Abstract
Few reports have examined the impact of HIV-1 transmitted drug resistance (TDR) in resource-limited settings where there are fewer regimen choices and limited pretherapy/posttherapy resistance testing. In this study, we examined TDR prevalence in Kampala and Mbarara, Uganda and assessed its virologic consequences after antiretroviral therapy initiation. We sequenced the HIV-1 protease/reverse transcriptase from n=81 and n=491 treatment-naive participants of the Uganda AIDS Rural Treatment Outcomes (UARTO) pilot study in Kampala (AMU 2002–2004) and main cohort in Mbarara (MBA 2005–2010). TDR-associated mutations were defined by the WHO 2009 surveillance mutation list. Posttreatment viral load data were available for both populations. Overall TDR prevalence was 7% (Kampala) and 3% (Mbarara) with no significant time trend. There was a slight but statistically nonsignificant trend indicating that the presence of TDR was associated with a worse treatment outcome. Virologic suppression (≤400 copies/ml within 6 months posttherapy initiation) was achieved in 87% and 96% of participants with wildtype viruses versus 67% and 83% of participants with TDR (AMU, MBA p=0.2 and 0.1); time to suppression (log-rank p=0.3 and p=0.05). Overall, 85% and 96% of study participants achieved suppression regardless of TDR status. Surprisingly, among the TDR cases, approximately half still achieved suppression; the presence of pretherapy K103N while on nevirapine and fewer active drugs in the first regimen were most often observed with failures. The majority of patients benefited from the local HIV care system even without resistance monitoring. Overall, TDR prevalence was relatively low and its presence did not always imply treatment failure.
Introduction
Transmitted HIV drug resistance (TDR) tends to impact therapeutic outcomes. Studies on North American and European patients infected with predominantly subtype-B HIV showed that treatment-naive patients infected with drug-resistant HIV who subsequently initiated antiretroviral therapy suffered a 2- to 5-fold higher risk of virologic failure and/or a faster rate of virologic failure compared to individuals infected with drug-susceptible viruses.1–4 Based on this evidence, treatment guidelines for treatment-naive patients in developed countries strongly recommend genotypic resistance testing before initiation of antiretroviral therapy.5–10
In contrast and despite an expanded access to antiretroviral therapy in developing countries where non-B HIV subtypes predominate, pretherapy genotypic resistance testing is generally not available for clinical use. Reports have shown an increase in TDR prevalence in countries with expanding access to antiretroviral therapy,11–15 though relatively little is known about the impact of non-subtype-B TDR on treatment outcomes specifically in resource-limited settings such as in sub-Saharan African countries, especially in rural communities. One study reports TDR prevalence in Kampala, the capital of Uganda, from 2009 to 2010 was 8.6%, but did not examine virologic response.16 Due to narrower regimen choices compared to developed countries and the lack of a posttherapy resistance monitoring system in Uganda, especially in rural settings, TDR is an important concern that should be monitored and addressed.
The Uganda AIDS Rural Treatment Outcomes (UARTO) cohort is a rollout setting in which patients were given antiretroviral regimens without guidance from pretherapy resistance testing. In this study, we retrospectively evaluated the prevalence, temporal changes of TDR, and virologic consequences of pretherapy genotypic drug resistance using stored plasma specimens collected between 2002 and 2004 from the capital city of Kampala and between 2005 and 2010 from a rural community of Mbarara, 280 km southwest of Kampala. These results provided a glimpse into the prevalence and impact of TDR in an African setting where drug resistance monitoring is not available for clinical decision making.
Materials and Methods
Cohort description
UARTO17 is a cohort of initially treatment-naive HIV-infected subjects followed primarily at the Immune Suppression Syndrome (ISS) Clinic in Mbarara, Uganda. Patient identifier “AMU” denotes subjects from the UARTO pilot study enrolled in Kampala between 9/3/2002 and 3/12/2004, and “MBA” denotes subjects from the main cohort in Mbarara between 6/27/2005 and 4/8/2010. These patient identifiers were chosen to be consistent with previous UARTO publications and for the ease of references within this article. AMU participants received HIV RNA monitoring at 12 and 24 weeks for up to 96 weeks. MBA participants received HIV RNA monitoring at ∼3 month intervals for up to 7.5 years. The AMU study was approved by the Makerere University Faculty of Medicine Human Subjects Committee. The MBA study was approved by the Mbarara University of Science and Technology Human Subjects Committee and Partners Healthcare Human Subjects Committee. Both studies were approved by the Uganda Council of Science and Technology. This study was approved by the University of British Columbia/Providence Health Care Research Ethics Board (H11-01642) and the University of California Human Research Subjects Committee.
Drug resistance genotyping by Sanger bulk sequencing
Total nucleic acid was extracted from 500 μl of plasma samples using NucliSENS easyMag (bioMérieux); reverse transcription and cDNA synthesis were performed with the SuperScript III One-Step RT-PCR System (Invitrogen) followed by “nested” second-round polymerase chain reaction (PCR) as previously described.18 Bulk sequencing was performed on an ABI 3730 DNA Sequencer using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Resulting chromatograms were aligned with HXB2 and base called using in-house software RECall.19 Resulting sequences corresponding to HXB2 pol 2253–3269 or 2253–3749 were uploaded to GenBank (accession number KJ906625–KJ 907196).
Definitions
The presence of transmitted drug resistance mutations was defined as the detection of any one of the mutations listed in the WHO 2009 surveillance mutations list20; this list contained only resistance mutations associated with protease and (non)nucleoside reverse transcriptase inhibitors [PI/(N)NRTI] adjusted for non-B subtype polymorphisms. Posttherapy PI/(N)NRTI resistance mutations were defined as the detection of any one of the mutations listed in the Stanford University HIV Drug Resistance Database updated in 2011/2012.21,22 HIV-1 subtypes were determined based on minimal Hamming distance compared to subtype reference sequences of HIV-1 pol downloaded from the Los Alamos HIV sequence database; all sequences in this study were also subtyped by and were confirmed to be 97% identical to the REGA HIV-1 Subtyping Tool Version 2.0 (http://dbpartners.stanford.edu/RegaSubtyping/) and also were 97% identical to the Los Alamos RIP 3.0 recombinant identification program (window size 400, confidence threshold 95%, www.hiv.lanl.gov/content/sequence/RIP/RIP.html). For consistency, virologic suppression was defined as having a viral load ≤400 copies HIV-RNA/ml as the viral load detection limit changed from 400 copies/ml in early samples to 40 copies/ml in 2006 onward.
Screening pretherapy samples for the presence of antiretroviral drugs
Since patients' self-reported therapy history was used to determine their antiretroviral drug-naive status, high-performance liquid chromatography coupled with tandem mass spectroscopy (HPLC-MS/MS) was used to measure drug concentrations in pretherapy plasma samples that were found to have baseline genotypic drug resistance. Drug level measurement was performed as previously described23 using an Agilent HPLC 1100, Applied Biosystem API 2000 mass spectrometer, Agilent XDB-C8 guard column, and Agilent Eclipse XDB-C18 column (50 mm). Briefly, 400 μl of acetonitrile was added to 100 μl of plasma samples to precipitate plasma proteins. Samples were filtered with a 30,000 MW cut-off spin column (Millipore, Bedford, MA) diluted 1:1 in ammonium acetate and subsequently put through the HPLC-MS/MS system. The antiretrovirals measured were nevirapine (NVP), amprenavir (APV), indinavir (IDV), nelfinavir (NFV), saquinavir (SQV), ritonavir (RTV), lopinavir (LPV), efavirenz (EFV), atazanivir (ATV), tipranavir (TPV), darunavir (DRV) and/or raltegravir (RAL), etravirine (ETV), maraviroc (MVC), lamivudine (3TC), and abacavir (ABA).
Results
Baseline characteristics
In the UARTO pilot study (Kampala/AMU), a total of 62% participants were female with a median age of 36 years (IQR 30–40), baseline log viral load of 5.5 (IQR 4.9–5.8), and baseline CD4 count of 60 (IQR 12–136). All AMU participants received generic fixed dose combinations of d4T/3TC/NVP as a first regimen. In the UARTO main cohort (Mbarara/MBA), a total of 70% of participants were female with a median age of 35 years (IQR 29–39), baseline viral load of 5.1 log10 HIV-RNA copies/ml (IQR 4.7–5.6), and baseline CD4 count of 131 (IQR 74–197). Initial regimens were primarily NVP based (86%) and EFV based (12%) in combination with lamivudine (3TC) and zidovudine (AZT) backbones. HIV subtypes were predominantly A and D in both communities (AMU 43% A, 44% D; MBA 49% A, 43% D). In the current study, baseline genotypic drug resistance profiles were successfully obtained for n=81 AMU and n=491 MBA subjects.
Prevalence of pretherapy drug resistance
Among the AMU (Kampala) subjects enrolled between 2002 and 2004, pretherapy mutations defined by the WHO 2009 surveillance mutations list20 were detected in 6/81 (7%) (Table 1). Three had NRTI-, one had NNRTI-, one had PI-, and one had both NRTI- and NNRTI-associated mutations. However, HPLC-MS/MS of these six resistant plasma samples revealed a detectable drug concentration (EFV) in one, suggesting at least one patient was not really drug naive. All six were female of age 27–41 years; all reported having child(ren) and/or a history of pregnancy, but pregnancy dates were not collected. Baseline viral load, CD4 counts, age, and gender between those with and without TDR were not significantly different (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid).
Table 1.
Kampala, Uganda (AMU, Enrolled 2002–2004, ntotal=81)
| Posttherapy virologic suppressionauntil study cutoff (n=4) | Virologic failurea(n=2) | |||||
|---|---|---|---|---|---|---|
| UARTO patient ID (AMU) | 001 | 012 | 055 | 071 | 037 | 049 |
| Baseline characteristics | ||||||
| Gender | F | F | F | F | F | F |
| Age | 37 | 30 | 31 | 27 | 37 | 41 |
| Baseline log VL | 4.8 | 4.5 | 5.6 | 5.1 | 5.4 | 4.4 |
| Baseline CD4 count | 47 | 269 | 22 | 62 | 7 | 25 |
| Ever pregnant? | Yes | Yes | Yes | Yes | Yes | Yes |
| Viral subtype | D | A | D | D | A | D |
| Pretherapy plasma resistance profiles | ||||||
| NNRTIb | — | — | — | K103N | — | K103N, Y181C |
| NRTIb | K70E/K | — | M41I/L/M | — | T69A/D/N/T | K65R, Q151M |
| PIb | — | M46L/M | — | — | — | — |
| HPLC-MS/MS drug level | ND | ND | ND | ND | ND | EFV (0.3 μg/ml) |
| Treatment and virologic consequences | ||||||
| Initial regimen (ccontraindicated by TDR) | d4T/3TC/NVP | d4T/3TC/NVP | d4Tc/3TC/NVP | d4T/3TC/NVPc | d4Tc/3TCc/NVP | d4Tc/3TCc/NVPc |
| Given TDR profile, was initial regimen appropriate? | Yes | Yes | No | No | No | No |
| Follow-up regimens | Unknown | Unknown | Unknown | Unknown | Unknown | Unknown |
| Duration of continuously suppressed VL (days) | 671, cutoff | 598, cutoff | 392, cutoffd | 256, cutoff | — | — |
| Duration of continuously unsuppressed VL (days) | — | — | — | — | 476, cutoff | 291, then ND |
| VL at study cutoff | ND | ND | ND | ND | log 3 | ND |
Virologic suppression was defined as ≤400 copies HIV-RNA/ml and detectable viral load and virologic failure were defined as >400 copies HIV-RNA/ml.
Transmitted drug resistance (TDR) mutations were defined by the WHO 2009 surveillance mutation list.
Drugs in initial regimen that were contraindicated with pretherapy resistance profiles.
Patient AMU055 had a single blip on day 294 posttherapy.
Detailed baseline characteristics, resistance profiles, treatment history, and virologic consequences of the six cases of WHO-defined transmitted drug resistance (TDR) reported in this study.
VL, viral load; NRTI, nucleoside and nucleotide reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors; F, female; ND, not detectable; EFV, efavirenz; NVP, nevirapine; d4T, stavudine; 3TC, lamivudine; last available follow-up time point (cutoff ).
Among the MBA (Mbarara) subjects with collection dates between 2005 and 2010, pretherapy mutations were detected in 15/491 (3%) (Table 2). Four had NRTI-, nine had NNRTI-, and two had both NRTI- and NNRTI-associated mutations. None had PI-associated mutations. Of these 15 TDR cases, 14/15 (93%) were females of age 22–37 years; two reported “pregnancy within 12 months prior to study enrollment.” HPLC-MS/MS of the 15 resistant samples revealed detectable drug concentrations (all NVP) in three female subjects (MBA 1118, 1309, and 1470, ages 22, 27, and 30 years; all harbored K103N), suggesting unacknowledged NVP use; however, none of these three subjects reported pregnancy in the previous 12 months. Baseline viral load and CD4 count between those with and without TDR were not significantly different (Supplementary Table S1). Patients with TDR were significantly younger (median 28 vs. 35 years; p=0.001) and were more likely to be female (93% vs. 69%; p=0.05).
Table 2.
Mbarara, Uganda (MBA, Enrolled 2005–2010, ntotal=491)
| Posttherapy virologic suppressiona throughout therapy (n=6) | Virologic failurea (n=4) | Initially suppresseda, but rebounded and stayed failedc (n=3) | No follow-up (n=2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UARTO patient ID (MBA) | 1323 | 1118 | 1161 | 1347 | 1466 | 1467 | 1057 | 1202 | 1300 | 1309 | 1450 | 1294 | 1470 | 1130 | 1306 |
| Baseline characteristics | |||||||||||||||
| Gender | F | F | F | F | F | F | F | M | F | F | F | F | F | F | F |
| Age | 31 | 22 | 33 | 31 | 22 | 25 | 23 | 33 | 22 | 27 | 37 | 25 | 30 | 37 | 28 |
| Baseline log VL | 4.9 | 5.7 | 3.9 | 3.6 | 5.3 | 5.9 | 5.4 | 5.0 | 4.9 | 3.9 | 3.4 | 5.9 | 4.2 | 3.9 | 3.6 |
| Baseline CD4 count | 160 | 271 | 316 | 226 | 159 | 30 | 7 | 97 | 426 | 174 | 198 | 7 | 12 | 36 | 14 |
| Pregnant within last 12 months? | No | Blank | No | No | No | No | Yes | — | No | No | Yes | No | No | No | No |
| Viral subtype | AE | D | D | D | A | D | D | A | A | A | A | D | D | A | D |
| Pretherapy plasma resistance profiles | |||||||||||||||
| NNRTIb | P225H | K103K/N | — | — | K103N | — | Y181C/Y | K103K/N, Y181C/Y | K101E/K | K103N | K103N | K103K/N/R/S | K103N | K103K/N,G190A | — |
| NRTIb | — | — | K219K/Q | M184V | — | K70K/E | T69A/D/N/T | — | — | M184V | — | — | — | — | T69A/D/N/T |
| PIb | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| HPLC-MS/MS drug level | ND | NVP (2.6 μg/ml) | ND | ND | ND | ND | ND | ND | ND | NVP (5.9 μg/ml) | ND | ND | NVP (2.1 μg/ml) | ND | ND |
| Treatment and virologic consequences | |||||||||||||||
| NNRTI | NVP | NVPc | NVP | EFV | NVPc | NVP | NVPc | NVPc | NVPc | NVPc | NVPc | NVPc | NVPc | — | — |
| NRTI | 3TC/AZT | 3TC/d4T | 3TC/AZTc | 3TCc/AZT | 3TC/AZT | 3TC/AZTc | 3TCc/d4Tc | 3TC/d4T | 3TC/AZT | 3TCc/AZT | 3TC/AZT | 3TC/AZT | 3TC/AZT | — | — |
| PI | — | — | — | — | — | — | — | — | — | LPV | — | — | — | — | — |
| Given TDR profile, was initial regimen appropriate? | Yes | No | No | No | No | No | No | No | No | No | No | No | No | — | — |
| Duration of continuously suppressed VL (days) | 1745 | 684 | 2184 | 1857 | 343 | 1425 | — | — | — | — | 416 | 754 | 168 | — | — |
| Duration of continuously unsuppressed VL without change in “bad” regimen (days) | — | — | — | — | — | — | 835 | 327 | 628 | 252 | 1015 | 1058 | 170 | — | — |
| Regimen modification (days posttherapy, new regimen) | — | — | — | 169, NVP/3TC/AZT | — | — | 932, LPV/TDF/FTC | 415, LPV/TDF/FTC | — | 337, LPV/TDF/FTC | — | — | — | — | — |
| Log VL at study cutoff | ND | ND | ND | ND | ND | ND | ND | ND | log 3 | ND | log 2 | log 4 | log 4 | — | — |
Virologic suppression was defined as ≤400 copies HIV-RNA/ml and detectable viral load and virologic failure were defined as >400 copies HIV-RNA/ml.
Transmitted drug resistance (TDR) mutations were defined by the WHO 2009 surveillance mutation list.
Drugs in initial regimen that were contraindicated with pretherapy resistance profiles.
Detailed baseline characteristics, resistance profiles, treatment history, and virologic consequences of the 15 cases of WHO-defined transmitted drug resistance (TDR) reported in this study.
M, male; AZT, zidovudine; LPV, lopinavir; TDF, tenofovir; FTC, emtricitabine; for other abbreviations see Table 1.
After stratifying samples by year of collection (Table 3) no statistically significant change in the prevalence of TDR was observed over time (AMU 2002–2004 ptrend=0.2, MBA 2005–2010 ptrend=0.9, Cochran–Armitage test for trend).
Table 3.
Percentage Prevalence of Pretherapy Drug Resistance Stratified by Year
| Year | No resistance (n)a | Yes resistance (n)b | Pretherapy resistance (%) |
|---|---|---|---|
| AMU Kampala | |||
| 2002 (n=17) | 15 | 2 | 11.8 |
| 2003 (n=51) | 48 | 3 | 5.9 |
| 2004 (n=12) | 12 | 0 | 0.0 |
| AMU total (n=80) | 75 | 5 | 6.3 |
| MBA Mbarara | |||
| 2005 (n=61) | 60 | 1 | 1.6 |
| 2006 (n=124) | 122 | 2 | 1.6 |
| 2007 (n=168) | 162 | 6 | 3.6 |
| 2008 (n=93) | 90 | 3 | 3.2 |
| 2009 (n=36) | 36 | 0 | 0.0 |
| 2010 (n=6) | 6 | 0 | 0.0 |
| MBA total (n=488) | 476 | 12 | 2.5 |
This table includes two individuals who were unenrolled later due to suspected prior antiretroviral use or for reasons other than not being antiretroviralnaive; pretherapy resistance was not detected in either subject.
Patients with pretherapy plasma drug concentrations detected by HPLC-MS/MS were excluded for a closer estimate of the prevalence of transmitted drug resistance.
Virologic consequences
We compared virologic outcomes in the first 6 months after therapy initiation between subjects with and without pretherapy resistant viruses. Of the 81 AMU subjects, 74 (68 wildtype, 6 TDR) had at least one pretherapy viral load measurement and one between >0 and ≤180 days posttherapy. Virologic suppression (at least one viral load ≤400 copies/ml within 180 days posttherapy) was achieved in 63/74 (85%) overall, or 59/68 (87%) pretherapy wildtype versus 4/6 (67%) TDR cases (Fisher's exact test, two-tailed, p=0.2, Fig. 1a). Of the 491 MBA subjects, 423 (411 wildtype, 12 TDR) had at least one pretherapy viral load measurement and one between >0 and ≤180 days posttherapy. Virologic suppression (at least one viral load ≤400 copies/ml within 180 days posttherapy) was achieved in 404/423 (96%) overall, or 394/411 (96%) pretherapy wildtype versus 10/12 (83%) TDR cases (Fisher's exact test, two-tailed, p=0.1, Fig. 1b).
FIG. 1.
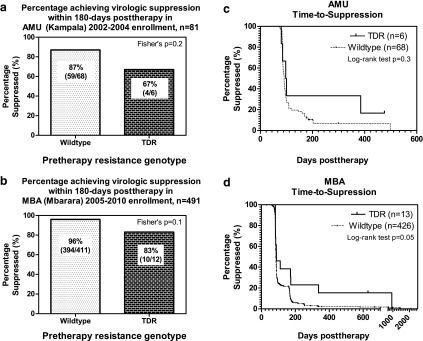
Virologic consequences of pretherapy resistance. (a) Percentage of AMU individuals who achieved virologic suppression (≤400 copies/ml) in the pretherapy wildtype versus the transmitted drug resistance (TDR) group. (b) Same as (a), MBA. (c) Kaplan–Meier plot showing time to virologic suppression in AMU individuals with pretherapy wildtype virus versus TDR. (d) Same as (c), MBA.
Next, we compared time to virologic suppression (duration between the start of therapy and the date of the first posttherapy viral load ≤400 copies/ml) between the pretherapy wildtype and the resistance groups using Kaplan–Meier methods in an intent-to-treat analysis. Of the 81 AMU subjects, 74 (68 wildtype, 6 TDR) had at least one viral load measurement pretherapy and one posttherapy; overall, 67/74 (91%) eventually achieved suppression with a median time to suppression of 90 days (IQR 85–99). Of the 68 patients with pretherapy wildtype HIV, 62/68 (91%) achieved suppression in a median of 90 days (IQR 84–99) and the remaining six were censored at 85, 176, 184, 188, 199, and 299 days posttherapy. In comparison, 5/6 (83%) of the TDR individuals achieved suppression in a median of 97 days (IQR 85–100) and the remaining 1/6 was censored at 476 days posttherapy (AMU037). This difference was not statistically significant (Fig. 1c, Kaplan–Meier curve, log-rank test, p=0.3). Of the 491 MBA subjects, 439 (426 wildtype, 13 TDR) had at least one viral load measurement pretherapy and one posttherapy; overall, 434/439 (99%) eventually achieved suppression with a median time to suppression of 85 days (IQR 83–97). Of the 426 patients with pretherapy wildtype HIV, 422 (99%) achieved suppression in a median of 85 days (IQR 83–97) and the remaining four were censored at 90, 92, 164, and 668 days posttherapy. In the pretherapy resistance group, 12/13 (92%) achieved suppression in a median of 89 days (IQR 83–172); the remaining individual never achieved virologic suppression while on continuous treatment and was censored at 628 days posttherapy. The difference between the two groups was of borderline significance (Fig. 1d, Kaplan–Meier curve, log-rank test, p=0.05.)
We further explored baseline characteristics that could introduce bias to our observations. In both the AMU and the MBA cohort, baseline viral load, CD4 count, age, and gender were not significantly different between the group that achieved and the group that did not achieve virologic suppression in both populations with one exception. A significantly higher baseline CD4 count (median 68 versus 19; Mann–Whitney test, two-tailed, p=0.04) was observed in AMU participants who achieved suppression within 180 days posttherapy (Supplementary Tables S2 and S3). Subtype bias may be introduced because subtype D has been shown with limited evidence to have a more rapid disease progression in treatment-naive individuals in Africa24–27; no statistically significant difference was found between D and non-D subtypes (Supplementary Tables S1–3).
Other definitions of virologic suppression (defined as having two consecutive viral loads ≤400 copies/ml) and exclusion criteria (excluding patients with pretherapy detectable drug levels and/or regrouping TDR cases that first regimen did not contraindicate with their TDR profile as non-TDR cases) were explored and showed similar results (results not shown).
Finally, we explored the details of posttreatment virologic and treatment history in the 6 AMU and 13 MBA TDR individuals with longitudinal viral load follow-up data. Among the six AMU TDR cases (Table 1, lower half ), 2/6 (33%) were prescribed initial regimens that were appropriate, given their TDR profiles (i.e., three active drugs); both achieved lasting virologic suppression until study cutoff (AMU001 and AMU012). In the remaining 4/6 (67%) who were prescribed initial regimens contraindicated by their TDR profiles, two experienced virologic failure (defined as unsuppressed viral load >400 copies/ml, AMU037 and AMU049; one or no active drug) while surprisingly the other two experienced lasting virologic suppression for 392 and 256 days until study cutoff (AMU055 and AMU071; two active drugs each, despite pretherapy K103N in AMU071). Once suppressed, we observed no cases of virologic rebound among these cases until study cutoff.
Of the 13 MBA TDR cases with longitudinal viral load follow-up (Table 2, lower half ), only one (MBA 1323) received an initial regimen that was not contraindicated by the pretherapy resistance profile (i.e., three active drugs). This patient achieved and maintained virologic suppression throughout the study duration. In the remaining 12/13 (92%) who were prescribed initial regimens contraindicated by their pretherapy resistance profiles, virologic failure was observed in 4/12 (33%) for 252–835 days (MBA 1057, 1202, 1300, 1309; all had NNRTI-associated mutations K101E, K103N, or Y181C; all received NVP; one to two active drugs). One of them never achieved virologic suppression and did not have their regimen modified until study cutoff, and three of them achieved suppression only when removed from NNRTI-containing regimens. Again surprisingly, 5/12 (42%) who received contraindicated first regimens (MBA 1118, 1161, 1347, 1466, 1467; two active drugs each) experienced continuously suppressed viral loads (despite the presence of K103N in two of them, and except for a single blip observed in MBA 1118). The remaining 3/12 (25%) patients receiving contraindicated regimens (MBA 1450, 1294, and 1470; two active drugs each) achieved initial suppression for 416, 754, and 168 days despite pretherapy K103N mutations and NVP in the first regimen, but later rebounded and remained with detectable viremia without regimen modification for 1015, 1058, and 170 days until study cutoff. In comparison, among the 397 pretherapy wildtype cases that had comparable data (at least one pretherapy and four posttherapy viral load measurements), 4/397 (1%) were never suppressed, 351/397 (88%) had suppressed viral load for the duration of follow-up, and 42/397 (11%) experienced postsuppression viral load rebound (defined as having two consecutive >400 copies/ml) at a median of 674 (IQR 416–1088) days posttherapy.
Posttherapy genotypic resistance (sequence) data were available for eight MBA TDR cases; all eventually developed other NRTI and/or NNRTI resistance mutations (Table 4). The most common additional resistance mutation observed was M184V; all eight developed this mutation after receiving 3TC/d4T and/or 3TC/AZT. Only 1/8 subjects received PI lopinavir (LPV), but resistance to LPV was not detected.
Table 4.
Posttherapy Resistance Profiles of MBA Patients with Pretherapy Resistance Mutations
| Patient ID | Date | Timing | NRTI | NNRTI | Regimen |
|---|---|---|---|---|---|
| MBA1057 | 11/1/2005 | Pre-ARV | T69A/D/N/T | Y181C/Y | — |
| MBA1057 | 5/22/2006 | Post-ARV | Wildtype | Wildtype | 3TC/d4T/NVPa |
| MBA1057 | 11/22/2006 | Post-ARV | D067N, T069D, K070K/R, M184V, K219N | K103N, Y181C | 3TC/d4T/NVP |
| MBA1057 | 6/14/2007 | Post-ARV | D067N, T069D, K070R, M184V, K219N | K103N, Y181C | 3TC/AZT/NVP |
| MBA1057 | 9/6/2007 | Post-ARV | D067N, T069D, K070R, M184V, L210L/V, K219N | K103N, Y181C | 3TC/AZT/NVP |
| MBA1057 | 2/14/2008 | Post-ARV | D067N, T069D, K070R, M184V, K219N | K103N, Y181C | 3TC/AZT/NVP |
| MBA1118b | 8/10/2006 | Pre-ARV | Wildtype | K103K/N | — |
| MBA1118 | 1/10/2008 | Post-ARV | Wildtype | Wildtype | 3TC/d4T/NVPa |
| MBA1118 | 5/27/2009 | Post-ARV | M184V | K103N | Untreated |
| MBA1202 | 1/18/2007 | Pre-ARV | Wildtype | K103K/N, Y181C/Y | — |
| MBA1202 | 6/11/2007 | Post-ARV | M184V | K103K/N, Y181C | 3TC/d4T/NVPa |
| MBA1202 | 7/4/2007 | Post-ARV | M184V | K103K/N, Y181C | 3TC/d4T/NVP |
| MBA1202 | 12/13/2007 | Post-ARV | M184V | Y181C | 3TC/d4T/NVP |
| MBA1294 | 6/28/2007 | Pre-ARV | Wildtype | K103K/N/R/S | — |
| MBA1294 | 11/30/2009 | Post-ARV | Wildtype | K103N | 3TC/AZT/NVPa |
| MBA1294 | 3/17/2010 | Post-ARV | M184V | K103N, M230L | 3TC/AZT/NVP |
| MBA1300 | 7/13/2007 | Pre-ARV | Wildtype | K101E/K | — |
| MBA1300 | 10/15/2007 | Post-ARV | M184V | K101E | 3TC/AZT/NVPa |
| MBA1300 | 3/26/2008 | Post-ARV | M184V | K101E/K, G190A | 3TC/AZT/NVP |
| MBA1300 | 6/19/2008 | Post-ARV | M184V | G190A | 3TC/AZT/NVP |
| MBA1300 | 8/28/2008 | Post-ARV | M184V | G190A | 3TC/AZT/NVP |
| MBA1300 | 4/1/2009 | Post-ARV | M184V | G190A | 3TC/AZT/NVP |
| MBA1309b | 7/31/2007 | Pre-ARV | M184V | K103N | — |
| MBA1309 | 10/24/2007 | Post-ARV | M184V | K103N, E138E/Q | 3TCa/AZT/NVPa/LPV |
| MBA1309 | 1/15/2008 | Post-ARV | M184V | K103N, E138E/Q | 3TC/d4T/NVP/LPV |
| MBA1309 | 4/8/2008 | Post-ARV | M184V | K103N, E138Q | 3TC/d4T/NVP/LPV |
| MBA1450 | 9/11/2008 | Pre-ARV | Wildtype | K103N | — |
| MBA1450 | 2/3/2010 | Post-ARV | M184V | K103N | 3TC/AZT/NVPa |
| MBA1470b | 11/4/2008 | Pre-ARV | Wildtype | K103N | — |
| MBA1470 | 7/20/2009 | Post-ARV | K070K/R, M184V | K103N | 3TC/AZT/NVPa |
| MBA1470 | 1/6/2010 | Post-ARV | M184V, L210L/W, T215Y | K103N, M230L | 3TC/AZT/NVP |
Drugs in the initial ARV regimens that the patients might not have received if their pretherapy resistance profiles were known.
Patients who had detectable plasma drug concentration pretherapy (see Table 2).
All other patients developed additional drug resistance mutations over time. No PI-associated resistance mutations were detected.
Only MBA, not AMU, patients had follow-up resistance genotyping. MBA 1130 and 1306 were lost to follow-up. MBA 1161, 1323, 1347, 1466, and 1467 had no longitudinal sequences available.
Discussion
Our study showed that in the UARTO cohort, the overall prevalence of HIV-1 TDR defined by WHO's 2009 transmitted drug resistance mutations list20 was 7% in Kampala's 2002–2004 pilot study (AMU, n=81) and 3% in Mbarara's main cohort 2005–2010 (MBA, n=491) with no statistically significant changes in prevalence over time. In both cities, regardless of TDR status, we saw high overall rates of virologic suppression (85% AMU and 96% MBA participants suppressed 180 days posttherapy; 91% AMU and 99% MBA participants eventually suppressed in a median of 90 and 85 days, respectively). We also observed that TDR cases were often prescribed first regimens that were contraindicated by their TDR profiles, but also showed a negative, but nonstatistically significant, impact of TDR on virologic outcome. Importantly, patients with TDR, even K103N, did not always experience virologic failure.
In the first part of the study, our reported TDR prevalence was comparable to WHO's 2006 Kampala surveillance estimate at <5%28; no rural community surveillance data are available for comparison with our Mbarara results. Mutation types observed were also consistent with claims that TDR in resource-limited settings is driven mainly by (N)NRTI resistance29,30 and with the fact that AZT/3TC/NVP or AZT/3TC/EFV is the recommended first-line regimen in the Ugandan national guidelines.31
The lack of time trends in pretherapy resistance prevalence, however, contrasted with reports that TDR is on the rise globally,3,29,30,32,33 with the most extreme being East Africa experiencing a 29% increase/year,34 but could be limited by our small number of TDR cases leading to a lack of statistical power, or differences in antiretroviral rollout staging compared to previous reports. These results were also limited by the cohort's heterogeneity in disease progression status,32 the reversion and/or persistence of selected mutations,35–41 as well as the often inaccurate self-reported treatment-naive status as shown by our HPLC-MS/MS analysis and the lack of pregnancy-related NVP usage information.
In the second part of the study, we reported that the majority of study participants achieved virologic suppression; patients with TDR defined by WHO's 2009 transmitted drug resistance mutations list, even those with K103N, did not always experience treatment failure when prescribed regimen(s) that were contraindicated by their TDR profiles. In other words, even though studies have shown that NNRTI-based regimens negatively affect treatment outcomes more than mutations from other drug classes,1 our results suggest that the detection of one or more of the mutations in the WHO TDR mutation list does not immediately imply treatment failure.
In fact, the total number of these observed TDR mutations, their identities, as well as the subsequent antiretroviral regimen prescribed all seemed to play a role in therapy outcome. For instance, P225H, the only TDR mutation from the WHO list observed in patient MBA 1323 (Table 2), is an NNRTI-associated nonpolymorphic mutation that usually occurs in combination with K103N.21,22 In vitro, P225H alone causes only a 3-fold increase in IC50 against NVP,42,43 but a 150-fold increase if it occurred in combination with K103N.43 Patient MBA 1323 subsequently received 3TC/AZT/NVP (two fully active drugs) and stayed on this same regimen with suppressed viral load for almost 5 years until the study cutoff, suggesting that the observation of a lone transmitted P225H in this particular case did not impact treatment outcome.
Similarly, K103N is an NNRTI-associated nonpolymorphic mutation that alone causes a 50-fold increase in IC50 against NVP in vitro44,45 but a >1,600-fold increase in combination with Y181C.42 In this study, when K103N was the only TDR mutation identified (in AMU 071, MBA 1118, 1466, 1450, and 1470, Tables 1 and 2), all patients experienced either sustained or prolonged but transient virologic suppression despite receiving an NVP-containing regimen, presumably due to the two other fully active NRTIs in the background. In contrast, when K103N was observed together with other TDR mutations (in AMU 049 and MBA 1202 and 1309), all patients failed to achieve virologic suppression with NVP-containing regimens. Note that although AMU 049 and MBA 1309 had zero or only one fully active drug, MBA 1202 did receive two fully active NRTIs, but this did not seem to be enough to overcome the effect of K103N in combination with Y181C. In addition, we observed that MBA 1202 and 1309 achieved immediate virologic suppression when NVP was switched-out and was replaced with a protease inhibitor (LPV).46,47
The same can be said about NRTI-associated mutations: the phenotypic effect of a lone detection of M41L, K70E, M184V, or K219Q (in AMU001, 071, MBA1161, 1347, and 1467) was simply not large enough to compromise the 3TC, d4T/AZT with NVP regimens (two fully active drugs); those who failed to achieve virologic suppression either had zero or one active drug in the regimen or had multiple TDRs. Taken together, these observations suggest that the mere observation of TDR does not doom a treatment regimen to failure; the net phenotypic effect on the treatment regimen needs to be considered.
Finally, it must be noted that although we did observe a slight but nonstatistically significant trend indicating that the presence of any TDR mutations would negatively impact treatment outcome, our statistical analysis was limited by the small number of cases in the TDR group. These results were also limited by the inability of bulk sequencing technology to detect low-frequency variants that had been shown to affect treatment outcomes,48–53 and that patients with HPLC-MS/MS detectable pretherapy plasma drug levels were included in all virologic outcome analyses because HPLC-MS/MS was not performed on plasma samples with wildtype viruses. Our results agreed with reports that subtype-D infection was not associated with a worse virologic outcome,54,55 which implied that the observed difference in suppression was not biased by subtype differences.
In terms of clinical management of TDR cases if pretherapy resistance testing were ever going to become available in these communities, our study provided the following insights: (1) for those failing therapy, a change to a potentially active drug led to immediate virologic improvement, (2) some patients were kept on the same failing regimens with a continuously monitored and detectable viral load for as long as 3 years, suggesting that viral load information was sometimes not used in clinical decision making or that regimen options were severely limited, and (3) three of the MBA TDR cases were initially suppressed and then experienced virologic rebound, demonstrating that antiretroviral-induced virologic suppression in individuals with TDR could be transient.
Conclusions
In conclusion, we showed that there was a low TDR prevalence in both Kampala and Mbarara with no significant time trend and that over 90% of the study participants benefited from the HIV therapy. Importantly, we showed that almost half of the TDR cases defined by WHO did not experience treatment failure. Together, these observations imply that even without pretherapy resistance testing, this health care system was largely beneficial at least until virologic rebound and/or until the further development of resistance. It also points out that although the WHO's 2009 list of transmitted drug resistance may be important for surveillance purposes, the presence of one or more mutations in the list did not predestine a patient to treatment failure even if the patient was put on a regimen that was partially contraindicated by the TDR mutation(s). Future studies should also examine the long-term impact of the lack of posttherapy resistance testing on clinical outcome and survival in such resource-limited settings.
Supplementary Material
Acknowledgments
This work was funded by the Canadian Institutes of Health Research (CIHR), National Institutes of Health (NIH) Centers for AIDS Research (CFAR) Program, National Institute of Mental Health RO-1 54907, P30 AI27763, and U01 CA066529, and National Institute on Alcohol Abuse and Alcoholism R-21 014784.
The work presented here was carried out in collaboration between all authors. The study was conceptualized and designed by D.R.B., G.Q.L., J.B., J.N.M., P.R.H., and P.W.H. Plasma samples as well as baseline and follow-up data were collected by C.M., J.H.O., N.E., and Y.B. Laboratory work was done by D.K. and G.Q.L. Results were analyzed by C.J.B. and G.Q.L. G.Q.L. wrote the manuscript; all authors contributed to, have seen, and have approved the manuscript.
Presented in part at the 17th Conference on Retroviruses and Opportunistic Infections (CROI), February 16–19, 2010, San Francisco, CA (Abstract M-224)
Author Disclosure Statement
P.R.H. has received consulting fees from ViiV/Pfizer and Quest and holds stock in Merck. The remaining authors have no competing interests.
References
- 1.Kuritzkes DR, Lalama CM, Ribaudo HJ, Marcial M, Meyer W, Shikuma C, et al. : Preexisting resistance to nonnucleoside reverse-transcriptase inhibitors predicts virologic failure of an efavirenz-based regimen in treatment-naive HIV-1-infected subjects. J Infect Dis 2008;197:867–870 [DOI] [PubMed] [Google Scholar]
- 2.Wittkop L, Günthard HF, de Wolf F, Dunn D, Cozzi-Lepri A, de Luca A, et al. : Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect Dis 2011;11:363–371 [DOI] [PubMed] [Google Scholar]
- 3.Little SJ, Holte S, Routy J-P, Daar ES, Markowitz M, Collier AC, et al. : Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002;347:385–394 [DOI] [PubMed] [Google Scholar]
- 4.Grant RM, Hecht FM, Warmerdam M, Liu L, Liegler T, Petropoulos CJ, et al. : Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA 2002;288:181–188 [DOI] [PubMed] [Google Scholar]
- 5.Asboe D, Aitken C, Boffito M, Booth C, Cane P, Fakoya A, et al. : British HIV Association guidelines for the routine investigation and monitoring of adult HIV-1-infected individuals 2011. HIV Med 2012;13:1–44 [DOI] [PubMed] [Google Scholar]
- 6.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents, 2013. www.ncbi.nlm.nih.gov/pubmed/12617573
- 7.Thompson MA, Aberg JA, Hoy JF, Benson C, Gu HF, Hammer SM, et al. : Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society–USA Panel. J Am Med Assoc 2012;308:387–402 [DOI] [PubMed] [Google Scholar]
- 8.Vandamme A-M, Camacho RJ, Ceccherini-Silberstein F, de Luca A, Palmisano L, Paraskevis D, et al. : European recommendations for the clinical use of HIV drug resistance testing: 2011 update. AIDS Rev 2011;13:77–108 [PubMed] [Google Scholar]
- 9.Montaner JSG: British Columbia Centre for Excellence in HIV/AIDS Therapeutic Guidelines: Antiretroviral (ARV) Treatment of Adult HIV Infection, 2013 [Google Scholar]
- 10.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. : Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2009;49:651–681 [DOI] [PubMed] [Google Scholar]
- 11.Aghokeng AF, Vergne L, Mpoudi-ngole E, Mbangue M, and Deoudje N: Evaluation of transmitted HIV drug resistance among recently-infected antenatal clinic attendees in four Central African countries. Antivir Ther 2009;14:401–411 [DOI] [PubMed] [Google Scholar]
- 12.Mosha F, Urassa W, Aboud S, Lyamuya E, Sandstrom E, Bredell H, et al. : Prevalence of genotypic resistance to antiretroviral drugs in treatment-naive youths infected with diverse HIV type 1 subtypes and recombinant forms in Dar es Salaam, Tanzania. AIDS Res Hum Retroviruses 2011;27:377–382 [DOI] [PubMed] [Google Scholar]
- 13.Bennett DE: The requirement for surveillance of HIV drug resistance within antiretroviral rollout in the developing world. Curr Opin Infect Dis 2006;19:607–614 [DOI] [PubMed] [Google Scholar]
- 14.Ndembi N, Abraha A, Pilch H, Ichimura H, Mbanya D, Kaptue L, et al. : Molecular characterization of human immunodeficiency virus type 1 (HIV-1) and HIV-2 in Yaounde, Cameroon: Evidence of major drug resistance mutations in newly diagnosed patients infected with subtypes other than subtype B. J Clin Microbiol 2008;46:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geretti AM: Epidemiology of antiretroviral drug resistance in drug-naive persons. Curr Opin Infect Dis 2007;20:22–32 [DOI] [PubMed] [Google Scholar]
- 16.Ndembi N, Hamers RL, Sigaloff KCE, Lyagoba F, Magambo B, Nanteza B, et al. : Transmitted antiretroviral drug resistance among newly HIV-1 diagnosed young individuals in Kampala. AIDS 2011;25:905–910 [DOI] [PubMed] [Google Scholar]
- 17.Kaida A, Matthews LT, Kanters S, Kabakyenga J, Muzoora C, Mocello R, et al. : Incidence and predictors of pregnancy among a cohort of HIV-positive women initiating antiretroviral therapy in Mbarara, Uganda. PLoS One 2013;8:e63411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brumme CJ, Harrigan PR, Preston EC, Dong WWY, Wynhoven B, Murphy C, et al. : Transmission of drug-resistant HIV-1 from an infected individual to a caregiver. Antivir Ther 2007;12:1139–1144 [PubMed] [Google Scholar]
- 19.Woods CK, Brumme CJ, Liu TF, Chui CKS, Chu AL, Wynhoven B, et al. : Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012;50:1936–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DE, Camacho RJ, Otelea D, Kuritzkes DR, Fleury H, Kiuchi M, et al. : Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009;4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafer RW: Rationale and uses of a public HIV drug-resistance database. J Infect Dis 2006;194(Suppl):S51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee S-Y, Gonzales MJ, Kantor R, Betts BJ, Ravela J, and Shafer RW: Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucleic Acids Res 2003;31:298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander CS, Montaner JSG, Asselin JJ, Ting L, McNabb K, Harris M, et al. : Simplification of therapeutic drug monitoring for twice-daily regimens of lopinavir/ritonavir for HIV infection. Ther Drug Monit 2004;26:516–523 [DOI] [PubMed] [Google Scholar]
- 24.Kuritzkes DR: HIV-1 subtype as a determinant of disease progression. J Infect Dis 2008;197:638–639 [DOI] [PubMed] [Google Scholar]
- 25.Kiwanuka N, Laeyendecker O, Robb M, Kigozi G, Arroyo M, McCutchan F, et al. : Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis 2008;197:707–713 [DOI] [PubMed] [Google Scholar]
- 26.Baeten JM, Chohan B, Lavreys L, Chohan V, McClelland RS, Certain L, et al. : HIV-1 subtype D infection is associated with faster disease progression than subtype A in spite of similar plasma HIV-1 loads. J Infect Dis 2007;195:1177–1180 [DOI] [PubMed] [Google Scholar]
- 27.Morgan D, Kaleebu P, Whitworth J, Yirrell D, Rutebemberwa a., Shier R, et al. : The stability between two HIV-1 RNA measurements one year apart and the relationship with HIV subtype in rural Uganda. Int J STD AIDS 2001;12:116–121 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization: Surveillance of transmitted HIV drug resistance. 2013. www.who.int/hiv/topics/drugresistance/surveillance/en/index.html Accessed May27, 2013
- 29.Frentz D, Boucher CA, van de Vijver DA, et al. : Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev 2012;14:17–27 [PubMed] [Google Scholar]
- 30.World Health Organization: The HIV drug resistance report–2012. Geneva, Switzerland, 2012 [Google Scholar]
- 31.Uganda Ministry of Health: Uganda Clinical Guidelines 2010. Kampala, Uganda, 2010 [Google Scholar]
- 32.Yanik EL, Napravnik S, Hurt CB, Dennis A, Quinlivan EB, Sebastian J, et al. : Prevalence of transmitted antiretroviral drug resistance differs between acutely and chronically HIV-infected patients. J Acquir Immune Defic Syndr 2012;61:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RJ, Okano JT, Kahn JS, Bodine EN, and Blower S: Evolutionary dynamics of complex networks of HIV drug-resistant strains: The case of San Francisco. Science 2010;327:697–701 [DOI] [PubMed] [Google Scholar]
- 34.Gupta RK, Jordan MR, Sultan BJ, Hill A, Davis DHJ, Gregson J, et al. : Global trends in antiretroviral resistance in treatment-naive individuals with HIV after rollout of antiretroviral treatment in resource-limited settings: A global collaborative study and meta-regression analysis. Lancet 2012;380:1250–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaugerre C, Morand-Joubert L, Chaix M-L, Picard O, Marcelin A-G, Schneider V, et al. : Persistence of multidrug-resistant HIV-1 without antiretroviral treatment 2 years after sexual transmission. Antivir Ther 2004;9:415–421 [PubMed] [Google Scholar]
- 36.Pao D, Andrady U, Clarke J, Dean G, Drake S, Fisher M, et al. : Long-term persistence of primary genotypic resistance after HIV-1 seroconversion. J Acquir Immune Defic Syndr 2004;37:1570–1573 [DOI] [PubMed] [Google Scholar]
- 37.Ghosn J, Pellegrin I, Goujard C, Deveau C, Viard J-P, Galimand J, et al. : HIV-1 resistant strains acquired at the time of primary infection massively fuel the cellular reservoir and persist for lengthy periods of time. AIDS 2006;20:159–170 [DOI] [PubMed] [Google Scholar]
- 38.Bezemer D, de Ronde A, Prins M, Porter K, Gifford R, Pillay D, et al. : Evolution of transmitted HIV-1 with drug-resistance mutations in the absence of therapy: Effects on CD4+ T-cell count and HIV-1 RNA load. Antivir Ther 2006;11:173–178 [PubMed] [Google Scholar]
- 39.Barbour JD, Hecht FM, Wrin T, Liegler TJ, Ramstead C a, Busch MP, et al. : Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 2004;18:1683–1689 [DOI] [PubMed] [Google Scholar]
- 40.Brenner B, Routy J-P, Quan Y, Moisi D, Oliveira M, Turner D, et al. : Persistence of multidrug-resistant HIV-1 in primary infection leading to superinfection. AIDS 2004;18:1653–1660 [DOI] [PubMed] [Google Scholar]
- 41.Gandhi RT, Wurcel A, Rosenberg ES, Johnston MN, Hellmann N, Bates M, et al. : Progressive reversion of human immunodeficiency virus type 1 resistance mutations in vivo after transmission of a multiply drug-resistant virus. Clin Infect Dis 2003;37:1693–1698 [DOI] [PubMed] [Google Scholar]
- 42.Bacheler L, Jeffrey S, Hanna G, D'Aquila R, Wallace L, Logue K, et al. : Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J Virol 2001;75:4999–5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang W, Limoli K, Sartoris K, et al. : Complex interactions involving multiple amino acid substitutions alter NNRTI susceptibility. Antiviral Ther 1999;4(Suppl 1):50–51 [Google Scholar]
- 44.Fujiwara T, Sato A, El-farrash M, Abe K, Isaka Y, Kodama M, et al. : S-1153 inhibits replication of known drug-resistant strains of human immunodeficiency virus type. Antimicrob Agents Chemother 1998;42:1340–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dueweke TJ, Pushkarskaya T, Poppe SM, Swaney SM, Zhao JQ, Chen IS, et al. : A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistant human immunodeficiency virus type 1 that confers increased sensitivity to other nonnucleoside inhibitors. Proc Natl Acad Sci USA 1993;90:4713–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, and Maartens G: Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med 2007;146(8):564–573 [DOI] [PubMed] [Google Scholar]
- 47.Paterson DL, Swindells S, Mohr J, Brester M, and Vergis EN: Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000;133(1):21–30 [DOI] [PubMed] [Google Scholar]
- 48.Johnson J a, Li J-F, Wei X, Lipscomb J, Irlbeck D, Craig C, et al. : Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naïve populations and associate with reduced treatment efficacy. PLoS Med 2008;5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson J. and Geretti AM: Low-frequency HIV-1 drug resistance mutations can be clinically significant but must be interpreted with caution. J Antimicrob Chemother 2010;65:1322–1326 [DOI] [PubMed] [Google Scholar]
- 50.Paredes R, Lalama CM, Ribaudo HJ, Schackman BR, Shikuma C, Giguel F, et al. : Pre-existing minority drug-resistant HIV-1 variants, adherence, and risk of antiretroviral treatment failure. J Infect Dis 2010;201:662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simen BB, Simons JF, Hullsiek KH, Novak RM, Macarthur RD, Baxter JD, et al. : Low-abundance drug-resistant viral variants in chronically HIV-infected, antiretroviral treatment-naive patients significantly impact treatment outcomes. J Infect Dis 2009;199:693–701 [DOI] [PubMed] [Google Scholar]
- 52.Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Metzner KJ, Kozal MJ, et al. : Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: A systematic review and pooled analysis. JAMA 2011;305:1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geretti AM, Fox Z V, Booth CL, Smith CJ, Phillips AN, Johnson M, et al. : Low-frequency K103N strengthens the impact of transmitted drug resistance on virologic responses to first-line efavirenz or nevirapine-based highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2009;52:569–573 [DOI] [PubMed] [Google Scholar]
- 54.Geretti AM, Harrison L, Green H, Sabin C, Hill T, Fearnhill E, et al. : Effect of HIV-1 subtype on virologic and immunologic response to starting highly active antiretroviral therapy. Clin Infect Dis 2009;48:1296–1305 [DOI] [PubMed] [Google Scholar]
- 55.Franzetti M, Violin M, Casazza G, Meini G, Callegaro A, Corsi P, et al. : Human immunodeficiency virus-1 B and non-B subtypes with the same drug resistance pattern respond similarly to antiretroviral therapy. Clin Microbiol Infect 2012;18:E66–70 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


