Abstract
Local and general hypothermia are used to treat spinal cord injury (SCI), as well as other neurological traumas. While hypothermia is known to provide significant therapeutic benefits due to its neuroprotective nature, it is unclear how the treatment may affect healthy tissues or whether it may cause undesired temperature changes in areas of the body that are not the targets of treatment. We performed 2-hour moderate general hypothermia (32°C core) or local hypothermia (30°C spinal cord) on rats that had received either a moderate contusive SCI or laminectomy (control) while monitoring temperatures at three sites: the core, spinal cord, and cortex. First, we identified that injured rats that received general hypothermia exhibited larger temperature drops at the spinal cord (−3.65°C, 95% confidence intervals [CIs] −3.72, −3.58) and cortex (−3.64°C, CIs −3.73, −3.55) than uninjured rats (spinal cord: −3.17°C, CIs −3.24, −3.10; cortex: −3.26°C, CIs −3.34, −3.17). This was found due to elevated baseline temperatures in the injured group, which could be due to inflammation. Second, both general hypothermia and local hypothermia caused a significant reduction in the cortical temperature (−3.64°C and −1.18°C, respectively), although local hypothermia caused a significantly lower drop in cortical temperature than general hypothermia (p<0.001). Lastly, the rates of rewarming of the cord were not significantly different among the methods or injury groups that were tested; the mean rate of rewarming was 0.13±0.1°C/min. In conclusion, local hypothermia may be more suitable for longer durations of hypothermia treatment for SCI to reduce temperature changes in healthy tissues, including the cortex.
Introduction
Hypothermia for treatment of patients with spinal cord injury (SCI) shows great promise as an acute neuroprotective therapy (Dietrich et al., 2009, 2011; Dietrich, 2012; Maybhate et al., 2012; Silasi et al., 2012). In addition to SCI, hypothermia has been shown to lead to functional improvements in many other injury types, including cardiac arrest (Arrich, 2007; Madhok et al., 2010; Beddingfield and Clark, 2012), stroke (Groysman et al., 2011; Chang and MarshAll, 2012; Lakhan and Pamplona, 2012), ischemia-reperfusion injury (Kawamura et al., 2006), traumatic brain injury (Tomura et al., 2012), and coma (Bouwes et al., 2012). Hypothermia works principally by lowering the overall metabolism of the body. For every 1°C reduction in body temperature, there is 6% reduction in metabolism as well as reduced inflammation (Reuler, 1978). Decreased metabolism brings about lower consumption of oxygen and glucose and reduced excitotoxic neurotransmitter release. These physiological changes lessen the risk of energy failure. On a cellular level, maintaining stores of energy helps to prevent the failure of sodium pumps and calcium influx, and the end result is prevention of cell death (Choi et al., 2012). For example, it has been shown that as little as a 1°C reduction in body temperature is enough to reduce gray and white matter damage during spinal cord ischemia (Saito et al., 2012).
In the clinic, core temperature is reduced, using either cold-water-circulating pads or intravascular cooling (Levi et al., 2010), inducing temperature changes ranging from 31°C to 34°C±1°C (88°F to 93°F±2°F) throughout the body. In the largest study to-date of cervical SCI patients, a striking 43% of patients who were treated with moderate hypothermia (33°C) showed improvement by at least one grade at follow-up, according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), which includes both sensory and motor evaluations (Dididze et al., 2012). Therefore, it is highly desirable to undertake studies that further reduce the temperature of the spinal cord after injury (to <33°C) in order to achieve the maximum therapeutic benefit. However, in doing so, the therapy must be administered safely to avoid complications.
We previously showed that 2-hour general hypothermia in rats with moderate contusive SCI leads not only to improved motor behavior and increased somatosensory evoked potential (SSEP) amplitude, but also to increased N1-latency of SSEP, for up to 4 weeks after injury (Maybhate et al., 2012). Because SSEPs are the response at the primary somatosensory cortex to stimulus of the periphery, a key question is how cortical temperature is affected during the period of hypothermia administration. Importantly, no study has been performed to measure the temperature differences of the cortex under local or general hypothermia. Nor has the effect that injury itself has on core and cortical temperature during hypothermia treatment been reported. Indeed, as patient shock is a primary response of injury, the SCI alone could affect how the body's temperature is regulated. Likewise, during application of general hypothermia, aberrant cooling of the brain, including the hypothalamus, which regulates temperature, may be undesired.
Thus, local hypothermia is attractive for its potential to selectively cool precise regions of the spinal cord while avoiding drastic temperature changes at the brain. In this work, we compare two methods of hypothermia, local and general, for the treatment of moderate contusive SCI. We sought to look at how three sites (core, spinal cord, and cortex) are cooled by comparing the two methods of hypothermia. Such information is critical for evaluating the safety and efficacy of hypothermia treatment, especially if it is to be administered for a longer period of time.
The mean temperature differences achieved at the core, spinal cord, and cortex were calculated for each group. We sought to answer three questions to better understand how modulation of central nervous system (CNS) temperatures differs depending on the injury state and treatment method.
(1) Do cortical and spinal cord temperatures during hypothermia significantly differ between rats that have undergone only laminectomy (control) and rats subjected to moderate contusion SCI?
(2) Do local and general hypothermia evoke significantly different temperatures at the spinal cord and cortex during hypothermia treatment?
(3) Does the rate of rewarming of the spinal cord differ based on the method of hypothermia induction or injury state?
These questions allowed us to determine whether there are changes in cortical and/or spinal cord temperatures that depend on the injury and to identify how each treatment may affect the temperature, not only at site of SCI, but also on the cortex. This is the first study to report the temperature profiles of the core, spinal cord, and cortex during 2-hour general or local hypothermia in rats following SCI.
Methods
All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. A total of 20 adult female Lewis rats (200–230 g, Charles River Laboratories, Germantown, MD) were used in this study. Five rats were randomly assigned into one of following four groups: (1) contusion injury followed by general hypothermia, (2) laminectomy followed by general hypothermia, (3) contusion injury followed by local hypothermia, and (4) laminectomy followed by local hypothermia. Rats were housed in individual cages and provided with free access to food and water for the experimental period.
Anesthesia
Anesthesia used for all surgical procedures included a mixture of 30.4 mg/kg ketamine, 4.3 mg/kg xylazine, and 0.9 mg/kg acepromazine maleate administered through intraperitoneal injection (0.12 mL). An adequate level of anesthesia was determined by monitoring the corneal reflex and limb withdrawal to pinch stimuli.
Laminectomy and contusion injury
Our SCI model has been previously described (Agrawal et al., 2008, 2009, 2010a, 2010b; Al-Nashash et al., 2009; All et al., 2009, 2010, 2012; Iyer et al., 2010; Kerr et al., 2010; Bazley et al., 2011, 2012a, 2012b). A laminectomy was performed by removing the T8 thoracic vertebra to expose the dorsal surface of the spinal cord without opening the dura mater. For the injury groups, the vertebrae at T6 and T10 were secured in stabilization clamps to reduce motion of the spinal column during impact of injury. An MASCIS impactor was used to induce contusion injury. The height of the impactor rod (weight=10 g) was precalibrated to 12.5 mm. The probe was then released to hit the dorsal exposed surface of the T8 spinal cord and induce moderate contusion injury. The impact trajectory, distance, velocity, and time were recorded to make sure consistency among all rodents. Animals for which the error in any of these parameters exceeded 5% were excluded from the study. This is a well-established model for contusion SCI and damage to the rat CNS caused at this height is comparable to moderate injury in patients.
Temperature probe implantation
A rectal probe of 5-cm long and 2 mm in diameter (RET-3; PhysiTemp, Clifton, NJ) was used to monitor core temperature. The accuracy with which rectal temperature parallels core temperature measured from thermistor probes implanted chronically into the chest cavity has been previously established (Poole and Stephenson, 1977). Two microprobe thermocouples (IT-24P Flexible Microprobes; PhysiTemp) were inserted, one in the T8 region of the spinal cord and another at the somatosensory area of the cortex.
To insert the probe at the cortex, under general anesthesia the head region was shaved and local anesthetic of 2% lidocaine HCl was injected under the skin. Next, a midline incision was made and the cranium bone was cleaned by removing the tissue underneath the skin. A dental drill was used to drill one small hole into the cranium. This hole was located on the somatosensory cortex corresponding to the left hindlimb region, which is located 2.5 mm posterior of the bregma and 2.8 mm laterally from the bregma. The temperature probe was inserted through the dura mater without causing compression to the brain tissue and dental cement was used to cover the small opening in skull. The second probe was securely placed on the spinal cord at the epicenter of injury in the T8 region. The paravertebral muscle and skin were then sutured closed.
Induction of general hypothermia
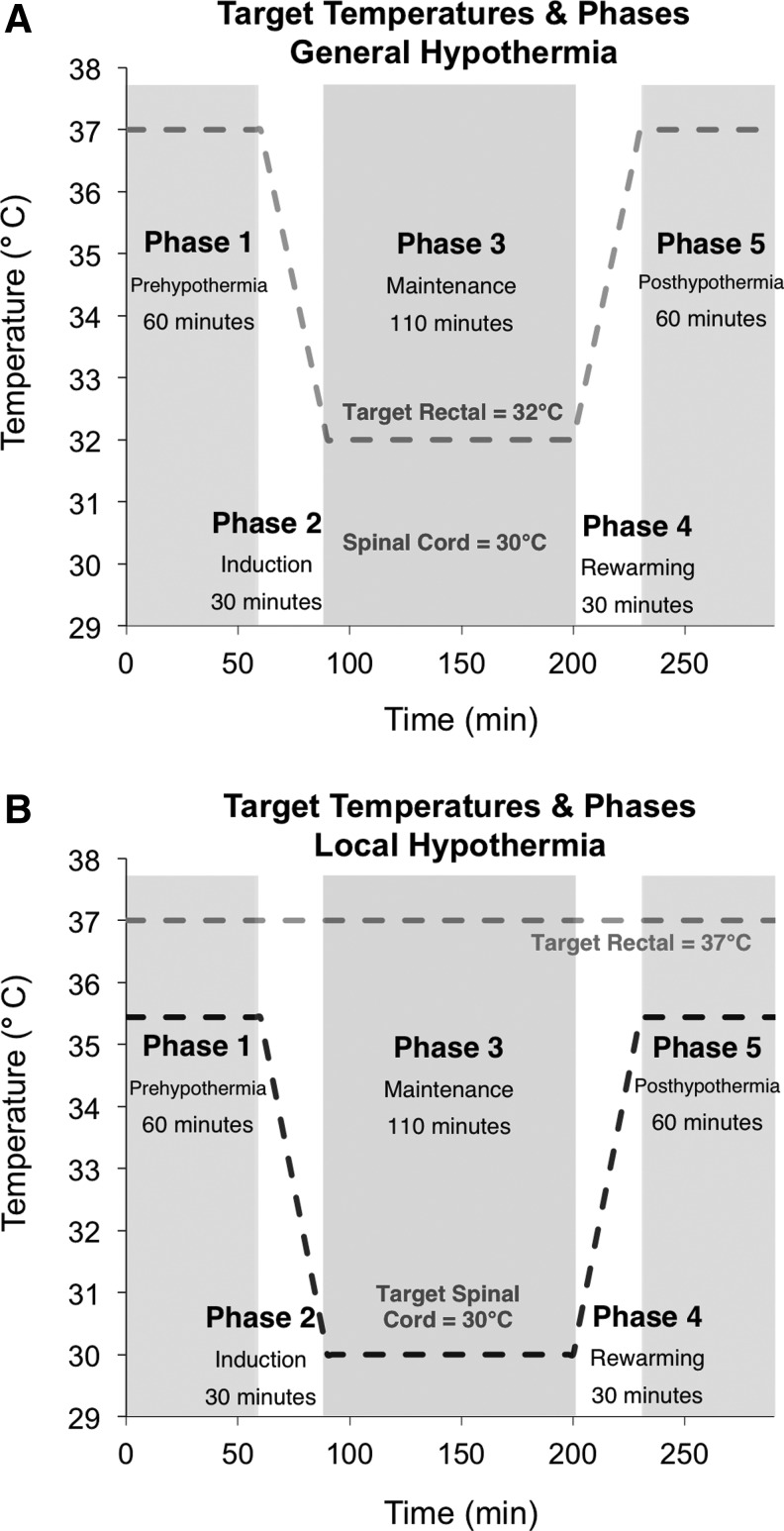
A group of randomly assigned rats underwent general hypothermia following the contusion injury or laminectomy. Figure 1 outlines the five phases and the target temperatures associated with each treatment group. Phase 1: Prehypothermia refers to the 1-hour period prior to cooling. Phase 2: Induction refers to the 30-minute hypothermia cooling phase. Phase 3: Maintenance refers to the following 2 hours during which hypothermia was maintained at the target temperature at either the spinal cord (local) or rectum (general). Phase 4: Rewarming refers to the 30-minute period during which the cooled body is rewarmed to the prehypothermia temperature. Phase 5: Posthypothermia refers to the 1-hour observation period following rewarming.
FIG. 1.
The five phases of hypothermia treatment and the corresponding target temperatures for general (A) and local (B) hypothermia. During general hypothermia, the rectal temperature was fixed at 32°C while the cortex and spinal cord temperatures were unconstrained. During local hypothermia, both the spinal cord and rectal temperatures were fixed at 30°C and 37°C, respectively, while the cortical temperature was unconstrained.
The phases of general hypothermia are shown in Figure 1A. The core temperature was maintained at 37°C±0.5°C with the help of a heating pad for 1 hour after the time of contusion or laminectomy and prior to inducing hypothermia. After 1 hour, general hypothermia was induced by spraying the rat with alcohol mist (70% ethanol) and using an electric fan until the core temperature dropped to 32°C±0.5°C, as previously described (Shin et al., 2006; Jia et al., 2008; Maybhate et al., 2012). The induction period lasted 25 to 30 minutes. The temperature was then maintained at 32°C±0.5°C for 2 hours. The temperature 32°C was used for general hypothermia because it is within the acceptable range of clinical applications. After 2-hour hypothermia, rats were rewarmed to 37°C±0.5°C using the heat pad. The rewarming period lasted 25 to 30 minutes. Rewarming was followed by a 1-hour observation period.
Induction of local hypothermia
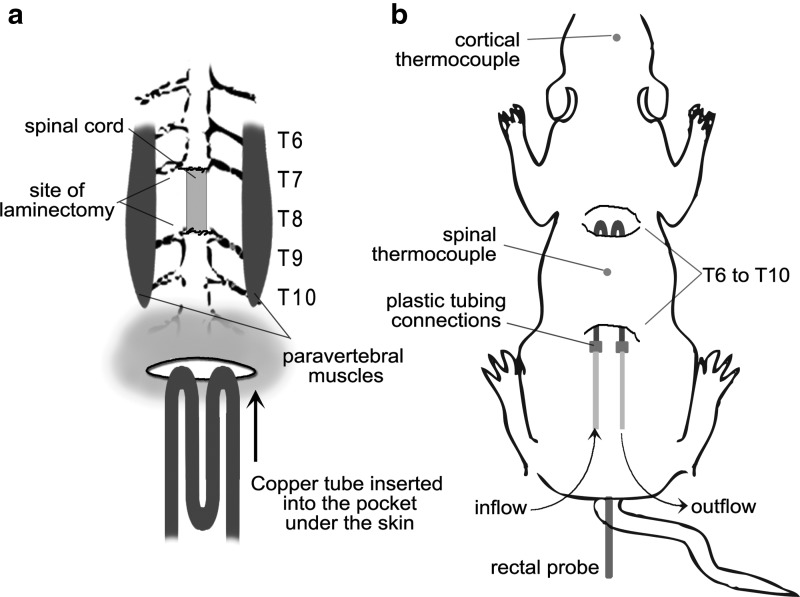
A group of randomly assigned rats underwent local hypothermia following moderate contusion injury or laminectomy. For local hypothermia, a heat exchanger was constructed from copper tubing, bent into four equal-length layers, 4.4 inches length and 0.8 inches in width. The heat exchanger was inserted over the paravertebral muscles in the dorsal region of the animal by a subcutaneous tunnel extending from T6 to T10 spinal segments. The small skin incision over the copper tubing was closed. Figure 2 outlines the placement of temperature probes and schematic of the cooling method.
FIG. 2.
Schematic of the experimental setup using the copper heat exchanger for local hypothermia. (a) The M-copper tubing unit was inserted under the skin over the paravertebral muscle extending from T6 to T10 spinal segments. (b) Inflow and outflow allowed for circulation of cold water. Temperature probes were inserted in the rectum, at the epicenter of injury at the spinal cord, and at the somatosensory cortex.
The phases of local hypothermia are shown in Figure 1B. The core temperature was maintained at 37°C±0.5°C with the help of a heating pad for 1 hour after the time of the contusion or laminectomy and prior to inducing the hypothermia. After 1 hour, local hypothermia was begun. To induce spinal cord hypothermia, cold water at 17°C±2.0°C was perfused through the heat exchanger at a rate of 129 mL/min using a peristaltic pump. The cooling period lasted 25 to 30 minutes. The temperature of the T8 region of the spinal cord was maintained at 30°C±0.5°C for 2 hours while the core temperature was maintained at 37°C±0.5°C. Following the 2 hours of spinal cord hypothermia, the heat exchanger was removed from the paravertebral muscle and the skin incision was closed permanently. The rewarming period lasted 25 to 30 minutes. Rewarming was followed by a 1-hour observation period. The temperature of the T8 region of the spinal cord and the cortex was recorded once every two minutes throughout the procedure.
Statistical analysis
The baseline period was defined as the average of Phases 1 and 5 because the core temperature and spinal cord temperatures were controlled during these periods. Paired t-tests revealed no statistical significance between Phases 1 and 5 for all experimental groups (data not shown). Therefore, these phases were combined and considered the baseline period for determining the temperature change at Phase 3.
Differences in means among groups for each phase were analyzed using a mixed-effect model analysis. This type of analysis takes into account both the fixed effects that result from differences attributed to the various treatments as well as any random effects that occur which are animal dependent. The fixed effects include differences due to the injury state (contusion or laminectomy) and the treatment status (global or local hypothermia). This particular method was used due to the multiple factors involved and the panel data measurements provided for each animal across each phase. Estimates of the difference in means among the temperature measurements were calculated with 95% confidence intervals (CIs).
All analyses were performed using STATA v 11.2 (2011; STATA Corporation, College Station, TX) and MATLAB 2011b (MathWorks, Inc., Natick, MA).
Results
Here we report the changes in temperature profiles of the core, spinal cord, and cortex following local and general hypothermia in rats with and without SCI. The raw data from these experiments showing the measured temperatures of the three sites for each group are shown in Supplementary Figures S1 and S2 (Supplementary Data are available online at www.liebertpub.com/ther). In addition, Supplementary Table S1 reports the mean temperatures and standard deviations at each phase of treatment. During both general and local hypothermia, the spinal cord during Phase 3 was decreased to approximately the same temperature, 30.3°C±0.8°C and 30.2°C±0.3°C, respectively. This illustrates the reliability of both methods for inducing precise cooling of spinal cord. During general hypothermia, the core temperature of rats was successfully reduced to 32.1°C±0.4°C. During local hypothermia, core temperature was successfully maintained at 37.3°C±0.7°C. Importantly, these data show that by using these methods, the core and spinal cord temperatures accurately recapitulated the target temperatures that were set within the experimental design.
General hypothermia resulted in larger temperature changes in injured versus uninjured group
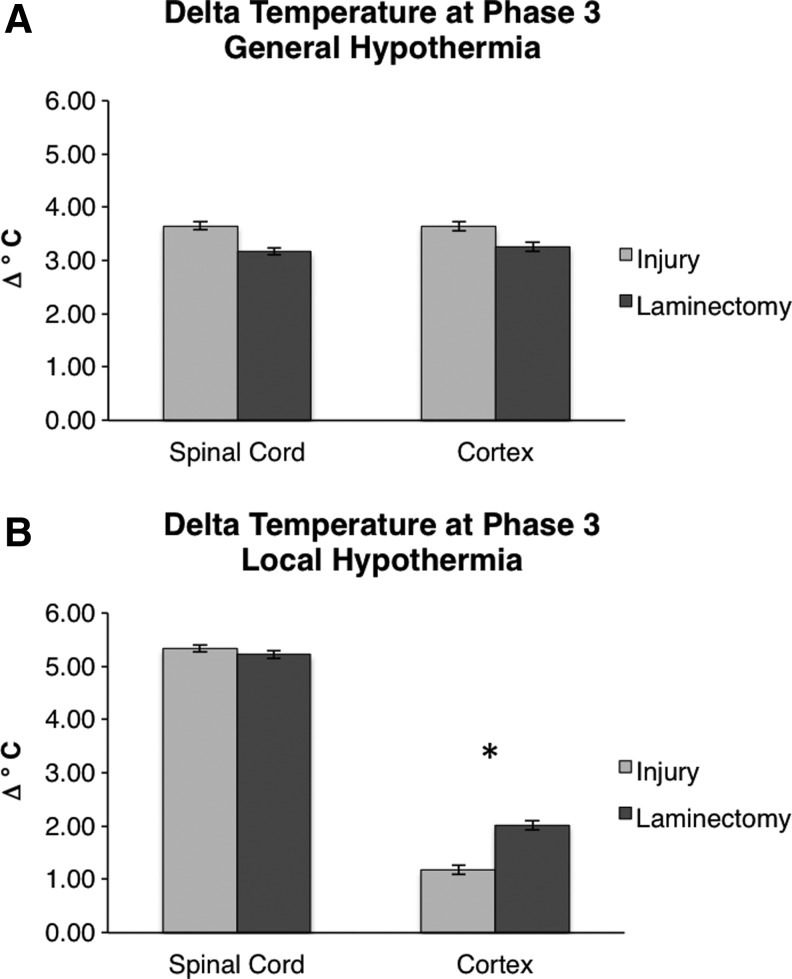
Our first question sought to answer whether the cortical and spinal cord temperatures that change during hypothermia differ between rats receiving a laminectomy (uninjured) and rats receiving a moderate contusion SCI (injury). We considered the cases of general hypothermia and local hypothermia separately for this analysis. First, the general hypothermia injured group was compared with laminectomy (control) to determine whether the effect of general hypothermia differs on injured versus uninjured subjects. During general hypothermia, core temperature was reduced to 32°C±0.5°C, which induced a constant spinal cord temperature of 30°C±0.5°C. Figure 3A shows that there were significant differences in the Phase 3 temperature decrease between injured and laminectomy groups. This difference was observed in both the spinal cord and cortex (p<0.001). At the spinal cord, the temperature of the injured group decreased by −3.65°C compared with the laminectomy group, which decreased by −3.17°C (p<0.001). Therefore, the spinal cord was cooled by an additional 0.48°C for injured group compared with the laminectomy group. Similarly, cortical temperature decreased by −3.64°C in the injured group but only −3.26°C in the laminectomy group (p<0.001). Therefore, the cortex was cooled by an average of 0.38°C more for the injured group compared with the laminectomy group.
FIG. 3.
Comparison of the changes in temperature between injury (light gray) and laminectomy (dark gray) groups. (A) During general hypothermia, both spinal cord and cortex have a larger temperature drop compared with laminectomy. (B) During local hypothermia, spinal cord temperature change is constant between groups while the laminectomy group exhibited a larger change at the cortex. The bars indicate observed mean temperatures and the error bars represent the 95% confidence intervals (CIs). *p<0.001 for an unpaired t-test between injury and laminectomy groups.
Local hypothermia resulted in smaller reduction in cortical temperature in injured versus uninjured group
Next, we analyzed the temperature reductions during local hypothermia (Phase 3) in the injured group compared with laminectomy (control). During local hypothermia, core temperature was maintained at 37°C±0.5°C while the spinal cord was maintained at 30°C±0.5°C. Figure 3B shows that the reduction in temperature at the spinal cord during local hypothermia was not significantly different between the injured group and laminectomy group (p=0.09). However, at the cortex, there was a measured significant difference between the injured and laminectomy groups. Cortical temperature was reduced by −1.18°C in the injured group and −2.02°C in the laminectomy group (p<0.001). Therefore, the cortical temperature in the injured group is less affected by local spinal cooling, resulting in the reduced temperature difference shown in Figure 3B. This effect is not observed during the general hypothermia condition most likely because the entire body is cooled, including the animal's head.
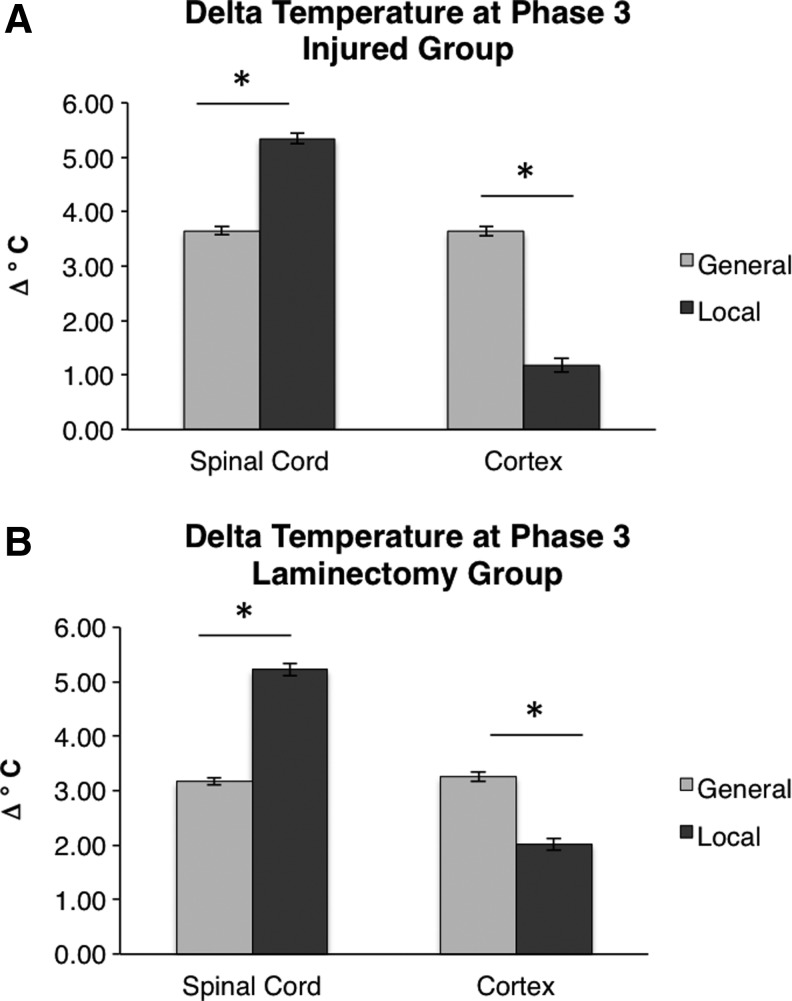
Local hypothermia induced a smaller change in cortical temperature compared with general hypothermia
The next question we sought to answer was whether local and general hypothermia induce significantly different temperatures at the spinal cord and cortex during Phase 3. Figure 4A shows the change in temperatures at the spinal cord and cortex, comparing general and local hypothermia in the injured group. During general hypothermia, the cortical temperature of the injured group was reduced by −3.64°C, whereas during local hypothermia the cortical temperature is only reduced by −1.18°C. Therefore, the cortex was cooled an average of 2.46°C more during general hypothermia compared with local hypothermia (p<0.001). Such a large reduction in cortical temperature that was exhibited during general hypothermia could have undesired effects on recovery postinjury. Figure 4B shows that the same result was found comparing local and general hypothermia in the groups receiving a laminectomy (p<0.001).
FIG. 4.
Comparison of the changes in temperature between general (light gray) and local (dark gray) hypothermia treatments. Results are shown for injury (A) and laminectomy (B) groups. Local hypothermia induced a larger temperature drop at the spinal cord, while general hypothermia induced a larger temperature drop at the cortex. The bars indicate observed mean temperatures and the error bars represent the 95% CIs. *p<0.001 for an unpaired t-test between general and local hypothermia.
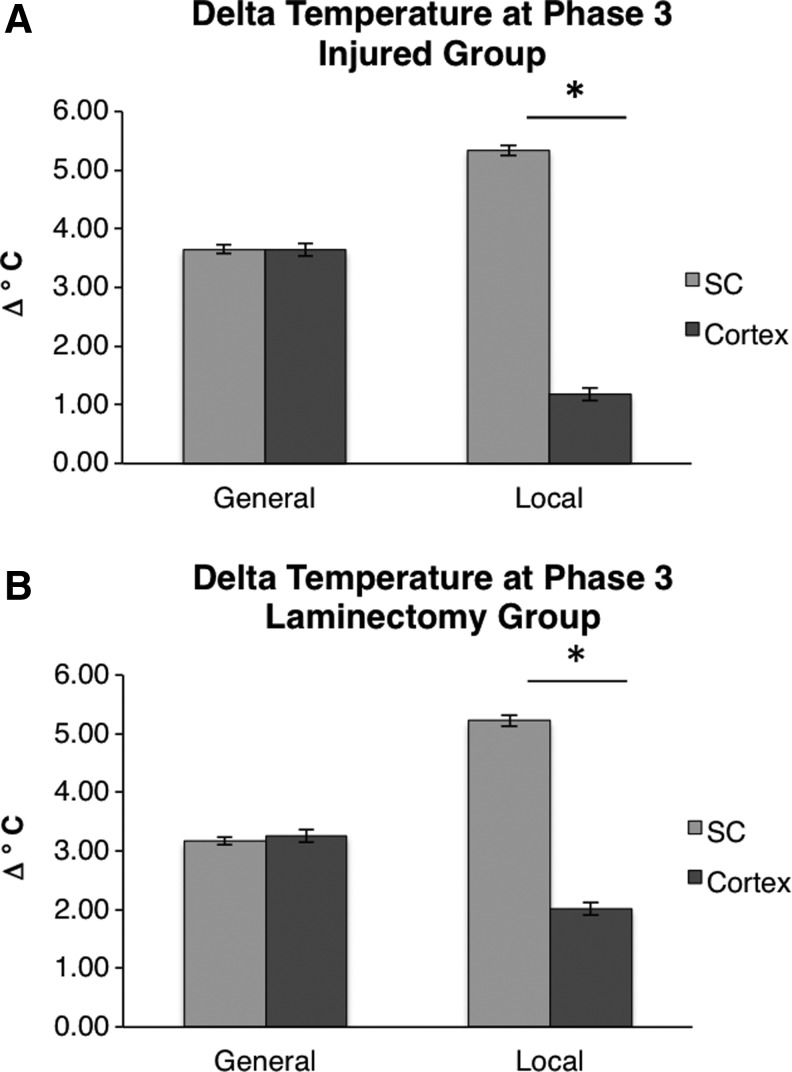
General hypothermia induces equal temperature changes in the cortex and spinal cord
Finally, we sought to evaluate the difference in temperatures between the cortex and spinal cord within the same group. Considering injured groups, Figure 5A shows that the spinal cord and cortex are cooled by the same degree during general hypothermia, whereas there is a large difference in the extent of cooling between the spinal cord and cortex during local hypothermia. During general hypothermia, the spinal cord and cortex are cooled by nearly equivalent amounts (−3.65°C and −3.64°C, respectively, not significant). In contrast, during local hypothermia, the spinal cord is cooled by −5.34°C while the cortex is only cooled by −1.18°C (p<0.001). Considering laminectomy groups, Figure 5B shows that the comparison of cooling at the spinal cord and cortex is similar. In local hypothermia, the cortex was cooled to a much lesser extent.
FIG. 5.
Comparison of the changes between the spinal cord and cortex. The spinal cord and cortex were reduced by the same amount during general hypothermia, but the spinal cord was reduced significantly more than the cortex during local hypothermia. Results are shown for injury (A) and laminectomy (B) groups. The bars indicate observed mean temperatures and the error bars represent the 95% CIs. *p<0.001 for an unpaired t-test between spinal cord and cortical temperatures.
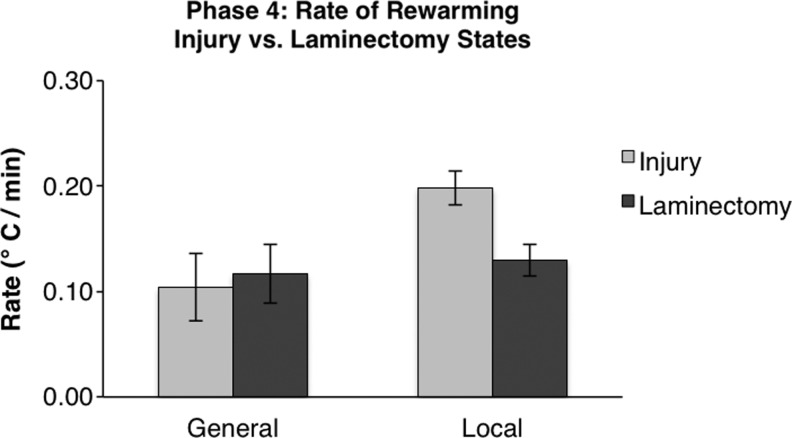
Rates of rewarming do not differ among injury status or treatment method
The final question we sought to address was whether the rate of rewarming of the spinal cord differs based on the method of hypothermia induction or injury state. A two-way ANOVA revealed no difference among the four groups (F=0.15). The rewarming rates for each group are shown in Figure 6. No statistically significant differences in rates of rewarming among groups were detected, showing that the method of hypothermia treatment nor injury affects the rewarming ability of the rat.
FIG. 6.
Rates of rewarming of the spinal cord. Rates between general and local hypothermia, as well as between injury and laminectomy groups, were compared. No comparisons were found to be significantly different. Error bars represent standard error.
Discussion
Although the complete mechanism of neuroprotective action of hypothermia is unknown, it is accepted that functional benefits of therapeutic hypothermia following SCI are due to a combination of neuroprotection, reduced inflammation, and a resulting decrease in secondary injury response. SCI induces many pathophysiological changes, and therefore the same hypothermia treatment may have a different effect on injured and healthy subjects. It is important to understand these differences prior to assessing the efficacy of the treatment.
Comparing injured with uninjured rats during general hypothermia, we first found that injured animals exhibited larger temperature drops at the spinal cord and cortex than uninjured rats, due to elevated baseline temperatures. This finding could be due to a number of mechanisms. Contusion SCI results in a cascade of secondary injury mechanisms within the CNS, including excitotoxicity, a breakdown of the blood–spinal cord barrier (BSCB), free radical production, and systemic inflammation (Hausmann, 2003). Secondary injury is not limited to the site of spinal injury; for example, the BSCB breakdown extends far away from the insult along the axis of the spinal cord (Schnell et al., 1999; Bartanusz et al., 2011). In fact, we found rats that received local hypothermia after SCI exhibited an elevated core temperature throughout all phases compared with those receiving a laminectomy. This elevated core temperature could be due to inflammation and increased blood flow as a result of the insult.
Next, we compared the cortical temperature of injured and uninjured groups during local hypothermia, and we found that cortical temperature was also higher in the injured group. This was opposite the case for general hypothermia in which injured animals had a lower cortical temperature than uninjured. This phenomenon may illustrate a greater suppression of the inflammatory response when treated with general hypothermia. As the cortex was minimally affected by local hypothermia, inflammatory processes due to injury may have allowed the cortex to remain elevated over uninjured controls. Few studies have directly compared local and general hypothermia and their effects on inflammation. A recent study by Ok et al. (2012) compared epidural hypothermia (EH) with systemic hypothermia in spinal-cord-injured rats and reported neuroprotective effects of both, but reported greater neuroprotective effects of general hypothermia based on measurements of inflammatory-related cells, including terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells and activated microglia (Ok et al., 2012). Although this study did not investigate inflammatory responses in the cortex, based on findings here, it would be interesting to determine whether there is an optimal cooling of the cortex, which could reduce inflammation without affecting neurological output.
Many studies have illustrated the benefits of hypothermia for neuroprotection after brain trauma. Moderate-to-mild general hypothermia comprises core temperatures in the range of 30–35°C, which can be induced in the clinic by whole-body cooling pads or immersion in cold water (Howes et al., 2010). A study conducted by Polderman et al. (2002) tested the effect of hypothermia treatment on 64 patients with severe head injury. Their study showed a marked decrease in intercranial pressure in 62 out of 64 patients within 4 hours of induction of hypothermia (Polderman et al., 2002). Another study by Clark et al. (2009) conducted 12 and 48 hours of general hypothermia (33°C) in adult rats 1 hour after permanent middle cerebral artery occlusion, a permanent stroke model. A significant reduction in lesion volume, behavioral, and functional impairment, such as that involving skilled reaching ability as well as enduring histological improvement, was observed in the hypothermia treatment groups (Clark et al., 2009). Numerous factors, such as the duration of hypothermia as well as the method of hypothermia employed, can determine the outcomes. Even the logistics of the hypothermia equipment are crucial for the assessment of safety and efficacy of a hypothermia procedure (Poli et al., 2013). Nevertheless, the effect of hypothermia on an otherwise healthy cortex if the hypothermia treatment is targeting the spinal cord remains to be elucidated.
Although general hypothermia may better suppress the inflammatory response, and has shown benefits for treatment of brain traumas and SCI, the associated hypothermic effect in other healthy tissues may cause complications (Swain, 1988; Hildebrand et al., 2004). These include vasoconstriction, which may cause kidney dysfunction and lead to problems with fluid management; bradycardia possibly leading reduction in cardiac output and even cardiac fibrillation; immunosuppression; mild coagulopathy and platelet dysfunction causing risk of heart attack and stroke; and thermoregulatory defense triggering shivering (Choi et al., 2012). General hypothermia can also induce the phenomenon of cutaneous vasodilation, known as the “hunting effect,” in which reduced contractility of arterioles at temperatures below 8°C causes a reversal of the vasoconstrictive response (Daanen et al., 1997). Such hematological changes can impair many other organs. Neural effects include loss of consciousness, a reduction in cerebral blood flow of about 6–7% per °C, and a loss of cerebrovascular autoregulation at about 25°C (Mallet, 2002). An additional complication that often occurs in trauma patients and the elderly, known as accidental hypothermia, is characterized by an unintentional fall in core temperature to <35°C (Mallet, 2002). The crucial core temperature for trauma patients is 34°C, below which mortality significantly increases (Jurkovich et al., 1987). At <32°C hypothermia in trauma patients, mortality is near 100%. This is highly applicable to SCI patients, as many such patients are likely to be trauma victims, especially due to motorcar accidents. In such cases, general hypothermia may not be a viable option, opening the door for local hypothermia treatment.
Local hypothermia, which focally cools the spinal cord, circumvents these major systemic side effects by maintaining the core temperature within an acceptable range while reducing spinal temperature to a therapeutic level. Therefore, we have developed a clinically relevant local hypothermia method that provides similar or even greater therapeutic benefits while preventing undesired cooling of the cortex or further damage to the injury or the surrounding vasculatures, peripheral nerves, muscles, and dura mater. Our semi-invasive method to induce local hypothermia of the spinal cord (Bazley et al., 2013) is similar to other local cooling methods like EH. EH is highly effective for locally cooling the spinal cord and involves circulating cold water through the epidural space using an epidural catheter and perfusion pump. Studies have shown that EH provides benefits similar to general hypothermia (Ha and Kim, 2008; Ok et al., 2012). Our semi-invasive method offers an alternative to EH whereby the dura is left intact and thus it offers a good trade-off between invasiveness and selective cooling.
Our previously published study (Maybhate et al., 2012) showed that treatment with 2-hour general hypothermia following acute moderate contusive SCI in rats led to improvements in SSEP amplitudes postinjury as well as functional recovery of motor behavior. The SSEP amplitude can be used to evaluate the number of intact, functional pathways between the periphery and cortex following an SCI. These increases in SSEP amplitudes were associated with beneficial outcomes, including an increase in motor behavior, measured by BBB score, and an increase in tissue sparing in the spinal cord. However, these same rats also exhibited increased N1-latency of SSEPs, or the time between sensory stimulus and the peak of the SSEP response. Other studies have also reported increased latencies in uninjured rats due to hypothermia treatment (Li et al., 2012; Madhok et al., 2012). One possibility is that the decrease in cortical temperature that was caused during the administration of general hypothermia induced long-term physiological changes in CNS conductivity. To the best of our knowledge, the temperature effect of hypothermia on the cortex, when used to treat SCI, has not been reported. In this study, we found that under general hypothermia, the cortex is cooled by 4°C. Because the cortex originates at a temperature of 34.3°C, it ultimately reaches a temperature of 30.3°C during treatment, even while the core temperature is maintained at 32°C. Thus, one cannot assume that the core temperature accurately reflects the temperature of the cortex. More importantly, such a low cortical temperature, which is intended to preserve spinal cord function, could result in adverse effects at the cortex. Poli et al. reported the effects on cortical temperature due to a head-neck cooling device in 11 patients for treatment of severe ischemic or hemorrhagic stroke. Although the treatment only minimally affected cortical temperature (−0.36°C after 49 minutes), patient side-effects included severe hypertension, intracranial pressure crisis (>20 mmHg), and decreased cerebral perfusion pressure (Poli et al., 2013). It is therefore imperative that the effects of general hypothermia on the cortex are well understood, even when used to treat SCI, as the treatment will inevitably induce temperature changes in other regions of the CNS.
Local hypothermia also induced a significant change in cortical temperature during treatment, although it was far reduced compared with general hypothermia. This comparison is critical because both treatment methods could be applied in the clinical setting for patients with traumatic brain injury or SCI, but their complications and side effects on the body in general, especially the cortex, have not been agreed upon universally by researchers as they are not well studied. The temperature at the cortex during local hypothermia was only reduced to 34.4°C, representing a drop of about 1°C from baseline. Although the effects on the cortex were limited, the temperature drop during local hypothermia was still significant, even while core temperature was maintained at 37°C. A possible explanation of this effect could be due to cooling of circulating cerebrospinal fluid (CSF). The CSF provides a medium of continuity between the brain and the spinal cord. Cooling of the spinal cord leads to the cooling of the CSF, parenchyma, and dura mater surrounding the spinal cord, which could in turn contribute to the decrease in cortical temperature. Therefore, given the significant differences in cortical temperature that are induced by the two methods of hypothermia, a complete study of electrophysiological outcomes comparing general and local hypothermia warrants future investigation.
Lastly, the rate of rewarming is now understood by clinicians to be a critical factor in the application of hypothermia treatment in patients, as recovery is sensitive to a steady and slow rate of rewarming. We found that there were no differences in the rate of rewarming of the spinal cord between the two methods of hypothermia treatment. These results suggest that the methods utilized for rewarming are highly efficient and that the body's thermoregulatory system to these methods is not affected by the cooling techniques employed. It is important for future studies to carefully consider the rate of rewarming, especially for longer durations of hypothermia, and to verify the rates are consistent with this report and do not cause impairment of thermoregulation posthypothermia.
Conclusions
In this study, we provide the temperature profiles of the core, spinal cord, and cortex during five phases of hypothermia treatment. First, we compared the temperature differences between injured and un-uninjured rats and found that injured animals were associated with elevated spinal cord and cortical baseline temperatures compared with laminectomy groups. Second, we found that the temperature of the cortex is significantly affected by both general and local hypothermia, but that local hypothermia significantly reduces the amount of cortical cooling compared with general hypothermia. Core and spinal cord temperatures do not necessarily reflect cortical temperature during therapeutic hypothermia. Finally, local hypothermia induced smaller changes in temperature of the cortex compared with general hypothermia while core temperature was maintained at 37°C throughout. In conclusion, these results strongly suggest that effects on cortical temperature should be carefully considered during administration of any method of hypothermia, even local hypothermia, and further studies are necessary to assess the efficacy of the treatment or whether there are any adverse effects to the cortex due to treatment. Nevertheless, for future studies that seek to increase the duration of hypothermia treatment, local hypothermia may provide the best option for reducing temperature effects throughout the body and minimizing side effects.
Supplementary Material
Acknowledgments
This project was supported by 2007-MSCRFII-0159-00, 2009-MSCRFII-0091-00, and 2013-MSCRFII-0109-00 from the Maryland Stem Cell Research Fund (PI: A.H.A.); R-175-000-121-133, R-175-000-121-733, R-175-000-122-112, and R-711-201-026-133 from National University of Singapore (PI: A.H.A.); and R21 HD057487 from National Institutes of Health (PI: C.L.K.). The authors thank Dr. Carol Thompson of the Johns Hopkins Biostatistics Center; Ms. Natasha Gupta of University of Texas-Dallas; Ms. Catherine Chia of RWTH AACHEN University, Germany; and Ms. Ashwati Vipin of National University of Singapore for help with the statistical analyses and article preparation.
Author Disclosure Statement
The authors declare no conflicts of interest.
References
- Agrawal G, Kerr C, Thakor NV, All AH. Characterization of graded multicenter animal spinal cord injury study contusion spinal cord injury using somatosensory-evoked potentials. Spine 2010a;35:1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal G, Sherman D, Maybhate A, Gorelik M, Kerr DA, Thakor NV, All AH. Slope analysis of somatosensory evoked potentials in spinal cord injury for detecting contusion injury and focal demyelination. J Clin Neurosci 2010b;17:1159–1164 [DOI] [PubMed] [Google Scholar]
- Agrawal G, Sherman D, Thakor N, All A. A novel shape analysis technique for somatosensory evoked potentials. Conf Proc IEEE Eng Med Biol Soc 2008;2008:4688–4691 [DOI] [PubMed] [Google Scholar]
- Agrawal G, Thakor NV, All AH. Evoked potential versus behavior to detect minor insult to the spinal cord in a rat model. J Clin Neurosci 2009;16:1052–1055 [DOI] [PubMed] [Google Scholar]
- Al-Nashash H, Fatoo NA, Mirza NN, Ahmed RI, Agrawal G, Thakor NV. Spinal cord injury detection and monitoring using spectral coherence. IEEE Trans Biomed Eng 2009;56:1971–1979 [DOI] [PubMed] [Google Scholar]
- All AH, Agrawal G, Walczak P, Maybhate A, Bulte JWM, Kerr DA. Evoked potential and behavioral outcomes for experimental autoimmune encephalomyelitis in Lewis rats. Neurol Sci 2010;31:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- All AH, Bazley FA, Gupta S, Pashai N, Hu C, Pourmorteza A, Kerr C. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One 2012;7:e47645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All AH, Walczak P, Agrawal G, Gorelik M, Lee C, Thakor NV, Bulte JWM, Kerr DA. Effect of MOG sensitization on somatosensory evoked potential in Lewis rats. J Neurol Sci 2009;284:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J. Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med 2007;35:1041–1047 [DOI] [PubMed] [Google Scholar]
- Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood–spinal cord barrier: morphology and clinical implications. Ann Neurol 2011;70:194–206 [DOI] [PubMed] [Google Scholar]
- Bazley FA, All A, Thakor NV, Maybhate A. Plasticity associated changes in cortical somatosensory evoked potentials following spinal cord injury in rats. Conf Proc IEEE Eng Med Biol Soc 2011;2011:2005–2008 [DOI] [PubMed] [Google Scholar]
- Bazley FA, Hu C, Maybhate A, Pourmorteza A, Pashai N, Thakor NV, Kerr CL, All AH. Electrophysiological evaluation of sensory and motor pathways after incomplete unilateral spinal cord contusion. J Neurosurg Spine 2012a;16:414–423 [DOI] [PubMed] [Google Scholar]
- Bazley FA, Pashai N, Kerr C, Thakor N, All AH. A simple and effective semi-invasive method for inducing local hypothermia in rat spinal cord. Conf Proc IEEE Eng Med Biol Soc 2013;2013:1445–1449 [DOI] [PubMed] [Google Scholar]
- Bazley FA, Pourmorteza A, Gupta S, Pashai N, Kerr C, All AH. DTI for assessing axonal integrity after contusive spinal cord injury and transplantation of oligodendrocyte progenitor cells. Conf Proc IEEE Eng Med Biol Soc 2012b;2012:82–85 [DOI] [PubMed] [Google Scholar]
- Beddingfield E, Clark AP. Therapeutic hypothermia after cardiac arrest: improving adherence to national guidelines. Clin Nurse Spec 2012;26:12–18 [DOI] [PubMed] [Google Scholar]
- Bouwes A, Binnekade JM, Kuiper MA, Bosch FH, Zandstra DF, Toornvliet AC, Biemond HS, Kors BM, Koelman JH, Verbeek MM. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 2012;71:206–212 [DOI] [PubMed] [Google Scholar]
- Chang M, Marshall J. Therapeutic hypothermia for acute air embolic stroke. West J Emerg Med 2012;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HA, Badjatia N, Mayer SA. Hypothermia for acute brain injury—mechanisms and practical aspects. Nat Rev Neurol 2012;8:214–222 [DOI] [PubMed] [Google Scholar]
- Clark DL, Penner M, Wowk S, Orellana-Jordan I, Colbourne F. Treatments (12 and 48 h) with systemic and brain-selective hypothermia techniques after permanent focal cerebral ischemia in rat. Exp Neurol 2009;220:391–399 [DOI] [PubMed] [Google Scholar]
- Daanen H, Van de Linde F, Romet T, Ducharme M. The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol 1997;76:538–543 [DOI] [PubMed] [Google Scholar]
- Dididze M, Green B, Dietrich WD, Vanni S, Wang M, Levi A. Systemic hypothermia in acute cervical spinal cord injury: a case-controlled study. Spinal Cord 2013;51:395–400 [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Therapeutic hypothermia for acute severe spinal cord injury: ready to start large clinical trials? Crit Care Med 2012;40:691. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Atkins CM, Bramlett HM. Protection in animal models of brain and spinal cord injury with mild to moderate hypothermia. J Neurotrauma 2009;26:301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WD, Levi AD, Wang M, Green BA. Hypothermic treatment for acute spinal cord injury. Neurotherapeutics 2011;8:229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groysman LI, Emanuel BA, Kim-Tenser MA, Sung GY, Mack WJ. Therapeutic hypothermia in acute ischemic stroke. Neurosurg Focus 2011;30:E17. [DOI] [PubMed] [Google Scholar]
- Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine 2008;33:2059. [DOI] [PubMed] [Google Scholar]
- Hausmann O. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003;41:369–378 [DOI] [PubMed] [Google Scholar]
- Hildebrand F, Giannoudis PV, van Griensven M, Chawda M, Pape H-C. Pathophysiologic changes and effects of hypothermia on outcome in elective surgery and trauma patients. Am J Surg 2004;187:363–371 [DOI] [PubMed] [Google Scholar]
- Howes D, Ohley W, Dorian P, Klock C, Freedman R, Schock R, Krizanac D, Holzer M. Rapid induction of therapeutic hypothermia using convective-immersion surface cooling: safety, efficacy and outcomes. Resuscitation 2010;81:388–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Maybhate A, Presacco A, All AH. Multi-limb acquisition of motor evoked potentials and its application in spinal cord injury. J Neurosci Methods 2010;193:210–216 [DOI] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DF, Thakor NV, Geocadin RG. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation 2008;76:431–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkovich GJ, Greiser WB, Luterman A, Curreri PW. Hypothermia in trauma victims: an ominous predictor of survival. J Trauma Acute Care Surg 1987;27:1019–1024 [PubMed] [Google Scholar]
- Kawamura N, Schmeichel AM, Wang Y, Schmelzer JD, Low PA. Multiple effects of hypothermia on inflammatory response following ischemia–reperfusion injury in experimental ischemic neuropathy. Exp Neurol 2006;202:487–496 [DOI] [PubMed] [Google Scholar]
- Polderman KH, Tjong Tjin Joe R, Peerdeman SM, Vandertop WP, Girbes AR. Effects of therapeutic hypothermia on intracranial pressure and outcome in patients with severe head injury. Intensive Care Med 2002;28:1563–1573 [DOI] [PubMed] [Google Scholar]
- Kerr CL, Letzen BS, Hill CM, Agrawal G, Thakor NV, Sterneckert JL, Gearhart JD, All AH. Efficient differentiation of human embryonic stem cells into oligodendrocyte progenitors for application in a rat contusion model of spinal cord injury. Int J Neurosci 2010;120:305–313 [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Pamplona F. Application of mild therapeutic hypothermia on stroke: a systematic review and meta-analysis. Stroke Res Treat 2012;2012:295906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi AD, Casella G, Green BA, Dietrich WD, Vanni S, Jagid J, Wang MY. Clinical outcomes using modest intravascular hypothermia after acute cervical spinal cord injury. Neurosurgery 2010;66:670. [DOI] [PubMed] [Google Scholar]
- Li N, Tian L, Wu W, Lu H, Zhou Y, Zhang L, Xu X, Zhang X, Cheng H. Regional hypothermia inhibits spinal cord somatosensory-evoked potentials without neural damages in uninjured rats. J Neurotrauma 2013;30:1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhok J, Maybhate A, Xiong W, Koenig MA, Geocadin RG, Jia X, Thakor NV. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: prognostication of functional outcomes. Crit Care Med 2010;38:1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhok J, Wu D, Xiong W, Geocadin RG, Jia X. Hypothermia amplifies somatosensory-evoked potentials in uninjured rats. J Neurosurg Anesthesiol 2012;24:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet M. Pathophysiology of accidental hypothermia. QJM 2002;95:775–785 [DOI] [PubMed] [Google Scholar]
- Maybhate A, Hu C, Bazley FA, Yu Q, Thakor NV, Kerr CL, All AH. Potential long-term benefits of acute hypothermia after spinal cord injury: assessments with somatosensory-evoked potentials. Crit Care Med 2012;40:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ok J-H, Kim Y-H, Ha K-Y. Neuroprotective effects of hypothermia after spinal cord injury in rats: comparative study between epidural hypothermia and systemic hypothermia. Spine 2012;37:E1551–E1559 [DOI] [PubMed] [Google Scholar]
- Poli S, Purrucker J, Priglinger M, Diedler J, Sykora M, Popp E, Steiner T, Veltkamp R, Bösel J, Rupp A. Induction of cooling with a passive head and neck cooling device effects on brain temperature after stroke. Stroke 2013;44:708–713 [DOI] [PubMed] [Google Scholar]
- Poole S, Stephenson J. Core temperature: some shortcomings of rectal temperature measurements. Physiol Behav 1977;18:203–205 [DOI] [PubMed] [Google Scholar]
- Reuler JB. Hypothermia: pathophysiology, clinical settings, and management. Ann Intern Med 1978;89:519–527 [DOI] [PubMed] [Google Scholar]
- Saito T, Saito S, Yamamoto H, Tsuchida M. Neuroprotection following mild hypothermia after spinal cord ischemia in rats. J Vasc Surg 2013;57:173–181 [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci 1999;11:3648–3658 [DOI] [PubMed] [Google Scholar]
- Shin HC, Tong S, Yamashita S, Jia X, Geocadin G, Thakor V. Quantitative EEG and effect of hypothermia on brain recovery after cardiac arrest. IEEE Trans Biomed Eng 2006;53:1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silasi G, Klahr AC, Hackett MJ, Auriat AM, Nichol H, Colbourne F. Prolonged therapeutic hypothermia does not adversely impact neuroplasticity after global ischemia in rats. J Cereb Blood Flow Metab 2012;32:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JA. Hypothermia and blood pH: a review. Arch Intern Med 1988;148:1643. [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab 2012;32:1939–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.