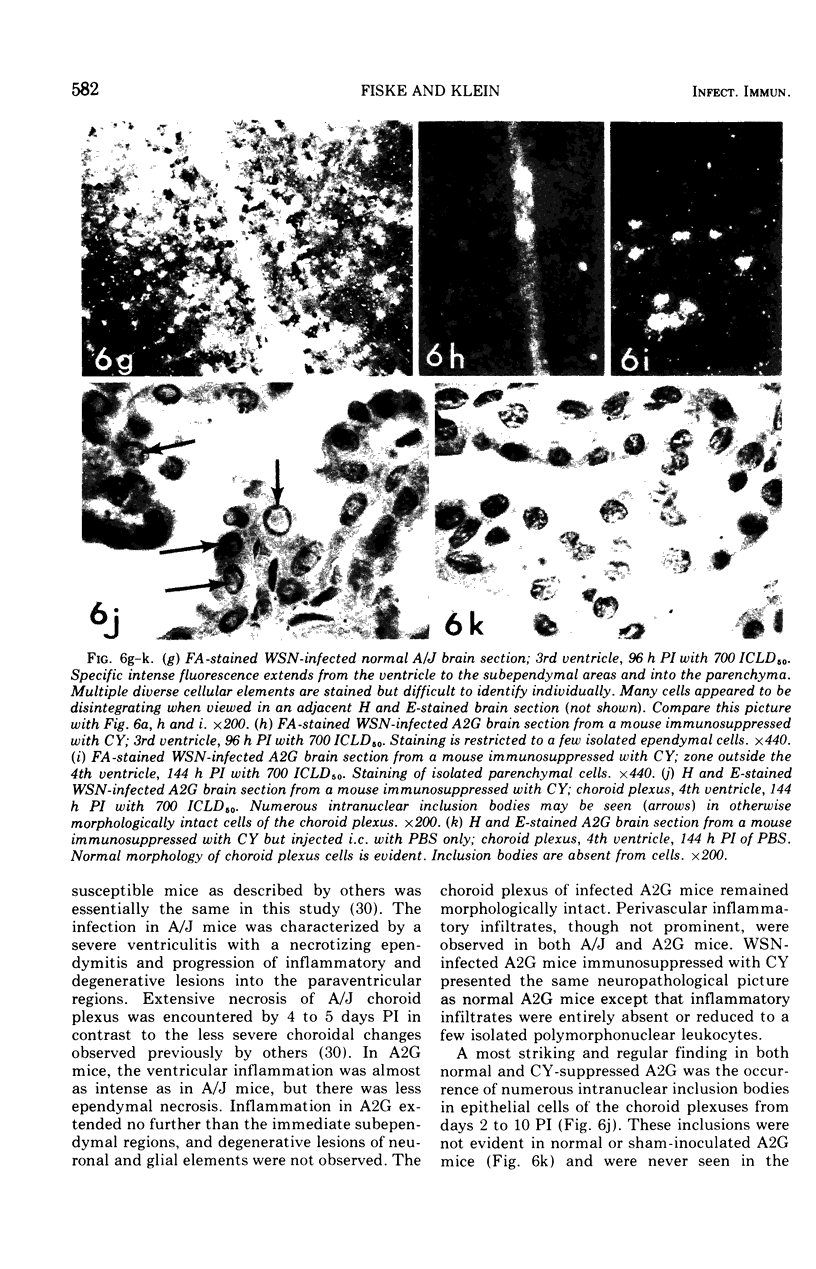
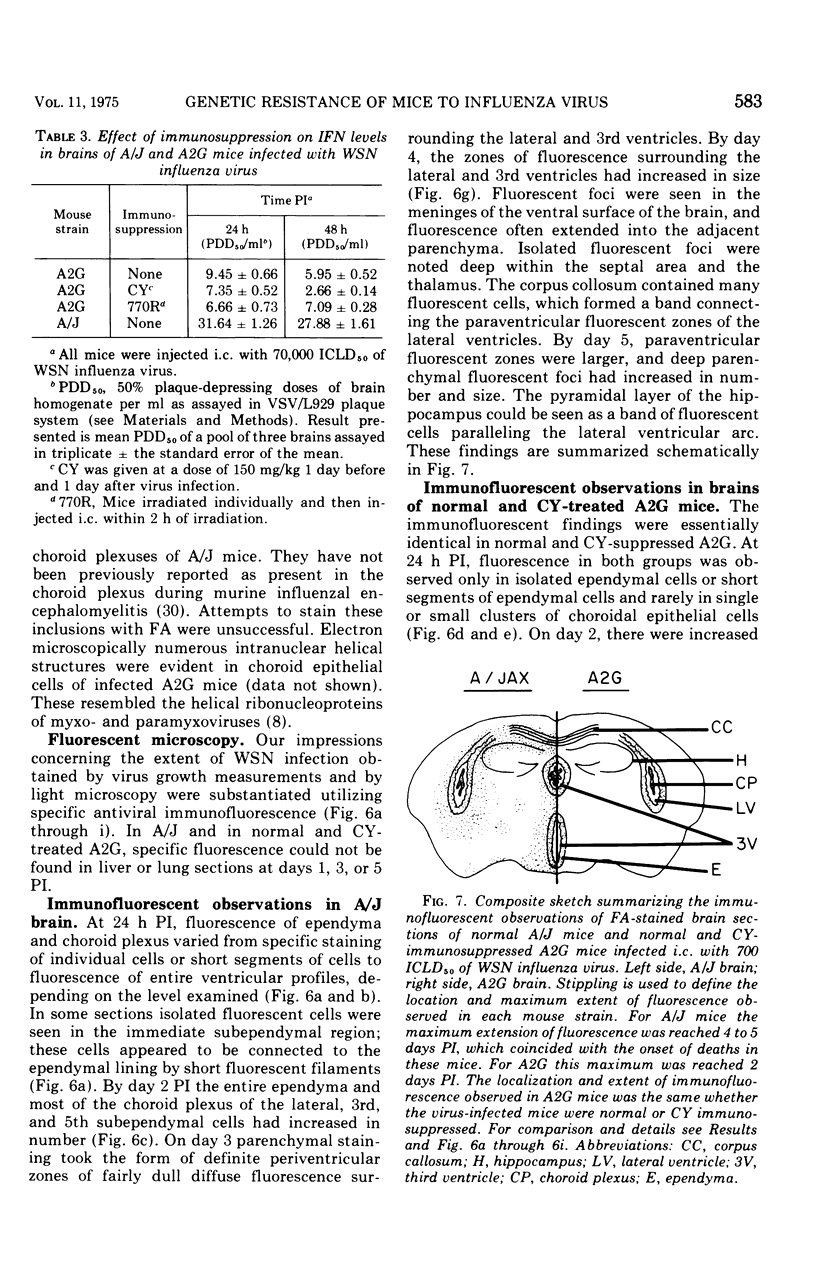
Abstract
A2G mice are genetically resistant to lethal infection with neurotropic and pneumotropic influenza viruses. A possible immunological explanation for this resistance was sought by assessing the effect of cyclophosphamide and X irradiation immunosuppression on the infection of A2G mice with lethal doses of neurovirulent virus. Immunosuppressed A2G mice survived lethal infection enen though rendered unable to produce specific antiviral antibody or to generate cell-mediated delayed-type hypersensitivity responses. Measurement of infectious virus replication and detailed observation of the infection by immunofluorescence microscopy show that immunosuppression does not potentiate or allow spread of the virus in A2G brains. Interferon levels were essentially the same in normal and immunosuppressed A2G brains but were 3 to 5 times lower than in the brains of susceptible mice dying of the infection. The results strongly suggest that the genetic resistance of A2G mice to the acute lethal effects of neurovirulent influenza virus infection does not depend on the induction of primary immune mechanisms as we currently understand them. Other possible explanations for this resistance are considered.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Interactions of antibodies, complement components and various cell types in immunity against viruses and pyogenic bacteria. Transplant Rev. 1974;19(0):3–55. doi: 10.1111/j.1600-065x.1974.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Law L. W. Effects of antilymphocyte serum on virus oncogenesis. Proc Soc Exp Biol Med. 1968 Jan;127(1):207–212. doi: 10.3181/00379727-127-32657. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Ptak W. Contact and delayed hypersensitivity in the mouse. I. Active sensitization and passive transfer. Immunology. 1968 Sep;15(3):405–416. [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS H. J. F. Intracerebral inoculation of mice; fate of the inoculum. Nature. 1950 Nov 25;166(4230):910–911. doi: 10.1038/166910b0. [DOI] [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- De Maeyer E., De Maeyer-Guignard J., Jullien P. Inter- feron synthesis in x-irradiated animals. 3. The high radiosensitivity of myxovirus-induced circulating interferon production. Proc Soc Exp Biol Med. 1969 May;131(1):36–41. doi: 10.3181/00379727-131-33799. [DOI] [PubMed] [Google Scholar]
- Duffy P. E., Wolf A., Harter D. H., Gamboa E. T., Hsu K. C. Murine influenza virus encephalomyelitis. II. Electron microscopic observations. J Neuropathol Exp Neurol. 1973 Jan;32(1):72–91. doi: 10.1097/00005072-197301000-00005. [DOI] [PubMed] [Google Scholar]
- HAMMOND C. W., TOMPKINS M., MILLER C. P. Studies on susceptibility to infection following ionizing radiation. I. The time of onset and duration of the endogenous bacteremias in mice. J Exp Med. 1954 May 1;99(5):405–410. doi: 10.1084/jem.99.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOAG W. G., STROUT J., MEIER H. EPIDEMIOLOGICAL ASPECTS OF THE CONTROL OF PSEUDOMONAS INFECTION IN MOUSE COLONIES. Lab Anim Care. 1965 Jun;15:217–225. [PubMed] [Google Scholar]
- Haller O., Lindenmann J. Athymic (nude) mice express gene for myxovirus resistance. Nature. 1974 Aug 23;250(5468):679–680. doi: 10.1038/250679a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Mims G. A. Pathogenesis for viral infections of the nervous system. N Engl J Med. 1968 Jan 11;278(2):84–concl. doi: 10.1056/NEJM196801112780205. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J. INHERITANCE OF RESISTANCE TO INFLUENZA VIRUS IN MICE. Proc Soc Exp Biol Med. 1964 Jun;116:506–509. doi: 10.3181/00379727-116-29292. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., LANE C. A., HOBSON D. THE RESISTANCE OF A2G MICE TO MYXOVIRUSES. J Immunol. 1963 Jun;90:942–951. [PubMed] [Google Scholar]
- Laver W. G., Webster R. G. The structure of influenza viruses. IV. Chemical studies of the host antigen. Virology. 1966 Sep;30(1):104–115. doi: 10.1016/s0042-6822(66)81014-0. [DOI] [PubMed] [Google Scholar]
- Lindenmann J., Klein P. A. Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med. 1967 Jul 1;126(1):93–108. doi: 10.1084/jem.126.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIMS C. A. Intracerebral injections and the growth of viruses in the mouse brain. Br J Exp Pathol. 1960 Feb;41:52–59. [PMC free article] [PubMed] [Google Scholar]
- Mayer V., Schulman J. L., Kilbourne E. D. Nonlinkage of neurovirulence exclusively to viral hemagglutinin or neuraminidase in genetic recombinants of A-NWS (HON1) influenza virus. J Virol. 1973 Feb;11(2):272–278. doi: 10.1128/jvi.11.2.272-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. F., Griffin D. E., Johnson R. T. Specificity of the inflammatory response in viral encephalitis. I. Adoptive immunization of immunosuppressed mice infected with Sindbis virus. J Exp Med. 1972 Aug 1;136(2):216–226. doi: 10.1084/jem.136.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K., Gamboa E. T., Harter D. H., Wolf A., Hsu K. C. Influenza virus encephalitis in squirrel monkeys receiving immunosuppressive therapy. J Immunol. 1971 Apr;106(4):1119–1121. [PubMed] [Google Scholar]
- Miyoshi K., Wolf A., Harter D. H., Duffy P. E., Gamboa E. T., Hsu K. C. Murine influenza virus encephalomyelitis. I. Neuropathological and immunofluorescence findings. J Neuropathol Exp Neurol. 1973 Jan;32(1):51–71. doi: 10.1097/00005072-197301000-00004. [DOI] [PubMed] [Google Scholar]
- Murphy B. R., Glasgow L. A. Factors modifying host resistance to viral infection. 3. Effect of whole body x-irradiation on experimental encephalomyocarditis virus infection in mice. J Exp Med. 1968 May 1;127(5):1035–1052. doi: 10.1084/jem.127.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson N., Cole G. A. Immunosuppression and experimental virus infection of the nervous system. Adv Virus Res. 1970;16:397–448. doi: 10.1016/S0065-3527(08)60028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974 Mar 30;11(1-3):478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Rager-Zisman B., Allison A. C. Effects of immunosuppression on coxsackie B-3 virus infection in mice, and passive protection by circulating antibody. J Gen Virol. 1973 Jun;19(3):339–351. doi: 10.1099/0022-1317-19-3-339. [DOI] [PubMed] [Google Scholar]
- Rose S. P. Preparation of enriched fractions from cerebral cortex containing isolated, metabolically active neuronal and glial cells. Biochem J. 1967 Jan;102(1):33–43. doi: 10.1042/bj1020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. I. Adsorption of virus and growth curves. J Exp Med. 1970 Apr 1;131(4):843–850. doi: 10.1084/jem.131.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shif I., Bang F. B. In vitro interaction of mouse hepatitis virus and macrophages from genetically resistant mice. II. Biological characterization of a variant virus MHV (C3H) isolated from stocks of MHV(PRI). J Exp Med. 1970 Apr 1;131(4):851–862. doi: 10.1084/jem.131.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. H., Noguchi P., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. II. Decreased virulence of PR8 influenza virus. Infect Immun. 1972 Jun;5(6):957–960. doi: 10.1128/iai.5.6.957-960.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg D. O., Shah K. V., Bang F. B. Effect of cyclophosphamide on the genetic resistance of C 3 H mice to mouse hepatitis virus. Proc Soc Exp Biol Med. 1973 Mar;142(3):762–766. doi: 10.3181/00379727-142-37111. [DOI] [PubMed] [Google Scholar]
- Worthington M., Rabson A. S., Baron S. Mechanism of recovery from systemic vaccinia virus infection. I. The effects of cyclophosphamide. J Exp Med. 1972 Aug 1;136(2):277–290. doi: 10.1084/jem.136.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]