Abstract
CD19 antigen is a major target for human B cell malignancies. Many studies have shown that the antibodies recognizing this antigen hold clinical therapeutic potential, while CD19 antibody of mouse origin requires genetic engineering to reduce the potential side effects of the antibody for their clinical use. There are many clones of CD19 antibodies available with different subclasses of immunoglobulin. IgM type antibody holds a high affinity and high complement activating capacities facilitating the targeting efficacy when it is used in targeting therapy. However, engineering the murine IgM antibody into a functional humanized antibody remains a challenge. The aim of this study was to construct a chimeric antibody composed of a CD19 specific murine IgM antibody 2E8 single-chain antibody fragment (scFv) and human IgG1 Fc region, which was named 2E8scFv-Fc or Hm2E8b. The function and the biological activities of this engineered antibody were characterized using a variety of approaches including cellular, immunological, flow cytometric, and molecular biological approaches. After switching from IgM- to IgG-like type antibody, Hm2E8b retained full antigen-binding activity to membrane CD19 antigen as its parental antibody 2E8, and the immune effector function analysis revealed that it could mediate complement-dependent cytotoxicity (CDC) to kill the target cells via IgG1 Fc domain. The yield of the engineered antibody Hm2E8b in the supernatant was 13.3 μg/mL expressed and secreted in the CHO cell system, which reached the secretory quantity of a regular mouse hybridoma cells. Our conclusion is that the IgM type of CD19 mouse antibody can be successfully engineered into an IgG1 type human-mouse chimeric antibody with similar affinity and biological activity. The yield of the Hm2E8b expression and secretion in CHO cell system was adequate to facilitate further development for therapeutic purpose.
Introduction
Immunotherapy using monoclonal antibodies (MAbs) is an effective and safe method for the treatment of human lymphoid malignancies.(1) In the last decade, CD20 is the major target for the B cell diseases. Many non-Hodgkin's lymphomas (NHLs) and some B cell leukemias have been successfully treated by combining chemotherapy with rituximab, a chimeric anti-CD20 antibody. However, some B cell tumors lack CD20 expression or lose it during the course of rituximab treatment,(2,3) which results in the poor response to rituximab in some patients; or in some cases, patients gradually lose responsiveness during continuous administration and end up in relapse.(4) Therefore, it is necessary to develop novel antibodies that recognize target proteins exclusively expressed on the malignant cells.
CD19 is a 95 kDa transmembrane glycoprotein and member of the Ig superfamily.(5) It is B lineage specific and is expressed on most B cells from the earliest stages of B progenitor development through the terminal differentiation into plasma cells.(6) As compared to CD20, CD19 is expressed on most acute lymphoblastic leukemias (ALL), chronic lymphocytic leukemia (CLL), and lymphomas of B lineage.(7) CD19 is rarely lost during the process of neoplastic transformation and is not expressed on normal hematopoietic stem cells or on normal tissues outside the B lineage. CD19 is not shed into circulation, therefore there is no soluble CD19 to compete for the binding of CD19-specific antibody to cell surface antigen. Several CD19-specific antibodies have been evaluated for the treatment of B lineage malignancies in vitro in both mouse models and clinical trials, including unconjugated antibodies,(8,9) antibody-drug conjugated,(10,11) and bi-specific antibodies targeting CD19 and CD3.(12,13) Anti-CD19 MAbs can induce growth arrest or death of tumor cells, recruit effector cells, reverse P-gp-mediated multi-drug resistance, and deliver organic compounds, toxins, and radioisotopes to target cells.(14–17) Despite recent clinical studies with anti-CD19 antibodies demonstrating encouraging results, challenges remain in optimizing anti-CD19 antibodies to achieve improved outcome.
Zhejiang Children's Hospital (ZCH)-4-2E8 (2E8), a murine IgM-type anti-CD19 antibody, was obtained in our laboratory previously. We demonstrated that 2E8 and antibody norcantharidin conjugated immunotoxin (2E8-NCTD) could specifically target the CD19 expressing B lineage leukemia cells.(18–21) However, as 2E8 is a murine MAb, it is immunogenic and does not mediate effector function in humans due to the murine origin of its constant region. In our previous study, a chimeric antibody Hm2E8 containing the murine antibody 2E8 variable domains and human IgG1 constant domains was constructed. However, the chimeric antibody Hm2E8 was only expressed in the cytoplasm of sf9 cells and lost antigen binding activity (unpublished data), which may be attributed to the possibility that the human IgG1κ leader used for Hm2E8 expression did not favor correct remodeling and secretion of murine IgM-type antibodies in the sf9 insect system, resulting in the absence of functional antibodies in the supernatant. In the present study, we amplified the 2E8 signal peptides from the parental IgM antibody 2E8 secreting hybridoma cell line by 5′RACE and connected the VH and VL domains by a short peptide linker to form a single-chain Fv (scFv) antibody fragment, which was then fused with the Fc (hinge, CH2, CH3) domains of human IgG1 to form human-mouse chimeric antibody 2E8scFv-Fc (Hm2E8b). The results revealed that the 2E8scFv-Fc fusion protein retained the specific antigen-binding affinity of the parental antibody 2E8 and was abundant and well secreted into supernatants.
Material and Methods
Construction of expression vector
The RNA was extracted from the 2E8 hybridoma cells and reverse transcribed into the cDNA by 5′RACE (Takara, Otsu, Shiga, Japan). The variable region genes of 2E8 were cloned into the pGEM-T easy vector (Promega, Madison, WI) and sequenced, and then the signal peptides were predicted by Signal P3.0 software. The VH containing signal peptide of 2E8VH and VL were ligated with the linker peptide (Gly4Ser)3 by splice overlap extension. The resulting PCR product was then digested with BamH I and EcoR I (Promega) and inserted into the same sites of pHMCH3 expression vector (constructed in our lab previously) containing hinge, CH2, CH3 of human IgG1, which was named pHMCH3-Hm2E8b (Fig. 1).
FIG. 1.
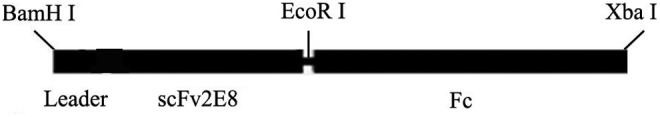
Details of cloning sites of pHMCH3-Hm2E8b.
Transfection into CHO cells
CHO cells were grown in RPMI-1640 medium with 10% newborn calf serum at 37°C in a humidified atmosphere of 5% CO2 and were split when they reached 70–80% confluency by trypsinization. CHO cells were plated at a density of 1×105 cells/well in 24-well plates 24 h before transfection without the addition of penicillin and streptomycin. CHO cells were transiently transfected with either purified pHMCH3-Hm2E8b or pHMCH3 vector using Lipofectamine™ 2000 Reagent (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. Positive clones were selected in the presence of 600 μg/mL G418 (BBI, Madison, WI), and single cell clones were isolated by limiting dilution. The highest antibody-producing clone was chosen after flow cytometric analysis for further experimentation.
Purification of Hm2E8b antibody
The supernatant was harvested and concentrated 20-fold with an Amicon ultra-15 centrifugal filter unit (Millipore, Darmstadt, Germany), then purified by a Bio-scale™ Mini Affi-prep Protein A cartridge (Bio-Rad, Hercules, CA). The eluted fractions were again concentrated five-fold with the Amicon centrifugal filter (Millipore).
Western blot analysis
Purified Hm2E8b was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), according to the method described by Sambrook and colleagues,(22) with 12% separation polyacrylamide gel and 5% condensed gel. The gel was then transferred onto the PVDF membrane. After blocking with milk for 2 h, the transferred membrane was incubated with 1:1000 dilution of HRP-conjugated goat anti-human IgG1 Fc antibody (Sigma, St. Louis, MO) for 2 h. Specific binding was detected with ECL Western blotting detection reagents (Bio-Rad).
Binding activity of Hm2E8b detected by flow cytometric analysis
Briefly, 5×105 Nalm-6 cells were added to each tube and incubated with 0.5 μg of the purified Hm2E8b for 30 min on ice. After washing three times with phosphate-buffered saline (PBS), the cells were stained with fluorescein isothiocyanate (FITC) labeled goat anti-human IgG Fc antibody (KPL, Gaithersburg, MD) for 30 min on ice in the dark, followed by another three rinses with PBS. The fluorescent signals were detected by a FACSCalibur™ flow cytometer (Becton-Dickinson, San Jose, CA) using Cellquest software.
The specificity of its binding to the CD19 antigen was further assessed by blocking test to its parental antibody 2E8. For this purpose, 5 μg of purified Hm2E8b was added to 5×105 cells and incubated for 30 min on ice. After washing three times with PBS, the cells were further incubated with parental antibody 2E8-FITC for 30 min on ice in the dark. The cells were washed and analyzed by flow cytometry.
Competitive inhibition assay
Nalm-6 cells were incubated on ice with the 2E8-FITC at a fixed sub-saturated concentration of 0.5 μg/mL either alone or in the presence of varying concentrations of either the Hm2E8b or the parental 2E8 antibody as inhibitors. After washing, the cells were analyzed by flow cytometry. Relative inhibition of binding by 2E8-FITC was calculated as follows: % of inhibition=[(% of positive cells without inhibitor – % of positive cells with inhibitor)/(% of positive cells without inhibitor)]×100%. The experiment was performed three times and the mean values were calculated. IC50 values (concentration of inhibitor producing 50% inhibition of binding by 2E8-FITC) were calculated using a sigmoidal dose-response curve fit.
Complement-dependent cytotoxicity assay
CDC assay was performed as described previously.(23) Briefly, CD19 positive human leukemia cell line Nalm-6 cells were used as target. Nalm-6 cells were washed and suspended in serum-free RPMI-1640 at a concentration of 1×106 cells/mL. Fifty μL (5×104) of the cell suspension were added to each well of a 96-well, flat-bottom plate and 25 μL different concentrations of the Hm2E8b and 2E8 antibodies were added per well. Cultures were performed in triplicate and plates were incubated for 1 h. As a source of complement, 25 μL normal human serum prepared from healthy volunteers was added and incubated at 37°C for 2 h. Wells omitting antibodies while retaining the same amount of culture medium and complement added or omitting Nalm-6 cells while retaining the same amount of both antibodies and complement were set as the negative controls. Ten μL of 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8) were added to each well and incubated for an additional 4 h at 37°C. The absorbance (A450–A650) of the formazan dye produced by metabolically active cells of each well was detected on a GeneQuant II (Biotech, Piscataway, NJ). Cytotoxicity was calculated according to the following formula:
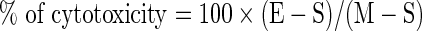 |
where E is the absorbance of experimental well, S is that in the absence of MAb (cells were incubated with medium and complement alone), and M is that of antibody and complement in the absence of target cells.
Results
Construction, expression, and purification of Hm2E8b
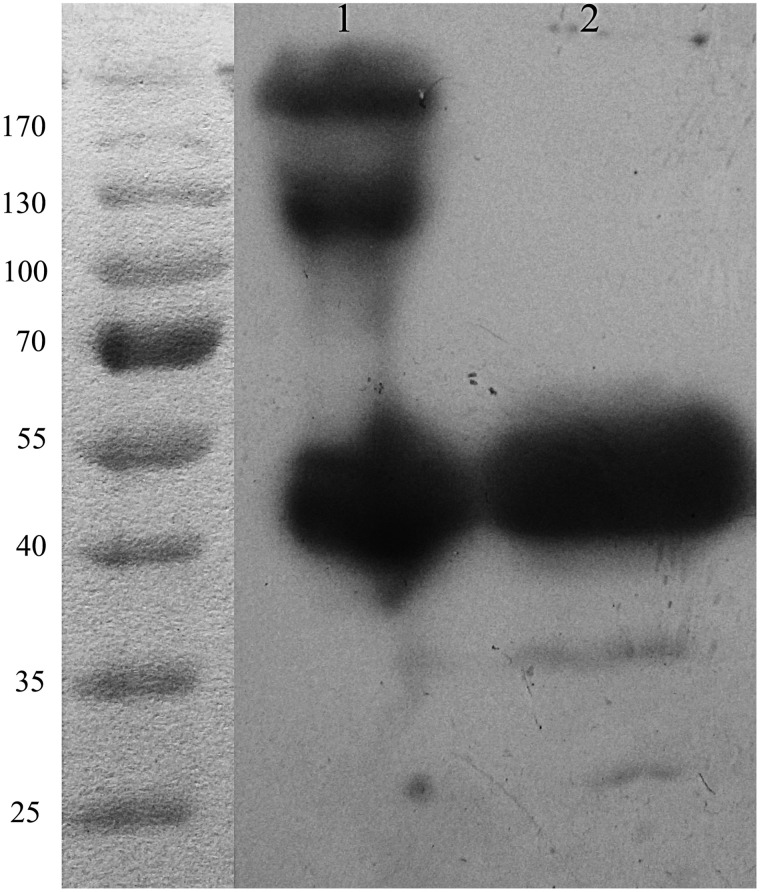
Hm2E8b was constructed by joining the murine scFv2E8 with the human IgG1-Fc fragment at the hinge region (Fig. 1). CHO cells were stably transfected with pHMCH3-Hm2E8b by Lipofectamine 2000 Reagent (Invitrogen) and selected with G418. Hm2E8b was successfully purified from culture medium by affinity chromatography using A-Sepharose. The yield of the purified antibody was at approximately 2 mg/mL in 1 mL after concentration from 150 mL of supernatant, which represented at least 13.3 μg/mL in unconcentrated culture supernatant. Western blot analysis showed that under the reducing condition only one band at 50 kDa was observed, which was in close agreement with the predicted molecular weight of 54kDa (Fig. 2, lane 2). Under non-reducing conditions, the purified protein showed three bands on the gel with molecular weights of 50, 130, and 200 kDa, respectively (Fig. 2, lane 1).
FIG. 2.
Western blot analysis of Hm2E8b. Protein ladder is shown in the left lane while Hm2E8b was run under non-reducing (lane 1) and reducing (lane 2) conditions detected with HRP-conjugated goat anti-human IgG1 Fc antibody.
Binding activity of Hm2E8b
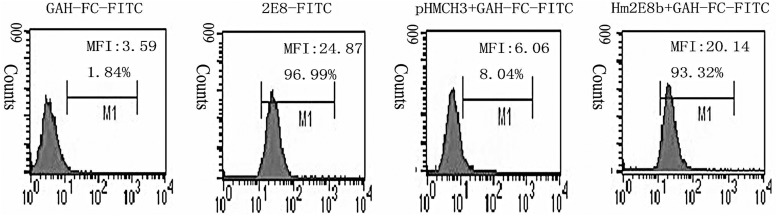
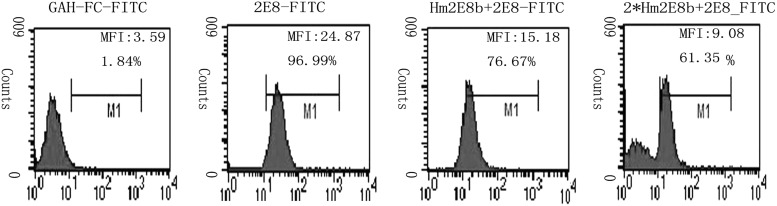
The specificity of Hm2E8b to recognize membrane CD19 antigen was examined by flow cytometry using a human pre-B leukemia cell line Nalm-6, which has been previously characterized to express CD19 on the cell membrane. As shown in Figure 3, Hm2E8b bound to Nalm-6 cells with comparable capacity to its parental murine antibody 2E8. The blocking test using our 2E8 MAb confirmed the above results (Fig. 4), which indicated that the engineered chimeric antibody Hm2E8b retained full antigen-binding activity as its parental murine antibody 2E8.
FIG. 3.
Flow cytometry analysis of Hm2E8b antibody. Four groups including background control (GAH-Fc-FITC), positive control (2E8-FITC), culture supernatant from CHO-pHMCH3 and culture supernatant from CHO-Hm2E8b. Two parameters as percentage of positive cells (%Gate) and mean fluorescence intensity (Geo mean) were used to assess the binding ability of the Hm2E8b antibody.
FIG. 4.
Blocking test of Hm2E8b antibody to 2E8-FITC. Binding activity of parental 2E8 antibody was partially blocked by chimeric antibody Hm2E8b. The panel includes four groups from left to right, representing background control (GAH-Fc-FITC), positive control (2E8-FITC), Hm2E8b+2E8-FITC, incubated with Hm2E8b twice+2E8-FITC. Two parameters as percentage of positive cells (%Gate) and mean fluorescence intensity (Geo mean) were used to assess the binding ability of the Hm2E8b antibody.
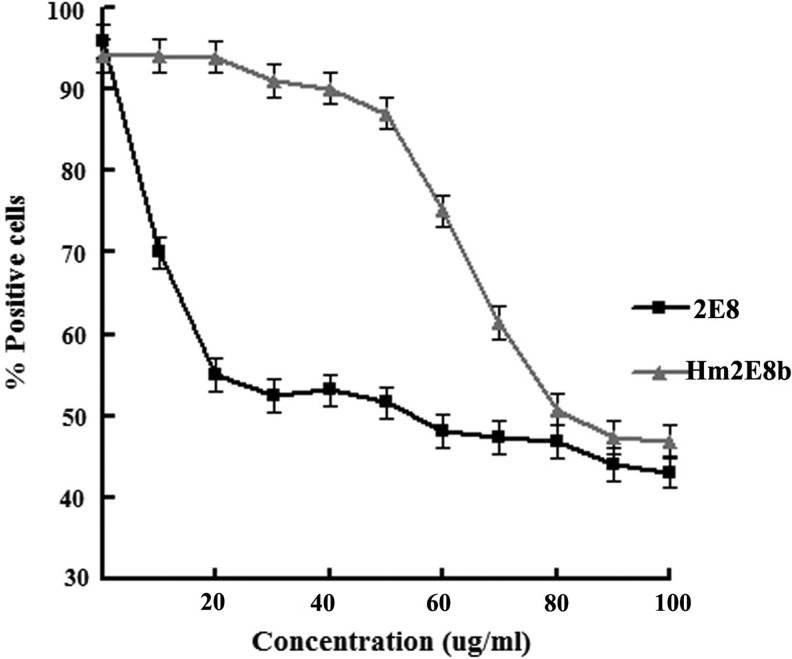
Relative affinity was assessed by competitive inhibition assay of the ability of the Hm2E8b or the 2E8 to compete with 2E8-FITC for binding to Nalm-6 cells. As shown in Figure 5, both Hm2E8b and 2E8 competitively inhibited the binding of 2E8-FITC in a concentration-dependent manner. To compare the affinity of Hm2E8b and that of parental 2E8, the IC50 values were determined. The IC50 values for the Hm2E8b and 2E8 were approximately 68 and 15 μg/mL, respectively. Thus the concentration of Hm2E8b (a monomer) to achieve half-maximum inhibition was slightly higher than that of 2E8 (a pentamer), indicating reduced binding affinity in comparison to its parental IgM antibody 2E8.
FIG. 5.
Competitive inhibition assay of Hm2E8b antibody. Decrease of 2E8-FITC binding with increasing concentrations of inhibitors Hm2E8b and 2E8 was monitored by flow cytometry. Approximately 4.5-times greater concentrations of Hm2E8b than that of the parental 2E8 antibody were needed to achieve half-maximum inhibition.
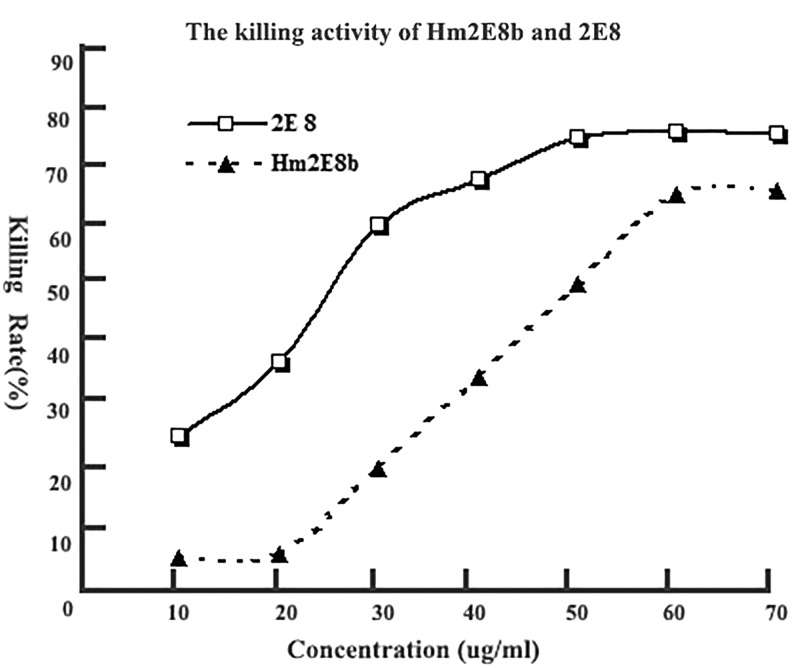
Killing target cells by complement-dependent cytotoxicity
While negative controls showed very limited killing cytotoxicity, both the murine IgM antibody 2E8 and the chimeric antibody Hm2E8b could kill CD19 positive Nalm-6 cells by CDC (Fig. 6) in a dose-dependent manner. The LD50 values of 2E8 and Hm2E8b to Nalm-6 cells were 25 and 52 μg/mL, respectively.
FIG. 6.
Complement-dependent cytotoxicity of Hm2E8b antibody. CD19+ B lineage leukemia cell line Nalm-6 was used as target cell. Results showed that Hm2E8b and 2E8 could kill Nalm-6 cells effectively in a dose-dependent manner.
Discussion
Antibody therapy has proven to be one of the most promising therapies for hematopoietic malignancies. CD19 has been identified as an excellent target for B lineage malignancy targeting. 2E8 is a novel murine IgM antibody that can specifically target the CD19 expressing B lineage leukemia cells. Unfortunately, murine antibody cannot be used in therapy due to the human anti-mouse antibody (HAMA) responses in clinical patients.(24) This obstacle can be overcome by antibody engineering or humanization to maximally reduce the antigenicity. On the other hand, how to humanize a murine IgM antibody into a functional antibody is still a critical issue. In 1994, Zebedee and colleagues generated a mouse-human IgG1 type chimeric antibody (ch2D10) by connecting the variable regions of a murine IgM antibody (m2D10) to the constant regions of a human IgG1. However the engineered antibody had significantly reduced binding affinity in comparison to its parental murine antibody.(25) Wang and colleagues constructed a similar chimeric antibody (C1-28), which lost its binding activity.(26) The loss of binding activity was considered to be a consequence of the fact that the Fv domain was apt to dissociate due to the change of constant region; the problem was resolved by fusing a scFv of 1-28 with the human IgG1 constant region to form a new chimeric antibody 5S, which was expressed in the 293T human embryonic kidney cell culture and yielded a supernatant of approximately 50 μg/mL IgG antibody. Most importantly, with this effort, the chimeric antibody retained the binding activity of its murine counterpart.(27)
In the present study, we have successfully constructed a novel chimeric antibody Hm2E8b by linking the anti-CD19 mouse IgM scFv2E8 to human IgG1 Fc region. Because the Fc region of human IgG1 contains a hinge region, Fc fusions can offer disulfide bonds for dimerization. The Western blot analysis has shown that the molecular weight of the homodimer was not strictly twice that of the monomer, which is possibly caused by extensive glycosylation of the antibody in CHO cells and/or a result of denaturation during electrophoresis.(28) The scFv-Fc Hm2E8b could also self-assemble into multimers; the cross-pairing of the variable regions may be the basis of the multimerization of the scFv-Fc constructs.(29) Hm2E8b has retained nearly full antigen-binding activity of its parental murine antibody 2E8.
Although the binding effects of the engineered Hm2E8b were reduced significantly after the IgM-type antibody was engineered into a IgG-type antibody, it is reasonable that the engineered IgG type chimeric antibody is only a monomer while the original IgM type parental antibody was a pentamer. So we considered that the antibody subtype switch most probably would not hamper the clinical application if it is used in patients in the future.
Another important issue for successful antibody engineering is the yields of the antibody expression after engineering and transfection in host cells. Using our pHMCH3 vector system, the yield of our Hm2E8b reached 13.3 μg/mL after several purification/concentration steps, which is comparable to the productive amount of a regular mouse hybridoma cell line in culture media. Mass production of Hm2E8b could be achieved by using a bioreactor with continuous perfusion, which can yield 1 g/L of chimeric antibodies.(30)
Taken together, our results have shown that by switching from an IgM- to IgG-like antibody is a viable option in the humanization of murine IgM antibody. The engineered antibody Hm2E8b presents a potent biological activity of recognition and kill of CD19 positive human leukemia cells, which warrants the further development of this antibody for clinical testing.
Acknowledgment
This work was supported in part by grants from the National Natural Science Foundation of China (nos. 30971283 and 81170502), the Zhejiang Provincial Natural Science Foundation (nos. Z205166 and Y2100070), and the Science Technology Department Foundation of Zhejiang Province (no. 2007C23007; Qianjiang project: 2009R10037). The authors would like to thank Mr. Hongqiang Shen, Mrs. Baiqin Qian, and Mr. Zhao Ning at the hematology-oncology laboratory in the Children's Hospital of Zhejiang University School of Medicine for their excellent technical support.
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Martin P, and Leonard JP: Targeted therapies for non-Hodgkin lymphoma: rationally designed combinations. Clin Lymphoma Myeloma 2007;7(Suppl 5):S192–198 [DOI] [PubMed] [Google Scholar]
- 2.Davis TA, Czerwinski DK, and Levy R: Therapy of B-cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res 1999;5(3):611–615 [PubMed] [Google Scholar]
- 3.Kennedy GA, Tey SK, Cobcroft R, Marlton P, Cull G, Grimmett K, Thomson D, and Gill D: Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br J Haematol 2002;119(2):412–416 [DOI] [PubMed] [Google Scholar]
- 4.Haidar JH, Shamseddine A, Salem Z, Mrad YA, Nasr MR, Zaatari G, and Bazarbachi A: Loss of CD20 expression in relapsed lymphomas after rituximab therapy. Eur J Haematol 2003;70(5): 330–332 [DOI] [PubMed] [Google Scholar]
- 5.Tedder TF, and Isaacs CM: Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes. A new member of the immunoglobulin superfamily. J Immunol 1989;143(2):712–717 [PubMed] [Google Scholar]
- 6.Sato S, Steeber DA, Jansen PJ, and Tedder TF: CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol 1997;158(10):4662–4669 [PubMed] [Google Scholar]
- 7.Uckun FM, Jaszcz W, Ambrus JL, Fauci AS, Gaji-Peczalska K, Song CW, Wick MR, Myers DE, Waddick K, and Ledbetter JA: Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood 1988;71(1):13–29 [PubMed] [Google Scholar]
- 8.Hekman A, Honselaar A, Vuist WM, Sein JJ, Rodenhuis S, Ten Bokkel Huinink WW, Somers R, Rumke P, and Melief CJ: Initial experience with treatment of human B cell lymphoma with anti-CD19 monoclonal antibody. Cancer Immunol Immunother 1991;32(6):364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vlasveld LT, Hekman A, Vyth-Dreese FA, Melief CJ, Sein JJ, Voordouw AC, Dellemijn TA, and Rankin EM: Treatment of low-grade non-Hodgkin's lymphoma with continuous infusion of low-dose recombinant interleukin-2 in combination with the B-cell-specific monoclonal antibody CLB-CD19. Cancer Immunol Immunother 1995;40(1):37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grossbard ML, Lambert JM, Goldmacher VS, Spector NL, Kinsella J, Eliseo L, Coral F, Taylor JA, Blattler WA, and Epstein CL: Anti-B4-blocked ricin: a phase I trial of 7-day continuous infusion in patients with B-cell neoplasms. J Clin Oncol 1993;11(4):726–737 [DOI] [PubMed] [Google Scholar]
- 11.Uckun FM, Messinger Y, Chen CL, O'Neill K, Myers DE, Goldman F, Hurvitz C, Casper JT, and Levine A: Treatment of therapy-refractory B-lineage acute lymphoblastic leukemia with an apoptosis-inducing CD19-directed tyrosine kinase inhibitor. Clin Cancer Res 1999;5(12):3906–3913 [PubMed] [Google Scholar]
- 12.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandi C, Wolf A, Kirchinger P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, and Kufer P: Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 2008;321(5891):974–977 [DOI] [PubMed] [Google Scholar]
- 13.Molhoj M, Crommer S, Brischwein K, Rau D, Sriskandarajah M, Hoffmann P, Kufer P, Hofmeister R, and Baeuerle PA: CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol 2007;44(8):1935–1943 [DOI] [PubMed] [Google Scholar]
- 14.Vallera DA, Elson M, Brechbiel MW, Dusenbery KE, Berns LJ, Jaszcz WB, Ramsay NK, Panoskaltsis-Mortar A, Kuroki DW, Wagner JE, Vitetta ES, and Kersey JH: Radiotherapy of CD19 expressing Daudi tumors in nude mice with Yttrium-90-labeled anti-CD19 antibody. Cancer Biother Radiopharm 2004;19(1):11–23 [DOI] [PubMed] [Google Scholar]
- 15.Zalevsky J, Leung IW, Karki S, Chu SY, Zhukovsky EA, Desjarlais JR, Carmichael DF, and Lawrence CE: The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood 2009;113(16):3735–3743 [DOI] [PubMed] [Google Scholar]
- 16.Herrera L, Stanciu-Herrera C, Morgan C, Ghetie V, and Vitetta ES: Anti-CD19 immunotoxin enhances the activity of chemotherapy in severe combined immunodeficient mice with human pre-B acute lymphoblastic leukemia. Leuk Lymphoma 2006;47(11): 2380–2387 [DOI] [PubMed] [Google Scholar]
- 17.Ghetie MA, Picker LJ, Richardson JA, Tucker K, Uhr JW, and Vitetta ES: Anti-CD19 inhibits the growth of human B-cell tumor lines in vitro and of Daudi cells in SCID mice by inducing cell cycle arrest. Blood 1994;83(5):1329–1336 [PubMed] [Google Scholar]
- 18.Chen YH, Tang YM, Shen HQ, Song H, Yang SL, Shi SW, Qian BQ, Xu WQ, and Ning BT: [Targeted killing of the Nalm-6 cells with 2E8-Genistein immunotoxin and its mechanism]. Zhonghua Er Ke Za Zhi 2009;47(1):57–61 [PubMed] [Google Scholar]
- 19.Zhang J, Tang Y, Qian B, and Sheng H: Preparation and evaluation of norcantharidin-encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8. J Huazhong Univ Sci Technolog Med Sci 2010;30(2):240–247 [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Tang Y, Li S, Liao C, and Guo X: Targeting of the B-lineage leukemia stem cells and their progeny with norcantharidin encapsulated liposomes modified with a novel CD19 monoclonal antibody 2E8 in vitro. J Drug Target 2010;18(9):675–687 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Tang Y, Shen H, and Qian B: Targeting and internalization of sterically stabilized liposome modified with ZCH-4-2E8. J Huazhong Univ Sci Technol Med Sci 2009;29(3):273–280 [DOI] [PubMed] [Google Scholar]
- 22.Neuhouser ML, Rock CL, Kristal AR, Patterson RE, Neumark-Sztainer D, Cheskin LJ, and Thornqiust MD: Olestra is associated with slight reductions in serum carotenoids but does not markedly influence serum fat-soluble vitamin concentrations. Am J Clin Nutr 2006;83(3):624–631 [DOI] [PubMed] [Google Scholar]
- 23.Natsume A, Shimizu-Yokoyama Y, Satoh M, Shitara K, and Niwa R: Engineered anti-CD20 antibodies with enhanced complement-activating capacity mediate potent anti-lymphoma activity. Cancer Sci 2009;100(12):2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller RA, Oseroff AR, Stratte PT, and Levy R: Monoclonal antibody therapeutic trials in seven patients with T-cell lymphoma. Blood 1983;62(5):988–995 [PubMed] [Google Scholar]
- 25.Zebedee SL, Koduri RK, Mukherjee J, Mukherjee S, Lee S, Sauer DF, Scharff MD, and Casadevall A: Mouse-human immunoglobulin G1 chimeric antibodies with activities against Cryptococcus neoformans. Antimicrob Agents Chemother 1994;38(7):1507–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang YG, Huang Y, Gu X, Yu M, Feng JN, Sun YX, Li Y, and Shen BF: [Construction and expression of chimeric anti-human CD20 monoclonal antibody]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2006;22(3):363–367 [PubMed] [Google Scholar]
- 27.Wang Y, Feng J, Huang Y, Gu X, Yu M, Feng JN, SunYX, Li Y, and Shen BF: The design, construction and function of a new chimeric anti-CD20 antibody. J Biotechnol 2007;129(4):726–731 [DOI] [PubMed] [Google Scholar]
- 28.Cao M, Cao P, Yan H, Lu W, Ren F, Hu Y, and Zhang S: Construction, purification, and characterization of anti-BAFF scFv-Fc fusion antibody expressed in CHO/dhfr- cells. Appl Biochem Biotechnol 2009;157(3):562–574 [DOI] [PubMed] [Google Scholar]
- 29.Wu AM, Chen W, Raubitschek A, Williams LE, Neumaier M, Fischer R, Hu SZ, Odom-Maryon T, Wong JY, and Shively JE: Tumor localization of anti-CEA single-chain Fvs: improved targeting by non-covalent dimers. Immunotechnology 1996;2(1):21–36 [DOI] [PubMed] [Google Scholar]
- 30.Rameez S, Mostafa SS, Miller C, and Shukla AA: High-throughput miniaturized bioreators for cell culture process development: reproducibility, scalability, and control. Biotechnol Prog 2014January22 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]